1 minute read
UP-developed Covid-19 test kits approved for commercial use
UP-developed Covid-19 test kits approved for commercial use COVID -19 News
By Joyce Ann L. Rocamora
Advertisement


MANILA - The Food and Drug Administration (FDA) has approved the coronavirus disease 2019 (Covid-19) test kits developed by the University of the Philippines-National Institutes of Health (UP-NIH).
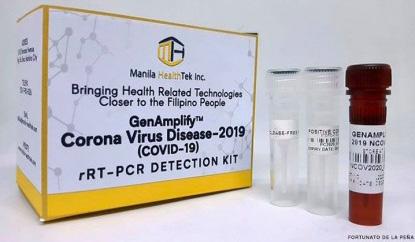
“The Food and Drug Administration (FDA) has now approved the Real-Time PCR for the detection of COVID-19 manufactured by the Manila HealthTek, Inc. for commercial use,” the FDA said in an advisory on Friday night. The UP-developed test kit was previously approved by the FDA for field trial. week with coronavirus detection kit
“This is the first locally made PCR based COVID-19 test kit approved by the FDA which was developed in collaboration with the University of the Philippines-National Institute of Health (UP-NIH), funded by the Department of Science and Technology (DOST),” the FDA said. (Image grabbed from DOST Secretary Fortunato dela Peña’s Facebook page)
Upon the company’s submission of necessary requirements, the FDA issued a certification for the COVID-19 test kit “to be allowed for commercial use”. Aside from the locally-made PCR based test kit, the FDA has also approved one additional rapid test kit on Friday, namely, “Hightop SARSCoV-2 IgM/IgG Antibody”, bringing the total number of Covid-19 approved test kits to 30. (PNA)





