6 minute read
NEWS
from REP March 21
Industry News
Vizient invests with PPE manufacturers to expand, stabilize U.S.-based supply
Vizient, Inc. (Irving, TX) has entered into strategic partnership agreements with Encompass, Standard Textile, and Prestige Ameritech in order to expand the available supply of a variety of needed personal protective equipment (PPE) items.
Building on an earlier agreement with Encompass Group, LLC for 19 million disposable gowns, in August, Vizient announced a new agreement with the company to provide an additional 65 million AAMI level 3 disposable isolation gowns manufactured annually in North America to its member hospitals.
Since then, Vizient has partnered to help Encompass start a new, automated manufacturing line for disposable gowns in the U.S., bringing both supply and onshore resiliency for these products, the company says.
The new manufacturing line is expected to be up and running in the first quarter of 2022.
Vizient has also partnered with Standard Textile to expand its US-based manufacturing operations for Vizient members. In the initial months of the pandemic, the agreement provided 1.4 million reusable fabric cover gowns (approximately 75 uses each), 1 million reusable fabric face masks and 500,000 reusable plastic face shields for Vizient members.
In August, Standard Textile joined the Novaplus Enhanced Supply Program, which provides Vizient members with globally diversified manufacturing and USbased stockpiles.
As a result, an additional 5.2 million reusable gowns, 1.3 million shoe covers, 1.5 million bouffant caps, 1.5 million skull caps and 1.3 million fabric face masks per year will be available for Vizient members who participate in the program.
Vizient also has a commitment to Prestige Ameritech for 9 million, U.S.-made N95 masks over a 12-month period. Since the agreement launched in April 2020, approximately 700,000 masks have been delivered monthly to program participants, Vizient said.
Sysmex, Roche renew agreement for global alliance
Roche (Basel, Switzerland) announced that, on December 14, 2020, it signed a Global Business Partnership Agreement (GBPA) with Sysmex.
Sysmex and Roche initially signed a GBPA on December 14, 2020 to form a framework for their alliance and commenced activities accordingly on January 1, 2021. The GBPA includes the DSSA and the TLSA, as well as an IT Solutions Collaboration Agreement, and the two companies have agreed to develop a next-generation global alliance.
Roche will continue to distribute and support Sysmex hematology products in selected countries in Central and South America, Europe, southern Africa, and Oceania. Sysmex plans to boost direct sales and support structures in Spain and India, both of which were added to their list of territories pursuant to the TLSA, in a bid to increase the resilience of the two companies’ global sales and service network.
This GBPA also introduced an IT Solutions Collaboration Agreement. In the newly defined collaboration, the two companies have agreed to utilize their respective IT platforms to improve customer experience in the short to mid-term, with a longer term ambition to use the IT systems to lead to improved clinical decision making.
The GBP agreement will run until the end of 2030.
BD announces data showing its antigen test may be more selective in detecting infectious COVID-19 patients than molecular tests
BD (Becton, Dickinson and Company) (Franklin Lakes, NJ) announced the publication of a peer-reviewed study that it says shows BD’s antigen test may be more selective than PCR (polymerase chain reaction) molecular tests at detecting people who are contagious and able to spread COVID-19 disease.
The study compared antigen and PCR test results to positive results using a viral cell culture test. Viral growth in the cell culture test indicates the presence of live virus in the patient sample, which may indicate the presence of infectious virus at the time the sample was taken. If no growth is present in the viral cell culture test, it is likely that there wasn’t enough viable virus for the patient to be contagious at the time the sample was taken.
Out of 38 positive PCR result specimens tested, only 28 were positive using the cell culture technique.
The antigen tests, conducted using the BD Veritor Plus System, were positive in 27 of the 28 cell culture positive tests. The company says that this data suggests that 10 of the 38 PCR positive results were potentially identifying non-infectious individuals, meaning PCR detected viral RNA fragments or small amounts of intact SARS-CoV-2 virus and that the patient wasn’t actually contagious at the time the sample was taken.
However, the BD antigen test agreed with all but one cell culture positive test.
“Point-of-care antigen tests, as demonstrated in this study with the BD Veritor Plus System, have the potential to significantly change the public health interventions needed to minimize the spread of COVID-19,” said Dr. Charles Cooper, study co-author and VP of Medical Affairs for BD. “By providing a more relevant test to identify individuals that are likely to be shedding infectious virus and therefore transmit SARS-CoV-2, we will be in a better position to contain its spread. Plus, the low cost and scalability of antigen-based testing makes it an important tool to contain and suppress COVID-19 community transmission.”
Medical drone startup to begin COVID vaccine delivery
Zipline Inc. (San Francisco, CA), a drone delivery service that specializes in medical supplies, announced that it plans to begin transporting COVID-19 vaccines in April.
The startup said in a release that it is partnering with “a leading manufacturer of COVID-19 vaccines” in all of the markets where its drones currently operate.
Zipline has been delivering medicine and supplies to rural clinics in Rwanda and Ghana since 2016 and, last year, began delivering personal protective equipment to hospitals and clinics in North Carolina. It plans to add operations in Nigeria later this year.
Zipline declined to specify its vaccine partner but said it has built a system that can deliver ultra-low temperature medical supplies, including “all leading COVID-19 vaccines,” according to Bloomberg News.
The vaccine developed by Pfizer Inc and BioNTech SE must be stored in extreme cold at temperatures of negative 70 degrees Celsius, requiring special freezers.
Zipline plans to add these ultra cold refrigerators at all of its distribution centers.
Pfizer, responding to an earlier request for comment about whether it is working with Zipline, said in a statement, “Pfizer supports Zipline’s efforts to expand access to vaccines and medicines to those in hard to reach geographies.”
Vaccine distribution sites without ultra-low refrigeration have limited options: They can forego Pfizer’s vaccine entirely opting for the Moderna Inc. alternative and hoping for additional vaccines from Johnson & Johnson and others to gain FDA approval; keep Pfizer’s vaccine vials on dry ice for up to 30 days; or keep them in standard refrigerators for up to five days. Zipline can help bypass the need for freezers – and prevent vaccines from spoiling – by repeatedly supplying a small numbers of doses on demand.
A clinic in its network, the company says, will be able to request a few dozen doses of a COVID-19 vaccine and receive them at an ultra low temperature in less than an hour.
Zipline’s fixed-wing, battery-powered drones navigate by GPS. They drop payloads of a few pounds each by parachute and can fly up to 100 miles round trip.
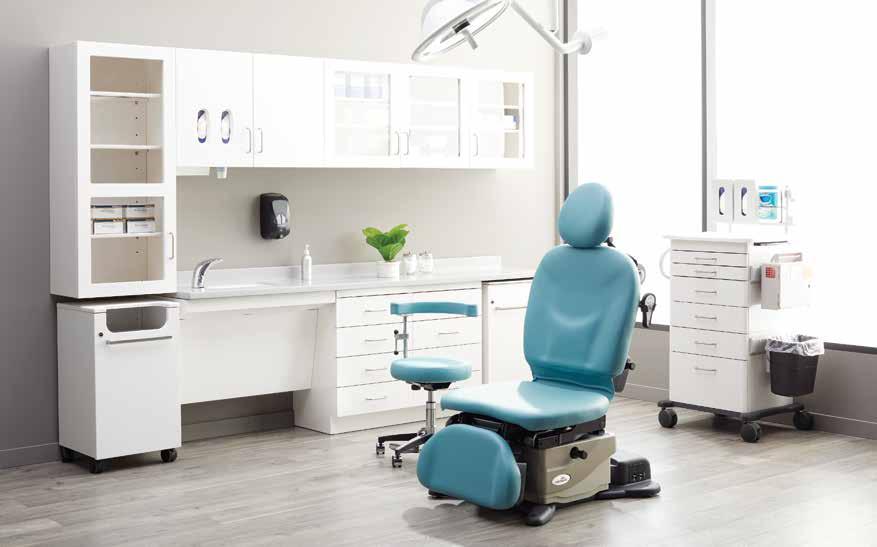
Download the promo details and a patient positioning infographic you can share with your customers at: midmark.com/ProcedureProgram
SAVE NOW!
2021 PROCEDURE ROOM PROGRAM
Your customers can SAVE NOW on Midmark® Procedure Chairs, Ritter® LED Lighting, Midmark® Mobile Procedure Carts and Ritter® Stools! Don’t miss this opportunity—be sure to tell your customers about these deals! (These offers end June 30, 2021.)
Don’t Be Fooled By Imitators
Don’t let imitators fool you in the new year, ensure you have authentic infection prevention products.
Increasing global demand for gloves and PPE created a tidal wave of inexperienced and unreliable entrepreneurs entering the marketplace, sourcing and marketing products without provenance, quality or proven compliance in the US.
“In times like these you must protect yourself and your customers by ensuring they have the authentic, reliable infection prevention products needed.”
Ventyv® is the premier brand of Sri Trang USA, Inc., a member of the Sri Trang Group. Sri Trang is a proven glove producer that has been protecting the world against infection since 1991.





