9 minute read
The Future of Personalised Medicine
Can T-cells be supercharged to fight cancer?
by Eleanor Langley
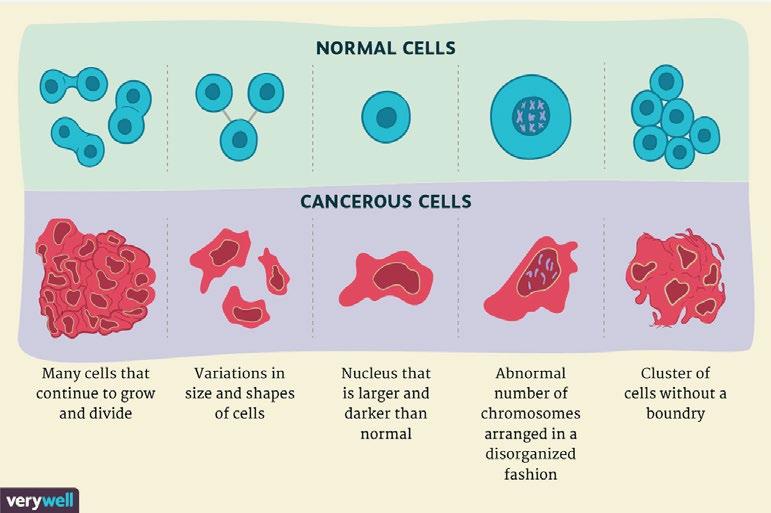
The term ‘cancer’ refers to more than 100 forms of the disease. Nearly all tissues and cells in the body have the ability to form malignant tumours. There were 17 million new cases of cancer in the world in 2017, and this is due to grow to 27.5m by 2040 (Cancer Research UK, 2019). The way that these tumours are formed are all quite similar, cancer is “a disease in which some of the body’s cells grow uncontrollably and spread to other parts of the body” (Weinberg, 1996). The formation of cancer is due to a mutation that occurs during cell division. During division, cells grow old or become damaged, they then die, and new cells take their place. If this order breaks down, meaning damaged or abnormal cells are able to continue growing and multiplying, a tumour can form. These tumours will either be malignant (cancerous) or benign (non- cancerous). The cancerous cells tend to spread uncontrollably, causing the tumours to invade tissues and other part of the body in order to form new tumours. This process is called metastasis.
in the treatment of cancer. Unfortunately, cancer treatments, such as chemotherapy, radiotherapy, targeted drugs and high doses of steroids, can also weaken the immune system. The balance between treatment and maintaining a healthy immune system is key in the fight against cancer (Cancer Research Institute, 2018).
As a result of this balancing act, recent technological and biological advancements in cancer treatments have focused on supporting a patient’s own immune system in its fight against cancer. These treatments are at the centre of hope in reducing death caused by cancer. This article explores these recent advancements and the impact they may have on healthcare for generations to come.
Recent advancements
In the early 2000s targeted drug therapies like Imatinib (Gleevec) and Trastuxumab (Herceptin) first emerged. These drugs kill cells that display the specific molecular changes that are first seen as cancer emerges. This treatment is a form of immunotherapy and has become more commonly used over the last decade due to their ability to shrink, and even remove, tumours in some patients with advanced cancer. These treatments can be effective for many years in a small percentage of patients.
Cancer is a genetic disease, this means that it is caused by a change in the genetic coding that controls how our cells function, grow and divide. These genetic changes can be caused by many things such as errors in cell division, damage to DNA due to environmental factors (e.g. smoking or UV radiation), as well as being inherited from parents. All these factors make you more vulnerable to getting cancer (National Cancer Institute, 2021).
One way in which cancer can be treated is by the immune system. The immune system protects the body against illness and infection caused by bacteria, viruses, fungi or parasites. This system is a collection of reactions and responses that the body makes in order to damage cells or infection and is pivotal
More recently, CAR T-cell therapy has become very prominent. Although T-cell therapies aren’t very widely used, they have shown the same ability to eradicate and prevent the return of cancer as other immune checkpoint inhibitors. Since 2017, six CAR T-cell therapies have been approved by the Food and Drug Administration (FDA) in the US. These forms of therapy are all able to treat various forms of blood cancer (National Cancer Institute, 2019). In the last 3 years, 130 U.S. based clinics have been authorised to provide this treatment, although these centres have treated fewer than 2,000 people to date. The cost of these treatments is also very high, with the newest costing more than $450,000. However, despite the cost and low success rate, CAR T-cell therapies have become part of the mainstream of cancer treatments (National Cancer Institute, 2019).
The Biology
Renier J. Brentjens, M.D., Ph.D., explained that CAR T-cells are the equivalent of ‘giving patients a living drug’. T-cells are the backbone of CAR T-cell therapy. T cells are a type of white blood cell, an important component of the immune system, and develop from stem cells in the bone marrow. They help to protect the body from infection and can help to fight cancer (Cancer Research, 2021). will help with the treatment. This will allow for a vast reduction in the time, materials and labour required to generate CAR T-cells, this will be extremely beneficial for patients with rapidly progressive diseases and those in poorly resourced healthcare environments (Penn Medicine News, 2022). If the researchers are able to do this, it will cut down on production time and allow for the treatment to become much more readily available. Therefore, allowing personalised medicine to be taken to the global market of healthcare.
Efficacy
In order for the FDA to approve these types of therapy in the US, the clinical trials have to show significant success in treating cancer; however, they don’t always work. Of the six CAR T-cell treatments that have been approved, the long-term success rate is less than 50% (National Cancer Institute, 2019).
At this stage in the research, it appears that CAR T-cell therapy may have limited efficacy because:
• CAR T-cells are T-cells with different genetic instructions. These T-cells are now able to create chimeric antigen receptors (CAR) and molecules. These new receptors need to be active for T-cells to find and kill the cancer cells. If this doesn’t happen T-cells cannot work and are unable to destroy the cancer.
T-cells are firstly collected by a cannula, one in each arm. One tube removes the blood and passes it into an apheresis machine, separating different parts of the blood. The T-cells are removed, and the rest of the blood cells and fluid go back into your body through the other tube. This process usually takes between 4 to 5 hours. The T-cells are then sent to a laboratory to make CAR T-cells. This process can take several weeks. While in the laboratory the patients’ T-cells are re-engineered to produce proteins on their surface. These proteins are called chimeric antigen receptors (CARs). The CARs recognise and bind to specific proteins or antigens on the surface of cancer cells. These receptors are synthetic molecules and do not exist naturally (National Cancer Institute, 2019).
Before undergoing CAR T-cell therapy patients undergo chemotherapy. This lowers the number of T-cells and prepares your body for the CAR T-cells. Once the specialised nurse has received your CAR T-cells, they are introduced back into the bloodstream through a drip. This process takes less than 30 minutes. You are then monitored for 2 weeks after CAR T-cell therapy (Cancer Research, 2021). The CAR T-cells will continue to multiply in the patient’s body and will recognise and kill cancer cells that have the target antigen on their surface.
Scaling up to commercial manufacturing of CAR T-cells presents one major challenge: the process needs to be simplified to help the transition from lab manufacturing to mass production (Eder. M, 2021). Due to T-cell therapy being unique to each individual patient, the ability to upgrade numbers of people being able to access it is very difficult.
Researchers at Penn Medicine are currently trying to manufacture shorter wait time to produce CAR T-cells, from the standard two-week-time to less than 24 hours. CAR T-cell therapy typically takes 9 to 14 days. A faster engineering time
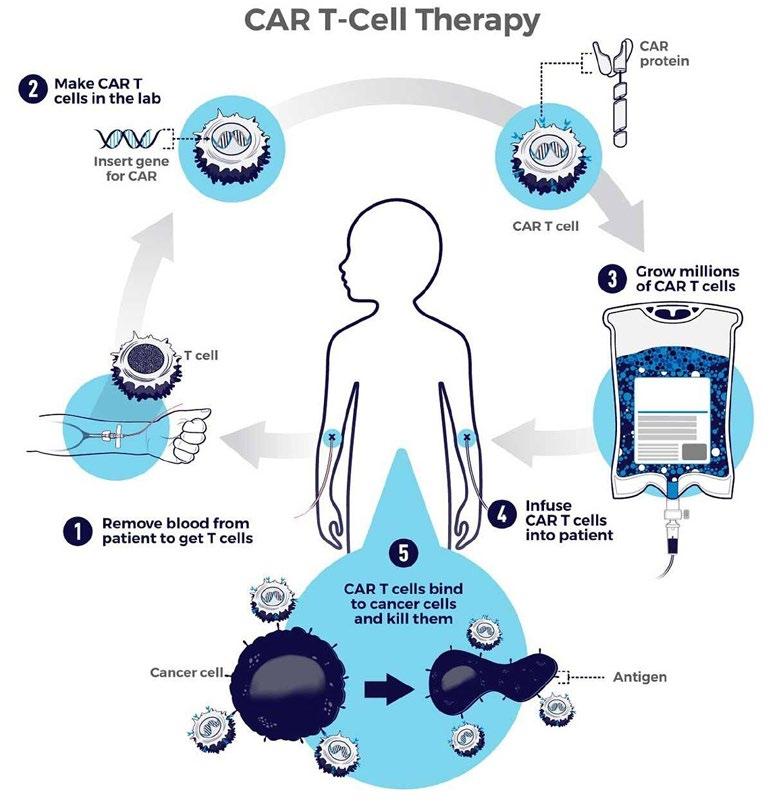
• Once in your body, CAR T-cells are supposed to multiply. When they don’t, they aren’t able to find and kill enough cancer cells to stop them from spreading.
• Sometimes CAR T-cells cannot kill cancer cells. This is known as T-cell exhaustion. Scientists believe that transcription factors (proteins that help turn genes on and off) are linked to this.
• Cancer cells are able to change or mutate. This can lead to the antigen, that the CAR T-cells were created to find, changing. If this happens, the T-cells are unable to locate the cancer cells (Cleveland Clinic, 2022).
The future
Personalised medicine is a type of medical care in which treatment is customised for an individual patient. Personalised medicine classifies tumours according to their genetic makeup instead of where they grow. People with the ‘same’ cancer can have different forms of the disease, meaning that their response to the treatment can vary. Using treatment that are specially tailored allows for more successful treatments. It gives patients a range of new, more effective treatments (Cancer Research, 2013).
At the moment, CAR T-cell therapy has been approved by the FDA and other institutions in the US, Europe, China, Australia and a few other countries. All of these countries are wealthy, developed countries. While there have been around 10,000 patients worldwide that have received this treatment in a clinical trial, only a small number of participants were from middle- and low-income countries. Africa, South America and India didn’t have a single registered member during this trial (Eder. M, 2021). This shows the need for this treatment to be more accessible as to allow people in lower-income countries to benefit from this treatment.
As more longitudinal and diverse sources of research emerge, the healthcare sector will be able to understand the long-term benefits of personalised medicine. Doctors will be able to use patients’ genetic and molecular information as part of routine medical care, which have the improved ability to predict which treatment will work better considering a deeper understanding of the underlying mechanisms by which various diseases occur. This will deliver improved approaches to preventing, diagnosing and treating a wide range of diseases (Medline Plus, 2022).
Conclusion
Personalised medicine will have a large impact on healthcare, but there are a number of influences which will impact patient health outcomes. Significant infrastructure investments are required to expediate the production process. The treatment will then have to reach at least 1 million patients – with all of their data collected and monitored – so that treatment plans are informed by historical case studies. This large amount of genomic data will have to be collected and stored securely. There is also the possibility of missing out certain groups of the population. This can lead to an over or under-representation of certain groups thereby limiting the validity of the data in the treatment of all. Finally, this treatment is very expensive. Although CAR T-cell therapy should reduce the cost of alternative long-term treatment plans, including surgery, chemotherapy and hormone therapy, for a patient, the upfront investment in this immunotherapy is beyond the budget of most public healthcare systems (Hospital Admin, 2016).
However, as Edward Wolton, Technical Consultant to Genomics England (a company of the Department of Health
References
Cancer Research Uk (2021). CAR T-cell therapy. [online] Cancer Research UK. Available at: https://www.cancerresearchuk.org/about-cancer/treatment/ immunotherapy/types/CAR-T-cell-therapy [Accessed 9/5/23]
Cancer Research UK (2013). Personalised medicine. [online] Cancer Research UK. Available at: https://www.cancerresearchuk.org/get-involved/donate/ become-a-major-donor/how-you-can-give/the-catalyst-club/personalisedmedicine [Accessed 9/5/23]
Cancer Research UK (2018). The immune system and cancer. [online] Cancer Research UK. Available at: https://www.cancerresearchuk.org/about-cancer/ what-is-cancer/body-systems-and-cancer/the-immune-system-and-cancer [Accessed 9/5/23]
Cancer Research UK (2019). Worldwide cancer statistics. [online] Cancer Research UK. Available at: https://www.cancerresearchuk.org/healthprofessional/cancer-statistics/worldwide-cancer [Accessed 9/5/23]
Cleveland Clinic (2022). CAR T-cell therapy. [online] Cleveland Clinic. Available at: https://my.clevelandclinic.org/health/treatments/17726-car-t-cell-therapy [Accessed 9/5/23]
Eder,M. (2021). Availability of CAR T-cell therapy: list of all countries. [online] Available at: https://www.support.com/knowledge/cell-gene-therapy/ which-countries-cell-therapy-available [Accessed 9/5/23]
Eder,M. (2021) Standardizing CAR-T therapy – Getting it scaled up! [online] Single Use Support. Available at: https://susupport.com/company/news/cellgene-therapy/car-t-therapy-getting-it-scaled-up [Accessed 9/5/23]
Eldridge, L. (2013) Cancer Cells vs. Normal Cells: How Are They Different? [online] Verywell Health. Available at: https://www.verywellhealth.com/cancer-cellsvs-normal-cells-2248794 [Accessed 9/5/23]
& Social Care) said “The difference between conventional and genetic medicine is like the difference between blood-letting medieval barbers and modern sterile surgery. Personalised medicines tailored to the patient’s genome is the future of targeted treatment for many diseases and is already saving countless lives; over time the cost per treatment will reduce to be far more affordable as the cost of genetic sequencing has already reduced 99.94% in 15 years!”
There are also exciting developments in the use of AI and machine learning in personalising medicine, as featured in a recent MIT Technology Review (Heaven. W, 2023). This research, published in 2022, illustrates that personalised drug treatment can also include the use of AI to select the most appropriate suite of drugs from those currently on the market, to provide the best patient outcome. As a result, technology may have its own answer on some of the problems it creates around cost and time – when many cancer patients have neither to spare.
Discussion questions
1. Will this treatment be accessible to lower income countries?
2. Is it worth investing in if only 50% of treatments have shown long term success?
3. Will life hacking or the editing of our unborn embryos be the natural next step in personalised medicine?
Interested in learning more?

Here is a TED talk on CAR T-cell therapy:
Heaven,W. (2023). AI is dreaming up drugs that no one has ever seen. How we’ve got to see if they work. [online] MIT Technology Review. Available at: https://www.technologyreview.com/2023/02/15/1067904/aiautomation-drug-development/?utm_source=engagement_email&utm_ medium=email&utm_campaign=wklysun&utm_content=02.26.23.subs_ eng&mc_cid=f5646a922c&mc_eid=18316ba0ad [Accessed 9/5/23]
Hospital Admin (2016). Advantages and Disadvantages of Precision Medicine [online] Health view X. Available at: https://www.healthviewx.com/ advantages-disadvantages-precision-medicine/ [Accessed 9/5/23]
Medline Plus (2022). What are some potential benefits of precision medicine and the Precision Medicine Initiative? [online] Medline Plus Genetics. Available at: https://medlineplus.gov/genetics/understanding/ precisionmedicine/potentialbenefits/ [Accessed 9/5/23]
National Cancer Institute (2022). CAR T Cells: Engineering Immune Cells to Treat Cancer. [online] National cancer Institute. Available at: https://www.cancer. gov/about-cancer/treatment/research/car-t-cells [Accessed 9/5/23]
National Cancer Institute (2019). NCI Dictionary of Cancer Terms. [online] National Cancer Institute. Available at: https://www.cancer.gov/publications/ dictionaries/cancer-terms/def/t-cell [Accessed 9/5/23]
National Cancer Institute (2021). What is Cancer? [online] National Cancer Institute. Available at: https://www.cancer.gov/about-cancer/understanding/ what-is-cancer [Accessed 9/5/23]
Penn Medicine News (2022). Penn Researchers Shorten Manufacturing Time for CAR T Cell Therapy. [online] Penn Medicine. Available at: https://www. pennmedicine.org/news/news-releases/2022/march/penn-researchersshorten-manufacturing-time-for-car-t-cell-therapy [Accessed 9/5/23]
Weinberg, Robert A. “How Cancer Arises.” Scientific American 275, no.3 (1996): 62-70.










