
15 minute read
COVID-19 & VACCINATIONS - WHAT YOU NEED TO KNOW AS A CAREGIVER OF OR A PERSON WITH T1D
COVID-19 & VACCINATIONS
WHAT YOU NEED TO KNOW AS A CAREGIVER OF OR A PERSON WITH T1D
BY DR. KIMBER SIMMONS
Coronavirus disease 2019, or COVID-19, is the contagious disease first identified in Wuhan, China in 2019 that is caused by the virus severe acute respiratory syndrome coronavirus 2, or SARS-CoV-2.1 SARS-CoV, or SARS-CoV-1, was a similar acute respiratory syndrome first reported in November of 2002 in the Guangdong province of Southern China that did not reach pandemic scale.2 The Centers for Disease Control and Prevention (CDC) confirmed the first case in the United States (US) on January 21, 2020.3 Because we did not yet fully understand how and to what extent the disease was spreading, people traveled, gathered, and went on in a way that quickly spread the virus around the world. We all remember how one day the virus seemed like a problem only in distant hotspots, and the next day our lives changed dramatically. After the World Health Organization (WHO) declared COVID-19 a global pandemic on March 11, 2020, and our president declared a national emergency on March 13, 2020, cities and states began imposing stay at home orders. As we sheltered in place, the new normal of social distancing, restrictions, masks, and other risk mitigations began.


COVID-19 most commonly spreads between people who are in close contact with one another through respiratory droplets or small particles, such as those produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose or mouth, then travel into the airways and lungs, causing infection. They can also enter through the eyes. The further away a person is from these droplets, the less likely they are to be infected. This is why social distancing guidelines suggest maintaining at least 6 feet of distance. While this is thought to be the main way the virus spreads, droplets can also land on surfaces or objects and be transferred by touch. A person may contract COVID-19 by touching a surface or object that has the virus on it and then touch their own mouth, nose, or eyes. Spread from touching surfaces is not thought to be the main way the virus spreads.
When viewed under a microscope, each coronavirus virion is surrounded by a halo or “corona” of what are called “spike proteins.”4 Infection occurs when these spike proteins attach to our cells that have ACE2 receptors. These receptors are most common in the nose, tongue, oral mucosa, heart, lungs and colon, but are also present on other tissues including beta cells in the pancreas. Common symptoms include cough, fatigue, loss of taste or smell, and fever, while less common symptoms include sore throat, headache, aches and pains, diarrhea, skin rash, discoloration of fingers or toes, and red or irritated eyes. While most illness is mild to moderate, serious COVID-19 symptoms that require immediate medical attention include shortness of breath or difficulty of breathing, loss of speech or mobility, confusion, and chest pain. There is a litany of confirmed and possible risk factors that increase risk for severe COVID-19 illness. The CDC currently states that there is limited evidence that T1D increases a person’s risk for severe COVID-19 illness. The biggest risk factor for severe illness is increased age. Interestingly, it is believed that 30-50% of people infected with SARS-CoV2 have no symptoms but can likely still infect others.
In the US, most states imposed stay at home orders between mid to late March 2020 until as late as May 2020, preventing a spike in new cases nationwide. While easing of restrictions in late spring and early summer 2020 resulted in a slight rise in new cases nationwide (with severe spikes in some sunbelt states), it was the onset of the fall and winter that the US saw its biggest increase in new cases. Positive cases of COVID-19 in patients at the Barbara Davis Center mirrored fall and winter spikes seen in Colorado.
Prior to the pandemic, we knew that people with T1D, especially those with poor glycemic control, were already at risk for certain infections. Adults with T1D are more likely than those without to have infections of the urinary tract, skin, lower respiratory tract, and serious bacterial infections. While adults see a higher risk, children and adolescents do not appear to have an increased risk of infections, including COVID-19. Although having type 1 diabetes doesn’t seem to be a risk factor for having a severe COVID-19 illness, we do know that people with T1D who have a hemoglobin A1c above target range or are a member of a minority race or ethnicity are at higher risk for diabetic ketoacidosis during COVID-19 illness.5

WHAT IS KNOWN ABOUT COVID-19 OUTCOMES IN PEOPLE WITH TYPE 1 DIABETES?
While there have been multiple studies identifying type 2 diabetes as a significant risk factor for hospitalization and death, the same risk has not been seen overall for patients with COVID-19 and T1D. The healthcare system in England reported in May 2020 that patients with T1D were 3.5 times more at risk for in-hospital COVID-19 related death than those without diabetes, while those were type 2 diabetes were 2 times more at risk. This sounds scary, but it is important to know that severe illness and death rates were greatly impacted by people having other risk factors. Of the people with T1D who died from COVID-19 illness, 62.3% had a history of cardiovascular disease or renal impairment. When taking this into account, the risk for COVID-19 related death in people with T1D is not very different than the risk of COVID-19 death in individuals without T1D. Importantly, the majority of people in this analysis were over 50 years of age, and the risk of COVID-19 related death is related to age as well non-white ethnicity, socioeconomic deprivation, and previous stroke and heart failure. Other risk factors that likely played a role such as obesity and hypertension could not be examined within this data set.6 In December 2020, another report was published in Diabetes Care stating that COVID-19 severity was tripled in the diabetes community. Again, this report did not clarify which additional risk factors were present in people with diabetes who had severe COVID-19 illness.7
Although risk for hospitalization is not likely different in people with T1D and no other risk factors when compared to healthy people in the community, maintaining glycemic control is an important tool for improving your chances of a better outcome in the event that you become hospitalized with COVID-19. One study saw better outcomes in those whose blood glucose levels were between 70 and 180 mg/dl more than 60% of the time, protecting them from diabetic ketoacidosis (DKA) and potentially negative outcomes with COVID-19.8 In the data from England, patients with a hemoglobin A1C >10% were 2.6 times more likely to have a COVID-19 related death compared to people with hemoglobin A1C at goal.
Those with T1D who are hospitalized with COVID-19 do require special care. Hyperglycemia, hypoglycemia, and high glycemic variability are independently associated with increased hospital morbidity and mortality. Some treatments can actually cause complications for people with T1D. Steroids are included in some COVID-19 management protocols and can increase blood glucose, which may necessitate the use of insulin drips. This can be complicated during isolation protocols that attempt to limit patient interaction and spread of the disease. Remdesivir, a nucleotide analog RNA polymerase inhibitor which has been shown to shorten the time to recovery in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection, can cause liver injury and the inability to store and release glucose normally, complicating diabetes management. Because the disease and treatment options may impact blood glucose levels, patients with T1D need to be closely monitored and may require more frequent adjustments in insulin dosing and/or insulin drips.
Overall, the rate of new diagnoses of T1D in the US remains similar to prior years. Prevalence of DKA upon new diagnoses of T1D, however, significantly increased during and after the stay at home orders. This is most likely due to fears of accessing healthcare during the beginning of the pandemic. It appears that people with T1D in the US in adequately resourced areas have been better able to maintain glycemic control since the pandemic began due to a more regularly scheduled lifestyle, reproducible meals, and more time for self-care.
HOW DO YOU AVOID CONTRACTING COVID-19?
Here are some simple practices: • Maintain a distance of 6 feet apart from individuals who are not in your household, more when indoors, and the further the better!
The CDC recently stated that individuals who are fully vaccinated (>2 weeks from last dose of vaccine) may visit with other fully vaccinated people indoors without wearing masks or staying 6 feet apart. Fully vaccinated people may also visit with unvaccinated people from one other household indoors without wearing masks or staying 6 feet apart if everyone in the other household is at low risk for severe disease. • Wear a mask! • Do not use a wet, dirty, or damaged mask. • Do not share your mask. • Do not use valved masks. You may be protecting yourself, but with a valve you are not protecting those around you. • Wear your mask so that it completely covers your mouth, nose, and chin, and so that is it fits tightly against your face. • Avoid the 3 C’s: spaces that are Closed, Crowded, or involve Close Contact. • Keep rooms well ventilated. When possible, open a window to increase the amount of natural ventilation indoors. • Wash your hands for 20 seconds – sing one round of “Happy Birthday” or the chorus to Dolly Parton’s “Jolene”! 9
NEXT QUESTION: SHOULD YOU GET A COVID-19 VACCINATION WHEN THEY’RE AVAILABLE TO YOU? YES!
There are currently three COVID-19 vaccinations authorized by the FDA for emergency use in the US: the Pfizer-BioNTech COVID-19 vaccine (authorized 12/11/2020), Moderna COVID-19 vaccine (authorized 12/18/2020) and Janssen COVID-19 vaccine (2/27/2021). All of these vaccines were authorized for emergency use after phase 3 clinic trials showed that the vaccines are highly effective at preventing severe COVID-19 illness and are safe. People with T1D participated in these trials. There is no reason to believe that people with T1D or other autoimmune diseases would be at increased risk for adverse effects from the vaccine – the health risks of COVID-19 illness are known and are far worse than any reported side effects from the COVID-19 vaccine. There have been anecdotal reports of both high and low blood glucose in those with T1D for a few days after receiving the vaccine. We would actually expect that when a person’s immune system is reacting to the vaccine; the resulting inflammation would result in temporary insulin resistance and higher glucose levels for a few days in someone with T1D.
You might wonder how the vaccines work. It is helpful to review information from biology class first. DNA is a long molecule that contains your unique genetic code for making all the proteins in your body. In order to make proteins, messenger RNA (mRNA) makes a blueprint of the DNA “genes” and carries this into cells in your body. Inside your cells, ribosomes read the mRNA and make proteins. Proteins are essential for all of the cells in your body to function.
Both the Pfizer-BioNTech and Moderna vaccines are COVID-19 mRNA vaccines. The mRNA instructs your own body to make a spike protein, which is harmless by itself. The Janssen vaccine uses a modified Adenovirus to carry spike protein DNA into cells in your body, which is made into mRNA, then spike protein. Adenoviruses are common viruses that typically cause colds or flu-like illnesses. The Janssen vaccine modified adenovirus so that it can enter your cells but cannot replicate inside your cells or cause illness. So, with any of the authorized vaccines, once the vaccine is injected into the muscle in your upper arm, your cells will make the SARS-COV2 spike protein. The mRNA or adenovirus vector is rapidly broken down by your body and is completely gone within days. Soon after the spike protein is made, your body will recognize that the protein doesn’t belong in your body and your immune cells will start getting rid of the protein and making antibodies to tag the protein for destruction. At the end of the process, your body will have

gotten rid of all adenovirus vector/ DNA, mRNA and spike proteins. Your body will have learned how to protect against future infection, similar to if you had been infected with COVID-19. The benefit of these vaccines, like all vaccines, is that you gain disease protection without ever risking the serious consequences of getting sick with COVID-19.10
All three vaccines are highly effective in preventing severe COVID-19 illness. PfizerBioNTech performed a double blind, randomized, placebocontrolled study in individuals 16 years of age and older. Participants received two vaccine doses, 21 days apart. Of the 43,548 participants, 21,720 received the vaccine and 21,728 a placebo. Only 8 cases of COVID-19 were diagnosed with onset at least 7 days after the second dose of the vaccine while 162 cases were diagnosed among the placebo group. The vaccine was thus 95% effective at preventing COVID-19 over the 2-month study and no one in the vaccine group had a severe COVID-19 illness causing hospitalization or death.11 Moderna also performed a double blind, randomized, placebo-controlled study, but in individuals 18 years of age and older. Participants received two vaccine doses, 28 days apart. Of the 30,420 participants studied, 15,210 received the vaccine and 15,210 received the placebo. Only 11 cases of COVID-19 were diagnosed with onset at least 14 days after the second dose of the vaccine and 185 cases were diagnosed among the placebo group. The vaccine was thus 94.1% effective at preventing COVID-19 illness and also did not allow for any severe COVID-19 illnesses or deaths in the vaccine group.12 Johnson & Johnson’s (Janssen vaccine) study (ENSEMBLE) was a randomized, double-blind, placebo-controlled clinical trial in 43,783 individuals 18 years of age and older where 21,895 received the vaccine and 21,888 received saline placebo. The trial was conducted in eight countries across three continents and included a diverse and broad population including 34% of participants over age 60 years old. In the US, the vaccine was 72% effective in preventing COVID-19 illness 28 days after the vaccine. The vaccine was 85% effective overall in preventing severe disease and showed protection against COVID-19 related hospitalization and death, beginning 28 days after vaccination.13 Clinical trials are currently being completed in children 12 years of age and older with plans to expand trials down to 1 year of age. We expect that there may be a vaccine available to children ages 12 and older by fall.
Side effects were similar with all vaccines, with the most common complaint being pain at the injection site. Other common side effects include headache, fatigue, chills, and muscle pain. Side effects were more common after the second dose in the Pfizer-BioNTech and Moderna vaccines. Fever was rarely reported and generally only after the second dose with the Pfizer-BioNTech and Moderna vaccines.

WHEN WILL YOU BE ELIGIBLE TO RECEIVE THE VACCINE?
Because vaccine distribution varies by state, it depends on where you live. Colorado has parsed out vaccine distribution into phases and multiple subphases. Phases 1A, 1B.1 and 1B.2 concentrated on healthcare workers, first responders, skilled nursing facilities, child-care facilities, preK-12 educators, and those over age 65 years. Phase 1B.3 opened March 5, 2021, and people age 16-64 with two or more high risk conditions, INCLUDING T1D, are eligible for vaccination! If you have T1D and also have one of the following risk factors, you are eligible for vaccination: cancer, chronic kidney disease, COPD, heart conditions, obesity, pregnancy, sickle cell disease, solid organ transplant, and/or a developmental disability that prevents you from wearing a mask. The next phase (Phase 1B.4) will open on March 19, 2021, and will allow for those 16-64 years of age with only one high risk condition, such as T1D, to be eligible for vaccination before the general public is vaccinated in phase 2. If you live in Colorado you can learn more about vaccine distribution phases by visiting www.covid19.colorado.gov/for-coloradans/vaccine/vaccine-for-coloradans. If you do not live in Colorado and want to learn more about vaccination timelines in your state, visit www.cdc.gov/vaccines/covid-19/covid19-vaccination-guidance.html.
If you live in Colorado you can learn more about where to get it by visiting covid19.colorado.gov/vaccine-providers. Are you a patient at the Barbara Davis Center who is 16 or older? Then make sure that you are signed up for MyChart! Because your chart already lists that you have T1D, you should be notified via email when you are eligible to schedule an appointment for vaccination. Don’t have MyChart? Sign up! You can sign up even if you’re not a patient by visiting mychart.uchealth.org/mhcweb/registration. If you are outside of Colorado or your healthcare system does not use MyChart, find your system’s electronic medical record and sign up. Most have a lottery system and will contact you when you become eligible. Visit covid.cdc.gov/covid-data-tracker/#vaccinations to view an interactive map with links to each state health department for more information on vaccine access outside of Colorado.
An important thing to remember is that just because we can see a light at the end of the tunnel does not signal an end to the pandemic. It is still all of our responsibility to mask up, wash our hands, and maintain six feet of distance when advised by the CDC to protect those who are not yet vaccinated and to reduce the spread of COVID-19 variants. These continued measures and increased vaccination will help ensure that in the near future our lives will look much like they used to before COVID-19.
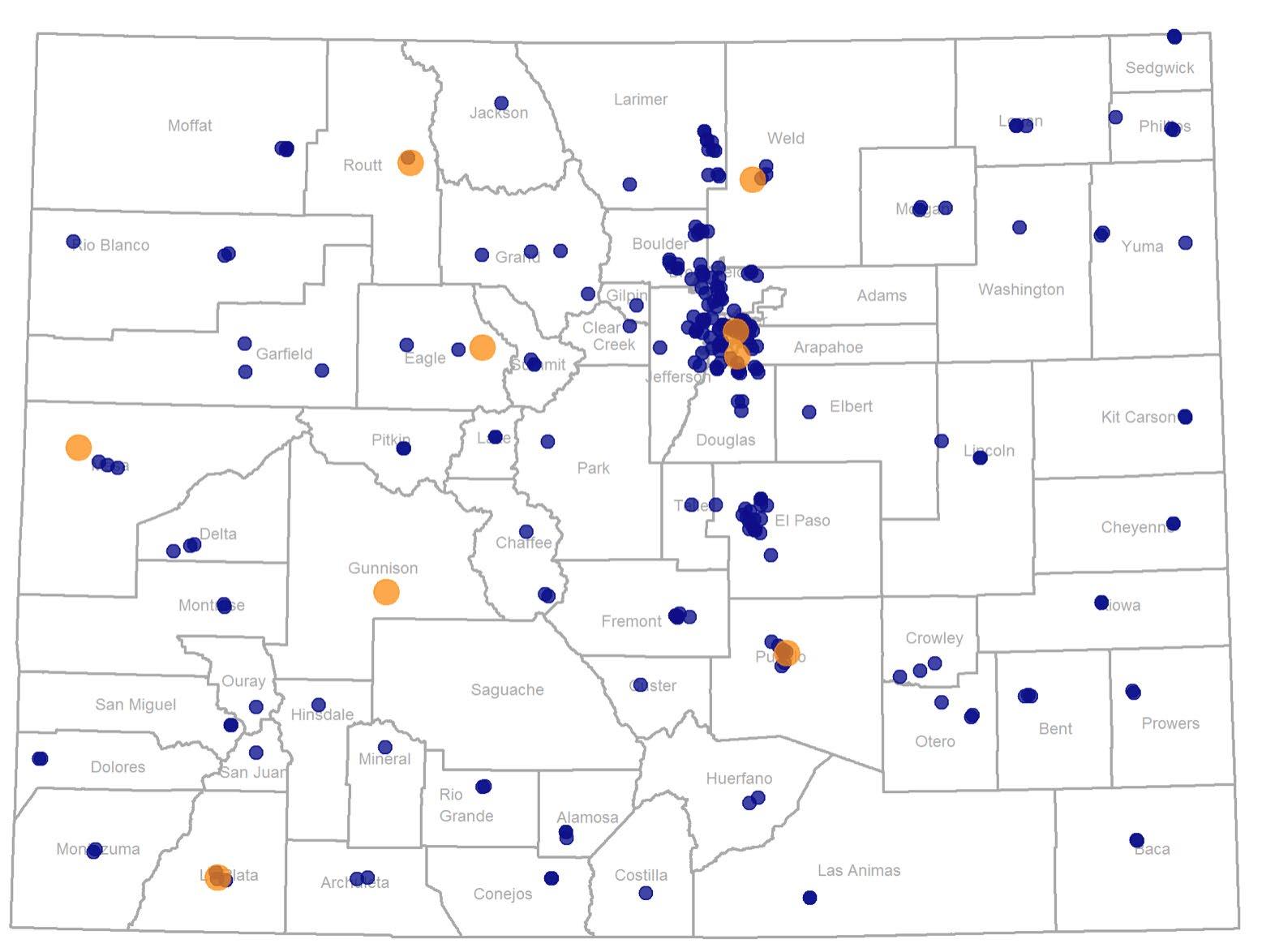
VACCINATION CENTERS IN COLORADO
1www.cdc.gov/coronavirus/2019-ncov/cdcresponse/about-COVID-19. html#:~:text=The%20new%20name%20of%20this,D%27%20for%20disease
2www.cdc.gov/about/history/sars/timeline.htm
3www.ajmc.com/view/a-timeline-of-covid19-developments-in-2020
4www.cdc.gov/coronavirus/mers/photos.html#:~:text=Coronaviruses%20 derive%20their%20name%20from,%2C%E2%80%9D%20or%20halo.
5Muller et al., Clin Infect Dis, 2005 Shah BR et al., Diabetes Care, 2003 Laffel LM et al., Pediatric Diabetes, 2018 Cengiz E et al., Pediatric Diabetes, 2013 Kahkoska AR et al., JAMA New Open, 2018 7Gregory et al, Diabetes Care, 2021
8Holman N, et al., Lancet, 2020
9www.npr.org/sections/goatsandsoda/2020/03/17/814221111/my-hand-washing-songreaders-offer-lyrics-for-a-20-second-scrub
10www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html
11www.nejm.org/doi/full/10.1056/NEJMoa2034577
12www.nejm.org/doi/full/10.1056/NEJMoa2035389
13www.fda.gov/media/146217/download






