3 minute read
Water and Biology
by AudioLearn
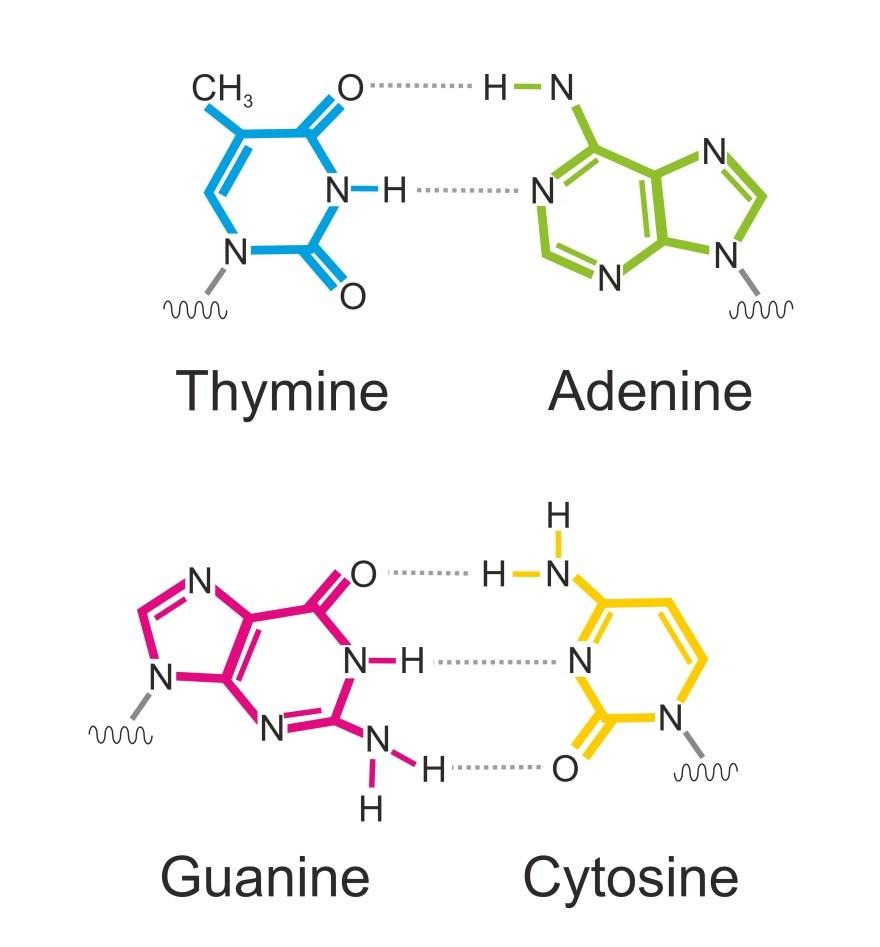
RNA is also a nucleic acid but is different from the DNA molecule. The sugar is a ribose sugar and not a deoxyribose sugar. The thymine nucleotide is not seen in the RNA molecule. Instead, there is guanine, which has a base pair with adenine in doublestranded RNA. Most RNA, however, is single stranded and does not have a base pair situation happening with it.
WATER AND BIOLOGY
Advertisement
Water is extremely important to life because it has a number of features that make it an excellent medium in which to have the biochemical reactions necessary for life occur. It is also very abundant in the earth; in humans, it involves 70 percent of the body. Perhaps there are life forms on other planets that do not contain water as part of their structure; however, life as we know it on earth is entirely dependent on it.
What features make water so good when it comes to life? As mentioned, water is a polar substance, which means that it is a molecule with a positive end electrically and a negative end electrically. This causes it to connect to or bind easily to other polar
molecules. What it doesn’t bind well to are fat-soluble molecules, like lipids. Fortunately, using certain biochemical “tricks” like carrier proteins, lipids can survive in an aqueous or water-containing solution. Molecules that can easily dissolve in water are called hydrophilic; molecules that cannot do this easily are called hydrophobic.
The formula for water is H2O. This means that two hydrogen atoms are covalently bonded to one oxygen atom. This is a bent molecule with a negative oxygen side and a positive hydrogen side (and thus its polarity as a molecule). Hydrogen bonds will connect water molecules together in solution; however, a hydrogen bond is considered ten times weaker than a covalent bond so it can be easily broken when necessary.
Water is a solvent that takes things up in solution. The compounds that are ionic (such as sodium chloride and other metal salts) will easily dissolve and break their bonding so they can be surrounded by water molecules, hydrating the ions. Both positive ions, like sodium, potassium, and calcium, and negative ions, like phosphate and chloride, have the ability to be hydrated by water. In fact, any polar molecule will dissolve easily in water, even larger molecules, such as polysaccharides, polypeptides, and nucleic acids.
Water is also a good temperature buffer. This is important because many enzymatic reactions in biochemistry require a specific temperature and cannot work outside of that temperature. Water has a high specific heat capacity, which is the heat required to raise a kilogram of water by one degree Celsius. This means that those reactions that give of heat as energy are also protected by the heat buffering capacity of water from raising the surrounding temperature too much.
Water acts as a metabolite in many enzymatic reactions involving metabolism. Metabolism is basically all of the chemical and physical processes within a cell, both those that build up molecules and those that break down molecules to form energy. Water is necessary for photosynthesis, aerobic respiration in the cells, and digestion of nutrients. It participates in hydrolysis (the breakdown of molecules) and condensation (the joining of two molecules together in a reaction).
Water participates in pH phenomena in its environment. The pH of a substance depends on whether it has a predominance of acids or a predominance of bases or alkaline substances. Solutions like stomach acid have a predominance of hydrogen ions,
which are highly acidic solutions like lye have a predominance of hydroxide (OH) ions, which are highly alkaline. The pH scale ranges from 1 (which is highly acidic) to 14 (which is highly alkaline). The pH considered to be neutral is in the middle at 7. In living things, the pH tends toward being neutral, with the pH of the blood in humans being strictly controlled at 7.35-7.45. This allows for most enzymatic reactions to take place and water is a part of what makes this environment hospitable for enzymatic reactions in most biochemical processes.
There are chemical buffers operating in cells and in the circulatory system that keep the pH within a narrow therapeutic range. This is necessary because, as mentioned, enzymes are tricky and don’t function outside of a certain pH range. In humans, there are protein buffers, bicarbonate buffer systems, and phosphate buffer systems. These will help maintain a pH balance in water so that there are not very large changes in the pH of body systems.



