Your Product Development in a Global and Rapidly Evolving Regulatory Environment



The food and beverage industry has to comply with an increasing number of guidelines and restrictions that vary by location. The industry is challenged continuously by evolving regulatory requirements related to substances, labeling, claims, origin of ingredients and packaging. Even though the industry encourages a global shift to strengthen and improve product labeling to help consumers make more informed choices, food and beverage manufacturers still need to follow country specific regulations. This can quickly become cumbersome and overwhelming.
Laws and regulations are continually evolving with significant differences from one country to another, impacting each and every department of a company. In fact, those regulations impact every step of a product development, from R&D to marketing through procurement and quality. Non conformity can be both costly and damaging for a brand image. In addition to these challenges, product development needs to be more and more innovative as it becomes increasingly complex to put products on the market.
Therefore, it is crucial for food operators to have a complete overview and control of a product development in order to be regulatory compliant, manage product information easily and efficiently, and considerably improve collaboration with suppliers and service providers. This can be achieved by implementing four simple best practices: 1) having a comprehensive knowledge of commercialization rules, 2) selecting the right information, 3) complying with regulation by design all along the commercialization process, and 4) collaborating efficiently with all external stakeholders.

The authorization of an ingredient or additive is strictly connected to the finished product’s legal name. In order to ensure compliance with applicable regulations, the following steps are recommended
1) define the product category
2) assess if all the ingredients, including additives, are allowed in this specific category
3) apply restrictions, such as, maximum levels
Allowance and limits for potential additives and compositional ingredients can be different for each country of commercialization of a product. Thus, it is compulsory for a company to know all the associated regulatory frameworks.
In the EU additive allowance is regulated under Regulation ( 1333 2008 Ingredient allowance is defined in vertical legislations specific to product categories. Food products such as chocolate, jam, fruits juices and canned tuna are covered by EU regulations or directives defining product composition. Other product categories depends on a country’s legislation.
Legislative requirements on food additives are different in the USA and in Canada. For ingredient allowance, federal standards define official names and composition requirements specific to certain food product
CA PROP 65 is a California law intended to protect the citizens from a list of chemicals that are known to cause cancer, birth defects or other reproductive harm. The program is administered by the Office of Environmental Health Hazard Assessment (OEHHA) of the California Environmental Protection Agency (CalEPA) OEHHA determines whether chemicals meet the criteria to be added to the CA PROP 65 list and administers labeling, warning and risk exposure requirements for these substances to be placed on the California market. It does not prohibit the sale of products containing hazardous substances at any level. To guide businesses in determining whether a warning is necessary or whether discharges of a chemical into drinking water sources are prohibited, OEHHA has developed “safe harbor levels” for many CA PROP 65 chemicals : “No Significant Risk Levels” for chemicals causing cancer and “Maximum Allowable Dose Levels” for chemicals causing birth defects or other reproductive harm. Depending on the level of exposure, penalties for violating CA PROP 65 can be as high as $2,500 per violation per day.
Examples of regulatory limits for specific substances in food products
Substance Product EU USA Canada
E160 B Annatto Flavored fermented milk products
10 mg/l
Good Manufacturing Practices
Sulphur dioxide Frozen vegatables 50 mg/kg Good Manufacturing Practices
Whey Chocolate Milk chocolate: Allowed, no limit, must be ≥ 14% for dry milk solids from partly or wholly dehydrated whole milk semi or full skimmed milk full, partly or wholly dehydrated cream, butter or milk fat.
Liquorice Confectionary containing glycyrrhizinic acid or its ammonium salt.
If addition of liquorice plant. Glycyrrhiza glabra at 4 g/kg, mention to be added after the list of ingredients or after the name of the food : ‘contains liquorice people suffering from hypertension should avoid excessive consumption’.
White chocolate: must be ≤ 5% (in weight)
Good Manufacturing Practices
Frozen mushrooms: 90 ppm
Milk chocolate: must be < 5%
Maximum levels (% glycyrrhizin content) 1.1 for chewing gum, 16 for hard candy and 3.1 for soft candy. FDA recommends to avoid eating large amounts of black licorice at one time to be informed about black licorice possible interactions with medications, herbs and dietary supplements.
No warning or limit for food products but only for Drugs and Health products cautions, warnings and contraindications are given for pregnant women (e.g. liver disorder, high blood pressure).
Examples of regulatory requirements for ingredients in relation to specific products
Substance EU USA Canada
Gluten-free products Gluten < 20 ppm Gluten < 20 ppm Gluten < 20 ppm
Organic products ≥ 95% of organic ingredients.
Exceptions: added water and salt.
GMO containing products Mandatory to declare if any ingredient is genetically modified. Exceptions GM food products in a proportion ≤ 0.9% of the individual food ingredients adventitious or technically unavoidable presence.
≥ 95% of organic ingredients.
Exceptions: added water and salt.
Federal legislation not mandatory to declare if the ingredient is genetically modified. Can be different in State Regulations.
≥ 95% of organic ingredients.
Exceptions: added water and salt.
It is not mandatory to declare if the ingredient is genetically modified.
› In Europe, food labeling is ruled by Regulation (EU) 1169/2011 covering all product categories of prepacked food and introducing specific new requirements for allergens and nanomaterials as well as mandatory nutrition labeling. Additional information for specific products (e.g. organic food, olive oil) can be found in other horizontal and vertical regulatory texts. Some national legislations are the transposition of these EU directives however each country can have specific regulations, from product conformity requirements to labeling rules. For instance, cheese is not regulated under an EU common regulatory framework fat on dry matter analysis is mandatory only in some countries.

› Entering the USA market successfully requires a complete and deep knowledge of each state and federal agency laws. Federal agencies dominate the regulatory oversight the US Department of Agriculture, Food Safety and Inspection Service monitors meat, poultry and egg products whereas the Food and Drug Administration is responsible for the remaining 75-80% of the U.S. food supply, including domestic and foreign food processing establishments. State agencies have an overseeing role at local level on food processing business in collaboration with federal agencies. Regarding food and nutritional labeling, food additives and product standards, almost all requirements are covered by federal law through the Code of Federal Regulations (CFR) Title 21 “Food and drugs”, Part 101 “Food labeling”. It covers various topics such as “Nutritional labeling of food” in section 9 in which the new Nutrition Facts declaration requirements are set out CFR part 101 also covers food additives and “Menu Labeling Requirements” in other dedicated sections.
› In Canada the CFIA (Canadian Food Inspection Agency) is the regulatory body responsible for food, animals, and plants safety and delivers federated regulatory programs Health Canada which is the health inspection department of CFIA, is setting out policies, regulations and standards for food and nutrition labeling, food additives, advertising and product standards under the Food and Drugs Act. It notably includes requirements for food allergens declaration in the list of ingredients, ‘Nutrition Facts’ table to support healthy eating and self management of health as well as instructions for a safe use, consumption, storage and handling of goods. Some new regulations are expected by 2021: food colors will be declared by their common name rather than the generic term “color”, the list of ingredients and allergens will be simplified and a front of pack labeling scheme will be developed.
Substance EU USA Canada
Graphic rules for legibility
› 1, 2 mm minimum for mandatory sentences
› 0, 9 mm if largest surface area of label <80 cm2
› Quantity statements must have minimum heights according to the net quantity
› 1, 6 mm minimum for mandatory sentences
› Name of the product and quantity statement to be emphasized with specific typing dimensions
› 1, 6 mm minimum for mandatory sentences
› Quantity statement typed in specific dimensions depending on the area of principal display surface
Product names and quantity
Product name and quantity statement to be written on the same visual field (not necessarily the main one)
Product name and quantity statement must be reported and shown to the public.
Product name and quantity statement must be reported and shown to the public.
How to indicate allergens
14 allergens with mandatory declaration to be emphasized through a specific typeset (e.g. in bold) in order to be clearly distinguished from the other ingredients of the list.


8 allergens to be mentioned with the name of the food source in bold and into brackets after the ingredient or with a ‘contains’ statement after the ingredients list same type size). Ex:“whey (milk)” in the list if ingredients or “Contains milk” at the end.
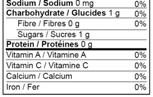
18 allergen food sources and 5 gluten food sources to be declared in the list of ingredients or with a contains/ contient statement at the end of the list of ingredients. Ex: “Contains Wheat”.
Allergens crosscontamination requirements
Minimum stability dates
Notdefined, even in Regulation (EU) 1169 2011
Not defined
The cross contamination ingredients can be indicated with a ‘may contain peut contenir’ statement.
Mandatory with fixed wordings. Few exceptions. (e.g. vinegar, candies, salt, sugar).
Not mandatory, with exception of infant formula.
Mandatory for prepacked products with a shelf life of 90 days, to be declared in both official languages with differences in formatting.
Nutrition information

Front of pack (FOP) nutritional labeling systems have been developed in various countries in order to increase transparency and help consumers to make informed choices. They can be voluntary or mandatory.
Voluntary FOP schemes generally include information about sugar, fat and salt contents per 100g or per portion of food as well as a scoring system. (e.g. stars, colour code, letters) indicating if these quantities are high or not, and/or the global quality of the nutritional profile of the product. While such national schemes have been used for many years in countries such as UK Australia and Scandinavian countries a new one has recently been adopted in France. The Official Journal of the French Republic recently published the order of October, 31st 2017 regulating the Nutri Score system as recommended by the French government under Articles L 3232 8 and R 3232 7 of the Public Health Code. This new labeling system consists in a graphic marker synthesizing the nutritional score of foods in 5 classes on a scale of 5 colors (from dark green to red), associated with letters ranging from A (“best nutritional quality”) to E (“poorer nutritional quality”). This system have been adopted after a consultation process that found it was the “best understood” labeling system. The graphic symbol of Nutri Score and its characteristics are provided in the appendix of the order. Any eligible company wishing to use the Nutri Score mark must notify its intention to French Public Health organization.
As a consequence of the e-commerce trend, European Regulation (UE) 1169/2011 states that all mandatory information on prepacked food products intended for distance selling must be provided before the consumer decides to purchase the article. Consumers should be given the necessary information to make informed choices as it is the case in a supermarket. This information must be accurate and provided in real time, thus introducing electronic exchange challenges between food operators (in particular between manufacturers and retailers). Compatibility of product information systems and management of recipe changes are among the key challenges.
FOP nutritional labeling can also be mandatory. For instance, in Mexico a compulsory FOP nutrition label with a declaration of sugar, sodium, fats, and caloric content per portion is in force since the amendment of the nutritional labeling regulation in 2014. The declaration of nutrients and caloric content must be done in the correct order from the left to the right. Some products are exempted of this FOP label requirement Herbs, spices, seasonings or mixture there of; extracts of pure coffee, whole beans, ground, decaffeinated or soluble or insoluble; herbal teas, decaffeinated or caffeinated tea, instantaneous and/or not containing soluble additives. Fermented vinegars and substitutes and products for sale in bulk. The FOP label can be complemented with a voluntary “nutritional stamp” based on very strict nutritional profiles developed by International Food Beverage Alliance (IFBA).
A nutrition claim is an indication referring to the content of one or more nutrients in a food product. Allowed nutrition claims are usually listed in relevant regulations and do not require any specific approval procedure. Nutrition and health claims significantly differ in terms of wording and conditions of use according to the country of commercialization.
In EU countries the conditions of use of such claims depend on nutrients content in 100 g or 100 ml of food.
In the USA and Canada they depend on nutrients content per portion (or serving) of the product.
Examples of nutrient content claim about calcium
Conditions of use The product must contain 15% per 100g or 100ml (or per package if it contains only a single portion) of calcium Recommended Daily Intake.
The food product must contain 10% to 19% of the Recommended Daily Intake per reference amount customarily consumed.
The food product must contain at least 5% of the Recommended Daily Intake per serving size.
A health claim refers to any indication stating, suggesting or implying that a relationship exists between a food category, a food product or one of its constituents and human health. Health claims are subject to different approval requirements depending on the country of commercialization and on the type of indication (it can notably be a structure/function claim, a disease risk related claim or a new scientific evidence based claim). Some of them require a premarket approval before their use, while other ones are listed in relevant regulations and can be generally used if the conditions of use are satisfied.
Food subject of the claim Sugar-free chewing gum Noncariogenic carbohydrate sweeteners xylitol, sorbitol, mannitol, maltitol, isomalt , lactitol, hydrogenated starch, hydrolysates, hydrogenated glucose syrups and erythritol isolated or combined), D tagatose, Isomaltulose Sucralose.
Wording of the claim Sugar-free chewing gum contributes to themaintenance of tooth mineralization
Frequent eating of foods high in sugars and starches as between meal snacks can promote tooth decay. The sugar alcohol used to sweeten this food may reduce the risk of dental caries.
Chewing 1 piece (2.7 g) of sugar free gum, 3 times per day after meals, helps reduce the risk of tooth decay.
Conditions of use Chewing gum must comply with the legislation for the nutrition claim SUGARS FREE
Food shall contain ≥ 1 of the noncariogenic carbohydrate sweeteners mentioned, with no minimum level required.
Sugar-free chewing gum must
› contain ≥ 0,8 g of sugar alcohol bulk sweetener per serving of stated size and reference amount
› meet the conditions for “free of sugars” and contains ≤0.25% starch, dextrins mono di and oligosaccharides or fermentable carbohydrates combined
Additional information required
Yes, consumer should be informed that the beneficial effect is obtained with chewing of 2-3g of sugar free chewing gum for 20 minutes, at least three times per day after meals.
No Yes, Total content of sugar alcohols must be declared in the Nutrition Facts table in grams per stated serving size.
The European Commission recently launched a public consultation on a regulation proposal for the establishment of methods of application of the Article 26.3 of Regulation (EU) 1169/2011. More specifically, this regulation would define rules to indicate the country or the place of origin of the primary ingredient of a food if it differs from the claimed origin of the main food. Interested stakeholders could send their comments by February 1st 2018. After the consultation, the act will be voted by a Committee with every Member State. The regulation is expected to enter into force on the 3rd day following the date of its publication in the Official Journal of the EU and will apply starting April 1st 2019. Food placed on the market or labeled before the date of application can be marketed until stocks are exhausted.
According to the regulation draft, the origin can be expressed with the following geographical areas
› “EU”, “non-EU” or “EU and non-EU” Member State(s) or third country(s)
› Region or any other geographical area within a Member State or a third country, which is clearly understandable by average informed consumers
› The country of origin or place of provenance in accordance with specific EU provisions applicable to the primary ingredient or ingredients as such (e.g. level of precision established for beef)
Alternatively, the food operators can put on the label an indication such as : ”X [X main ingredient] does not come from (the country of origin or place of origin of the food)” or a similar formulation that can still have the same meaning for the consumer.
The origin of the primary ingredient must be expressed with a font size not less than the minimum size required by Article 13.2 of Regulation (EU) 1169/2011 (1.2 mm) and, in any case, in the same field of vision in which the indication about the country or place of origin of the food is written. Furthermore, it must not be less than 75% of the height of the indication of the country or place of origin of the food, if expressed in writing.
Also at the European level country of origin regulations are already in place for meat, honey, olive oil, wine, fish and organic food.
Starting from requirements of Regulation (EU) 1169/2011 and waiting for European Commission implementing regulations on ingredients origin, the Italian government published several laws regulating the labeling of the country of origin of certain finished products (e.g. milk, pasta, rice, tomato pasta and wine) as well as the ingredients they may contain.
A French Decree 2016-1137 setting up labeling mandatory indications for prepacked food was published on August 21st 2016. Only food intended for final consumers manufactured and marketed in France is concerned. Prepacked food manufactured in other countries (including EU Member States) and products with protected geographical indication are out of scope. The targeted products are milk used as an ingredient in dairy products (yogurts, butter, cheese) and meat (beef, pork, sheep, goat, poultry) used as an ingredient in processed food. For meat, the country of birth, rearing and slaughter of animals has to be stated with specific wordings that depend on whether the meat has a single country of origin or more (“EU origin”, “non-EU origin”). For milk, the country of collection, packaging or processing has to be specified. This information is stated in the list of ingredients just after the relevant ingredient or with a note at the end of the ingredient list. Order of September 28th, 2016 related to Decree 2016-1137 defines specific thresholds for milk and meat used as an ingredient 50% and 8% respectively above which the indication of their origin is mandatory. Product traceability will last for five years and some financial penalties are defined in case of non compliance with provisions.
Food labeling is not only ruled by food sector legislations it can also be covered by regulations on packaging and its recycling.
In Europe, the regulatory framework is laid down by the Packaging Directive 94/62/EC of December 31st 1994 and has been revised in 2004 It gives targets that countries need to meet with their own strategy and legislation. As a result, regulatory texts can be very different from country to country.
In Germany, the legal basis is the Packaging Ordinance of 1991 that aims at preventing, reducing, reusing and/or recycling packaging waste and, consequently, to return it to the production loop.
On January 1st 2019, a new German Packaging Act will come into force with additional ecological waste management requirements.
Different types of packaging are considered:

› Sales packaging
› Overpacks
› Transport packaging
› Beverage packaging
› Reusable packaging
It defines the following principles:
› Packaging waste should be avoided
› If packaging waste can not be avoided, reuse and recycling must be given priority over energy recovery
› Waste removal compatible with public welfare must be put in place
Therefore, it is mandatory for manufacturers to set up a collection and disposal system to manage one way packaging placed on the market for “private consumers”. In particular, a deposit is mandatory for beverage single use packaging. Moreover, specific symbols and logos have to be added on the packaging in order to allow refund system.
In France, the Triman logo must be visible on all recyclable products collected separately and subject to an extended producer responsibility principle after use. If not possible, it has to be added to the packaging, package insert or any other medium, even when a product is sold online. This requirement is the result of the implementing decree 2014-1577 of January 1st 2015 and is subject to a deposit. The generalization of this signage is gradually implemented.
The use of the Triman logo and related signs is free and authorized by ADEME (French Environment and Energy Management Agency) for informational purposes. In fact, it aims at supporting consumers in clearly and unambiguously identify recyclable products, collected separately and subject to an extended producer responsibility principle after use. The Triman logo does not replace the Green Dot or the “Info tri” Green Dot.
Eco emballage and Adlephe are two State recognized organizations with the missions of organizing, supervising and supporting the recycling of household packaging in France. Industrials that are supporting and contributing financially to the waste management system monitored by Eco Emballage or Adelphe must print a Green Dot on all the packaging they place on the French market. Thus, the presence of the Green Dot on a packaging does not necessarily mean that it will be recycled It only means that the manufacturer has to contribute financially to the collection, sorting and reprocessing of its products’ packaging. The “Info tri” Green Dot completes and gives more sense to the Green Dot by providing to consumers additional information it specifies for each item of packaging if it has to be discarded or recycled, and how.

Food contact materials (FCMs) are materials which are or are intended to be in contact with food during its production processing storage preparation and serving such as food packaging, kitchenware, processing machines and containers. Thus, they can have an impact on food safety and quality throughout the whole food supply chain. FCMs cover a wide range of different materials such as plastic, paper, glass and metal, but also adhesives, printing inks and coatings used in the finishing of final goods.
To ensure the safety of FCMs, a series of legal requirements and controls are in place in the European Union Commission Regulation (EC) 1935-2004 is the European framework legislation for FCMs. It sets out the general principles of safety and inertness. Article 3 states that substances present in FCMs shall not migrate into food in concentrations that could endanger human health or imply an unacceptable change in the food composition or a deterioration in its organoleptic properties.
The regulation also sets out general requirements for FCMs manufacturing as the obligation to follow Good Manufacturing Practices (GMP) laid down in Commission Regulation (EC) 2023/2006 as well as specific requirements for the authorization process.
FCMs must be demonstrated to be compliant with the rules through appropriate tests part and be subject to the Declaration of Compliance DoC.
A material or article is considered “safe” when it respects specific migration limits or other regulatory restrictions. When such a limit is not established, toxicological evaluations made by EFSA are considered as the reference. For non evaluated substances, the manufacturer has the responsibility to demonstrate the product safety.
EU Regulation on plastic materials and articles Regulation (EU) 10/2011 is the most comprehensive and effective European regulation with specific measures for plastics, processes for recycling plastics, regenerated cellulose film, ceramics, and active and intelligent materials and articles.
In the absence of specific EU measures, Member States may maintain or adopt their own national provisions that comply with the rules of the treaty.

In the USA and Canada, the approach towards FCMs is different, notably regarding definitions, laws, and official bodies involved in the processes of risk assessments and authorizations.
Main actors Risk Assessment by EFSA, Risk Management by European Commission.
Risk Assessment and Risk Management by FDA.
Voluntary submission to Health Products and Food Branch (HPFB) for food packaging assessment of chemical safety.
Authorization Dossier for substances to be authorized.
FCMs are considered as indirect additives chemicals that might be transferred to the food by its packaging or processing equipment.
FCM is only food packaging and materials that come in contact with food during production and processing but not other consumer products such as kitchen tools, utensils, etc.
Regulatory framework Commission Regulation (EC) 1935/2004 is the main reference. Requirements are based on migration.
Reference framework regulation is the Code of Federal Regulation (CFR), Title 21. A Food Contact Notification is necessary for compliance. Requirements are based on exposure, Threshold of Regulation (ToR) are given.
Division 23 of the Food and Drug Regulations is the main framework. It contains a dmission and non-admission, limits of substances.
Labels must comply with increasingly complex legislations throughout the world. Ensuring products are labeled correctly and completely with applicable local national and international laws is one of the major challenges of food and consumer good businesses.
An important aspect to consider when exporting to a foreign country is that a mere literary translation of labels, despite being done by a native speaker, is not always sufficient. Specific wording is required that may not come across in the translation. Regulations on labeling define specific wordings that are mandatory and can differ even in the same language the wording for the “best before date” in Canadian French “meilleur avant” is not the same as in France (“à consommer de préférence avant le”). Moreover cultural and linguistic specificities must be taken into consideration, especially to make the consumer feel confident with the product.
Even in the case of common regulations, each single country has its own interpretation according to the local level of tolerance and sensitivity on specific topics.
For instance, in Italy authorities and control bodies will scrutinize information such as the country of origin (“made in Italy” label.) This is due to the high amount of domesticfood products In contrast, their approach is less challenging for products coming from other EU countries.
In many countries specific guidelines as defined by national bodies or professional associations apply for various and numerous food product categories, besides official regulations. These labeling guidelines are not only for product composition but also for “marketing” in terms of names and pictures that appear on the packaging. As an example, printing a vanilla flower on a ice cream packaging is allowed only for products made with natural flavoring.

A non-conformity can arise from different aspects of a product such as:
› Its composition it can contain banned ingredients or additives
› Hygiene aspects contaminants can be present in higher levels than authorized
› Labeling issues mandatory information can be missing, marketing texts can be misleading
If the non-conformity is detected, it can involve different types of penalties notably product recalls, criminal sanctions and financial costs. Each country has its own legal framework on penalties. However, it is difficult to foresee what are the risks associated. In fact, many factors such as the size of the industry/retailer, the exposition of the product to the market and interpretation by local authorities have to be taken into account for a non conformity linked with marketing issues.
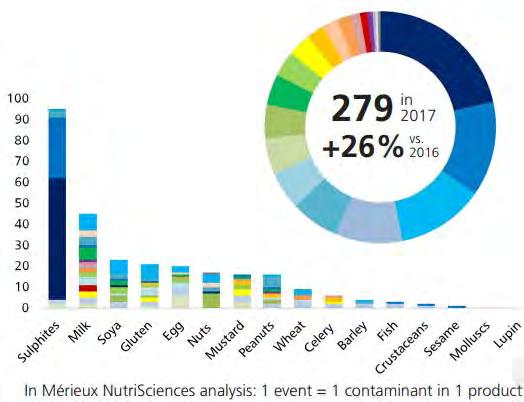
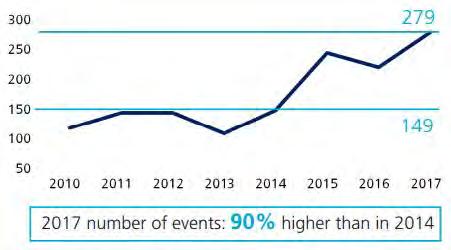
Presence of an undeclared allergen in a food product is a serious food safety hazard with high risk for final consumers. If it happens in Europe a withdrawal procedure in line with regulation (EC) 178/2002 principles must be immediately initiated, associated with huge costs (several millions) and damages on brand image. Nevertheless, undeclared allergen remains a major cause of food product recalls worldwide, with an increasing trend over the past few years. Their presence is largely due to the occurrence of cross contamination in factories and supply chain, or to labeling errors (wrong label or erroneous allergen declaration). Allergen related recalls may also be caused by food fraud. In Europe, a 26% increase in notifications due to allergens was observed in 2017 according to the Rapid Alert System for Food and Fee (RASFF) database, which is managed by the European Commission. A total of 279 notifications was made by European Member States However, this number does not properly reflect the real number of allergen alerts in European countries. Most allergen recalls are not actually linked with cross border situations, and therefore are not reported in RASFF system.
One of the pain points for the food industry is the management of food labeling due to small adjustments of recipes such as the substitution of a raw material due to a supplier change. If the introduction of a new allergen must obviously be declared and requires the switch to a new pack, the change in nutritionals do not necessarily result in the immediate change of label artwork. Food operators need to know how tolerant the local authorities are to take an appropriate decision regarding label stocks. Therefore, anticipation, foresight and good knowledge of local rules and their interpretation are key to minimize risks and costs.
Mérieux NutriSciences analysis
It is clear that product compliance requires a strong knowledge of local rules and their interpretation, as well as the ability to always be up to date regarding these rules in order to anticipate the application of any possible change.
Typically, small companies that lack a dedicated regulatory department are challenged by compliance/ regulatory issues and must rely on external specialists or guidance provided by industry trade associations. But even for medium size and big companies which export products to a large number of countries, monitoring those countries without any people based in the region is time consuming and quite a big challenge.
› How to be aware in real time of new regulations?
› How to know the interpretation and tolerances by local authorities, as well as by professional organizations?
› How to be aware of ongoing discussions?
Whatever the situation, relying on local regulatory experts is essential to answer the key questions and ensure product compliance.
Having access to a network of food regulatory specialists spread out in the countries where the products are intended to be commercialized, is a key element of food companies strategy. This is the best way to maximize product and label compliance while minimizing the risks related to it. Moreover, using specific dedicated tools to facilitate country monitoring and be alerted in case of impacting new rules is suitable to gain in efficiency and reactivity. No need anymore to spend time on identifying the right sources and monitoring the related websites. Instead, time is spent applying the appropriate rules with the support of experts, maximizing product compliance and meeting the marketing targets as well as consumer expectations.
How to adapt the labeling of my product to this country?
How to know the new and emerging regulations?
How to know if a regulatory change impacts my products?
What are the rules for this product on this market? Which specifications for the related ingredients?
Does my product and its ingredients comply with local rules of this country?
How to implement a new regulation and consolidate with existing rules ? How to know the local interpretation of the text?
Information is key during product development, but having the right information, at the right place and at the right time is better. However, managing a large amount of data can be difficult when there is no proper procedure in place. For instance, if the R&D team creates a new recipe or modifies an existing one, there is a large amount of information that will be created, such as cost simulation data, final weight of ingredients, health claims, etc. in addition to the one that already needs to be taken into account, like the Bill of Materials (BOM).
Another example is the compliance/regulatory and quality departments, which are also impacted by the large amount of data. They have to screen all this information from recipe to labeling, and end up spending too much time trying to sort the data instead of quickly ensuring a product compliance. Moreover, if there is no structure or process in place to manage, classify and save the data in a consistent way, the different teams will not be efficient It is then clear that resources are spent on non value added tasks.
These examples illustrate how it can be challenging to handle all the information and, efficiently apply the appropriate regulations and guidelines at the different stages of a product lifecycle. However, several steps can be implemented to solve these challenges.
Creating a unique source of information will enable you to quickly access and find the right information at the right time. Once the product information is gathered and consolidated, it is easy, when needed, to retrieve, re-use and share this information. Product information includes data, requirements, claims, certifications, specifications, documents and information related to the product development, product changes/modifications, and quality and compliance/regulatory management.
For all product development stages, from idea generation to end of a life, a product life cycle generates various data and documents Managing all this information can rapidly be overwhelming. In addition, the modification or substitution of one element, such as a raw material containing a new allergen, requires additional attention as a small change can impact a large number of finished products.
So to understand the various elements’ relationships, to have all specifications readily available at any stage of a product lifecycle, and to avoid duplication of information, it is necessary to gather and structure all the data and documents in a consistent way. The different elements also need to be organized and have clear and established links between them.
Being regulatory compliant requires companies to create and generate various documents and information, which may have to follow specific frameworks defined by regulations. Some examples include ingredient statements, nutritional facts labels or simply a product datasheet. To effectively manage these documents, it is important to have all information dematerialized in one place, to create templates defining which information is needed, and to automatically fill in those templates with the required information.
Businesses need to ensure the compliance of their products during their entire lifecycle.
Every department of a company R&D, quality, compliance/regulatory, marketing, procurement and so on is impacted by regulations therefore, information continuity and collaboration are crucial.
To ensure compliance, each department needs to work with the most up to date and accurate information. This can become challenging and frustrating when there is a large volume of information and no process in place that clearly defines how and where to find the right information.
The different departments, especially the compliance/regulatory and quality teams, lose time requesting product information and analyzing it, while they could spend it being more regulatory proactive, (i.e.: anticipating new regulations, taking actions before non-conformities arise, etc.)
The R&D, compliance/regulatory and quality teamwork illustrates this challenge the first one should be able to use and apply pre-defined guidelines and enforced regulations when creating and testing recipes, without having to wait for the compliance/regulatory and quality departments to notice an error or non-conformity. This is also true during the NPD process, the regulatory affairs team is usually involved in the later stages, when a product is ready and needs safety assessment, which can slow down time to market if a non-conformity is detected at that time.
On the other hand, the compliance/regulatory and quality teams should have the latest version of a recipe and not be overwhelmed by trial and error from the R&D.
In addition to that, dealing with multiple markets adds other challenges as a company faces multiple country specific regulations various suppliers, different languages, etc.
The examples mentioned previously demonstrate how important information continuity is different departments working on different steps of a product life-cycle, with numerous exchanges, tests, versions controls, product modifications, etc. and they all need to use and share product information from one department to another.
So to facilitate a product compliance, it is essential to create strong collaboration through information continuity to quickly track information and data, easily establish a link between the various product’s elements from raw materials to finished product, and automate tasks and processes in order to avoid human error, (e.g. automate nutritional values calculations, ingredient statements and lists, etc.). This can be achieved by sharing a single source of information, enforcing collaboration through processes and workflows and structuring similar product information in a consistent way.
Information continuity is relevant not only during product development but also to the different IT systems used by a business, especially an e-commerce platform. A company re uses information generated during the product development phase and pushes it to its e-commerce platform. However, manual keying or a non structured source of information can lead to the update of the wrong information. Hence, the importance of having a structured and single source of information, with easy to find product data, that can be seamlessly and automatically pushed towards an e-commerce website.
Streamlining communication and collaboration with partners such as suppliers, food testing specialists, regulatory experts and creative agencies, enforces compliance from the beginning.
Let’s take the example of the requests for proposals sent by clients food and beverage manufacturers often have to deal with a precise list of requirements or bill of specifications from those clients. They must quickly respond to these proposals. They should not have to spend time chasing supplier information, products sheets or double checking information accuracy. It is then necessary to centralize all this information, receive notifications and leverage the current product portfolio to look for existing products that exactly meet the requirements or could be re used and modified.
If a new product needs to be developed, it is also important to have a single source of information to perform an advanced search in order to easily find specific raw materials which meet the client’s needs, (e.g. allergens, health claims, etc.).
Additionally, workflows should be implemented to streamline internal and external processes. Internally, to gain time searching for product information raw materials’ certifications, etc. and, externally, to streamline communication with partners.
› How to ensure the timeliness and accuracy of your information
» The first step is to gather all data and documents received from external stakeholders,stakeholders, (e.g. requirements, certifications, documents, etc.
» Similar information should follow the same structure for example if your suppliers submit different raw materials’ datasheets, the information of those datasheets should ultimately follow the same order.
» It is essential to ensure that your single source of information is always up to date. This will optimize and speed up any information search and ensure data accuracy.
› How to enforce the compliance of your product
» The quality team should implement workflows, processes and/or a portal to easily track quality and tests results. They should also have access to any documentation that will help them determine, for instance, that the labeling and ingredient statement are regulatory compliant. However, to be more efficient, the quality department should determine, in advance, a detailed control plan with the different tests that need to be performed.
» Working with food regulatory experts will provide additional support in improving product compliance They will deliver test and consultancy services to ensure the safety and compliance of a product. They have the knowledge and skills to give, for example, their approval on products and labeling conformity.
» It is possible to automate through an IT solution those processes. It allows you to easily track samples and collect tests results, but more importantly, it enables you to automatically link all these results to your finished product and to optimize this relationship.
Some easy steps can be taken to streamline communication with your suppliers and partners.
» The first one is to always structure the information received and send from/to your external stakeholders in the same way. For example, similar test results received from a laboratory should have a consistent structure.
» Additionally, every time your suppliers submit new information, such as a price change, new characteristics of an existing raw material, or a certification’s update, you should immediately update the element concerned by the modification. The following options will support the implementation of these different steps create, use and share with your partners your own templates or set up a supplier portal. The last one will enable authorized partners to log on to a secured platform and fill in all product’s details, upload certificates, etc following templates defined by your company. They will become more proactive in submitting information which will improve collaboration. It will also enforce their accountability as they will be responsible for updating the required elements. The same processes should be implemented for packaging development to ensure that the required characteristics, such as dimensions and composition, are respected.
Compliance also relies on collaboration around the artwork the marketing department and/or the packaging team manages the development of an artwork however, it is the quality and compliance/regulatory team which validates the final output. Having a common platform where they can all exchange while developing the artwork will speed up its creation. Authorized stakeholders, including the marketing department, packaging team and external creative agency, should also have access to regulatory guidelines while developing the artwork.

Food and beverage manufacturers face important regulations when launching their products. From ingredients to labels to packaging, it is challenging to comply and be up to date with the various laws, guidelines and restrictions. New factors such as the needs for traceability and transparency emphasize this challenge and stress the importance of having the right processes, workflows and support in place. Globalization also highlights the need to have a complete understanding of the local rules.

Therefore, to ensure an efficient product development and a faster go to market, it is essential to set up and follow regulatory frameworks It requires structured information, data continuity from suppliers to consumers, and efficient collaboration facilitated by optimal processes. Those frameworks will not only facilitate each stage of a product development, but also support the entire product life cycle, from marketing brief to end of life.
So, Compliance by design is the optimal methodology to be regulatory compliant. The agility factor is peer to peer expertise from a regulatory ecosystem. This can also be described as collective intelligence, with the sharing and use of experts’ knowledge and skills. Collective intelligence is the ability to collaborate and share expertise and knowledge between every stakeholder (e.g. regulatory experts, IT solution providers, company’s departments, etc.) to facilitate product development and speed up product launch.
Aptean is one of the world’s leading providers of purpose-built, industry-specific software that helps manufacturers and distribu tors effectively run and grow their businesses. With both cloud and on-premise deployment options, Aptean’s products, services and unmatched expertise help businesses of all sizes to be Ready for What’s Next, Now®. Aptean is headquartered in Alpharetta, Georgia and has offices in North America, Europe and Asia-Pacific.
To learn more about Aptean and the markets we serve, visit www.aptean.com.