
20 minute read
A Year Like No Other
Unlocking the Promise of Precision Medicine
The COVID-19 pandemic has created often unmanageable volumes of patients requiring acute care for a disease that’s never been previously seen. Early on, clinicians were observing that severe COVID disease and death are more prevalent among patients who are elderly and/or who have certain underlying health conditions. There were little data about what factors contributed to those poor outcomes, and what therapies are effective. Drs. Brian Garibaldi (Pulmonary and Critical Care), Scott Zeger (former Provost and Chair of Biostatistics), and Amita Gupta were tapped to establish the Precision Medicine Center of Excellence for COVID-19 to begin to identify patient-tailored care solutions. Drs. Matt Robinson and Bob Bollinger from the CCGHE were also enlisted to help support the COVID Precision Medicine Center’s work. This center is the newest of 17 precision medicine centers throughout the Johns Hopkins Health System.
Advertisement
Systemwide Patient Data Repository and Severe COVID Risk Calculator
The first effort of the Center was to establish a COVID-19 data registry (JH-CROWN) from patient records across 5 of the Hopkins-affiliated hospitals—Johns Hopkins Hospital, Bayview Medical Center, Howard County General Hospital, Sibley Memorial Hospital, and Suburban Hospital. Garibaldi, Gupta, Robinson and colleagues then analyzed the data to identify specific health factors that predict the likelihood that a patient admitted with COVID-19 will progress to severe disease or death within 7 days. “We identified a few readily measurable demographic and clinical factors that, when assessed on admission to the hospital, can predict if someone has a 5% or a 90% risk of developing severe disease or dying from COVID-19” says Dr. Gupta. “It’s incredibly useful information to have when communicating with patients and families and for informing resource allocation in the hospital.”
Published Findings:
Patient Trajectories Among Persons Hospitalized for COVID19: A Cohort Study
Garibaldi BT, Fiksel J, Muschelli J, Robinson ML, Rouhizadeh M, Perin J, Schumock G, Nagy P, Gray JH, Malapati H, Ghobadi-Krueger M, Niessen TM, Kim BS, Hill PM, Ahmed MS, Dobkin ED, Blanding R, Abele J, Woods B, Harkness K, Thiemann DR, Bowring MG, Shah AB, Wang MC, Bandeen-Roche K, Rosen A, Zeger SL, Gupta A. Annals of Internal Medicine. 2020 Sep 22. doi: 10.7326/M20-3905. Epub ahead of print. PMID: 32960645; PMCID: PMC7530643.
Dr. Amita Gupta
Dr. Matthew Robinson
Dr. Bob Bollinger
Continued
Dr. Matthew Robinson
Published Findings:
Development of Severe COVID-19 Adaptive Risk Predictor (SCARP), A Calculator to Predict Severe Disease or Death in Hospitalized Patients with COVID-19
Wongvibulsin S, Garibaldi BT, Antar AAR, Wen J, Wang MC, Gupta A, Bollinger R, Xu Y, Wang K, Betz JF, Muschelli J, BandeenRoche K, Zeger SL, Robinson ML. Ann Intern Med. 2021 Mar 2. doi: 10.7326/M20-6754. Epub ahead of print. PMID: 33646849.
Smart Tool for Patient-Tailored COVID Clinical Projections
Building on the initial risk prediction findings, Dr. Matthew Robinson applied artificial intelligence computer learning tools to make an even more powerful tool. With a team of colleagues in precision medicine, biostatistics, and data science, he designed and developed an adaptive clinical tool that predicts patienttailored clinical trajectories, which was validated using data from the JH-CROWN registry. The Severe COVID-19 Adaptive Risk Predictor Prediction Model (called SCARP) provides clinicians with real-time, evidence-based projections about a patient’s risk for severe disease or death within the next 24 hours and next 7 days of hospitalization. SCARP is a smart tool that continuously adjusts with changing clinical factors. In other words, it updates an individual patient’s 1 and 7 day risk scores, based on data entered at the bedside into Hopkins’ patient medical records system. SCARP’s forecasting provides advance notice for positioning treatments, ICU beds, and other resources, and it provides information critical to patient and family communications and decision making. SCARP is felt to be so useful, that it is now fully integrated into the Johns Hopkins Health System electronic medical records system (EPIC).
The SCARP effort has just been published, and it marks the quickest timeline from research to implementation across all of Johns Hopkins’ 17 Precision Medicine Centers of Excellence. Robinson’s work hasn’t gone unnoticed. He was recently honored with the Johns Hopkins 2020 Clinician Scientist Career Development Award, and the SCARP tool is garnering attention from clinicians around the world seeking a more tailored approach to COVID-19 patient care. Additionally, the FDA has approached him about potentially using its crowdsourced CURE-ID platform to help identify which COVID-19 therapies are showing the greatest promise. CURE-ID is an internetbased case study repository in which members of the clinical community report new uses of existing drugs for difficult-to-treat infectious diseases. With SCARP’s capability of taking thousands of data points, crunching them rapidly, and returning patient-tailored care options, there is certainly no shortage of applications.
According to Dr. Robinson, “As healthcare providers, we know what information we need to deliver high quality care. As researchers, we are in a position to look critically at what’s possible and meet the need. Applying research to practical use in improving patient care is tremendously gratifying.”
Precision Medicine
Continued
COVID-19 Treatment Outcomes among Minority Populations
A randomized clinical trial demonstrated that use of the drug remdesivir was associated with a shorter inpatient stay for hospitalized COVID patients. However, the benefit of prescribing remdesivir in the community setting outside of clinical trials is not clear. In addition, COVID-19 is having a disproportionate and devastating impact on risk of infection, hospitalization, and death among Black and Latino communities in the U.S. However, since a small percentage of participants in the remdesivir clinical trial were Black or Latino, it is also important to confirm that remdesivir is being prescribed for, and is equally beneficial in, all COVID patients. Therefore, the Hopkins COVID Precision Medicine team is conducting a retrospective study of remdesivir use that analyzes data from Black and Latino patients in the JH-CROWN registry.
“Treatments that are effective in one slice of the population don’t necessarily help everyone,” says Dr. Bollinger. “Our job as physicians is to provide patients with care that gives them the best chances for quick and full recovery. Our job as researchers is to figure out exactly what that involves for each individual patient. Our goal is to optimize treatment outcomes for all patients, especially for patients from communities most affected by COVID-19.”
Published Findings:
Comparison of Time to Clinical Improvement With vs Without Remdesivir Treatment in Hospitalized Patients With COVID-19
Garibaldi BT, Wang K, Robinson ML, Zeger SL, Bandeen-Roche K, Wang MC, Alexander GC, Gupta A, Bollinger R, Xu Y. JAMA Netw Open. 2021 Mar 1;4(3):e213071. doi: 10.1001/jamanetworkopen.2021.3071. PMID: 33760094. Dr. Bob Bollinger

Medical records from 1,972 COVID-19 patients were analyzed for mortality and clinical improvement

Dr. Yuka Manabe

Dr. Matt Robinson

Dr. Kathleen Page
Rapid COVID Diagnostics
A new disease poses rapidly evolving challenges for care providers. Recognizing a need for accurate, reliable, rapid, and accessible diagnostic tests, NIH established the Rapid Acceleration of Diagnostics (RADx) initiative “to promote development, commercialization, and implementation” of COVID testing technologies as well as strategies that enhance access to testing among those most in need. CCGHE faculty have taken on 2 RADx efforts.
Evaluating Rapid Testing Technologies
The standard COVID test is the polymerase chain reaction, or PCR test, which detects COVID RNA to confirm the presence of disease. It’s highly accurate and sensitive, but it’s an expensive process that can take days to return results. Drs. Yuka Manabe and Matt Robinson are evaluating the sensitivity of COVID antigen tests, which instead pick up specific proteins from the surface of the virus and can return results rapidly. Study participants get the standard nasal pharyngeal swab and a PCR test, and they also get a less invasive intra-nasal swab and provide a saliva sample. The testing platforms are then compared. The goal of the the study is to answer questions about the role that rapid antigen testing can play in identifying COVID quicker and easier, thereby mitigating community spread and getting patients into care quickly.
Customized Testing Strategies for Baltimore’s Latino Community
Nationally, Latinos have been disproportionately affected by COVID. Within the Johns Hopkins Health System, for example, the COVID positivity rate between March 11 and May 25, 2020 was 43% among Latinos, compared to 18% among Blacks and 9% among Whites. To address the disparities, Centro SOL, led by Dr. Kathleen Page, partnered with the Johns Hopkins Medicine Office of Diversity, Inclusion and Health Equity, several community-based organizations that serve Latinos, media outlets, and local officials to develop outreach strategies to expand access to free testing. Approaches include a mobile testing site, a bilingual hotline to link patients to testing services, and support for the JH Emergency Department to provide timely delivery of results to Spanish-speaking patients and rapidly link them to aftercare and social services. Support is also provided for the health department contact investigation team, and a team of bilingual clinicians to facilitate complex communications with patients and families. Finally, a weekly, bilingual “Ask Your Doctor” Facebook live session is held to answer community questions about COVID-19. The team is evaluating testing uptake, positivity rates, household contacts tested, and characteristics of testers stratified by approaches and by campaign exposure. This effort leverages lessons learned from the ongoing JHHS Latino COVID-19 response, our team’s experience developing campaigns in partnership with community members to enhance HIV testing among Latinos, and a mature network of local partners
Above, team members register people seeking COVID-19 testing at the mobile clinic

Changing Course to COVID-19
When COVID arrived in Baltimore, Dr. Kathleen Page sprung into action to provide testing services and outreach to Baltimore’s Latino immigrant community. This is a role she knows well, serving as Co-Director of Baltimore’s Centro SOL, an organization that promotes equity in health and opportunity for Latinos through clinical care, research, education, and advocacy at Johns Hopkins in active partnership with the Latino community.
Dr. Page has long provided community health screenings and services to Baltimore’s Latino community, but making the leap to screening for a highly contagious disease, with no cure, and with which many people who are infected don’t have symptoms, was no small feat. Additionally, restrictive immigration policies have created conditions in which people don’t feel safe seeking healthcare services without risking deportation.
“There is no shortage of challenges,” Page notes. “At the beginning it was was getting the testing site up and running, training staff, and ensuring their safety. Our amazing team didn’t hesitate when asked to staff the mobile testing site. In the colder months we have had to ensure that we can safely provide the same services indoors.”
The team offers testing and then contacts people with results within 48 hours. Upon being tested, people are offered critical public health information about isolation and other precautions for keeping their contacts safe.
Dr. Page noted the impact of community partnership: “Our longstanding work in health services and advocacy has helped encourage people to seek testing. We also have a great partner in Father Bruce Lewandowski at the Sacred Heart of Jesus parish, who has graciously provided space for us to serve the community. 3,500 people have been screened for COVID and referred into care, as needed, so we know our team is meeting a vital need in the community. ”

Dr. Kathleen Page
India’s Mental Health Outcomes
Dr. Nishi Suryavanshi
Dr. Nikhil Gupte
Dr. Gauri Dhumal

Smita Nimkar
Mental Health Outcomes among Health Care Professionals Involved in the Treatment and Care of COVID-19 Patients in India
Frontline healthcare workers are under tremendous pressure in their role as COVID patient care providers. With shortages of personal protective equipment and other resources in India, providers have also faced a prolonged period of increased personal risk to themselves and their families. The pandemic’s toll on mental health among clinical care providers hasn’t been well documented, so Drs. Nishi Suryavanshi, Nikhil Gupte, and team launched a study to characterize the impact. The team is conducting a structured survey among healthcare professionals working in flu clinics and hospital COVID-19 units in Maharashtra. The team seeks to characterize the severity of symptoms associated with depression and anxiety, understand the specific factors having an impact on mental health, and assess the pandemic’s impact on quality of life for healthcare workers. The results could help guide the development of tailored interventions that improve mental health in the short- and long-term. Study Team: Nishi Suryavanshi, Nikhil Gupte, Abhay Kadam, Smita Nimkar, Gauri Dhumal

Pandemic Planning in Pune
The COVID-19 pandemic presented challenges for research teams around the world conducting clinical trials. The experience with tuberculosis studies underway at the Byramjee Jeejeebhoy Government Medical College-Johns Hopkins University Clinical Research Site (BJGMC-JHU CRS) in Pune, India, required swift action, agility, and innovation in ensuring that study participants continued to receive lifesaving care in the midst of a national lockdown.
The WHO has prioritized TB preventive therapy, including for household contacts of people with multidrug-resistant tuberculosis (MDR-TB), as a key strategy for controlling the TB epidemic. One study to prevent MDR-TB among household contacts of confirmed cases underway at the BJGMC-JHU CRS is a multinational, phase 3, randomized clinical trial to assess therapies for preventing confirmed or probable active TB in high-risk household contacts.
Participants on study routinely receive enough medication to last until their next scheduled clinical visit. With visits cancelled due to India’s national lockdown, and with recovery from COVID-19 likely to be prolonged, the team needed to find alternate ways to distribute medication. Mail delivery was not an option and asking research participants to pick up medication from the CRS or at designated locations within the community was deemed too risky. With the lockdown imminent, the team was under considerable pressure to develop and implement a plan to deliver enough medication to participants. While it might appear fairly straightforward, there were considerable logistical and safety issues to address:
• Specific measures were planned for, and the team notified participants of what to expect. • Online prescriptions were sent to the pharmacist for filling. • Personal protective equipment (PPE)—including gloves, N-95 masks, and hand sanitizer—was provided for staff going into the field. The team decided to use glasses/goggles and helmets rather than full PPE kits, because of the optics. There is considerable stigma associated with disease, and teams presenting to households in such gear could cause problems for participants in their communities. • Online training was provided on safe use and disposal of PPE. • The BJGMC Dean provided a letter to outreach counselors that granted them authority to travel during the lockdown. • Each Outreach Counselor was assigned a specific route to reach participants, and a “drop and run” protocol was implemented in order to minimize personal interactions. • The day before the drop, participants were contacted by the Outreach team, and phone screenings were conducted to assess COVID-19 related symptoms.
The planning that went into ensuring participants for one study were provided for during a health emergency served as a template for other studies at the BJGMC-JHU CRS. Clear communication with study participants inspired further confidence in the team’s commitment to their health and wellbeing.
Dr. Vidya Mave
Dr. Nishi Suryavanshi
Dr. Nikhil Gupte
Dr. Sandesh Patil

Dr. Natasha Chida
318 Participants per session, on average
3,100+ Subscribers Making the Rounds
Medical Grand Rounds, during which physicians discuss clinical issues and research, are a core methodology of inpatient care and medical education. Developed by Drs. Natasha Chida and Michael Melia, who lead the Johns Hopkins Infectious Diseases Fellowship Program at Johns Hopkins, ID COVID-19 Grand Rounds was established early on as a virtual seminar series to help build knowledge about research and patient care as it emerges. It has been wildly successful.
First convened April 7, 2020 as a weekly series through June, it was subsequently convened twice in July and went monthly as of August. A wide range of topics covered by top experts from around the world has drawn a large and faithful following that traditional in-person Grand Rounds does not afford.
Topics have ranged from diagnostics, therapeutics including Remdesivir and the controversial use of chloroquine/hydroxychloroquine, the role of convalescent plasma in recovery, vaccine development, ICU outcomes, achieving herd immunity, multisystem inflammatory syndrome in children, the impact of COVID by race and ethnicity, antibodies, the efficacy of protective measures like masking and social distancing, public health policy for reopening schools, and the science about long-term effects of COVID.
Drs. Chida and Melia have built a library of timely information about COVID-19 in a rapidly changing landscape. As Chida notes, “Sharing lessons from research and clinical care—across institutions and disciplines—informs improvements in patient care. It’s central to Johns Hopkins’ mission.”
10,600+ Unique visitors Archived online: https://bit.ly/2KBcgZh
Right, Drs. Michael Melia, Kathleen Page, Natasha Chida, and Damani Piggott discuss the disparate impact of COVID-19 by race and ethnicity

New Efforts to Tackle Tuberculosis

Tackling TB
One of CCGHE’s primary areas of research is to improve care and long-term outcomes for people with tuberculosis (TB). We launched several new and exciting efforts in 2020 that build on our long-standing work.
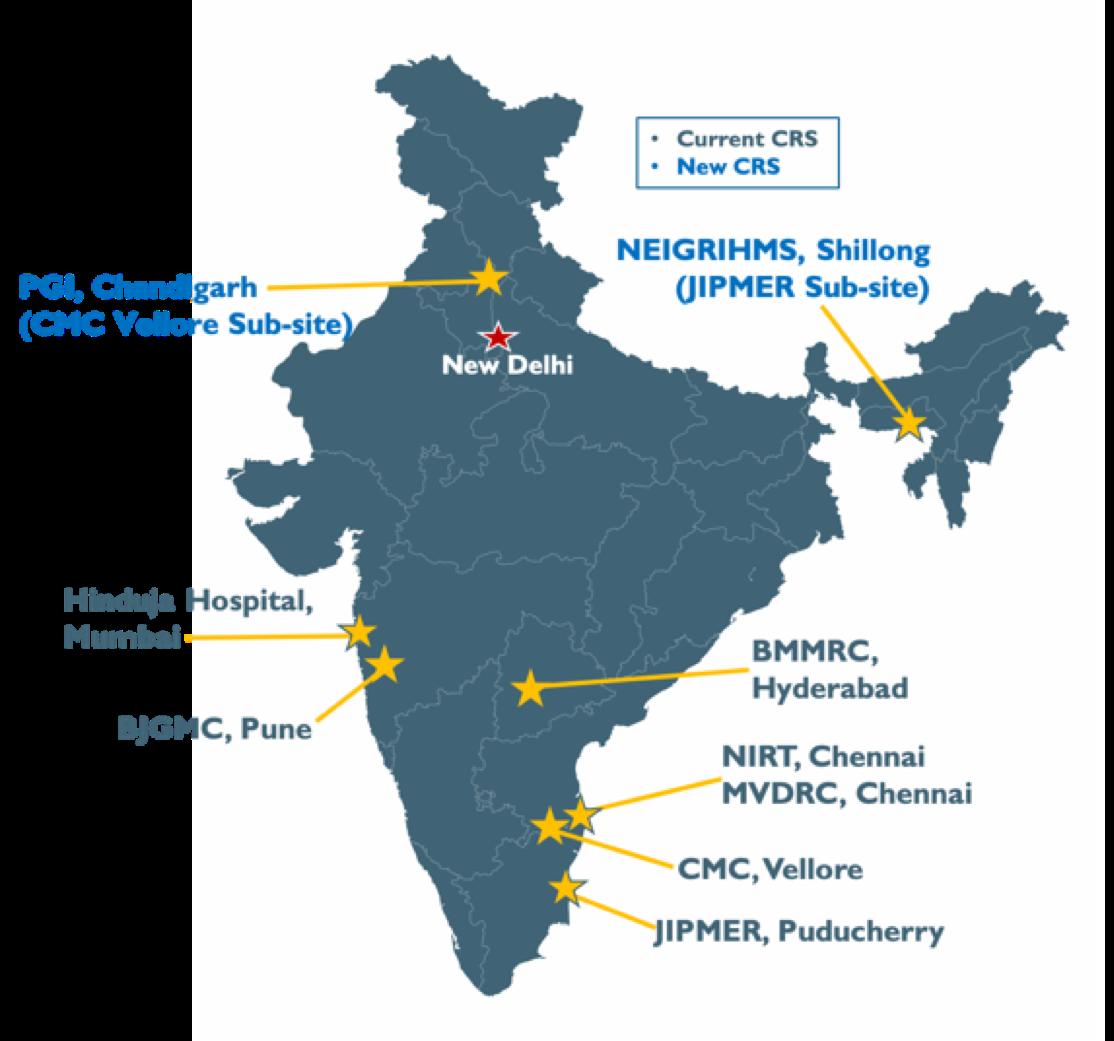
RePORT India
While we have participated in the Regional Prospective Observational Research in Tuberculosis (RePORT) consortium since 2014, Phase II of this important effort was launched during 2020.
Background
The mission of RePORT International is to advance TB science globally, with emphasis on translational research to provide new tools for TB control. India’s countrywide consortium (RePORT India): • Advances regional TB science in India, towards fulfilling the TB strategic goals of the country; • Strengthens TB research capacity and infrastructure; and • Fosters research collaboration within India and with other countries focused on research that can lead to clinically important biomarkers, vaccines, drugs, and diagnostics.
Partnerships
RePORT International supports countrywide consortia in India, Brazil, South Africa, China, Indonesia, and the Philippines for the purpose of conducting basic and clinical research to identify clinically important TB biomarkers, which are basically signals in the body that raise flags about a change in disease or that one is likely to occur. Research is also conducted on promising vaccines, therapeutic drugs, and diagnostics. The partnerships comprise in-country and U.S research institutions. Since 2014, Dr. Amita Gupta has served as the U.S. Chair for RePORT India, which represents the largest program of all participating countries.
Bilateral Support
RePORT India is supported with bilateral funding from the Government of India’s (GOI) Department of Biotechnology (DBT) and the U.S. National Institutes of Health’s (NIH) National Institute of Allergy and Infectious Diseases (NIAID), Division of AIDS (DAIDS), and Office of AIDS Research (OAR). CRDF Global administers and oversees the funding from the U.S. government.
RePORT India Partnerships
• Bhagawan Mahavir Medical Research Center (BMMRC) | University of Texas • Byramjee Jeejeebhoy Government Medical College (BJGMC) | National Institute for Research in Tuberculosis
(NIRT) | Johns Hopkins University
• Christian Medical College-Vellore (CMC-Vellore) | University of California-San Francisco (PGI Subsite) • PD Hinduja Hospital | Johns Hopkins University • Jawaharlal Institute of Postgraduate Medical Education & Research (JIPMER) | Boston Medical Center | Boston
University | Rutgers University (NEIGRIHMS Subsite) • Prof. M. Viswanathan Diabetes Research Centre (MVDRC) | University of Massachusetts | NIRT International
Centre for Excellence in Research (ICER)
RePORT India Phase I (2014-2020)
The objective for the first phase was to provide data and specimens to biomarker researchers and collaborators to better understand the prognosis of TB disease in India and how it progresses from TB exposure to active disease among 2 cohorts of patients: those who have active TB disease, and those who are household contacts (HHCs) of an active case of TB. To support the research, the RePORT India consortium established a Central TB Sample Biorepository at the National Institute of Research in Tuberculosis (NIRT) in Chennai, and a Statistical/Data Management Center at the Society for Applied Studies-Centre for Health Research and Development (CHRD) in New Delhi. Pharmaceutical Product Development, LLC (PPD) was contracted to provide technical support.
RePORT India Phase II (2020-2025)
Leveraging the data, specimens, infrastructure and scientific partnerships established in Phase I, RePORT India will focus on five specific scientific aims in Phase II: 1. Evaluate Novel Diagnostics & Biomarkers of Diverse
States of Infection 2. Identify Markers of Lung Injury Associated with
Unfavorable TB Treatment Outcomes 3. Identify Markers of Treatment Response 4. Identify Mechanisms of Protection against TB in
Exposed Persons 5. Identify Immunologic Markers of Persons at Highest
Risk of Progress of Latent TB Infection to TB
RePORT India will also assess cross-cutting epidemiologic and COVID-19 related aims.
In addition to the Phase I cohorts of confirmed TB cases and their household contacts, Phase 2 introduces a new study cohort of people who are suspected but not confirmed to have TB.
CCGHE plays a central role in facilitating scientific collaboration and coordination both internationally and across the India consortium. Dr. Amita Gupta is a member of the Executive Leadership Group for RePORT International, and Dr. Nikhil Gupte is lead Biostatistician. Dr. Bob Bollinger is the U.S. Protocol Chair for the RePORT India Phase II Common Protocol, and CCGHE staff and faculty serve in coordination and working group leadership roles. 2
47 publications from our 2 RePORT India research partnerships, including these high impact findings
Baseline IL-6 is a biomarker for unfavorable tuberculosis treatment outcomes: a multi-site discovery
and validation study. Mave V, Kadam D, Gaikwad S,, et al (Submitted: American Journal of Critical Care Medicine)
Measuring tuberculosis drugs in hair in adults and children as a tool to monitor exposure and outcomes.
Mave V, Kadam D, Gaikwad S, et al. Int J Tuberc Lung Dis. 2021 Jan 1;25(1):52-60. doi: 10.5588/ijtld.20.0574. PMID: 33384045.
Tuberculosis preventive treatment should be considered for all household contacts of pulmonary TB
patients in India. Paradkar M, Padmapriyadarsini C, Jain D, et al. PLOS One. 2020 July 29;15(7):e0236743. PMID: 32726367; PMCID: PMC7390377.
Whole genome enrichment approach for rapid detection of Mycobacterium tuberculosis and drug resistanceassociated mutations from direct sputum sequencing
Soundararajan L , Kambli P , Priyadarshini S et al. Tuberculosis (Edinb). 2020 Mar;121:101915. doi: 10.1016/j.tube.2020.101915. Epub 2020 Feb 20. PMID: 32279871.
Transcriptomic profiles of confirmed pediatric tuberculosis patients and exposed household contacts identifies tuberculosis disease, infection, and response
to treatment among Indian patients. Tornheim JA, Madugundu A, Paradkar M et al. J Infect Dis. 2019 Dec 4. pii: jiz639. doi: 10.1093/infdis/jiz639. [Epub ahead of print]. PMID: 31796955.
Delamanid central nervous system pharmacokinetics in tuberculous meningitis in rabbits and humans
Tucker EW, Pieterse L, Zimmerman MD et al. Antimicrob Agents Chemother. 2019 Sep 23;63(10). pii: e00913-19. doi: 10.1128/AAC.00913-19. Print 2019 Oct. PMID: 31383662; PMCID: PMC6761520.
Sub-therapeutic rifampicin concentration is associated with unfavourable tuberculosis treatment outcomes.
Ramachandran G, Padmapriyadarsini C, Gaikwad S et al. Clin Infect Dis. 2019 May 10. pii: ciz380. doi: 10.1093/cid/ciz380. [Epub ahead of print]. PMID: 31075166.
Effect of diabetes mellitus on the pharmacokinetics and
pharmacodynamics of tuberculosis treatment. Alfarisi O, Mave V, Gaikwad S et al. Antimicrob Agents Chemother. 2018 Oct 24;62(11). pii: e01383-18. doi: 10.1128/AAC.01383-18. Print 2018 Nov. PMID: 30126955; PMCID: PMC6201087. 15

2020 RePORT India Annual Meeting in Mumbai





