





● to find a cure
● to help patients live better


● to improve lives
● to provide access regardless of age, socioeconomic status, race or gender











Vision: To be a nationally recognized leader in innovative clinical research that transforms healthcare for the communities we serve.


Purpose: to provide research infrastructure support and serve as resource for CommonSpirit Health and aligned partners
Source: Guardian Research Network




Core Values

Major Accomplishments
●Integrity
●Diversity and Equity

●Accountability

●Respect
●Compassion
●Collaboration
Key Accountabilities

●CommonSpirit Health Research Institute Board of Directors


●Research compliance and operational standards
●Organized and aligned research programs across system
●Sole owner, non-profit subsidiary
●Single IRB
●Single CTMS
●One contracting party
●Academic Partnerships
●DE&I (MICA)




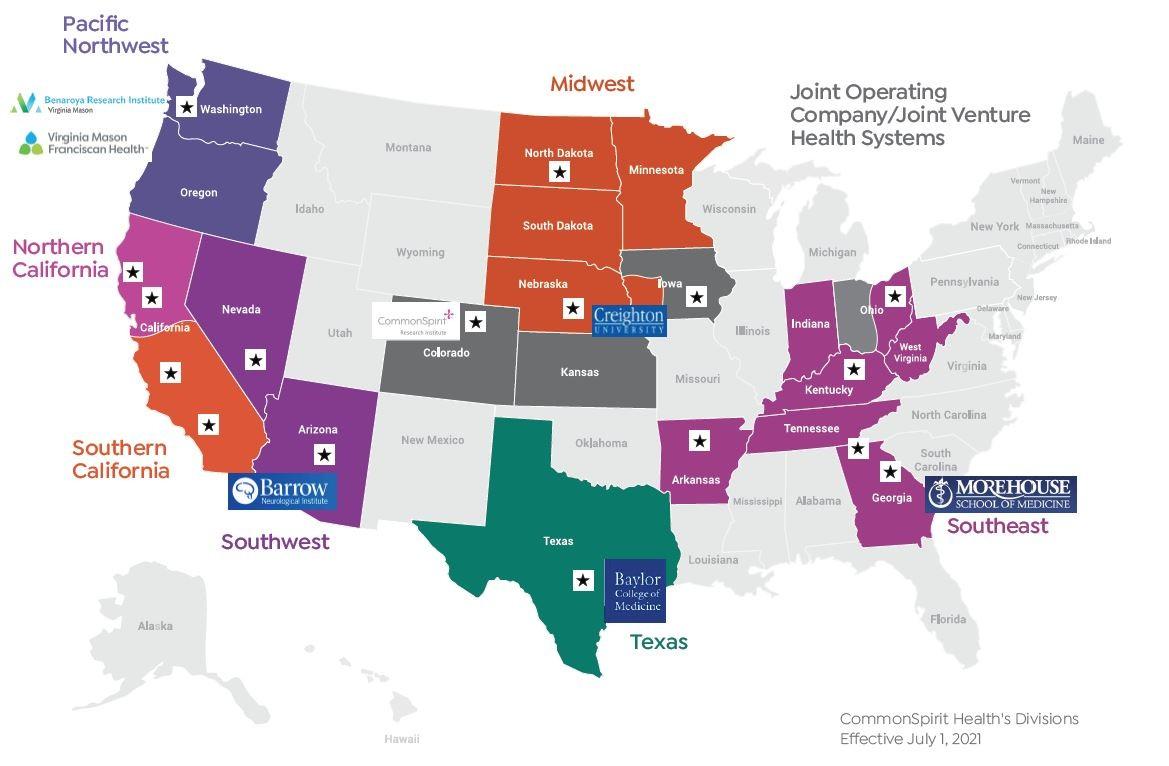
Freenome Trial 7 sites, >6K patients, 6M revenue potential



● Cancer- NCORP (NCI funded) ● Cardiovascular Research ● Neuroscience



Emerging Areas- Population health, Implementation Science, Women and Infants

53 locations
262 Investigators

282 studies
$6.8M
Resource for NCI trials ● Access for cancer patients


● Centralized Regulatory ● Centralized QA
























CSHRI provides the pathway to partner with industry and federal organizations



Sponsored Programs

Integrity and Compliance
RACE Research Analytics Center of Excellence
Research
Finance Research Operations

IRB
● Federal Wide Assurance
● IRB (internal and External)
● PRivacy Board Reviews
● IIR, GME and Student Research
QA
● COI reviews
● Post Approval Monitoring
● NCORP monitoring
● CAPA
● Regulatory Affairs Training and Education
● Staff Training & Onboarding
● CITI
● Education
● Policies and Procedures
Contracts
● Master CTAs/Agreements
● Single site CTAs/Agreements
● Just In Time Contracts
● Comprehensive Contracting Services (DUA, BAA, CDA etc)
Grants
● Research Grants
● Federal Grants
● Foundation Partnership
● Policies and Procedures
NCORP

● Program Management
● Centralized Regulatory
● Centralized contracting
● Infrastructure
● Financial clinical trial compliance oversight, which is a focus of the OIG
● Research clinical trial Budget Management & Negotiation
● Medicare Coverage Analysis (MCA)
● Clinical trial Research Revenue Cycle Management

● Clinical trial Financial Reporting
● Operational Finance
● Signal Path Finance
Research Site Operations
● Research Managers
● Research Coordinators

● Compliance to SOPs
● PI and Patient Facing
● Trial Path Implementation
New Business Development
● Preliminary Clinical Trial Feasibility
● Recruitment of PIs
● Study Implementation with New Site Onboarding
Data Support tools
● CTMS
● REDCap
● PI Tools analytics
● Statistical tools
● Clinical trial operation insights
Data Lakes for Research
● Data extraction
● ETL & Governance
● Registry integration
AI and Machine learning
● Automation of data extraction
● NLP and ML studies
● Clinical data mapping
GME Program Support
Taking Care of Our People
Practice
Environment of the Future
Culture and PurposeDriven Leadership
Job Accountability & Standard
Staff Training & Education
Vision and Strategy, communication
Our Future
Quality and Clinical Enterprise
Operational Excellence
Academic Partnerships
Health Equity
More In Common Alliance - Research
One CommonSpirit
Org Alignment, Systemness, standardized process, system wide tools
(CTMS, IRB, SAS)
Financial Stewardship
Grants, Contracts, Philanthropy
Cost Savings
Convener and Thought Leader in Catholic Healthcare
Best in class national community research enterprise
ConsumerFocused Patient engagement
Decentralized at home trials.
DEI



















Integrated Delivery Network
Growth and Diversification
Org Alignment
IIR/CT growth
Value-Based Care and Risk
National and local focus on strategy for research?? C o m m o n S p i r i t 2 0 2 6 Our People Our Excellence


● Staff Recruitment and Retention

● Education and Training

● Organizational Alignment
● Functional Reorganization - position alignment

● Diversity, Equity and Inclusion
● Address burnout and wellbeing


















CSHRI




● Driving health system change based on who we include
● 1-2 research projects each year
● Focus areas include cardiovascular, cancer, neuroscience, women and infants, population health
Principles
● Patient Centered
● Help improve diversity, equity and inclusion in clinical research (distributive justice)
● Enhance joint clinical trials and investigator initiated research


● Secure grant funding
● Lead innovation together
● Research Data Scientist Recruitment

● Grant Specialist recruitment

● Data Infrastructure Alignment (EDP) ●
Internal data capabilities

● REDCap and CTMS
● Research Analytics and Data Council
● Governance and provisioning
● External collaborations (academic and vendors)
Patient Intelligence
Patient Intelligence is a valuable tool that saves lives and expands access to critical care by enabling physicians to connect high-risk patients to cutting edge medical interventions and clinical trials
● 40 M unique lives
● 44 M distinct encounters
● 211 M diagnosis
● 24K patients with genetic data

● 3 Molecular Lab Caris, Neogenomics and Tempus Elements of Patient Intelligence
Searchable Patient Repository to create Patient Cohort for Research, Study, Quality Improvement



Hydrate Data Lake with Molecular data from Tempus, Caris and Neogenomics







Queryable doctors notes on terms
Patient Intelligence uses data from Research Data Hub, enabling population analysis (de-identified patient cohort analyses), identification of candidates for new drugs and building pursuit lists for care coordinators



EHR Matching with Molecular data

● Operational Excellence- best in industry
● Academic Partnerships

● National Recognition (scholarship,grants)



● DE&I
● Annual Report
● Research Summit

Academic Partnerships
External Industry Partnerships
Organizational Alignment
Operational Excellence
Growth and Value
●Clinical Institute support

Leading edge device and drug therapies
Precision Medicine
CSL and PE partnership
Scientific Support

Student/IIR
Diversity, Equity and Inclusion
● One CommonSpirit
●PE support
Value, Growth Research
Prioritization
Revenue, IP
CSHRI
●Standardization of Process
●Data infrastructure, resources
●People
Visibility and Reputation
Recruitment Retention
Training/Education
Digital Strategies
Research Informatics and Analytics
Patient Experience
●Academic Partnerships
●Revenue Generation
●Investigator Initiated Research
Diversity, Equity, and Inclusion
●Website
●Annual Report
●Research Summit
●National Partnerships
●Scholarship and Grants
●Diversity in Clinical Trials
●Patient Engagement
●Employee Experience
●Student Research
●Philanthropy (REEF)

● Vani Nilakantan, PhD System VP Research, CSHR) , vani.nilakantan@commonspirit.org
● Mary Rydman, System Director, Research Operations, CSHRI; mary.rydman@commonspirit.org
● Julia Link, System Director, Sponsored Programs Office, CSHRI julia.link@commonspirit.org
● Vino Raj, MD, Clinical Research Informatics Scientist, CSHRI, vino.raj@commonspirit.org

● Melissa Aigner, System Director, Research Finance, melissa.aigner@commonspirit.org

● Russell Stolp, System Manager, Research Integrity-IRB, russell.stolp@commonspirit.org
● Lauren Bacon, System Manager, Research Integrity-Quality Assurance, lauren.bacon@commonspirit.org
● Bradford Williams, Program Coordinator, CSHRI, bradford.williams@commonspirit.org
https://sites.google.com/commonspirit.org/clinicalinstitutes/clinical-institutes-home/research


