
37 minute read
COVER STORY





THE PATIENT EXPERIENCE
Anthony, Retiree, Long Island, NY
“You really shouldn’t have to go through an unwieldy process for something that saves a life,” said Anthony, who learned of his Hepatitis C diagnosis upon his retirement. To secure coverage for treatment, Anthony needed an “ombudsman” to work on his behalf and to navigate his insurance plans. He found just the right person to help him in Susan Lee, PharmD, a pharmacist who works with Dr. David Bernstein and Dr. Henry Bodenheimer. In an interview with ACG MAGAZINE, Anthony recalled that Susan was a “catalyst to make things happen” when it came to dealing with Medicare and the union health plan he has in retirement.
The cost of treatment was a big issue for Anthony. “All kudos to Susan for finding ways to find a medication that was right for me and that was affordable,” he added. “Without Susan, forget it!” Susan not only navigated successfully with Anthony’s health plans, she also followed up with him by phone and checked in frequently. Anthony credits her with helping to make sure he completed the full course of treatment for HCV so he can be there for his children and grandchildren.
Zachary, Student, Age 18, Florida
Newly diagnosed with severe ulcerative colitis this spring, Zachary was admitted to the hospital and started on steroids. Zachary's insurance declined vedolizumab. He was subsequently re-admitted to the hospital for a 14-day stay and received his first dose of infliximab. The insurance company declined an outpatient course of 10 milligrams/kilogram of infliximab. His gastroenterologist at the Mayo Clinic in Jacksonville, Florida, Dr. Francis Farraye, submitted a peer-to-peer request which took so much time that Zachary's condition worsened, requiring a third hospital admission where he again received infliximab. Zachary described this experience as “terrible, scary, not fun” in an interview with ACG MAGAZINE. For this high school junior, his diagnosis changed everything, “in a bad way.” Zachary's mother, Rhonda, is a nurse who was a health care finance major in college. She shared that she is, “still perplexed how insurance companies can sit on the phone and dictate to you patient care, I never agreed about it, never understood it.” For her, the hardest part was seeing the mental toll that the fight to get medication coverage took on Zachary, but she found working with Dr. Farraye and his team one of the things that was a “ray of sunshine” for their family. Coverage was ultimately obtained and Zachary is doing much better.
Stories, such as the ones outlined above, are unfortunately a common occurrence in gastroenterology practices. Prior authorization (PA), which is a cost containment and quality control process mandated by many health insurance plans, requires providers to submit documentation for review by the insurer to determine if a mutually agreed upon treatment plan by the patient and the medical provider is medically necessary for the patient and warrants coverage. The process of submitting PAs and awaiting determination is often tedious, time-consuming, and anxiety-provoking. It has also been linked to negative outcomes, including delays in initiating treatment, worsening clinical outcomes, patient dissatisfaction, and even abandonment of optimal treatment plans.1 Gastroenterologists are constantly dealing with the PA process due to the increased frequency of prescribing high-cost medications such as biologics and direct-acting antivirals, and insurers failing to recognize updated treatment pathways. Per a survey of 156 gastroenterologists, approximately 63% of gastroenterology practices spend more than 25% of their time communicating with payers.2 Additionally, one-third of providers endorse switching from their preferred treatment plan once a week or more due to the arduous PA process.3 Given the time spent and the profound impact of the PA process on patient care, it is critical for gastroenterologists to know how to successfully conquer the PA process and advocate for reform.
AN OVERVIEW OF THE PA PROCESS
Gastroenterologists typically collaborate with their patients when designing optimal and patient-centered treatment plans. Depending on the medical condition being treated and the gastroenterologist’s experiences with medication acquisition, the gastroenterologist should inform patients of a potential alternative treatment plan in case payers deny coverage of the preferred treatment. This may alleviate any unforeseen stress when the patient is informed a PA is required or the medication is not covered. Regardless, this scenario is not ideal as it can generate patient confusion and/or dissatisfaction with the treating gastroenterologist, given that most patients lack awareness and understanding of medication coverage and the acquisition process within the U.S. healthcare system. Once it is determined that a PA is required for the treatment, the gastroenterologist or staff may complete an electronic or paper version of the PA form to submit to the payer with the necessary accompanying documents such as progress notes, lab results, and imaging. The payer then reviews the materials and may request additional information prior to deciding coverage outcomes. If the PA is approved, the requested medication can be dispensed or provided to the patient.
It is in the best interest of the patient and the office staff for the gastroenterologist to set a realistic expectation of the approval timeline. The time frame of approval is highly variable and can range from a few hours to several weeks. Explaining this process to the patient at the time of prescribing the medication will help set realistic expectations for the patient and foster a good relationship with the provider’s office.
COMMON CHALLENGES IN ACCESSING DISEASE-SPECIFIC TREATMENTS
Inflammatory Bowel Disease Commonly, PAs for inflammatory bowel disease (IBD) are initially denied because payers enforce step therapy, in which patients must try and fail more than one lower-cost medication before payers are willing to cover the requested medication.4 While step therapy used to be the standard of care, it is no longer acceptable based on disease severity and the supporting data for early use of high-cost medications in subsets of patients with IBD to effectively induce and maintain remission.5,6
SURVEY OF 156
GASTROENTEROLOGISTS
SHOWED APPROXIMATELY 63%
OF GASTROENTEROLOGY PRACTICES
SPEND MORE THAN 25%
OF THEIR TIME COMMUNICATING WITH PAYERS
Hepatitis C Medication access for hepatitis C treatment is highly dependent on the type of insurance plan and the state in which the prescriber or patient resides. For the purpose of this article, insurance types will be generalized to federal insurance programs (Medicaid and Medicare) and private commercial insurance.7 Medicaid, which is state- and federally-funded, has significant variation in approval criteria for hepatitis C medication across the country. When approved by Medicaid, copayment costs tend to be low.8 Federally-funded Medicare plans are more or less uniform in their approval criteria, but the copayment costs vary significantly across the country and may be too costly for most.9 Therefore, Medicare approval does not always guarantee patient access to the prescribed medication.
Adding to the challenge of access, each private commercial insurance plan has its own criteria for medication approval. Copay costs may be high, but patients with these plans can use manufacturer-sponsored coupons which can substantially decrease copay costs. Rarely, commercial plans may be self-funded or self-insured, which may require the patient to reach out to the employer’s human resource or benefits manager instead of the insurance company to determine coverage.
The documentation necessary for PA submission may include office notes, lab results, and degree of liver fibrosis. Insurance payers may request additional information such as drug testing, HIV testing, a written patient attestation letter, and/or an alcohol and illicit drug use questionnaire.10 The top three reasons for denial of hepatitis C therapy are a lack of advanced hepatic fibrosis, failure to document abstinence from alcohol or recreational drugs, and the prescriber lacking experience with hepatitis C treatment.
For denied PA requests, the gastroenterologist or their staff may submit an appeal with a letter of medical necessity. If the payer is Medicare and the appeal is denied, the gastroenterologist or their staff may submit an appeal for internal review and then a contracted external company for review, who then makes the final decision. If the external company denies the appeal, the gastroenterologist will need to use an alternative medication. If the payer is not Medicare and the appeal is denied, the gastroenterologist can request a peer-to-peer to provide a verbal appeal as to why the requested medication is necessary for the patient. The payer then makes a decision, and, if denied, the gastroenterologist will have to use an alternative medication, but may resubmit a PA for the original requested treatment in six months. The appeal process may differ slightly depending on the state and plan type. Approved PAs are often valid for a certain amount of time and once expired, a new PA will need to be submitted. Additionally, if patients change insurance plans, a new PA may be required by the new insurer.
CONQUERING THE PA PROCESS
While the PA process can be tedious and dealing with denials can lead to frustration and delays in patient care, there are certain approaches that can be used to successfully obtain PA approvals.11
Identify one individual or a team to oversee and own the PA process from beginning to end. One of the most essential steps is to designate an individual or team of individuals to own the process. Depending on the resources available in the gastroenterology practice, the PA process may be allocated to a pharmacy team (pharmacists and pharmacy technicians) located in the practice or in an on-site or outside specialty pharmacy. In this model, the pharmacy technicians can oversee all gastroenterology PAs and employ pharmacists’ assistance as needed to provide further rationale as to why the selected treatment is medically necessary. “While the PA process can be tedious and dealing with denials can lead to frustration and delays in patient care, there are certain approaches that can be used to successfully obtain PA approvals.”
Having an on-site specialty pharmacy permits for easier direct communication about PA approvals and faster processing of medications to further minimize delays in treating patients. If pharmacy support is not available in the gastroenterology practice, the PA process may be delegated to medical assistants, registered nurses, or an external specialty pharmacy or manufacturer hub. When outsourcing the PA process, the gastroenterology office and its staff remain responsible for providing the external pharmacy or manufacturer hub with patient-specific information, given that these entities do not have direct access to patients’ medical charts.
Know the top payers in the practice, obtain their formularies and PA forms, and establish a point-of-contact. Once an individual or team is identified to own the PA process from beginning to end, it is important for that individual or team to identify the most common payers in the practice and become familiar with those payers’ formularies and PA processes. Formularies and PA forms can often be obtained online and kept on file for organizational and time-saving purposes. Additionally, when possible, the person or team responsible for the PA process should identify a contact person within each insurance company and obtain his or her contact information. If a PA follow-up is required, the office can reach out directly to the contact person so time is not wasted on automated phone prompts. Having a point of contact with each payer also permits for escalation of grievances to appropriate individuals if the PA process becomes difficult to navigate.
Verify benefits and identify if the requested treatment will be covered by medical or pharmacy. Prior to starting the PA process, the individual or team responsible for the PA process should be comfortable with performing benefit verification to determine which plan a patient has, so that the correct PA form and information can be submitted without causing further delays in the patient’s treatment. Benefit verification can be performed via telephone or the specific insurer’s website. It is also important to verify if the requested
medication should be billed through medical or pharmacy benefits to identify the correct PA form to use. If patients are planning to acquire their medications through pharmacy benefits, insurance eligibility may also be confirmed by contacting the patient’s preferred pharmacy to verify the prescription coverage they have on file.
Spend time completing the initial PA request and include all pertinent information justifying why the requested treatment is medically necessary. Once the correct PA form has been acquired for the patient’s insurer, ensure that all necessary fields are completed and pertinent information justifying why the requested treatment is medically necessary has been included with the PA form. Consider including progress notes, pertinent laboratory results, reports of imaging studies, procedure findings, and a list of previous treatments. Providing comprehensive information will allow the insurer to make a more informed decision and may prevent unnecessary denials. Attaching a letter of medical necessity may escalate the PA to a clinical pharmacist or nurse for direct review and expedite the process by bypassing the algorithm that most insurers use to make an initial determination for PA request. If electronic PA (ePA) is used, this still requires paper documents to be printed and converted into PDF format to be uploaded to a specific insurance plan or general electronic PA platform.12,13 The ePA process may save time in obtaining medication approval.
Track when the PA was submitted and follow-up as needed to ensure appropriate outcomes. Upon submitting a completed PA request, the individual or team responsible for the PA process should document the submission in an Excel spreadsheet (Figure 1) or similar digital document to allow the office staff to record whether a decision from the insurance company is received in a timely manner. This should serve as the reference tool for the individual or team members responsible for the PA process. It should record the submission of each patient’s PA and the current status. Maintenance of the “Date to follow up” column will assist in the efficiency of the process. If a decision is not received within 3 business days, the individual or team should follow up with the insurer. A database should also be maintained for all approved PAs, so that a new PA can be submitted in a timely manner when the original PA expires. “The prescribing gastroenterologist should request to speak with a gastroenterologist representing the insurance company and emphasize the consequences of treating the patient with other treatment options or letting the disease worsen or progress.”
If the PA is rejected, submit an appeal. If the PA is denied, an appeal should be submitted. This may require submitting the original PA form with more information or requesting a peer-to-peer review, in which the gastroenterologist speaks with a representative of the insurance company to provide a verbal or written appeal as to why the requested medication is medically necessary. The written appeal should be concise and to the point, addressing each insufficient reason for denial. The prescribing gastroenterologist should request to speak with a gastroenterologist representing the insurance company and emphasize the consequences of treating the patient with other treatment options or
letting the disease worsen or progress. Additionally, information about why other medication alternatives covered by the insurer are not appropriate for the patient should be provided. Lastly, clinical information should be included again, with supporting references of practice guidelines or clinical studies justifying why the requested treatment option is preferred.14
Once the appeal is approved, the story is not over. The out-ofpocket costs may be too high for the patient and deter them from starting treatment. Financial assistance counseling may not be in the list of services provided by the prescriber’s office, but for such situations an informational tool should be prepared to be given to patients as a financial resource guide.15,16
Figure 1. Fields to Track PA Submissions
Date to Follow-up Patient Name Date of Birth Medication Date PA Sent Comments
While the PA process may be arduous for the provider and their office staff, it can also be traumatizing for patients. For example, patients who failed previous HCV treatments may have experienced difficulties with older interferon-based treatment regimens and anxiously await initiating the newer HCV therapies, which have higher cure rates and fewer side effects. Patients starting IBD treatment may be apprehensive about possible adverse effects and needle injections. Unfortunately, the insurance plan-mandated PA process can prolong patients’ wait time for these medications and increase their anxiety, also delaying medically-necessary therapies.
ADVOCATING FOR PA REFORM
The PA process is intended to minimize use of excessive drug spending and encourage appropriate prescribing practices. However, when not executed properly by insurers, this process can end up causing harm to patients. Given that healthcare reform begins with legislation, it is crucial that gastroenterologists participate in advocacy efforts. The American College of Gastroenterology (ACG), as well as various other organizations in GI and hepatology are committed to helping gastroenterologists and their patients with advocacy and policy.17,18 ACG and others are currently advocating for payers to standardize the PA requirements and criteria, improve transparency and accessibility of the PA process, and reduce PA requirements and physician administrative burden. Furthermore, there is a push for payers to approve PAs for a minimum of one year and to develop true “peer-to-peer” interactions in which gastroenterologists can speak with a representative who practices in a similar location and manages similar conditions. Given the numerous barriers surrounding hepatitis C treatment as discussed previously, the AASLD has focused more on improving access to hepatitis C medications, and still meet with patient advocates to determine where to focus future advocacy efforts.19,20
HOW YOU CAN PARTICIPATE
Gastroenterology practices should promote advocacy efforts to encourage reform with the PA process: 1. Contact your local legislator or government regulators via telephone or email to voice the impact of the PA process on your practice and patient care. 2. Become active in various medical organizations and get involved in their public policy activities. 3. Educate your patients on the PA process and equip them with resources to engage in advocacy efforts at the patient level. 4. Social media is a very powerful and effective platform for informing and mobilizing communities to participate in advocacy.21 Social media platforms like Twitter, Instagram, and Facebook permit gastroenterologists to comment in real time and tag appropriate individuals to foster awareness and conversation.
RESOURCES
1. American Medical Association (AMA). 2018 AMA prior authorization (PA) physician survey. https://www.ama-assn.org/system/files/2019-02/priorauth-2018.pdf. Accessed February 25, 2019. 2. Rubin D, Patel S. Integrated specialty pharmacy improves access to IBD drugs. https://www.healio.com. Accessed November 15, 2018. 3. RN Sights. Prior authorization headaches for pharma brand managers. www. rnsights.com. Accessed November 15, 2018. 4. Crohn’s and Colitis Foundation. Step Therapy. http://www. crohnscolitisfoundation.org/. Accessed February 25, 2019. 5. American Gastroenterological Association. Ulcerative colitis clinical care pathway. https://www.gastro.org/guidelines/ibd-and-bowel-disorders.
Accessed February 25, 2019. 6. American Gastroenterological Association. Crohn’s disease clinical care pathway. https://www.gastro.org/guidelines/ibd-and-bowel-disorders.
Accessed February 25, 2019. 7. United States Census Bureau. Health insurance coverage in the United States: 2017. https://www.census.gov/content/dam/Census/library/publications/2018/ demo/p60-264.pdf Accessed February 25, 2019. 8. Hepatitis C: The state of Medicaid Access. The National Summary Report. https://stateofhepc.org/report/. Accessed February 25, 2019. 9. Medpagetoday. Huge spread in patient copays for HCV drugs. https://www. medpagetoday.com/MeetingCoverage/ACG/60839?xid=nl_mpt_DHE_2016-1018&eun=g605133d0r&pos=1 Accessed February 25, 2019. 10. SAMHSA. CAGE-AID Questionnaire. https://www.integration.samhsa.gov/ images/res/CAGEAID.pdf February 25, 2019. 11. Bhat S, Zahorian T, Robert R, Farraye FA. Advocating for Patients With
Inflammatory Bowel Disease: How to Navigate the Prior Authorization Process.
Inflamm Bowel Dis. 2019 Feb 8. doi: 10.1093/ibd/izz013 12. Expressscript. Electronic Prior Authorization. https://www.express-path.com/ login.aspx February 25, 2019. 13. Covermymeds. Electronic Prior Authorization. https://www.covermymeds. com/main/ February 25, 2019. 14. Empire Liver Foundation. Fighting for your patients: Successful prior authorization tips from the pros. https://hepfree.nyc/wp-content/ uploads/2017/11/ELF-Fighting-for-your-Patients-Successful-Prior-
Authorization-Tips-from-the-Pros-6-7-16-2.pdf Accessed February 25, 2019. 15. American Liver Foundation. Financial Assistance Resources. https:// liverfoundation.org/wp-content/uploads/2017/07/ALF-Financial-Resources-
Guide.pdf February 25, 2019 16. Crohn’s & Colitis Foundation. Financial Help. https://www. crohnscolitisfoundation.org/living-with-crohns-colitis/financial-resources.html
February 25, 2019. 17. American Gastroenterological Association. Advocacy & Policy. https://www. gastro.org/advocacy-and-policy Accessed February 25, 2019. 18. American College of Gastroenterology. Public Policy. https://gi.org/publicpolicy Accessed February 25, 2019. 19. American Association of the Study of Liver Diseases. AASLD deepens its relationship with patient group. https://www.aasldnews.org/aasld-deepens-itsrelationship-with-patient-groups/ Accessed February 25, 2019. 20. American Association of the Study of Liver Diseases. Hepatitis C guideline. https://www.hcvguidelines.org/ Accessed February 25, 2019. 21. Scott JT, Maryman J. Using social media as a tool to complement advocacy efforts. Global Journal of Community Psychology Practice. 2016;7:1-22.
An Eye-Opening and Edifying Experience: ACG’s Young Physician Leadership Scholars Program

“Leadership is a way of thinking, a way of acting, and, most importantly, a way of communicating” – Simon Sinek
By Elizabeth R. Paine, MD, Madison, MS
AS THE PLANE BEGAN ITS SLOW, TILTING DESCENT into
lighted bundles of buildings and streets, I found a familiar old fluttering stirring in my gut. After years of comprehensive, intensive training in medical management, endoscopic practice, and diagnostic evaluation, I was very ready for just about any clinical conundrum that I might face. Yet some of the knowledge now most necessary to my professional practice had been, up to now, learned through trial and error and without formal instruction. The jerk of tires on pavement pulled me forward out of my seat and hit me with a sudden realization: I am now an intern in the fellowship of leaders.
In 2018, I was honored to be chosen as part of the inaugural class of the American College of Gastroenterology’s new Young Physician Leadership Scholars Program (YPLSP). This program provides a vital resource for early-career gastroenterologists to learn and practice leadership strategies, complex communication, and professional advocacy. Having been in practice for close to five years, I can emphatically and unequivocally say that this program fills a huge gap in typical medical training.
As physicians, we are asked to lead medical teams, services, programs, divisions, and sometimes even departments with little to no formal training or practical tools. Much like being a third year medical student again, we are forced to figure it out as we go. Thankfully, the YPLSP is changing all of that.
CHALLENGING PRECONCEPTIONS ABOUT THE ROLE OF PHYSICIANS AS ADVOCATES
The program began in the fall of 2018 with stimulating online modules and readings that guided us through various facets of modern leadership, advocacy preparation, financial wellness, and emotional intelligence. Each module expanded my understanding of the complexity of our changing healthcare system and challenged my preconceptions about the role that physicians can play in advocating for impactful and important transformation of our healthcare landscape.
It was October 2018, and the plane was landing in Philadelphia – the site of the first in-person training component of the program at the ACG Annual Scientific Meeting. It was here that the rubber really met the road for me—figuratively speaking this time. I met my fellow participants—sisters and brothers in the trenches of early career and trial-and-error leadership learning. Soon we would meet Dr. Mark Pochapin and Dr. David Hass as they led us through an informative and open discussion of state-of-the-science leadership, networking, organizational development, and models of individual influence.
Particularly poignant was when I stood in front of a large blank paper with instructions to start creating my own leadership model. As I grouped valued characteristics into categories, my model began to take shape as a kite. As I looked around the room at each of the scholars’ models, I paused as I recognized what an amazing experience this was—to be in a group of leaders just starting their careers with so many gifts and so much promise.
ADVOCACY TRAINING AND EXPERIENCE IN WASHINGTON, DC
When the plane landed again it was April 2019 and I was in Washington, DC. What was a VA gastroenterologist from Mississippi doing in the national power-seat—what could I do? That question was soon to be answered, and, in the answering, this program and this experience fundamentally changed how I see my role as an ACG diplomat. I walked through buildings named for luminaries of our national discourse and met with fascinating and passionate Representatives and Senators from my state. I found, more surprisingly, that the YPLSP had given me an awareness of my own passion for my profession and the patients for whom we provide care. More importantly, the training of the program had given me the language, the understanding, and the voice for the role I was about to play. Specifically, when I met with Senator Cindy Hyde-Smith with my ACG Governor for Mississippi, Dr. Stephen Amann, I remember sharing about the importance of eliminating barriers to colon cancer screening among residents of our home state. I had become an advocate, and in so doing, a leader.
In closing, this experience has been one of the most eye opening and edifying of my entire journey in medicine. The founders of the YPLSP have much of which to be proud. They have provided critical and timely training for physicians looking to be leaders and advocates for the field of gastroenterology specifically and the ACG and the practice of medicine more broadly. The skills I have learned and tools I have been given will serve me life-long, and I have been inspired to be a change agent in the rapidly evolving state of national healthcare. Thank you for affording us such an amazing opportunity to grow!

—Dr. Paine

(Page 35) Dr. Paine at the United States Capitol, April 4, 2019. This page (Photo top) YPLSP attendees at a Congressional Briefing with ACG President Dr. Sunanda V. Kane and Rep. Kim Schrier (D-WA) (center front). (Photo bottom) L to R: Dr. Pooja Singhal, Dr. Sunanda Kane, Dr. Sadeea Abbasi, and Dr. Elizabeth Paine.
REFLECTING on MY EXPERIENCE
as an ACG Fellow at the FDA
ACG’s FDA Visiting Fellowship Program gives current first- or second-year GI fellows the opportunity to apply for a one-month rotation at the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research, Division of GI and Inborn Error Products. Fellows gain valuable experience in the drug/ medical device registration and approval process and observe the interaction between FDA, academia, and industry.
Recently, ACG MAGAZINE invited Dr. Amanda Cartee and Dr. Yasmin Hernandez-Barco to offer reflections on their experiences as ACG-FDA fellows and the insights they gained.
What was your specific project/assignment?
Throughout the five-week fellowship, I attended meetings (internal and with industry), courses, and a public workshop on pediatric drug development for Inflammatory Bowel Disease. During the public workshop, the FDA, investigators, and industry came together for a full day of discussion about barriers to drug approval for pediatric IBD patients, emerging topics (i.e., therapeutic drug monitoring), and solutions to difficulties in pediatric drug approval. The division also arranged for me to meet one-on-one with people from different groups within and that work with the division: toxicology, chemistry, statistics, labeling, epidemiology, devices, and over-the-counter (OTC). This gave me an opportunity to see the depth and breadth of the FDA’s role in drug approval and drug regulation.
In addition, I completed a literature review project on histologic small bowel assessment for celiac disease to review the role of biopsy in diagnosis and monitoring response to treatment, proper biopsy technique, and pros and cons to various histologic scoring systems for celiac disease.
I have a new appreciation of the FDA’s role in regulation post-drug approval. I learned that drugs and industry are regularly monitored after approved in several ways. First, drugs manufactured are audited and tested to ensure that the chemical composition and active metabolites are the same as when the drug was going through the approval process, ensuring safety and efficacy of the product itself. There is also an epidemiology group who monitors for side effects reported by patients, providers, and industry as part of post-marketing surveillance. I learned about how this can be more difficult for the FDA than other regulatory bodies in countries with a unified health system. Lastly, I learned how important clinical trial design is to drug labeling and the FDA’s role to ensure that claims remain true to study findings.
—Dr. Cartee
Amanda K. Cartee, MD, GI Fellow, University of Michigan Dr. Cartee was an ACG FDA Fellow during her training at Mayo Clinic Rochester.
What motivated you to apply to serve as an ACG fellow at the U. S. Food & Drug Administration?
My main clinical interest is in celiac disease and gluten-related disorders. One of the emerging areas in celiac disease is non-dietary therapeutics in addition to or in place of a gluten-free diet. There are several agents in the early phases of drug development. I thought that visiting the FDA would give me greater insight in to the process of drug development from non-clinical to approval, post marketing surveillance, and the interaction between the agency and industry. The fellowship exceeded my expectations. From the moment that I arrived, all of the staff were welcoming and eager to teach me. I could not have imagined a better, more fulfilling experience.
One of my first activities was learning about the history of the FDA and its interaction between Congress. The main tenets of the FDA are to ensure that drugs are both safe and effective. Previously, I had not known
how the FDA is entrusted to protect vulnerable populations, including healthy volunteers and children. Until my rotation at the FDA, I was not familiar with the laws requiring industry to perform studies in children after adult approval to again ensure safety and efficacy for this group.
I also learned about the emphasis the FDA places on patients and their role in drug approval. This was evident in the requirement for patient reported outcome measures in clinical studies. In addition, listening sessions were held between patient advocacy groups and the agency to help increase communication of the important aspects that new treatments can improve quality of life and effects to measure.
After this experience, I have a greater appreciation for the thoughtfulness and effort that goes in to ensuring the drugs we prescribe are safe and effective for our patients.
I would absolutely encourage other GI fellows to apply to this program. Whether interested in clinical medicine, research, and/or regulatory medicine, this program has something to offer everyone. The program allows you to get a glimpse of the nuances of drug labels, clinical trial design, and the various components of drug approval. and the factors which matter to this important body. I gained so much more from this fellowship than I ever imagined and can definitely say I left a better physician and researcher.
What was your specific project/ assignment?
My project involved describing the natural history of pancreatic diseases and the unique challenges in pancreatic disease drug development. Currently, no guidance exists for pancreatic disease drug development, and it is my hope that my work at the FDA will help to inform the evaluation and development of potential treatments for patients with these diseases.
As part of my project, I had the opportunity to work closely with the entire team at the Division of Gastroenterology and Inborn Errors Products (DGEIP) in the Center for Drug Evaluation and Research (CDER). I was immediately welcomed by my mentor, associate director, and extended team. From the beginning of my time there, I was fully immersed as a team member and had the opportunity to attend meetings with research and industry sponsors. I also met with experts from each of the labeling subdivisions and had the opportunity to work in the Center for Devices and Radiological Health (CDRH), where I was able to learn about the latest devices and radiographic developments for the treatment and diagnosis of GI diseases.
I learned that the FDA operates through an incredible network of individuals, comprised of physicians, scientists, statisticians, pharmacologists, epidemiologists, and more. These individuals form teams, which work together to ensure that industry and researchers can effectively and safely develop new drugs and treatments for patients across the country. These teams work within the parameters of outlined regulations and guidance to ensure that safe and efficacious treatments reach patients.
The FDA represents the doctors and safety gatekeepers for the country. The most surprising thing about the FDA is the organizational structure leveraged for the drug development process. These robust teams of incredibly intelligent individuals work tirelessly with researchers and industry to ensure that clinical trials are well-designed and that the indications, ultimately printed on drug labels, are informative and useful for payers, physicians, and patients.
I developed a greater understanding of the labeling process and the indications printed on drug labels (both over-the-counter and prescribed). There is a wealth of information included on each label, which is carefully included by FDA experts after their careful review of available clinical trial data. I will certainly be able to better counsel patients as a result of understanding the incredible amount of information included on each label.
Absolutely. Moreover, I specifically recommend this program to individuals interested in regulatory science, population health, or clinical trial research.
This experience came at a pivotal moment in my career. I am very grateful for this tremendous opportunity and wish to thank all of the incredible individuals who I met during my time at the FDA. I hope to have the opportunity to continue to be a part of the development of FDA guidances, specifically those for pancreatic diseases.
Yasmin G. Hernandez-Barco, MD, GI Fellow, Massachusetts General Hospital, Research Fellow, Harvard School of Medicine
As a physician-scientist, my research focuses on making a significant impact on the lives of patients through medical advances. The FDA shares my goal and plays a critical role in the approval of ground-breaking treatments found through major laboratory and clinical discoveries.
I applied for this fellowship to gain insight into the way the FDA works, “The FDA represents the doctors and safety gatekeepers for the country. The most surprising thing about the FDA is the organizational structure leveraged for the drug development process.”
—Dr. Hernandez-Barco
EDUCATION
THE ACG EDGAR ACHKAR VISITING PROFESSORSHIP

Providing Noteworthy Speakers for Training in Your Communities
(EAVP), the ACG Institute is committed to creating opportunities for speakers to serve as faculty for medical grand rounds presentations and to enhance the educational experience of GI fellows-intraining while providing clinically relevant presentations that also include ACG member physicians in the community.
EAVP visits continue to reach diverse GI programs across the country and to spark excellent discussions while inspiring and educating fellows. Here ACG MAGAZINE highlights visits by David T. Rubin, MD, FACG, to Brown University; Brian E. Lacy, MD, PhD, FACG, to New York Presbyterian/Weill Cornell; William D. Chey, MD, FACG, to the University of Colorado; and ACG Institute Director Nicholas J. Shaheen, MD, FACG, to Wayne State University/Detroit Medical Center. “This was an incredible opportunity to learn from a world-renowned expert on IBD in an intimate and friendly setting in our own backyard... the ACG Edgar Achkar Visiting Professorship is an innovative and effective way to foster the exchange of knowledge at the forefront of GI and furthers the education of gastroenterology trainees directly from highly regarded subspecialty experts. ”
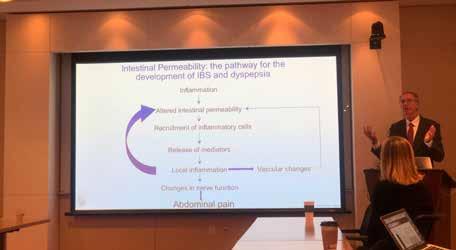
“Dr. Lacy's talk on functional bowel disorders (i.e., in particular, irritable bowel syndrome) was incredibly thought-provoking and insightful. Perhaps, what was most impressive and distinct from any prior functional bowel talk, is the way he presented a cogent argument for better understanding the underlying pathobiology of irritable bowel syndrome in helping guide treatment options. It was a transformative talk in making a condition, that is often a great source of frustration for both patients and providers alike, into a disease process akin to inflammatory bowel disease or gastroesophageal reflux disease.”
—Shawn L. Shah, MD, Gastroenterology Fellow


“The most interesting and engaging lecture was on Thursday evening when multiple metro Detroit GI fellowship programs came together for this lecture. There were engaging discussions on screening, surveillance, and endoscopic management of Barrett’s as well as on some of the newer diagnostic modalities.”





—William D. Chey, MD, FACG 2019ACG EDGAR ACHKAR
VISITING PROFESSORSHIPS
EAMONN M. M. QUIGLEY, MD, MACG
Mount Sinai Beth Israel, St. Luke and West FEBRUARY 26–27
AMY S. OXENTENKO, MD, FACG
NYU School of Medicine APRIL 2
STEPHEN B. HANAUER, MD, FACG
Lehigh Valley Health Network APRIL 3–4
DAVID T. RUBIN, MD, FACG
Brown University MAY 8–9
BRIAN E. LACY, MD, PHD, FACG
New York Presbyterian/Weill Cornell MAY 9
NICHOLAS J. SHAHEEN, MD, MPH, FACG
Wayne State University/ Detroit Medical Center MAY 9–10
WILLIAM D. CHEY, MD, FACG
University of Colorado JUNE 6–7
DAVID J. HASS, MD, FACG
Cooper University Hospital JUNE 13
DOUGLAS K. REX, MD, MACG
Texas Tech University Health Sciences Center El Paso JUNE 14
ASHWANI K. SINGAL, MD, MS, FACG
University of Iowa Hospitals & Clinics JULY 25
EDWARD V. LOFTUS JR., MD, FACG
University of California, Davis SEPTEMBER 19
MARIA T. ABREU, MD
Mountain Vista Medical Center/ Midwestern University NOVEMBER 12-13
TRAINING IN ONCOLOGIC ASPECTS

of Gastroenterology and Endoscopy at MD Anderson Cancer Center, Houston, Texas
Irina M. Cazacu, MD, ACG International Training Grant Recipient
RECEIVING THE ACG GRANT AND TRAINING AT MD ANDERSON CANCER
CENTER was, for me, a dream come true. I was honored to have the opportunity to train and perform research under the mentorship of the renowned gastroenterology expert, Dr. Manoop S. Bhutani, who has offered incredible support throughout the process of my application and during my fellowship.
I received my medical degree from the University of Medicine and Pharmacy in Craiova, Romania four years ago. There is a significant cancer burden in Romania and an acute need for oncologists, so I decided to begin my training in medical oncology to fight against one of the world’s most dreaded diseases. My plan is to develop a clinical and research career with a focus on oncologic aspects of gastroenterology.
With this goal in mind, I started my PhD studies with an emphasis on molecular profile and early detection of pancreatic cancer. I am fortunate to have an outstanding mentor, Dr. Adrian Saftoiu, who shared with me his continuous excitement of practicing in the field and all the challenges faced during attempts to improve the diagnosis of gastrointestinal cancers. He encouraged me to apply for the ACG International Travel Grant that would offer me the chance to train abroad and acquire clinical and research experience from a leading cancer center in the United States.
FINDING A ”SECOND HOME” AT MD ANDERSON
Houston and MD Anderson became my second home very quickly. I was pleasantly surprised with the philosophy of this prestigious institution, with its focus on excellence, respect, and value of diversity. I came to understand the pure dedication this institution has toward patients with cancer, and it really dawned on me that the simplest things can make a huge difference in the lives of our patients.
During my training at MD Anderson, I was exposed to a wide variety of complex oncological cases and gained experience from the highvolume of patients I saw. I shadowed and observed various complex endoscopic procedures performed by Dr. Bhutani in cancer patients, enriching my medical knowledge by understanding the indications, findings and clinical implications for each case.
Dr. Bhutani is part of the multidisciplinary expert panel who developed the pancreatic cancer screening algorithm. As part of his team, I was involved in research projects regarding pancreatic cancer screening and this experience offered me a better understanding of this concept, from clinical benefits to psychological impact.
LEARNING FROM MULTIDISCIPLINARY TUMOR BOARDS
I attended various multidisciplinary tumor boards related to gastrointestinal cancers, where I gained first-hand knowledge from experts in the field. At the moment in Romania, there are only vague recommendations for the use of multidisciplinary teams. At MD Anderson, I became familiar with the principles and practice of multidisciplinary teams and my plan is to promote interdisciplinary interactions that will facilitate effective teamwork at my home institution. I had also the chance to attend various educational sessions, teaching rounds, journal clubs, and lectures from world-class faculty. I grew so much as a doctor in this past year due to the sheer number of opportunities afforded to me.
A TURNING POINT IN MY RESEARCH CAREER
My training at MD Anderson under the mentorship of Dr. Bhutani was a turning point in my research career. I was placed in a stimulating environment with an outstanding team who facilitated my scientific training and productivity. I was able to extend the output of my research papers by a considerable amount, both in qualitative and in quantitative terms. I participated in several research projects, from study design, Institutional Review Board submission, data collection, data analysis, to drafting of the manuscripts. My work resulted in six manuscripts accepted for publication and two presentations at international meetings, DDW 2018 and ACG’s Annual Scientific Meeting in 2018.
EUS-guided fiducial markers placement for image-guided radiotherapy in gastrointestinal malignancies was another area of research during my training at MD Anderson. We have conducted a meta-analysis showing that EUS-guided placement of fiducial markers is safe and has a high rate of technical success. Furthermore, I was part of the ongoing research projects looking at the clinical and survival benefits of fiducial markers in patients with pancreatic cancer. I had also the

—Irina M. Cazacu, MD
incredible opportunity to observe new treatment modalities for pancreatic cancer that are under research at MD Anderson and are likely to change the clinical practice in the future.
PLANS FOR THE FUTURE
My dream and future career plan is to be actively involved not only in the treatment of gastrointestinal cancers but also in the diagnosis of these devastating diseases by training in interventional gastroenterology. I plan to become a pioneer of the pancreatic cancer screening program in Romania upon my return.
Being part of Dr. Bhutani’s team was a tremendous experience which will inspire and encourage me as a person and as an oncologist/ interventional gastroenterologist for the rest of my professional career to achieve and to provide the best possible care for my patients. Dr. Bhutani was not only my mentor but also my biggest career advocate. I am very grateful to the ACG for providing me with an awesome learning experience that is invaluable to me and the patients I will care for. Without question, training at MD Anderson Cancer Center under the mentorship of Dr. Manoop Bhutani was a wonderful, lifechanging experience that I will carry with pride and honor for the rest of my medical career.






