Intellectual Property, Innovation and Clinical Research
Manoja Ratnayake Lecamwasam, PhD MBA, System VP, Intellectual Property and Life Sciences Innovation
February 24, 2023

What is Intellectual Property (IP)?


Intellectual property refers to creations of the mind. It can be an invention or design (utility patent or design patent), a brand name (trademark, tradename), or literary works, artistic works, software (copyright).
https://www.ipophil.gov.ph/what-is-intellectualproperty
2

What is a Copyright?




4
What is a Trademark/Tradename?



5






6
Know-How and Expertise

Intellectual Know-How and Expertise –intangible and has no physical characteristics; no one can touch it, but it is still someone’s intellectual asset!

7
Institution-owned IP can be generated from
Clinical Research
● Investigator- initiated research (Protocols, methods, inventions etc)

● IP that does not fall under the scope and funding of Sponsor
● Co-development from feasibility studies
● Pilots or validation studies
8
Why Protect IP?

9




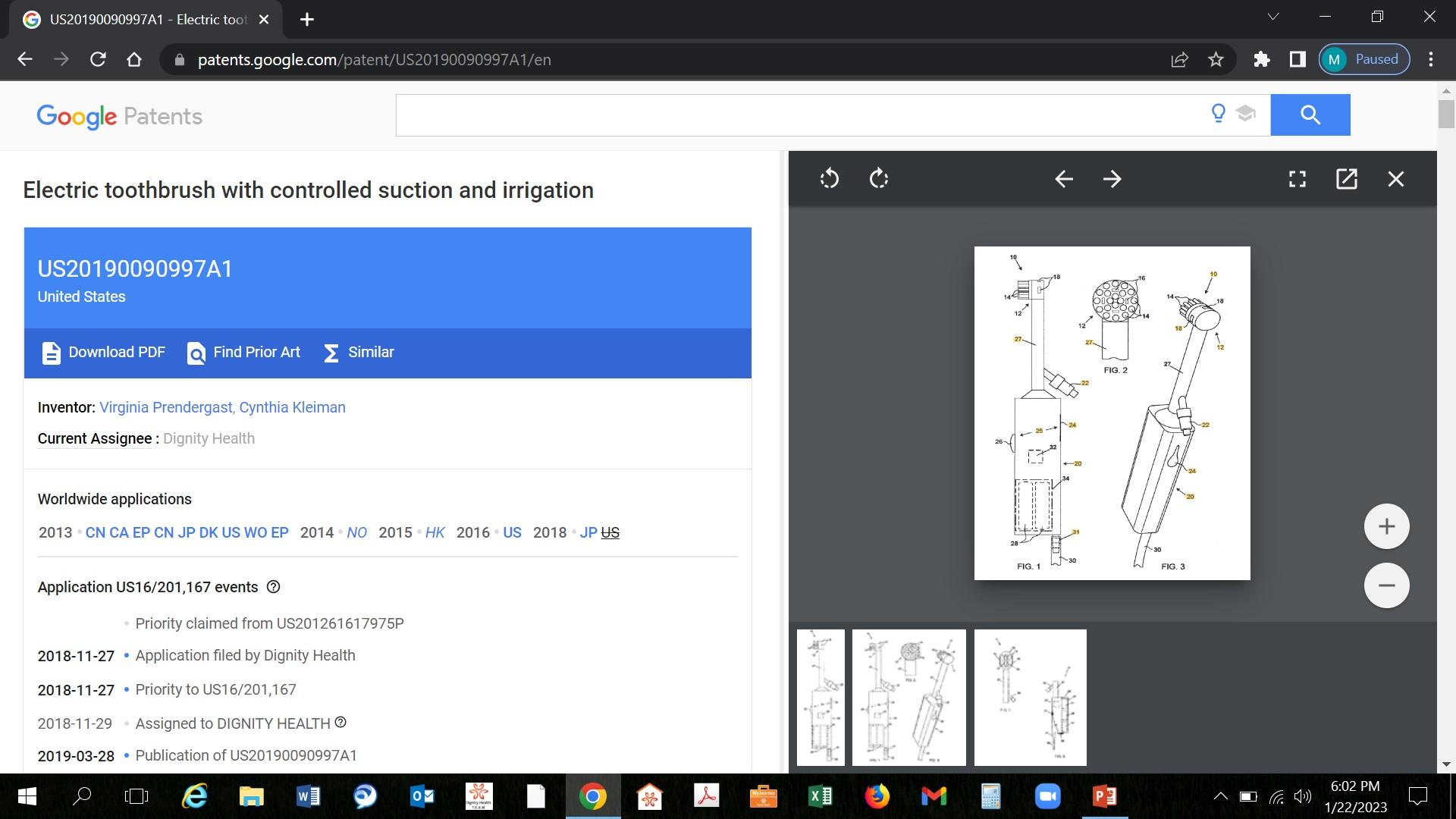



10
IP POLICY
Dignity Health IP Policy – current version effective as of February
2019 for all Dignity Health employees
- employees or users of Dignity Health resources
- Assignment of IP to Dignity Health
- Profit sharing provisions for inventors, departments and facilities included
CommonSpirit Health IP Policy – currently in progress

11
Innovations in Clinical Trials

12



Use of Personal Health Records to Match to Clinical Trials
•
Platforms that consolidate a person’s multiple medical records and allows people to contribute their de-identified data to multiple research programs – securely and anonymously

15
Technology to synchronizes research and clinical care
• Reduce administrative burden
• Increase participation of smaller sites

• Open clinical trials to diverse and underrepresented communities
• Speed up patient screening for recruitment
• Reduce cost of clinical trial set up and infrastructure
16
Thank you.

17
Questions?

18


























