













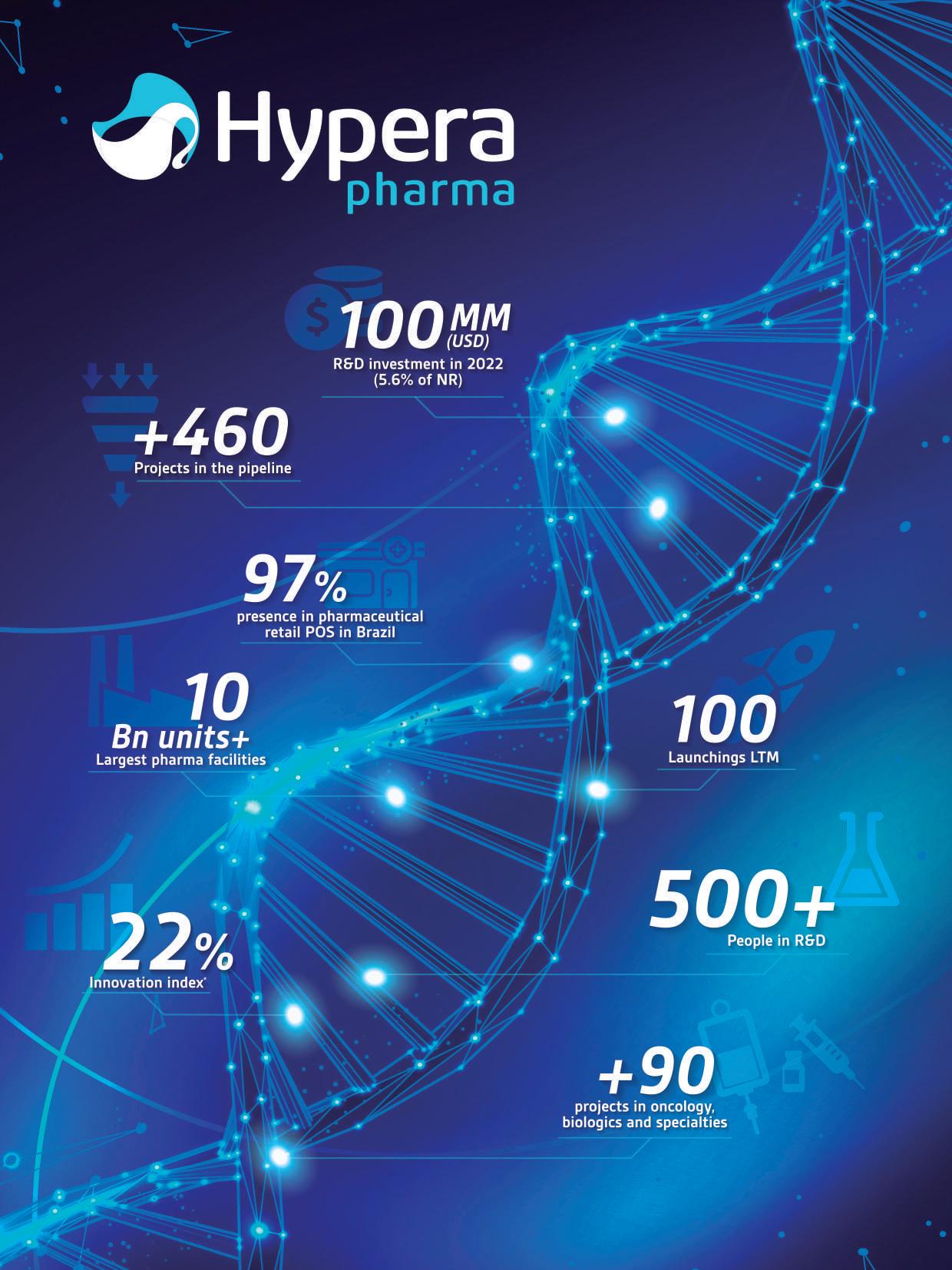
7 bioBr
Promoted by
Organized by
The Brazilian Pharma & Health Project is an initiative coordinated by the Brazilian Industry Association of Active Pharmaceutical Ingredient (Abiquifi) with the support of the Brazilian Trade and Investment Promotion Agency (ApexBrasil), that aims to expand the participation of companies from Brazilian pharmaceutical and APIs productive chain into the international scenario, seeking to increase exports, technology transfer, investment attraction and the internationalization of the sector.

It promotes actions in business intelligence, international marketing, prospective missions, international fairs, business rounds, and trade missions directed to different target markets, including countries from all five continents.

https://abiquifi.org.br/projeto-setorial/ jessica.cerqueira@abiquifi.org.br
+55 (11) 2638-4487
8 bioBr


Abiquifi (Brazilian Industry Association of Active Pharmaceutical Ingredient) was founded in 1983 to represent and defend the pharmaceutical and API industry. At the beginning of 2010, it also represented companies engaged in the production of pharmaceutical adjuvants, non-active pharmaceutical inputs, and service providers in the sector.
The main objective of Abiquifi is to stimulate production, technological development, and exports of active pharmaceutical ingredients manufactured in Brazil.

Given the nature of the provider of the drug segment, the entity brings together the various links in this chain, promoting internationalization, research and development actions, and working with the sanitary control and promotion agencies.

www.abiquifi.org.br
abiquifi@abiquifi.org.br
+55 (11) 2638-4487
The Brazilian Trade and Investment Promotion Agency (ApexBrasil) works to promote Brazilian products and services abroad and attract foreign investments to strategic sectors of the Brazilian economy. In order to achieve its goals, ApexBrasil carries out several trade promotion initiatives aimed at promoting Brazilian products and services abroad, such as prospective and trade missions, business rounds, support to the participation of Brazilian companies in major international fairs, visits of foreign buyers and opinion makers to learn about the Brazilian productive structure, among other business platforms that also aim at strengthening the Brazil brand.
www.apexbrasil.com.br
apexbrasil@apexbrasil.com.br
+55 (61) 3426-0202
10 bioBr


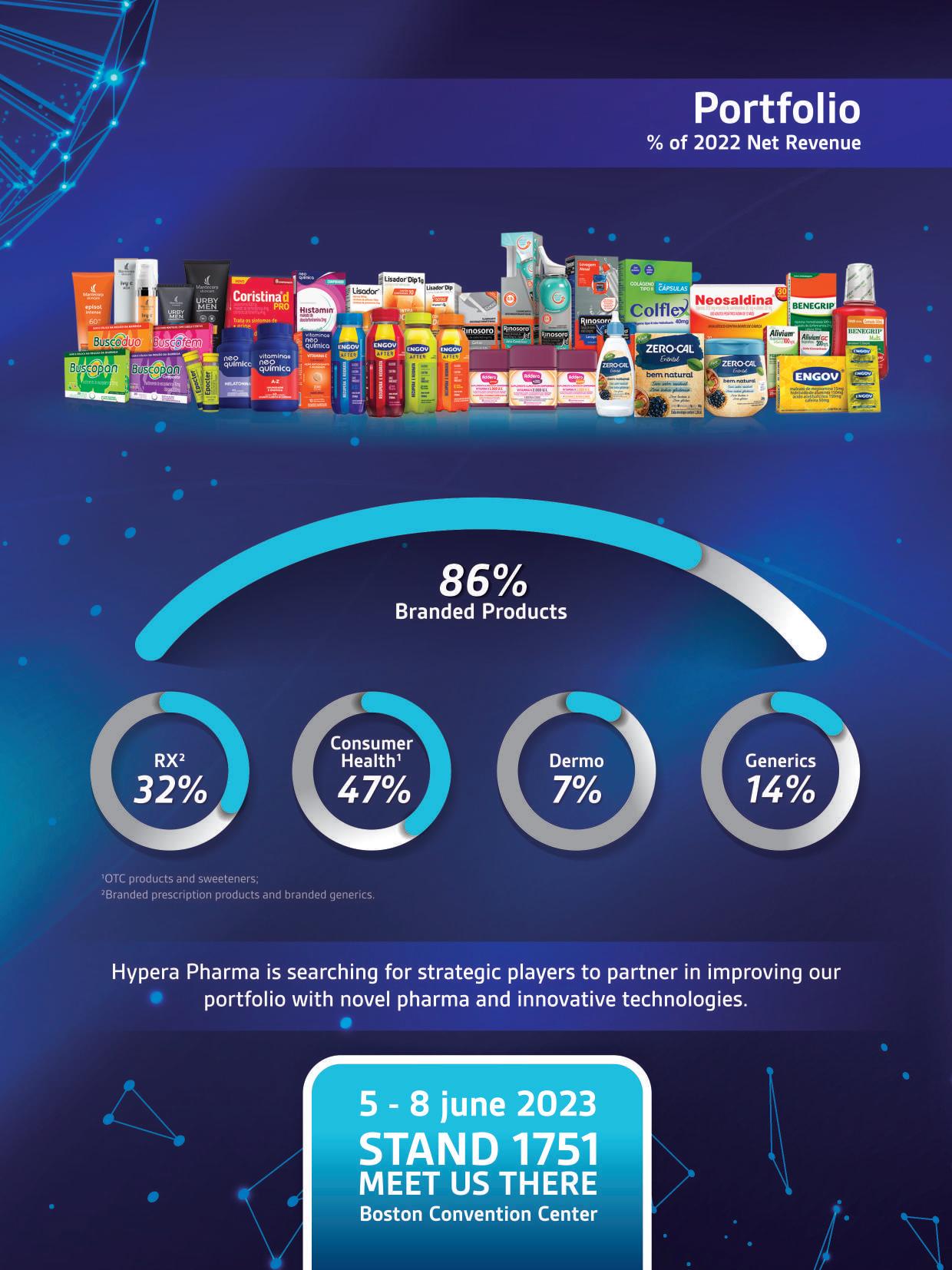
Gold partners







Silver partners














Exhibitors

Brazilian Delegation

12 bioBr
















13 bioBr








SUMMARY
20
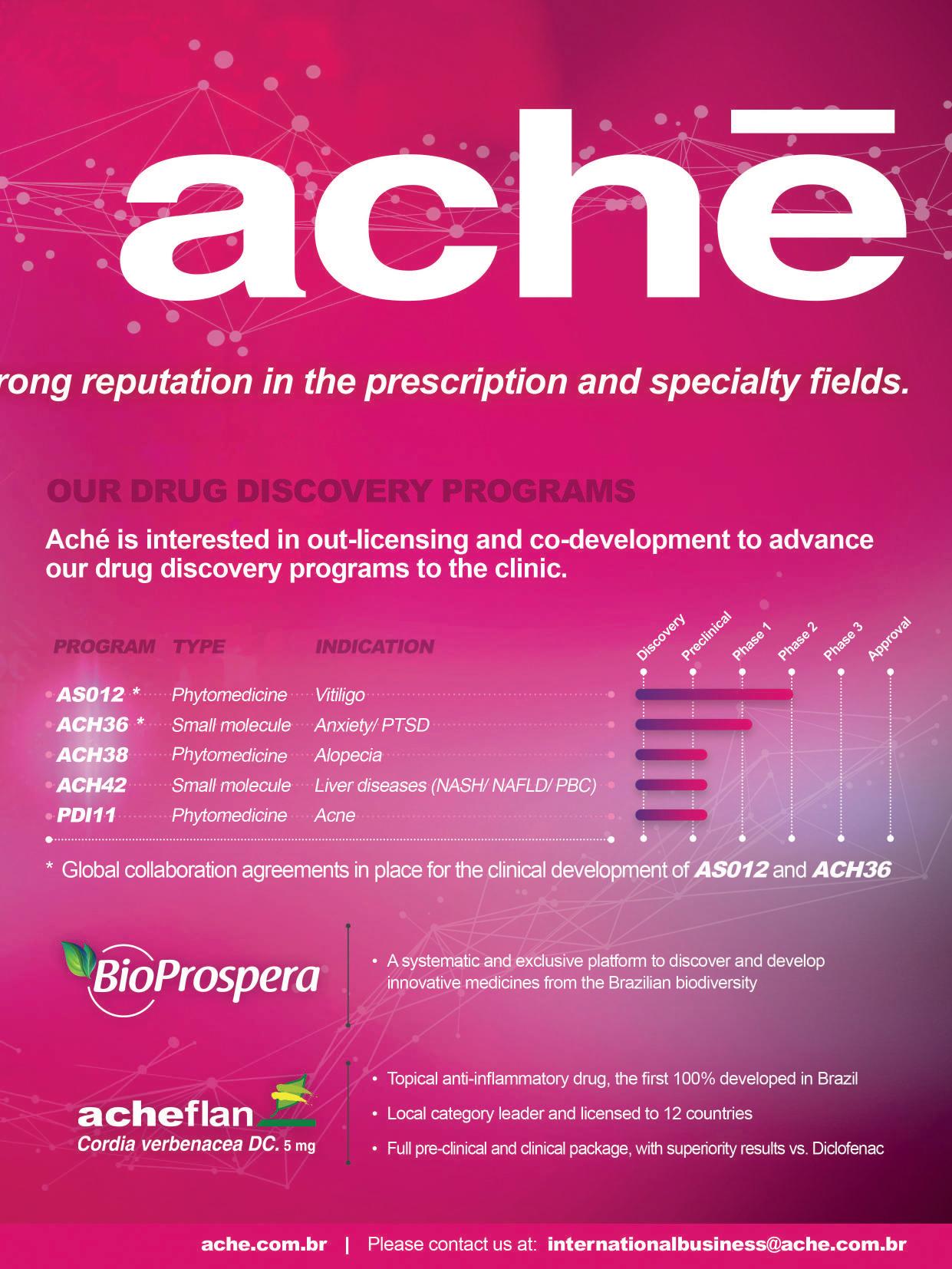
HEALTH INNOVATION
From Lab to Market: Programs
Bridging the Gap for Radical Innovation Development in Brazil
44 62 54 70 72 76
INCENTIVE
Navigating Brazil’s Innovation Ecosystem and Public Policies for Growth

MANAGEMENT
ESG: Advancing Brazil’s Health Sector and Socioeconomic Development
DEVELOPMENT
The Intersection of Health and Industrial Development: Strengthening Brazil’s Unified Health System and Competitiveness

INNOVATIVE SCIENTIFIC INSTITUTION
New Butantan vaccines promise to impact global public health
HEALTH TECHNOLOGIES
The Brazilian Ecosystem of Biotechnology Innovation

PRODUCTIVE CAPACITY
The role of local vaccine manufacturers to overcome health emergencies
SCIENTIFIC KNOWLEDGE
Shaping the Future: A Closer
Look at Brazil’s Science and Technology Capabilities and
BIO Br Project Management by: Reporter: BIOMINAS Brasil / Planning, Control and Operation: Carolina Sellani, Jéssica Cerqueira and Gabriel Keniti
18 bioBr
/ Contact us at: abiquifi@abiquifi.org.br / Visit our
Art Editor: Daniel Guedes
site: bph.org.br 28


From Lab to Market:


Programs Bridging the Gap for Radical Innovation Development in Brazil

To encourage collaboration between the various actors in the biotechnology sector in Brazil, the government has implemented a series of mechanisms to encourage health innovation, which are fundamental for the growth of this market. The Ministry of Science, Technology and Innovation (MCTI) created the Brasil-Biotec initiative whose purpose is to create an environment to bring academia and companies closer together to intensify innovation processes, and consequently, strengthen government actions to accelerate the development of new technologies and products arising from investment in research in the areas of biotechnology. In
addition, to provide greater regional inte gration in Latin America, the Latin Ameri can Biotechnology Center (CABBIO) aims to consolidate cooperation ties between Brazil, Argentina, and Uruguay. These ini tiatives reinforce the importance of inno vation and the need for cooperation be tween different actors to ensure that highly complex medicines, such as biotechnolog ical medicines, are increasingly accessible.
Marcia C. Barbosa, Secretary
of
Policies and Strategic Programs of the MCTI, says that the public policies developed by the MCTI are of a fiscal, financial, and commer cial nature, as well as to foster the formation of qualified human capital and stimulate an entrepreneurial culture in science and

20 bioBr HEALTH INNOVATION
learn from the challenges faced in the early stages of innovation development. The project also involves national pharmaceutical companies, sharing their experiences




 Norberto Prestes, the President of Abiquifi
Norberto Prestes, the President of Abiquifi
HEALTH INNOVATION

and needs to complement the ecosystem’s vision and suggest adjustments to leverage local investment in radical innovation. According to Norberto Prestes, the President of Abiquifi, “the idea is to align everyone’s goals and remove bottlenecks that impede startups’ development in Brazil.” The purpose is to establish a promising ecosystem of startups capable of interacting with companies interested in innovation, attracting new investors, reducing the time and costs of importing and exporting research materials, and investing in projects that serve the Unified Health System (SUS). The project is expected to result in new guidelines and the alignment of interests between ongoing developments and market offerings.

The strengthening of the innovation ecosystem, supported by companies, universities, the government, and regulatory bodies, directly contributes to increasing the global competitiveness of the Brazilian pharmaceutical industry, affirms Uilberson Carlos da Silva, RD&I Director of Blau Farmacêutica. In agreement, Rogerio Almeida, R&DI Vice President at Cristália, declares that Brazil has made significant progress in training professionals and modernizing its industrial park in recent years. In that regard, the know-how accumulated allows us to seek more daring innovation challenges. Given this, several initiatives have enabled new startups to emerge more structured, as is the case of the incubator Eretz.bio and the Senai Institute of Innovation in Biotechnology, which provide training and enable the creation of research and development processes in Brazil. For Silva (Blau), These programs help startups grasp the language of the market and the importance of conducting structured innovative projects, such as drug discovery, in a collaborative and market-driven manner, increasing the attractiveness of private companies to collaborate
in that ecosystem. That said, It is relevant to highlight the need for greater approximation between academia, research centers, startups, and industry in the health area. According to Almeida (Cristália), although there are development initiatives, the connection between those involved in this field can be amplified, which can improve the success of discoveries and the availability of medicines resulting from the process. At this point, he also emphasizes the role of Abiquifi, representing the private sector, as a vital agent in the articulation between the relevant actors in the pharmaceutical ingredients market.

Embrapii is a mixed fundraising model (public and private) to support RD&I projects in Brazil. The access to this investment model is agile and flexible. Companies present their technological challenges to one of the 96 Embrapii Units (centers of excellence in science and technology) spread across the country, which collaborate in the development of solutions. By combining different skills, Igor Nazareth, interim president of Embrapii, states that the Em-

22 bioBr
Rogerio Almeida, R&DI Vice President at Cristália
Uilberson Carlos da Silva, RD&I Director of Blau Farmacêutica
Marcia C. Barbosa, Secretary of Policies and Strategic Programs of the MCTI


HEALTH INNOVATION

brapii Units have the ideal profile for cooperation on drug discovery initiatives because co-investment allows sharing costs and risks associated with innovation, which drives the sector forward. He additionally highlights examples of good initiatives from various Embrapii Units for the drug discovery process in Brazil, such as CQMED, from the State University of Campinas (Unicamp), the CNPEM (National Center for Research in Energy and Materials), the CIEnP (Center for Innovation and Pre-Clinical Trials) and CEINFAR, Embrapii Unit at the University of São Paulo (USP). Furthermore, the Brazilian Development Bank (BNDES) also provides resources for Embrapii through a partnership with FUNTEC, the bank’s instrument for strategic innovation projects with non-reimbursable resources, which aims to support the performance of pre-clinical and clinical trials (phase 1 and 2) in new drugs, pharmaceuticals, biopharmaceuticals, and Active Pharmaceutical Ingredients (APIs). According to José Luis Gordon, Director of Productive Development, Innovation, and Foreign Trade at BNDES, public innovation funding initiatives must comply with public policies, and, in the case of health projects, innovations must


meet the needs of SUS. In this way, BNDES seeks to align the funding of new medicines and strategic inputs with the Ministry of Health’s strategy. For the coming years, the intention is to increase the focus on innovation and double the portfolio, together with the national innovation ecosystem and public policies for productive and industrial development, to Gordon (BNDES) investments in the health sector will be crucial to this effort. Health innovation programs have proven to be fundamental for the growth of the biotech sector in
Brazil, both in the public and private spheres. As a result, biotechnology has played an increasingly important role in the Pharmaceutical Industry, which in return, has promoted a significant increase in investments in the area. With the adoption of public policies to encourage research and development, on top of an increasingly favorable regulatory environment, Brazil offers several opportunities for companies and investors interested in collaborating with the sector. ●
24 bioBr
José Luis Gordon, Director of Productive Development, Innovation, and Foreign Trade at BNDES
Igor Nazareth, interim president of Embrapii
Biotechnology has played an increasingly important role in the Pharmaceutical Industry, which in return, has promoted A SIGNIFICANT INCREASE IN INVESTMENTS IN THE AREA





Shaping the Future:
A Closer Look at Brazil’s Science and Technology Capabilities and Infrastructure for Innovation


According to the Brazilian Legal Framework for Science, Technology, and Innovation (Law No. 13,243/2016), Science and Technology Institutions (ICTs) are public or private non-profit entities whose mission is to execute basic or applied research. Therefore, these institutions play a fundamental role in the production of scientific knowledge, in the development of new technologies, and the formation of highly qualified human resources in the most diverse segments. In addition, they are also responsible for the production of goods and services with high value, thus contributing to the country’s economic development.

Although Brazilian federal universities
are considered one of the main scientific institutions in the country, there are other organizations that promote science, technology, innovation, and entrepreneurship through planned activities, such as SENAI Institute of Innovation in Biotechnology (SENAI Biotech) and Supera Parque. SENAI Biotech was founded by the National Service for Industrial Learning (SENAI) to promote biotechnology innovation and competitiveness. The institute provides technology services and innovative solutions to businesses and industries, with an emphasis on health, food, the environment, and energy. They are responsible for conducting research, developing innovations, providing consulting and training for human resource certification in the field of biotechnology. On the other hand, Supera Parque emerged from a partnership between the
SCIENTIFIC KNOWLEDGE 28 bioBr



29 bioBr
Butatan Institute possesses an Industrial Park, a complex of laboratories, and animal facilities that allow research in several areas, such as biotechnology, immunology, and biochemistry

University of São Paulo (USP), the City Hall of Ribeirão Preto, and the State Secretariat for Economic Development, Science, Technology and Innovation to promote entrepreneurship, innovation, and science. Supera Parque is an important player in health and biotechnology in the country, housing companies, and research institutions that work with drug development, diagnosis, and plant biotechnology. In addition to them, several ICTs and Brazilian universities are developing innovation programs to stimulate the development of new services and products that can reach the market.
According to information from the Ministry of Science, Technology, and Innovation (MCTI), Brazil reached the mark of more than 300 ICTs, highlighting the importance attributed to scientific research and technology in Brazil’s growth. Although each of these institutions presents its specialty, they all play a fundamental role in promoting science and technology in the country, with different objectives and areas of activity. In this context, this article will address three of the main institutions with ICTs in the country: Butantan Institute, Oswaldo Cruz Foundation, and Sociedade Beneficente Israelita Brasileira Albert Einstein.
BUTANTAN INSTITUTE is a Science and Technology Institution (ICT) of the State of São Paulo linked to the State Health Secretariat and is one of the leading organizations responsible for immunobiologicals production in Brazil. With more than 100 years of history, it has a large installed capacity for vaccines and serum production. Butatan possesses an Industrial Park, a complex of laboratories, and animal facilities that allow research in several areas, such as biotechnology, immunology, and biochemistry. In addition, it has the Science Park formed by museums and visitation spaces. The institution’s objective is to contribute to the popularization of science in society through research, clinical and scientific development, production, and commercialization of biological products.
THE SOCIEDADE BENEFICENTE ISRAELITA
BRASILEIRA ALBERT EINSTEIN has a modern infrastructure for research and development in the area of biotechnology applied to health, covering all stages from technological development, from discovery to clinical phases of new products. For the initial stages of research, there is the Experimental Re-

30 bioBr
SCIENTIFIC KNOWLEDGE


SCIENTIFIC KNOWLEDGE
search Center, a core facility with many areas, platforms, and cutting-edge equipment. In addition, there are the Pre-Clinical and Clinical Research Centers, as well as the Academic Research Organization (ARO), which was the first institution in South America to receive accreditation from the Association for the Accreditation of Human Research Protection Programs – AAHRPP. This structure is associated with Eretz.bio, an innovation hub at Einstein that aims to assist health techs in developing their products and businesses. Eretz. bio recently launched a program devoted to supporting biotechnology startups, the Einstein Innovation in Biotechnology Program, that offers training and mentoring in addition to its close relationship with more than 10,000 clinical staff physicians who work at the hospital. In addition, it promotes partnerships with universities and industry in the sector,


intending to encourage innovation and the development of solutions to health problems.
INSTITUTE OF TECHNOLOGY

ON IMMUNOBIOLOGICALS / OSWALDO CRUZ FOUNDATION (FIOCRUZ)

is an institution of science and technology in health with several capacities installed throughout Brazil, including substantial technological parks for immunobiologicals (Bio-Manguinhos/Fiocruz) and medicines production (Farmanguinhos/Fiocruz). The institution has research laboratories in areas such as molecular biology, genomics, and bioinformatics. Additionally, it has research institutes, reference centers in infectious diseases, hospitals, museums, and public health information and documentation centers. Fiocruz, through Bio-Manguinhos, is also responsible for manufacturing most vaccines used in Brazil’s National Immunization
The Sociedade Beneficente
Israelita Brasileira
Albert Einstein has a modern infrastructure for research and development in the area of biotechnology applied to health, covering all stages from technological development, from discovery to clinical phases of new products
32 bioBr


SCIENTIFIC KNOWLEDGE
Program. With the new Health Biotechnology Industrial Complex that is being built in Santa Cruz/Rio de Janeiro, Bio-Manguinhos intends to introduce new products, and increase operations in the international market, mainly through the United Nations agencies. This Institute also has an advanced pilot plant to scale-up new products, manufacture clinical batches and offer biotechnological services to national and international partners.
With such a wide-reaching infrastructure of Science and Technology Institutions (ICTs) in the country, partnerships are opportunities to make better use of installed capacities and optimize the time-to-market of new products. Currently, the Butantan Institute is organizing itself to offer its structures to provide services to the academic community and leverage cooperation projects, carrying out an exchange of competencies to meet the external and internal needs of the institute.
To this end, it has mapped the infrastructure and skills of the Institute to identify installed capacities and presenting options for collaboration with businesses and ICTs, thereby harnessing the innovation ecosystem. Cristiano Gonçalves, Innovation, and Tech Licensing Manager at Butantan, states that this contributes to establishing more strategic partnerships, both from a scientific and industrial point of view, to promote open innovation and foster collaborative relationships. For Mauricio Zuma, Bio-Manguinhos’ CEO, beyond to strategically contributing to the country with the introduction of immunobiologicals in the public health programs through R&D and technological transfer partnerships, the institution also has an important potential to promote innovation in the country through its pilot plant, helping to fill the existing gap between development and manufacturing. Eretz.bio, on the other hand, makes its infrastructure available for startups to develop and validate new technological solutions through its preclinical and
clinical research structures and interface with the hospital’s clinical staff; it serves more than 100 national and international health techs in its ecosystem. That demonstrates that the established capacities are used efficiently and complementary, which can lead to an acceleration of the development of new biotech solutions and products applied to health.

The Butantan Institute operates from the perspective of the public policy of Partnerships for Productive Development (PDPs), which consists of purchasing mechanisms promoted by the Ministry of Health, to enable technology transfer lined up with
Fiocruz, through Bio-Manguinhos, is also responsible for manufacturing most vaccines used in Brazil’s National Immunization Program

34 bioBr


SCIENTIFIC KNOWLEDGE

the strategies of large multinational pharmaceutical companies. Furthermore the institute establishes cooperation in the form of a co-development code, which is an approach in which the institute acts in a complementary and collaborative manner to promote the joint development of technologies of public health interest, as seen in the cases of the vaccines Dengue, Chikungunya, and COVID-19 (Butanvac and Coronoavac), all of which are co-development strategies. The aim is to achieve common development goals strengthening the country in the generation of new technologies.
An example of this is the dengue vaccine, in Phase 3 of clinical development, in partnership with the National Institute of Health (NIH), a research agency of the US government, whichwhichButantan Institute’s data were licensed to Merck Sharp Dome as part of a co-development plan. According to Gonçalves (Butantan), partnerships and co-development agreements should be complementary in the perspective of the stakeholders’ contributions. Other Butantan initiatives include the CoronaVac (Phase 3) pivotal study that obtained promising results for approval of the vaccine, a key tool in the fight against COVID-19, which immunized a large part of the Brazilian population during the health crisis. Currently, the institute develops ButanVac, a fully national vaccine against Covid-19 (phase 2 study), as well as the Valneva vaccines for Chikungunya (Phase 3).
Zuma (Bio-Manguinhos) claims that the well-built capacity for clinical and industrial development has made Fiocruz and Butantan vital players in the supply of vaccines for COVID-19 in Brazil, highlighting the importance of investing in the infrastructure that already exists in the country. Furthermore, the public-private partnership strategy has been a great driver for the advancement of biotech-
nology applied to health in Brazil. According to Zuma (Bio-Manguinhos), partnerships with private companies are important to promote learning and knowledge exchange, allowing the institution to develop its own innovation processes in the future. As a result, they can leverage previous experiences and technical training to easily adapt to new technology transfer projects with new institutions. Zuma (Bio-Manguinhos) emphasizes that the collaborations established with private partners improve the institution’s quality standards since it undergoes several audits, gaining greater agility in identifying and solving problems.
Bio-Manguinhos CEO’s also mentions that the Institute’s growth in the area of molecular diagnostics is based on previous autochthonous projects. The NAT kit for HIV/ HBV/HCV and malaria, developed, in partnership with the Federal University of Rio de Janeiro (UFRJ), the Institute of Molecular Biology of Paraná (IBPM) and a German company, serves the entire Brazilian network of public blood centers, analyzing more than 3 million blood bags annually, and has stood out for its rapid ability to develop new kits for screening. With this technology, even before the WHO declared


36 bioBr
Cristiano Gonçalves, Innovation, and Tech Licensing Manager at Butantan
Mauricio Zuma, Bio-Manguinhos’ CEO






SCIENTIFIC KNOWLEDGE
the coronavirus pandemic, Bio-Manguinhos was able to develop tests for COVID-19. Recently the technology was also used to develop kits to diagnose monkeypox, among others.

The collaboration with the pharmaceutical industry is essential for creating biotech startups, as they will be the leading developers of technologies originated within programs such as those at Einstein. The Einstein Biotechnology Innovation Program also focuses on translational research, which supports the development of research that can be transformed into new human health related products through various shapes of collaboration, such as co-development of technologies, sponsorship in training programs for entrepreneurs, investment in public research notices, incubation of startups of common interest, among others. Through Eretz.bio and the Einstein Biotechnology Innovation Program, various agents (universities, startups, large companies, development agencies, chambers of commerce, investors, and researchers) come together to promote the development of a strong national industry. Dr. Rodrigo Demarch, Chief Innovation Officer at Hospital Israelita Albert Einstein, mentions that international partnerships now cover more than 23 countries, boosting the development of domestic products with global appeal, as well as recruiting cutting-edge technologies to Brazil.
Academia is essential for the continuity of initiatives in ICTs.

Gonçalves (Butantan) states that this movement arises mainly in universities through the training of mas-
ters and doctors. On the other hand, programs such as Eretz.bio, for example, are necessary for training researchers and entrepreneurs to learn about innovation and how to apply that knowledge into products and, eventually, businesses. According to Dr. Demarch (Einstein), developing skills related to entrepreneurship may be the key to success for having more researchers working on de-
velopment projects. For Gonçalves (Butantan), these professionals, in addition to having the opportunity to enter the industry, can develop academic spin-offs, in which they will have the opportunity to form strategic alliances for the business. Zuma (Bio-Manguinhos) adds that although today’s arrangement is still not ideal, it is heading towards a promising future, with opportunities and initiatives flourishing, making it necessary for public policies in science and technology, industries, and health to be outlined robustly and constantly, to train quality professionals in areas related to biotechnology. In this manner, it creates an environment conducive to the emergence of new startups with transformative technologies based on this human capital.
The installed capacities in science, technology, and innovation in Brazil are strong indicators of the country’s development in this area. The expertise of researchers, along with the creation of new infrastructure for research and development, has resulted in cutting-edge technologies and innovative products, with the potential to improve both public and private health, and consequently the quality of life of Brazil’s population. These facilities are an important resource to boost the biotechnology sector and attract new national and international investments. With the continuous evolution of these capacities, Brazil can establish itself as a center of excellence in science and technology, contributing to the improvement of the global population’s well-being and the country’s economic development. ●
40 bioBr
Dr. Rodrigo Demarch, Chief Innovation Officer at Hospital Israelita Albert Einstein
The expertise of researchers, along with the creation of new infrastructure for research and development, HAS RESULTED IN CUTTING-EDGE TECHNOLOGIES AND INNOVATIVE PRODUCTS, with the potential to improve both public and private health, and consequently the quality of life of Brazil’s population






Navigating Brazil’s Innovation Ecosystem and Public Policies for Growth

The National Innovation Policy created by the MCTI (Ministry of Science, Technology, and Innovation) in 2020 aims to convert knowledge into innovative products, processes, and services, as well as foster the training of professionals to raise the levels of innovation in the economy. Furthermore, it seeks to encourage research, development, and innovation in companies, research centers, and private non-profit entities, besides promoting the coordination and alignment of public policies related to innovation. As a result, the Brazilian innovation ecosystem is made up of a broad participation of public authorities, through the financing of universities, companies, and investment funds, in addition to institutions that promote the ecosystem, such as accelerators and incubators.
In the last ten (10) years, the number of

technology parks in Brazil has more than doubled (as Informed by the Ministry of Science, Technology, and Innovation), with a significant increase of 175%, going from 20 to 55 in this period, with the most relevant areas being information and communication technology (82%), energy (61%) and health (46%). In Latin America, Brazil attracts the highest percentage of investments in the region as it has one of the most robust ecosystems. According to Rodrigo Secioso, Innovation Superintendent at Finep (Finance Agency for Studies and Projects), Brazil stands out mainly due to its technical capacity and relevant experience within research centers and companies, which favors the country’s growth as a research and development hub.
Several ongoing initiatives aim to create technology parks specialized in biotechnology to promote interaction between research centers and companies to strengthen the sector’s innovation outcomes. Embrapii (Brazilian Company of Research and Industrial In-
44 bioBr INCENTIVE
novation) has been successful in this cooperation model, supporting 150 initiatives that had biotechnology as an enabling solution, resulting in R$ 220 million (USD 44 million) in R&D&I. According to Igor Nazareth, interim president of Embrapii, “This model of cooperation between companies and research groups is the best strategy that Brazil can adopt to strengthen the entire support network for innovation, regardless of the sector.”.

In 2023, Embrapii joined the World Bioeconomy Forum, a network of development agencies that brings together 90 nations with the goal to map urgent issues and consolidate data. Bioeconomy has been considered a key point for Brazil to stand out regionally and globally due to the country’s resources and relevant scientific production, being one of the countries with the highest number of publications on the subject. Through cooperation, all this potential can be shared through partnerships that allow the attraction of talents to Brazil. According to Naz-


45 bioBr
INCENTIVE
areth (Embrapii), while the bioeconomy is seen as an important and expanding field, one of the obstacles it faces is a lack of offthe-shelf solutions, which makes the path to innovation in the sector more challenging. As a result, Embrapii prioritizes this agenda, investing additional resources and forming partnerships to bridge the gap between market demand and technological advancement.
Marcia C. Barbosa, Secretary of Strategic Policies and Programs at the MCTI (Ministry of Science, Technology, and Innovation), claims that biodiversity is a resource that has to be used to develop new biotech technologies, a sector considered strategic for the socioeconomic development of the country. Brazil has a high number of research institutions and universities, as well as a highly qualified workforce. However, the government must continue to invest in public policies, such as tax incentives, financing programs for companies and startups, pub lic-private partnerships, and training pro grams. For the growth of the biotechnology and health sector to be accelerated, Secioso (Finep) highlights the importance of estab lishing a priority national agenda with clear long-term objectives and obtaining a social consensus on the relevance of innovation in the sector. Due to the long time required for the development of these technologies, there must also be a commitment from civ il society to guarantee a continuous flow of public and private investments therefore, state policies can be aligned with society demands. In this sense, public policies that help the development of innovation are seen as valuable and attractive tools to engage in vestors interested in the country’s potential.
The current Action Plan on Science, Technology, and Innovation for Health (PACTI SAÚDE) is the result of a collabo ration between the MCTI, the Ministry of Health, and development agencies such as CNPq (National Council for Scientific and

Technological Development) and Finep, in addition to other entities representing the sector. Its main objective is to promote science, technology, and innovation in health through basic, applied, and translational research. For the year 2023, the Ministry of Science, Technology, and Innovation (MCTI) has plans to update its public policy on R&D&I in Health, maintaining the thematic lines and strategic actions for the area, to expand the population’s access to health services, strengthen the prevention, diagnosis, and treatment of diseases, and decreasing external dependence on products and technologies in the sector. These guidelines serve as orientations for public policies to avoid overlapping actions and abrupt changes in funding lines, allowing a consistent direction of efforts among the various actors involved, such as civil society, academia, and private and public sectors.
Brazil is at a favorable moment for biotechnology in the healthcare sector since the National Fund for Scientific and Technological Development (FNDCT), the leading fund for promoting innovation in the country with non-reimbursable resources, will have R$ 9.6 BILLION (USD 1.9 BILLION) IN 2023, and this figure should grow in the coming years.

46 bioBr
Rodrigo Secioso, Innovation Superintendent at Finep (Finance Agency for Studies and Projects)


INCENTIVE
As thematic lines, the following were highlighted:
1. Pre-clinical tests, including alternative methods to animal experimentation;
2. Prevention, control, diagnosis, and treatment of emerging and re-emerging transmittable diseases;
3. Diagnosis and treatment of non-transmittable chronic diseases;
4. Science Frontier, particularly in personalized medicine and regenerative medicine, including stem cells and cell therapy;
5. Health supplies (drugs, biopharmaceuticals, immunobiological, diagnostic kits, biomaterials, equipment, and devices) and technological domain for their production;
6. Strengthening of clinical research.
The MCTI guidelines for health innovation have a significant impact on the Brazilian biotech market in terms of public policies to encourage research. Some of the Brazilian government’s initiatives in recent years have aimed to foster scientific research and innovation by bringing academia and companies closer together, developing new technologies and therapies for public health, and sharing infrastructure for scal ing production, which demonstrates the effort of the federal government to invest in biotechnology. In this scenario, according to Barbosa (MCTI), the creation of public policies to encourage research and develop ment of health inputs in Brazil strengthens the universality of the healthcare system and can contribute to the country becom ing less dependent on geopolitical changes.
Brazil is at a favorable moment for bio technology in the healthcare sector since the National Fund for Scientific and Technologi cal Development (FNDCT), the leading fund for promoting innovation in the country with


non-reimbursable resources, will have R$ 9.6 billion (USD 1.9 billion) in 2023, and this figure should grow in the coming years. In addition, the Executive Groups of the Economic-Industrial Health Complex (Ministry of Health) were restored, and they are responsible for discussing strategies to strengthen the domestic production of health inputs, with the ambitious objective of reaching 70% of it. In this way, the inter-ministerial alignment favors Brazil’s technological independence. According to Secioso (Finep), these resumptions impact the market from two main perspectives: the first is to provide the continuity of public investments in R&D&I in the long term, and the second is to demonstrate public interest in innovation, encouraging the private sector to create its own innovation structures, exhibiting greater investment security in the health sector. According to Barbosa (MCTI), the S&T&I legal framework was created precisely with the purpose of encouraging the national pharmaceutical industry to incorporate innovation as a means of growth, differentiation, and strengthening of the entire sector.
In addition to investments in biodiversity, which can be used to develop phar-
48 bioBr
Igor Nazareth, interim president of Embrapii


maceuticals, cosmetics, and food, Brazil has other possibilities for investment in crucial areas, such as biopharmaceuticals and immunobiologicals production, a growing field that Brazil already has production facilities and trained professionals. Furthermore, the development of diagnostic kits and mRNA therapies, which are legacies of the pandemic, are promising areas growing worldwide and may represent an opportunity for the Unified Health System (SUS). Finally, the personalization of medicine is a prominent theme in which Brazil still is in an early stage but has the potential to change the dynamics of the Brazilian pharmaceutical market if there is a focus on more complex diseases.
Biotechnology research advances have a significant impact on both agribusiness and the health sector. According to Nazareth (Embrapii), there are many biomass residues that are burned to generate energy that could be used to manufacture sustainable chemicals via biotechnological processes. Embrapii’s strategy to invest in research projects in bioeconomy includes a R$ 40 million (UDS 8 million) non-refundable fund available in the Basic Funding Alliance (BFA) to develop new technological routes, which include biotech. BFA contributes up to 90% of the total value of the projects, using resources from the Ministry of Science, Technology and Innovation (MCTI). To apply, private companies must join with at least two Embrapii Units and two startups. In this model, companies present their technological challenges to the Embrapii Units, which are science

and technology institutes in the country, and work in collaboration to develop solutions.

Although there are still obstacles in the sector itself, related to technological risks, and in the Brazilian context related to better integration of ecosystem actors, regulatory framework, infrastructure, and geographic concentration of resources, public policies established in recent years have started to change the scenario. The narrowing of these sectors can lead to an increase in private investment in research, development, and innovation, which, in return, will bring more resources to the health sector as a whole. Biotechnology has the potential to improve public health in Brazil in several ways. Some of the main contributions include the development of new drugs and therapies to treat a wide variety of diseases, increasingly accurate and rapid tests, and new vaccines. Furthermore, it can be used to develop technologies from biodiversity and promote the bioeconomy as a strategic sector for Brazil. Biotechnology can bring significant benefits to Brazilian society, and it is vital to create continuous measures to enhance the population’s well-being. ●

50 bioBr
INCENTIVE
Marcia C. Barbosa, Secretary of Strategic Policies and Programs at the MCTI (Ministry of Science, Technology, and Innovation)




The Intersection of Health and Industrial Development:

Strengthening Brazil’s Unified Health System and Competitiveness

Brazil has one of the world’s largest health markets, ranking sixth in the pharmaceutical sector with revenues of approximately $20 billion in 2022. Industrial policies, such as the Industrial, Technological, and Foreign Trade Policy (Pitce), recognized the importance of medicines and the Health Economic-Industrial Complex (CEIS) for the country’s development. The industrial development in Brazil can be integrated with the health sector, benefiting both the population’s well-being and the country’s economy, especially given that the Brazilian Unified Health System (SUS) serves more than 190 million people, with 80% relying solely on it for all healthcare services. Associating health as a vector of industrial and social development is essential to create an enabling environment for technological development. Due to this, Brazil was able to produce and distribute vac-
cines to fight the COVID-19 pandemic. Therefore, it is essential to continue investing in this area to support technological development and strengthen Brazil’s competitiveness.
In recent years, the presence of biotechnology businesses in Brazilian technology parks has expanded significantly, rising from 59% in 2014 to 80% in 2021. Among the major developments recognized in the field of healthcare, advanced therapies such as cell therapy, gene therapy, and tissue bioengineering, as well as nanotechnology, stand out. Regarding the industrial segments, there are macro trends relating to on-site in vitro diagnostics, prostheses and orthoses, and imaging diagnosis. According to representatives of the Ministry of Health (MS) and the Ministry of Development, Industry, Commerce, and Services (MDIC), it is fundamental to strengthen the productive chains in the health economic-industrial complex, seeking
54 bioBr
DEVELOPMENT
insertion in global value chains, and using the State’s purchasing power, increased by the high SUS (Brazilian Unified Health System) demand, to boost technological development in the sector. In this manner, Brazil may improve its competitiveness in the sector by forming strategic alliances with companies and laboratories to promote the development of solutions.
As reported by the Ministry of Health (MS), the Health Economic-Industrial Complex (CEIS) mobilizes around 10% of GDP, represents 1/3 of scientific and research effort, has strong alignment with 4.0 technologies, and is a privileged space for the generation of investment, income, and employment opportunities, representing 10% of job occupations and 25 million direct and indirect jobs. Despite having a robust industry, the health sector has an increasing trade deficit, with imports reaching a record US$ 20 billion. Given this context and the importance of the health industry, it is critical to consider the sector’s neo-industrialization process. In this regard, the Brazilian Government restored the Executive Group of the Health Economic-Industrial Complex (Geceis), which strives to boost production, innovation, and universal access to health through the coordination of a multisectoral agenda.
According to the Ministry of Health (MS), this systemic agenda helps to structure and coordinate Brazil’s industrial, science, technology, innovation, and social programs. The main objective defined by the Government is meeting 70% of SUS (Brazilian Unified Health System) needs in medicines, equipment, vaccines, and other medical materials



within ten years. Neo-industrialization has been viewed not only as a means of reducing the country’s manufacturing and technology dependence but also as a means of ensuring long-term universal access to healthcare.
According to the Ministry of Health (MS), the Health Economic-Industrial Complex (CEIS) is being strengthened to fulfill the demands of the SUS (Brazilian Unified Health System) and thereby guarantee equitable and full access to health. As a result, the formulation of public policies, the development of programs, and other incentive actions aim to support the guidelines, which include support for research, technological development, the establishment of a legal framework, incentives for local production, the use of the State’s purchasing power, investments in productive segments, and regional and global cooperation. Geceis is coordinated by the Ministry of Health, led
55 bioBr
DEVELOPMENT
by Minister Nísia Trindade Lima, and jointly coordinated by the Ministry of Development, Industry, Commerce, and Services (MDIC), led by Vice-President Geraldo Alckmin, contemplating more ministries, bringing together a total of twenty government entities in the social, economic, science and technology, promotion and regulation areas, as well as the collaborative participation of civil society.
For the Ministry of Health, Geceis is the main locus of institutional articulation and social participation for the construction of the multisectoral agenda focused on development, innovation and production in health. According to Rodrigo Rollemberg, Secretary of Green Economy, Decarbonization and Bioindustry at the Ministry of Development, Industry, Commerce, and Services (MDIC), there is an effort to listen to representatives from the many sectors of the Health Economic-Industrial Complex (CEIS), including national and global enterprises. There is constant communication between the Ministry of Health, in particular the Secretariat of Science, Technology, Innovation and Health Complex, with the Ministry of Development, Industry, Commerce and Services. In addition, partners in this initiative are the MCTI (Ministry of Science, Technology and Innovation), MRE (Ministry of Foreign Affairs), MAPA (Ministry of Agriculture and Livestock), Ministry of Finance, BNDES (Brazilian Development Bank), INPI (National Institute of Industrial Property), Anvisa (Brazilian Health Regulatory Agency), Embrapa (Brazilian Agricultural Research Corporation), Embrapii (Brazilian Company of Research and Industrial Innovation), FINEP (Finance Agency for Studies and Projects), and others interested in contributing to the implementation of actions and policies focused on sustainable industrial growth in the Health sector are also partners in this effort. Furthermore, it has been planned the construction of a database to help choices on In-
For these products to reach the Brazilian population, RD&I
dustrial Policy strategies in the field of Health.

resulting in integration between the country’s various health research centers, startups that develop innovative products, and Brazilian companies
The collaboration between the Ministries of Health (MS), Science, Technology, and Innovation (MCTI), and Development, Industry, Commerce, and Services (MDIC) is critical for building productive and innovative capacities in the health sector. This inter-ministerial partnership ensures that new national products fit the needs of the health system while also providing universal and high-quality access to the Brazilian population. The goal of this strategy, which is based on Ministry of Health guidelines, is to stimulate innovation in key areas for the SUS (Brazilian Unified Health System), such as the SUS’s list of strategic products, thus improving the entire chain of the country’s Health Economic-Industrial Complex.
Innovation catalyzes increased local production of strategic products for the SUS (Brazilian Unified Health System), such as active pharmaceutical ingredients, medicines, vaccines, and medical devices. However, for these products to reach the Brazilian population, RD&I processes must be operationalized by the sector’s agents resulting in integration between the country’s various health research centers, startups that develop innovative products, and Brazilian companies. According to Secretary Mr. Rollemberg (MDIC), long-term planning, continuous investments, and regulatory policies that promote innovation are essential. According to the secretary, the “government’s role is to create a favorable environment for the development of biotechnology,” and to accomplish this, there will be an articulation between industry and government bodies to carry out a situational diagnosis, the goal of which is to assist in decision-making related to innovation processes in the sector.
According to Norberto Prestes, President of the Brazilian Industry Association of Active Pharmaceutical Ingredients (Abiquifi), it is vital to build an even more favorable
56 bioBr
PROCESSES MUST BE OPERATIONALIZED BY THE SECTOR’S AGENTS


DEVELOPMENT
atmosphere that attracts the interest of investors to focus on radical health innovation. In this manner, Abiquifi’s Radical Innovation Program articulates several actions with strategic partners such as the Ministry of Health, MDIC, and MCTI, private companies, development agencies, and regulators, as well as startups and sector researchers, to promote improvements in the Brazilian population’s ecosystem, always following Brazilian Unified Health System guidelines and priorities. According to Prestes (Abiquifi), it is also crucial to examine the global market and current technological routes to align the development ambitions of Brazilian institutions and businesses with market trends. Still, according to Abiquifi’s president, much of what is required to achieve the goals of industrialization of health products in Brazil has already begun, and there are many examples around the world from which we can be inspired so that we have a more robust ecosystem with new solutions coming to SUS (Brazilian Unified Health System). Norberto expresses his desire to see concrete results from Abiquifi’s Radical Innovation Program within the next eight months.

According to Mr. Rollemberg (MDIC), it is important to create innovation platforms focused on the needs of the healthcare sector. As a result, Research Networks could be established as a partnership between technology-based companies and medium and large anchor companies to service key projects for the Brazilian Unified Health System. So that initiatives can obtain expert technical assistance, accelerating the process from the lab to patient care through improved regulatory, financial, and innovation management.
The Ministry of Health (MS) states that “promoting the industrial scalability of active pharmaceutical ingredients (APIs) and products to fully meet the demands of the SUS (Brazilian Unified Health System) in an economically viable way and with external independence is an important mission for the Ministry of Health.”
In this manner, a more robust health industry reduces prices, increases job opportunities, preserves Brazilian know-how, and prepares Brazil for future geopolitical changes. Brazil’s biodiversity provides chances for the development of novel products and solutions that are highly competitive in the global market, especially in the health sector. According to Secretary Mr.Rollemberg (MDIC), the growth of biotechnology to diverse regions of the country, such as the northern region, continues to offer excellent prospects for industrial development for healthcare in Brazil. There is an Industrial Pole placed in the Manaus Free Trade Zone (ZFM) (in Amazonas) with about 500 industries in operation. According to Rollemberg (MDIC), these companies benefit from the tax incentives provided by the Information Technology Law and, in exchange, must invest 5% of their total revenue from sales of the incentivized goods in Research, Development, and Innovation (RD&I) activities in order to modernize their processes and produce innovative goods and services that, can be applied in the health sector. Furthermore, the Amazon Biobusiness Center (CBA), which will have a new administrative management partnership, is at an appropriate time for new prospects, which can promote research, development, and project transformation into significant businesses for the Amazon region and Brazil, both in health, as well as other segments. As a result, the Amazon Biobusiness Center (CBA) has the potential to become an important Brazilian business and technology hub, cooperating with other technology centers in the country. These initiatives demonstrate that industrial development for healthcare remains constantly evolving. The combination of economic development, investments in research and innovation, and appreciation for biodiversity raises Brazil’s potential as a health sector leader. ●
58 bioBr






Advancing Brazil’s Health Sector and Socioeconomic Development
ESG is an abbreviation for Environmental, Social, and Governance, which means environment, social responsibility, and corporate governance. The term’s origin dates back to the report “Who Cares Wins” by The UN Global Compact, whose purpose was to present improvements in the integration of the financial market with initiatives related to the theme. Companies that adopt ESG practices seek to establish a transparent and reliable relationship with their stakeholders, employees, consumers, and investors. The subject has gained relevance because it represents a risk management measure related to social impact, external environmental factors, and internal governance practices. All of this translates into the enhancement of
the institutional image, attraction of investments, and the acquisition of new customers.



The theme of sustainability and ESG guidelines has an intrinsic relationship with the productive sector in health, covering several aspects. First, naturally, every industrial process requires consideration related to the environmental impact of infrastructure and operations, such as water and energy consumption and waste generation. However, the health industry has enormous potential to contribute to the bioeconomy, which includes the sustainable use of biological resources. This premise includes the development of medicines and inputs from natural sources such as plants and microorganisms, as well as appreciating biodiversity and traditional communities that rely on these resources.

62 bioBr MANAGEMENT
ESG


MANAGEMENT
A few years ago, the focus was only on the production of the product, however given the scarcity of resources, there is now a concern with efficiency and production optimization. According to Meiruze Freitas, Director, Second Directorate (Brazilian Health Regulatory Agency), biotechnology plays a key role in promoting the circular economy model, which aims to minimize waste and maximize the use of resources. For example, the use of bioreactors in the production of biopharmaceuticals, which can reduce the amount of waste generation, as well as the development of drug delivery systems based on biodegradable and biocompatible nanoparticles produced by microorganisms.
According to Ana Paula Repezza, Business Director at ApexBrasil (Brazilian Trade and Investment Promotion Agency), the adoption of ESG practices reduces cost through increased production efficiency, which improves market competitiveness. In addition, it attracts international investors and boosts the brand image, which may lead to new business opportunities and partnerships. Repezza (ApexBrasil) also points out that by complying with environmental, social, and governance standards, companies gain advantages to access even international markets that have been historically more difficult to access, such as Europe and the United States.

Bearing in mind the position that a large part of the industry was in a few years ago, sustainability as a purpose is innovative, according to Antônio Carlos Teixeira, CEO of Globe Química. Similarly, Repezza (ApexBrasil) agrees that companies that incorporate ESG practices are regarded as leaders in their sectors because they demonstrate to investors a long-term vision and reveal the potential to outperform the competition and expand globally. According to Teixeira (Globe), the pharma-chemical industry is already well-advanced regards direct environmental impacts, such as air, soil, and water, and states
that today the main goal is to search for energy efficiency and the reduction of the footprint of greenhouse gasses. Still, according to the CEO of Globe, although this mentality is considered transforming, “the enlargement of ESG practices in companies is the materialization of a duty”. For ApexBrasil, the transversal inclusion of ESG practices is seen as a guiding principle in all its actions, because of that, these practices were included in the new guidelines announced by ApexBrasil.
With the growing need for an interdisciplinary approach to complex topics, according to Freitas (Anvisa), although the primary role of Drug Regulatory Authorities is to ensure safe, effective, and high-quality products for public consumption, a more significant interaction between different regulatory spheres is needed for the promotion of human, animal, and environmental health. In this way, ESG guidelines are rapidly becoming part of the day-to-day regulation of products and services for human health. Within

64 bioBr
Meiruze Freitas, Director, Second Directorate (Brazilian Health Regulatory Agency)

MANAGEMENT
the current context, biological products and processes are subject to regulations that can limit their development since they are not yet prepared for effective risk management. According to Freitas (Anvisa), proper advancement in this industry requires that the regulation goes hand in hand with the development of these products, with a lot of dialogue and interaction between regulators and developers. By overcoming these challenges and taking advantage of opportunities, biotechnology can play a crucial role in creating a more sustainable future, says the Anvisa director. Nonetheless, ESG practices are still marginally addressed concerning the central issues of medicines and biotechnology products regulation. However, Freitas (Anvisa) mentions that among some possible measures to be explored would also be to take into account the environmental impact during the evaluation process that precedes the registration of products such as medicines and Active Pharmaceutical Inputs (API). As a result, the regulatory agency eventually could require manufacturers to make public the disclosure of information about their ESG practices and policies in order to encourage the development of more sustainable products. Nevertheless, Freitas (Anvisa) points out that, to move in this direction, it is necessary to consolidate legal frameworks and adopt specific policies to provide predictability and legal certainty to those involved. For Teixeira (Globe), public policies can be created to assemble audit mechanisms to monitor companies that claim to use sustainable practices. Besides that, the government can also adopt complementary policies that encourage companies to embrace such practices, in this regard, one can explore tax incentives or taxation to level the competitiveness of the national market and encourage multinational companies to also be motivated to adopt them.
Commitment to sustainability generates economic benefits, and enhances pro-


ductivity and innovation capacity, resulting in a more dynamic and resilient market. This development is particularly important for Brazil because of its biodiversity potential. In this way, incorporating sustainable practices into the health sector brings benefits not only to the population but also to the Brazilian State, promoting the country’s sustainable socioeconomic development. ●

66 bioBr
Antônio Carlos Teixeira, CEO of Globe Química
Ana Paula Repezza, Business Director at ApexBrasil (Brazilian Trade and Investment Promotion Agency)





New Butantan vaccines promise to impact global public health
 By Esper Georges Kallás M.D., Infectious Disease Specialist, Full Professor at the Department of Infectious and Parasitic Diseases at the School of Medicine of the University of São Paulo (FMUSP) and Director of Instituto Butantan
By Esper Georges Kallás M.D., Infectious Disease Specialist, Full Professor at the Department of Infectious and Parasitic Diseases at the School of Medicine of the University of São Paulo (FMUSP) and Director of Instituto Butantan

Butantan is internationally renowned as a pioneering and innovative scientific institution, and it is not a recent thing. In addition to the centenary production of hyperimmune sera, our Influenza vaccine was pre-qualified by the World Health Organization (WHO) in 2021 and we were able to export more than 7 million doses to Uruguay, Ecuador, Nicaragua, Colombia, Bolivia and Cuba – on top of that, 80 million doses are delivered every year to the Brazilian Ministry of Health. All this amid the greatest pandemic of the 21st century, when we provided the population with over 100 million doses of CoronaVac.
Our work is guided by research and production of immunizing agents that can highly impact global public health, such as the dengue and chikungunya vaccines, both diseases transmitted by the Aedes aegypti mosquito. In the Americas alone, 500 million individuals live at risk of contracting the dengue virus, while chikungunya is present in 115 countries.
Under development since 2010, Butantan’s dengue vaccine stood
out for being single dose and containing the four subtypes of the attenuated virus. It is also the first technology transfer of a Brazilian vaccine to a multinational corporation, the US-American pharmaceutical company Merck. Preliminary data from phase 3 clinical trials showed that the vaccine is nearly 80% effective – an excellent result that depicts the quality of our work.
The chikungunya vaccine developed with the Franco-Austrian pharmaceutical company Valneva will be distributed by Butantan to countries in Asia, Africa and Latin America. The research is currently in
phase 3 of clinical trials and involves 750 Brazilian adolescents. Another study carried out in the United States in 2020, attested to 98.5% of seroconversion in adults and the elderly. Engaged in expanding our production capacity and the diversity of products offered to Latin America, the factory that will produce the vaccines against the two arboviruses is ready, proving our ability to combine basic and applied research to benefit public health. Consolidating Butantan as a reference in the production of immunobiologicals means fulfilling our commitment to the lives of Brazilians and, from now on, to people worldwide. ●

70 bioBr INNOVATIVE SCIENTIFIC INSTITUTION


The Brazilian Ecosystem of Biotechnology Innovation


Biotechnology is a rapidly developing field with tremendous potential to revolutionize healthcare and disease treatment. In Brazil, biotechnology is gaining attention not only for its clinical and care potential but also as a promising economic development lever, following in the footsteps of countries such as Israel, the USA, and Singapore.
The development of new therapies, diagnostic tools, and vaccines can benefit the national health context have a global impact, contributing to the advancement of health technologies worldwide and positioning Brazil as a leader in the sector.
Brazil has several unique factors that enable this to happen. With a population of over 210 million people, Brazil has a large domestic market and abundant natural and human resources to develop new health products. The country also boasts one of the largest biodiversities on the planet, with a wide variety of plants and microorganisms that canto develop new bioactive compounds, generating innovative solutions with a robust differential. The development of products based on Brazilian biodiversity can have a sig-
nificant positive impact on environmental preservation and create job opportunities for local populations, establishing a full value chain of cultivation, production, and genetic conservation processes for natural assets.
Brazil’s extensive and diversified public healthcare system, which serves a large population of lower-income individuals, creates a demand for low-cost and accessible treatments. Startups developing innovative and inclusive solutions can address this demand. Furthermore, Brazil has a favorable regulatory environment for research and development of biotechnological products, with a strong and well-established regulatory agency, Anvisa, ensuring safety and efficacy of the new solutions created. The country also holds a qualified workforce of researchers with knowledge in this field, trained by internationally recognized research institutions and universities.
Recently, the FDA recommended conducting clinical studies in populations with genetic diversity, making Brazil an important site for the development of new drugs and tests. Brazil’s unique set of advantages has attracted startups from around the
globe, recognizing the opportunity to accelerate their pre-clinical and clinical research while also having their technologies incorporated by Brazilian medical centers. These companies have access to state-ofthe-art facilities for the validation and co-development of new technologies, comparable to those found in world-renowned medical centers, with the added benefit of conducting tests at more cost-effective rates.
Hospital Israelita Albert Einstein, a non-profit private institution based in São Paulo, is one such medical center that has been leading the way in health innovation initiatives. Einstein has gained global recognition as a center of excellence in healthcare, having received numerous awards and certifications, and was the first institution accredited by Joint Commission International (JCI) , in 1999, which is the main certification body for hospitals worldwide. Recently, it also achieved the prestigious Magnet recognition, considered the most significant recognition globally for nursing services.
In addition to providing excellent patient care, Einstein serves as a leading center for clinical and translational research in Brazil and Lat-

72 bioBr HEALTH TECHNOLOGIES
Dr. Sidney Klajner, President of Sociedade Beneficente Israelita Brasileira Albert Einstein


HEALTH TECHNOLOGIES
in America. The institution is engaged in innovative projects in diverse areas, including oncology, neurology, and cardiology. Einstein stands out due to its strategic partnerships with biotechnology companies, universities, and research institutions worldwide. Through these collaborations, the center supports the development of new therapies and medical devices, contributing to advancements in healthcare.
Einstein’s innovation hub, Eretz. bio, is a vital pillar of the institution, celebrating its fifth anniversary at the end of 2022. The hub leads more than 150 concurrent partnership projects between the hospital’s internal departments and startups from over 20 countries. This collaboration is critical to ensure that innovations reach patients as quickly as possible, with the added assurance of having undergone rigorous scientific testing and evaluation.
In 2022, Eretz.bio launched the Biotechnology Innovation Program, with the aim of boosting the Brazilian biotechnology industry and accelerating the country’s potential to develop new solutions. This program offers startups comprehensive support, including mentoring, networking, access to financing and infrastructure, as well as regulatory assistance, intellectual property guidance, and preclinical and clinical studies.
The Einstein Biotechnology Innovation Program is focused on developing national and international technologies in the area of Bioconvergence. In recent years, the healthcare market has undergone a significant digital transformation process, with the convergence of biotechnology with multiple areas of science, such as big data, nanotechnology, biomaterials
engineering, and computer science. Bioconvergence is a concept that has been seen in countries that stand out in the innovation scenario, and Einstein is betting on it for the upcoming years.
Bioconvergence drives the Einstein Biotechnology Innovation Program, which aims to accelerate the development of new biopharmaceutical products and regenerative medicine, such as cellular and gene therapies, organ transplantation using 3D bio-printing, and non-invasive diagnostic tests. These developments have the potential to transform the treatment of diseases such as can-
cer, diabetes, and neurodegenerative disorders. Furthermore, advances in synthetic biology, bioinformatics, and genomics, associated with big data, can facilitate the development of personalized medicine solutions that are accessible to most of the population.

Einstein is confident that Brazil has all the necessary ingredients to become a major player in the biotechnology industry, particularly in human health. The development of global startups could lead to the creation of groundbreaking solutions, generating job opportunities, and driving economic growth for the country. ●

74 bioBr
The development of global startups could lead TO THE CREATION OF GROUNDBREAKING SOLUTIONS, generating job opportunities, and driving economic growth for the country


RESOURCES
Investment environment in bio and health in Brazil
 Eduardo Emrich, CEO & President - Biominas
Eduardo Emrich, CEO & President - Biominas

The venture capital and private equity industry in Brazil is relatively recent. Normative Instruction CVM no. 391, which instituted equity investment funds (FIPS), similar to the limited partnerships in the United States, dates from 2003, therefore only 20 years ago. During this period, the sector grew strongly, reaching in 2018 the number of 268 funds raised, surpassing R$ 84 billion in joint funding.
The support of public institutions such as the Funding Authority for Studies and Projects (FINEP) and The Brazilian Development Bank (BNDES) was essential to encourage the initial stages of development of the investment environment in the country. Both institutions made considerable investments in funds and articulated with other national and foreign institutional investors to increase resources available. In 2006, BNDES approved the creation of the Criatec funds, aimed at supporting innovative start-ups. The three Criatec funds had resources close to US$ 100 million.
A new venture capital development cycle occurred more recently with the boom in the creation of startups in Brazil and worldwide.
The number of startups went from 4,000 in 2015 to more than 14,000 in 2021. The expressive growth of new companies, especially in information technology, attracted a significant number of new investors, enhancing the creation of the first unicorns. In less than 5 years, Brazil reached 16 unicorn companies.
The wave of IT startups was followed by a wave of health techs, companies that use information technologies to develop new healthcare solutions. In 2021, more than half of Brazilian health techs were less than 5 years old. More recently, a smaller wave of high technology companies (deep techs) in bio
A new venture capital development cycle occurred more recently with the boom in the creation of startups in Brazil and worldwide. The number of startups went FROM 4,000 IN 2015 TO MORE THAN 14,000 IN 2021
and health has been consolidating.
In a way, Brazil had prepared itself for these waves. In 2004 the Technological Innovation Law was enacted and updated in the following decade. The law encouraged: (i) the creation of innovation environments, such as incubators and accelerators; (ii) the participation of research and technology institutions, in addition to medium and large companies, in the innovation process; and (iii) the constitution of investment funds. These points created a favorable environment for the waves of entrepreneurship and innovation that followed.
In health and biotechnology, a new factor played a significant role in attracting investors to the numerous bottlenecks identified: the COVID-19 pandemic. Investors previously averse to investing in these areas began to clearly visualize the existing market opportunities, with the lack of health inputs and products (diagnostic tests, equipment, vaccines, drugs and others).
Pre-acceleration programs focused on bio and health, such as Biominas Brasil’s Biostartup Lab, emerged in 2016. They were essen-
76 bioBr
tial for discovering and structuring new business entities from academic projects. In fact, many startups that have gone through the program have received funding in recent years. Individuals, angel investor groups or startup acceleration programs have come to fund the initial validations of these startups and spin-offs.

At almost the same time, seed capital and venture capital investment initiatives dedicated to bio began to emerge via equity investment funds or venture builders.
The Sociedade Beneficente Israelita Brasileira Albert Einstein was one of the first to allocate resources to startups, generally accelerated by its Eretz.bio incubator. The success of the initiative leveraged the constitution of a Corporate Venture Capital (CVC) fund in 2021 under the management of Vox Capital. In 2022, Einstein expanded its innovation activities by creating the Biotechnology Innovation Program to support startups and entrepreneurs focused on health.
Other CVCs were also constituted. Kortex Ventures is a fund that brings together three large Brazilian companies: Grupo Fleury, Grupo Sabin, and Bradesco Saúde, with approximately US$ 40 million for investment in companies aiming to transform healthcare through technology. The fund has already invested in 8 startups, including biotechs and health techs.
Neuron Ventures, launched in 2019 with US$ 10 million, is Eurofarma’s Corporate Venture Capital. The fund invests primarily in health techs in the initial stages.
Among the venture builders, Vesper Ventures stands out for its portfolio made up of disruptive startups
focused on major global challenges. The initiative has already supported the creation of 7 startups.
In 2022, the fund manager Pitanga announced its first investment in biotechnology, through its Pitanga Redux fund, in Next Innovative Therapeutics (Nintx), a startup dedicated to the development of new medicines based on Brazilian biodiversity.
The Biotec fund, managed by FIR Capital, together with Health Invest, although focused on health companies in the growth stage, invested in 2 high potential health techs, ISA and Mevo. Incidentally, both companies received investments from other funds, which shows an already existing synergy among investment managers in health and bio.
The cases shown above are indicative of the venture capital vitality in biotechnology and health in Brazil. Funds have been growing in numbers and becoming more diversified, covering the spectrum of business development phases. That reflects investors’ growing trust in the startups scientific and technological capacity to generate innovations and solid financial returns. ●
Startups
Brazil has a blooming startup and investment ecosystem. The country is known for its quality in research and publications, now it’s time to show the world the outputs of our scientific production. This year at the BIO Convention two Brazilian startups will be, for the first time, at the Startup Stadium.
Mirscience Therapeutics


Mirscience Tx has a RNA platform that develops precision medicine for the treatment of muscle wasting diseases like cancer cachexia and sarcopenia exploring undruggable targets.
Imunotera
ImunoTera is contributing to a new era of therapeutic vaccines. The female founders developed an “on-demand platform” to treat chronic and infectious diseases. The first product was designed to tackle HPV-induced cancer.
The role of local vaccine manufacturers to overcome health emergencies
Mauricio Zuma
The discussion about equitable access to vaccines gained even more light during de Covid-19 pandemic, when developed countries with local manufacturing capacities guaranteed, even before registration, excessive number of doses for their own populations, reflecting in an unbalanced supply worldwide.
If, on one hand, vaccination advanced more quickly in these countries, on the other, different instances involving cooperation between governments and international organizations mobilized to establish initiatives to expand the access to immunizers to LMIC (Low and Middle Income Countries) populations in face of this and future health emergencies.
Such demand came in the direction of establishing new local manufacturers in LMIC to ensure supply to the 82% of the world’s population living in these countries. While this may be considered an important initiative at first glance, it is not an easy one in the short to medium term given the challenges of immunobiological manufacturing.
It is a fact that the existence of productive capacity and skilled workforce in vaccine manufacturing, specifically speaking of Bio-Manguin-
hos/Fiocruz, represented an advantage that allowed the complete incorporation of the Covid-19 (recombinant) vaccine technology and the production of a large number of doses in less than a year - which is an unprecedented achievement. This was possible in large part due to some institutions present in the Brazilian biotechnology ecosystem, policies at the federal government level supporting self-sufficiency in immunobiological production as strategic to national sovereignty, and the existence of a strong National Health Surveillance Agency (ANVISA), that was able to implement the “rolling submission” process to speed up product registration.
Vaccine manufacturing is a highly complex process. It requires high investments in infrastructure, including the ones for the incorporation of new technologies and processes, and for expansion of areas. The operations of the facilities also have a high fixed cost, being necessary to ensure high demands, national and/or international, to provide economies of scale and make the process sustainable.
In addition to being economically viable, vaccine manufacturing must be technologically renewable, enabling the incorporation of new,


high-quality products. For this, the company needs to establish a robust innovation ecosystem, investing heavily in R&D and process improvements to build its own competencies. In parallel, technology transfer partnerships should be contemplated in the strategic plan as a way to accelerate the availability of vaccines for public health actions. This business model, safeguarding the full dissemination of information and technical assistance, also contributes to the establishment of local capabilities.
Other requirements must be considered, such as the availability of highly qualified professionals at all stages of development, manufacturing and quality control, and the compliance with increasingly stringent regulatory standards, considering that high quality standards require a strong quality system.
All these issues highlight the importance of seeking different partnerships not to duplicate efforts, but to leverage the best capabilities of each institution in an integrated and complementary way, so that manufacturers can strengthen their contribution to emergency preparedness and response, based on their core competencies, for equitable access to vaccines. ●
78 bioBr PRODUCTIVE CAPACITY
CEO - Bio-Manguinhos / Fiocruz / Brazilian MOH













































































































































 By Esper Georges Kallás M.D., Infectious Disease Specialist, Full Professor at the Department of Infectious and Parasitic Diseases at the School of Medicine of the University of São Paulo (FMUSP) and Director of Instituto Butantan
By Esper Georges Kallás M.D., Infectious Disease Specialist, Full Professor at the Department of Infectious and Parasitic Diseases at the School of Medicine of the University of São Paulo (FMUSP) and Director of Instituto Butantan












