Otosclerosis in a nonendemic population: Utility of CT scan and correlation with audiometry and surgical outcome
Study of steroid effects on graft and inner ear outcomes in tympanoplasty: Randomized controlled trial
Antibiotics, steroids, and combination therapy in chronic rhinosinusitis without nasal polyps in adults
Nasal airway obstruction: Prevalence and anatomic contributors
www.entjournal.com A Vendome Publication JUNE 2018 • VOL. 97, NO. 6
MEETING HIGHLIGHTS:
• Surgical failures after a textbook surgery: The chronically infected sinus
• Technical tips for successful orbital decompression
Course Directors:
• Signature Social Event –Chihuly Glass Museum
• Cadaver Prosections
• Primary frontal sinus surgery: To do, or not to do?
• Second chances: Finding success in revision sinus surgery
• Would you do this in your office?
• Nasal polyps, our nemesis
• Complications of endoscopic sinus surgery: Managing the worst-case scenario
• Coding controversies. How would I code this? A case based panel
• Cough, throat clearing, and postnasal drip; tips for treatment of these challenging symptoms
• I don’t have migraines, Doc, I have sinus headaches
• Balloon Dilation: From sinuses to eustachian tubes
• Topical therapies for chronic rhinosinusitis
• Prednisone: Friend and foe
• Epistaxis, hemostasis and HHT
• Epiphora – I’m really not crying
ANCILLARY NON-CME & Social Events
THURSDAY, 7/12/18
5:15 - 6:15 pm
Acclarent Evening Symposium
Leveraging New Advancements in 3D ENT Navigation
FRIDAY, 7/13/18
7:30 – 8:30 am
Intersect ENT Breakfast Symposium
Advancing Care for Recalcitrant Polypoid Patients with Evidencebased Innovation
12:00 – 1:00 pm
Arrinex Lunch Symposium Chronic Rhinitis: Neurophysiology and New Treatment Paradigms
12:00 – 1:00 pm
Cook Medical Lunch Symposium
Nasoseptal Flap Donor Site Repair Using Biologic Grafts
12:00 – 1:00 pm
Entellus Medical Lunch Symposium
Office Based Sinus Surgery for Chronic Sinusitis, Eustachian Tube Dysfunction and Nasal Airway Obstruction
• Defining Appropriate Medical Therapy for CRS
• Understanding the International Consensus on Allergy and Rhinology Statements…and the most recent Allergic Rhinitis installment
• Controversies in allergy testing and immunotherapy: Challenging traditional practice
• The functional nose: When to do more than septoplasty and turbinate reduction
• Contemporary approaches to the turbinates, nasal septum, and nasal obstruction
1:00 – 5:00 pm
Entellus Medical Cadaver Lab
Approaches to Office-Based Sinus Surgery: A Hands-On Lab

1:00 – 3:00 pm &
4:00 - 6:00 pm
Medtronic Cadaver Lab
Navigating In-Office Sinus Surgery
1:00 – 5:00 pm
Olympus Cadaveric Lab
Enhanced Visualization in Advanced Surgery Techniques for Practicing Rhinologists
• Endotypes matter in CRS management
• Asthma update: What every ENT should know about state of the art asthma treatment
• Runny noses: A comprehensive approach to the medical and surgical treatment of pediatric sinusitis
• Management of CSF Rhinorrhea
• Frontal drill out: When, why and how
• Complex inflammatory sinusitis cases: Case presentations
• Pituitary surgery: Pearls and Pitfalls
• Skull base cases: Case presentations
12:00 – 1:00 pm
Stryker Lecture and Mobile Lab
Frontal Sinus Masterclass Using Building Blocks® Anatomy Planning and Target Guided Surgery Dissection
6:30 – 8:00 pm
Women in Rhinology Networking Event
SATURDAY, 7/14/18
7:30 – 8:30 am
OptiNose Breakfast Symposium
Details at http://www.american-rhinologic.org/sss
7TH Annual Summer
Symposium
Sinus
www.american-rhinologic.org Contact: Wendi Perez, Executive Administrator, ARS, PO Box 269, Oak Ridge, NJ 07438 | Tel: 973-545-2735 | Fax: 973-545-2736 | wendi@amrhso.com
The Best Sinus Course in the World: Improving Rhinology from Office to OR July 12-14, 2018 The Westin Seattle, Seattle, WA
• Keynote Speaker: Albert Merati, President Elect AAO
Registration & Housing Opens 3/1/18!
Greg Davis, MD, FARS; Marc Dubin, MD, FARS; Douglas Reh, MD, FARS

EDITORIAL BOARD
EDITORIAL BOARD MEMBERS
Editor-in-Chief
Robert T. Sataloff, MD, DMA, FACS
Professor and Chairman, Department of Otolaryngology–Head and Neck Surgery, and Senior Associate Dean for Clinical Academic Specialties, Drexel University College of Medicine Philadelphia, PA
Jean Abitbol, MD
Jason L. Acevedo, MD, MAJ, MC, USA
Jack B. Anon, MD
Gregorio Babighian, MD
Peter C. Belafsky, MD, PhD
Bruce Benjamin, MD
Gerald S. Berke, MD
Michael J. Brenner, MD
Kenneth H. Brookler, MD
Karen H. Calhoun, MD
Steven B. Cannady, MD
Ricardo Carrau, MD
Swapna Chandran, MD
Chien Chen, MD
Dewey A. Christmas, MD
Nicolle T. Clements, MS
Daniel H. Coelho, MD, FACS
David M. Cognetti, MD
James V. Crawford, MD
David H. Darrow, MD, DDS
Rima Abraham DeFatta, MD
Robert J. DeFatta, MD, PhD
Hamilton Dixon, MD
Paul J. Donald, MD, FRCS
Mainak Dutta, MS, FACS
Russell A. Faust, PhD, MD
Ramón E. Figueroa, MD, FACR
Charles N. Ford, MD
Paul Frake, MD
Marvin P. Fried, MD
Richard R. Gacek, MD
Andrea Gallo, MD
Frank Gannon, MD
Emilio Garcia-Ibanez, MD
Soha Ghossani, MD
William P. R. Gibson, MD
David Goldenberg, MD
Jerome C. Goldstein, MD
Richard L. Goode, MD
Samuel Gubbels, MD
Reena Gupta, MD
Joseph Haddad Jr., MD
Missak Haigentz, MD
Christopher J. Hartnick, MD
Mary Hawkshaw, RN, BSN, CORLN
Garett D. Herzon, MD
Thomas Higgins, MD, MSPH
Jun Steve Hou, MD
John W. House, MD
Glenn Isaacson, MD
Steven F. Isenberg, MD
Stephanie A. Joe, MD
Shruti S. Joglekar, MBBS
Raleigh O. Jones, Jr., MD
Petros D. Karkos, MD, AFRCS, PhD, MPhil
David Kennedy, MD
Seungwon Kim, MD
Robert Koenigsberg, DO
Karen M. Kost, MD, FRCSC
Jamie A. Koufman, MD
Stilianos E. Kountakis, MD, PhD
John Krouse, MD
Ronald B. Kuppersmith, MD, MBA, FACS
Rande H. Lazar, MD
Robert S. Lebovics, MD, FACS
Keat-Jin Lee, MD
Donald A. Leopold, MD
Steve K. Lewis, BSc, MBBS, MRCS
Daqing Li, MD
Robert R. Lorenz, MD
John M. Luckhurst, MS, CCC-A
Valerie Lund, FRCS
Karen Lyons, MD
A.A.S. Rifat Mannan, MD
Richard Mattes, PhD
Brian McGovern, ScD
William A. McIntosh, MD
Brian J. McKinnon, MD
Oleg A. Melnikov, MD
Albert L. Merati, MD, FACS
Joseph P. Mirante, MD, MBA, FACS
Ron B. Mitchell, MD
Steven Ross Mobley, MD
Jaime Eaglin Moore, MD
Thomas Murry, PhD
Ashli K. O’Rourke, MD
Ryan F. Osborne, MD, FACS
J. David Osguthorpe, MD
Robert H. Ossoff, DMD, MD
Enrique Palacios, MD, FACR
Michael M. Paparella, MD
Kourosh Parham, MD, PhD
Arthur S. Patchefsky, MD
Meghan Pavlick, AuD
Spencer C. Payne, MD
Kevin D. Pereira, MD, MS (ORL)
Nicolay Popnikolov, MD, PhD
Didier Portmann, MD
Gregory N. Postma, MD
Matthew J. Provenzano, MD
Hassan H. Ramadan, MD, FACS
Richard T. Ramsden, FRCS
Gabor Repassy, MD, PhD
Dale H. Rice, MD
Ernesto Ried, MD
Alessandra Rinaldo, MD, FRSM
Joshua D. Rosenberg, MD
Allan Maier Rubin, MD, PhD, FACS
John S. Rubin, MD, FACS, FRCS
Amy L. Rutt, DO
Anthony Sclafani, MD, FACS
Raja R. Seethala, MD
Jamie Segel, MD
Moncef Sellami, MD
Michael Setzen, MD, FACS, FAAP
Stanley Shapshay, MD
Douglas M. Sidle, MD
Herbert Silverstein, MD
Jeffrey P. Simons, MD
Raj Sindwani, MD, FACS, FRCS
Aristides Sismanis, MD, FACS
William H. Slattery III, MD
Libby Smith, DO
Jessica Somerville, MD
Thomas C. Spalla, MD
Matthew Spector, MD
Paul M. Spring, MD
Brendan C. Stack, Jr., MD, FACS
James A. Stankiewicz, MD
Jun-Ichi Suzuki, MD
David Thompson, MD
Lester D.R. Thompson, MD, FASCP
Helga Toriello, PhD, FACMG
Ozlem E. Tulunay-Ugur, MD
Galdino Valvassori, MD
Emre Vural, MD
Donald T. Weed, MD, FACS
Neil Weir, FRCS
Kenneth R. Whittemore, MD
David F. Wilson, MD
Ian M. Windmill, PhD
Ian J. Witterick, MD,MSc, FRCSC
Richard J. Wong, MD
Naoaki Yanagihara, MD
Eiji Yanagisawa, MD, FACS
Ken Yanagisawa, MD, FACS
Anthony Yonkers, MD
Mark Zacharek, MD
Joseph Zenga, MD
Liang Zhou, MD
CLINIC EDITORS
Dysphagia
Jamie A. Koufman, MD
Peter C. Belafsky, MD, PhD
Gregory N. Postma, MD
Facial Plastic Surgery
Anthony P. Sclafani, MD, FACS
Geriatric Otolaryngology
Kourosh Parham, MD, PhD, FACS
Karen M. Kost, MD, FRCSC
Head and Neck
Ryan F. Osborne, MD, FACS
Paul J. Donald, MD, FRCS
Reena Gupta, MD
Imaging
Enrique Palacios, MD, FACR
Ramón E. Figueroa, MD, FACR
Laryngoscopic
Robert T. Sataloff, MD, DMA, FACS
Otoscopic
John W. House, MD
Brian J. McKinnon, MD
Pathology
Lester D.R. Thompson, MD, FASCP
Pediatric Otolaryngology
Rande H. Lazar, MD
Rhinoscopic
Eiji Yanagisawa, MD, FACS
Dewey A. Christmas, MD
Joseph P. Mirante, MD, MBA, FACS
Ken Yanagisawa, MD, FACS
Special Topics
Robert T. Sataloff, MD, DMA, FACS
Thyroid and Parathyroid
David Goldenberg, MD
138 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
Nasal Valve Collapse Sufferer, Jennifer:
Help your patients achieve a new normal
Add LATERA® to your Nasal Airway Obstruction procedures and relieve patients of the symptoms of Nasal Valve Collapse. LATERA provides predictable outcomes and proven results1,2, for the breathing room they seek.

Learn more at: go.ent.stryker.com/latera0618
INDICATIONS FOR USE: The Latera Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. Risks include temporary symptoms such as mild bruising and inflammation, awareness of the implant, and mild pain or irritation. Other risks related to the LATERA implant included discomfort, infection, reaction to material, and device retrieval.
MKT30266 Rev A
ENTELLUS, SPIROX, LATERA and it logos are trademarks of Entellus Medical, Inc. ©2018 Entellus Medical, Inc.
1. San Nicoló, et. al. 2017. Absorbable Implant to Treat Nasal Valve Collapse. Facial Plast Surg, 32:233-240.
2. Stolovitzky, P. , Sidle, D. M., Ow, R. A., Nachlas, N. E. and Most, S. P. (2018), A prospective study for treatment of nasal valve collapse due to lateral wall insufficiency: Outcomes using a bioabsorbable implant. The Laryngoscope. doi:10.1002/lary.27242
“
When I run, it’s like there’s a clothespin on my nose.”
a part of Stryker
Now
Editor-in-Chief Robert T. Sataloff, MD, DMA, FACS 219 N. Broad St., 10th Fl., Philadelphia, PA 19107 entjournal@phillyent.com Ph: 215-732-6100
Managing Editor Linda Zinn
Manuscript Editors Martin Stevenson and Wayne Kuznar
Associate Editor, Reader Engagement Megan Combs
Creative Director Eric Collander
National Sales Manager Mark C. Horn mhorn@vendomegrp.com Ph: 480-895-3663
Traffic Manager Eric Collander
Please send IOs to adtraffic@vendomegrp.com
All ad materials should be sent electronically to: https://vendome.sendmyad.com
Customer Service/Subscriptions
www.entjournal.com/subscribe Ph: 888-244-5310 email: VendomeHM@emailpsa.com
Reuse Permissions Copyright Clearance Center info@copyright.com Ph: 978-750-8400 Fax: 978-646-8600
Chief Executive Officer Jane Butler
Chief Marketing Officer Dan Melore
Vice President, Finance Bill Newberry
Vice President, Custom Media Jennifer Turney Director, Circulation Rachel Beneventi
ENT-Ear, Nose & Throat Journal (ISSN: Print 0145-5613, Online 1942-7522) is published 9 times per year in Jan/Feb, Mar, Apr/May, June, July, Aug, Sept, Oct/ Nov and Dec, by Vendome Group, LLC, 237 West 35th Street, 16th Floor, New York, NY 10001-1905.

©2018 by Vendome Group, LLC. All rights reserved. No part of ENT-Ear, Nose & Throat Journal may be reproduced, distributed, transmitted, displayed, published, or broadcast in any form or in any media without prior written permission of the publisher. To request permission to reuse this content in any form, including distribution in education, professional, or promotional contexts or to reproduce material in new works, please contact the Copyright Clearance Center at info@ copyright.com or 978.750.8400.
EDITORIAL: The opinions expressed in the editorial and advertising material in this issue of ENT-Ear, Nose & Throat Journal are those of the authors and advertisers and do not necessarily reflect the opinions or recommendations of the publisher, editors, or the staff of Vendome Group, LLC. ENT-Ear, Nose & Throat Journal is indexed in MEDLINE/PubMed and Current Contents/Clinical Medicine and Science Citation Index Expanded. Editorial offices are located at 812 Huron Rd., Suite 450, Cleveland, OH 44115. Manuscripts should be submitted online at www.editorialmanager.com/entjournal. Instructions for Authors are available at www.entjournal.com.
SUBSCRIPTIONS: For questions about a subscription or to subscribe, please contact us by phone: 888-244-5310; or email: VendomeHM@emailpsa.com. Individual subscriptions, U.S. and possessions: 1 year $225, 2 years $394; International: 1 year $279, 2 years $488; Single copies $28; outside the U.S., $40.
POSTMASTER: send address changes to Ear, Nose & Throat Journal, PO Box 11404 Newark, NJ 07101-4014.
140 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
ADVERTISER INDEX Pages American Rhinologic Society ............... CV2 Arbor Pharmaceuticals 157 CANT Corp 145 Compulink Business Systems .............. CV3 Entellus Medical .................................... 139 Eosera, Inc............................................. 159 Fyzical Therapy and Balance Centers 137 InHealth Technologies 141 McKeon Products................................. CV4 MTI, Inc.................................................. 153 Shire PLC 143 Spectrum Audiology 162





The new two-piece magnetically coupled solution for non-surgical closure Round Oval www.inhealth.com We speak ENT ©2017 InHealth Technologies Manufactured by Freudenberg Medical, LLC (161010.06) The voice of experience since 1978 Magnetic
Nasal Septal Perforation Prosthesis
4
3Closure
ORIGINAL ARTICLES
156 Otosclerosis in a nonendemic population: Utility of CT scan and correlation with audiometry and surgical outcome
Lu Hui Png, MBBS, MRCS, MMed(ORL);
Jing-Yin Pang, MBBS, MRCS(Edin), MMed(ORL); Amit Karandikar, MBBS, FRCR(UK); Julian Park Nam Goh, MBBS, FRCR(UK); Seng Beng Yeo, MBBS, FRCS(Edin), FAMS(ORL); Heng Wai Yuen, MBBS, MRCS, MMed(ORL)
163 Study of steroid effects on graft and inner ear outcomes in tympanoplasty: Randomized controlled trial
Ali Kouhi, MD; Sasan Dabiri, MD; Amin Amali, MD; Nasrin Yazdani, MD; Mahboubeh Baroodabi, MD; Taha Kouchakinejad, MD; Alireza Mohseni, MD
167 Antibiotics, steroids, and combination therapy in chronic rhinosinusitis without nasal polyps in adults
Yuan F. Liu, MD; Clare M. Richardson, MD; Stewart H. Bernard, MD; Christopher A. Church, MD; Kristin A. Seiberling, MD
173 Nasal airway obstruction: Prevalence and anatomic contributors
David W. Clark, MD; Anthony G. Del Signore, MD, PharmD; Roheen Raithatha, MD; Brent A. Senior, MD
ONLINE EXCLUSIVES
E1 Revision stapes surgery after stapedotomy: A retrospective evaluation of 75 cases
Enrico Maria Amadei, MD; Claudio Cola, MD
E5 The incidence of revision adenoidectomy: A comparison of four surgical techniques over a 10-year period
Nipun Bhandari, MPH; Debra M. Don, MD; Jeffrey A. Koempel, MD, MBA
E10 Paraganglioma of the larynx diagnosed with maneuvered three-phase contrastenhanced computed tomography
İrfan Çelebi, MD; Gülpembe Bozkurt, MD; Abdullah Soydan Mahmutoğlu, MD
E14 Histopathologic evaluation of Ecballium elaterium applied to nasal mucosa in a rat rhinosinusitis model
Can Mehmet Eti, MD; Yusuf Vayısoğlu, MD; Berkan Kardaş, MD; Rabia Bozdoğan Arpacı, MD; Elif Sahin Horasan, MD; Arzu Kanık, PhD; Neslihan Eti, MD; Serap Yalın, BScPhm; Derya Ümit Talas, MD
E18 Mastoid obliteration, scutum plasty, and ossiculoplasty without staging after canalwall-up attico-mastoidectomy in adults
Shao-Cheng Liu, MD; Shyi-Gen Chen, MD; Chih-Hung Wang, MD; Bor-Rong Huang, MD
E24 Endoscopic surgery for primary sinonasal malignancies: Treatment outcomes and prognostic factors
Yan Huang, MD; Qian-hui Qiu, MD, PhD; Shui-xing Zhang, MD, PhD
E31 Hemifacial spasm secondary to middle ear cholesteatoma
Maheep Sohal, MD; Nicholas Karter, MD; Marc Eisen, MD, PhD
E33 Prevalence of mutations in the GJB2, SLC26A4, GJB3, and MT-RNR1 genes in 103 children with sensorineural hearing loss in Shaoxing, China
Hong Yu, MM; Dan Liu, MM; Jingqun Yang, MM; Zhiqiang Wu, MM
142 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018 EDITORIAL OFFICE Robert T. Sataloff, MD, DMA, FACS, Editor-in-Chief • 219 N. Broad St., 10th Fl. • Philadelphia, PA 19107 CONTENTS JUNE 2018 • VOL. 97, NO. 6
DEPARTMENTS 140 Advertiser Index 144 ENT Journal Online 146 Guest Editorial 148 Otoscopic Clinic 149 Imaging Clinic 151 Facial Plastic Surgery Clinic 154 Pediatric Otolaryngology Clinic E39 Rhinoscopic Clinic E41 Laryngoscopic Clinic E43 Geriatric Otolaryngology Clinic
* Consider
Age 1-2 years Age 3-4 years
78% of HOS patients developed an abdominal hernia1
72% of HOS patients had otitis media2
A rare combination of common childhood complaints could indicate Hunter syndrome1,2 REFER TO
For more information, visit: MPS2Syndrome.com intended for audiences outside the U.S. hunterpatients.com intended for audiences within the U.S.

SPECIALIST TODAY Silas, 1.5 Silas, 5 © Shire 2017 C-ANPROM/INT//0074 March 2017 © 2017 Shire S30749 03/17
A
ARE YOU MISSING HUNTER SYNDROME? Median age of onset and prevalence data from HOS (Hunter Outcome Survey). 1. Wraith JE et al. Genet Med 2008; 10(7): 508–516. 2. Keilmann A et al. J Inherit Metab Dis 2012; 35(2): 343–353.
the
68% of HOS patients were diagnosed with enlarged tonsils or adenoids1 of early
importance
assessment, diagnosis, and follow-up by a specialist ACT EARLY* HUNTER SYNDROME IS A PROGRESSIVE GENETIC DISEASE
ONLINE EXCLUSIVES
Revision stapes surgery after stapedotomy: A retrospective evaluation of 75 cases
Enrico Maria Amadei, MD; Claudio Cola, MD
We retrospectively evaluated a series of 75 surgical revisions after stapedotomy for the treatment of otosclerosis, carried out between 2001 and 2015. Intraoperative findings, causes of failure, and surgical solutions using an angular prosthesis, Causse prosthesis, and glass-ionomer cement were reviewed. Audiometric results performed the day before revision surgery and 1 to 2 months postoperatively were also examined. An incus necrosis was discovered in 65 patients; 55 of whom had partial necrosis of the long process of the incus and 10 with total necrosis. In 5 patients, a dislocation of the foot of the piston alone....
The incidence of revision adenoidectomy: A comparison of four surgical techniques over a 10-year period
Nipun Bhandari, MPH; Debra M. Don, MD; Jeffrey A. Koempel, MD, MBA
Approximately 130,000 adenoidectomies are performed each year in the United States. Few studies have examined adenoid regrowth and the incidence of revision surgery or have compared four different surgical instruments commonly used for adenoid surgery within the same institution. This study aimed to determine the incidence of revision adenoidectomy after the use of microdebrider, Coblation, suction cautery, and curette instruments over a 10year period at a single major tertiary children’s center in the United States. A retrospective chart review was performed for all patients who underwent primary and/or revision adenoidectomy at the Children’s Hospital Los Angeles (CHLA)....
Paraganglioma of the larynx diagnosed with maneuvered three-phase contrast-enhanced computed tomography
İrfan Çelebi, MD; Gülpembe Bozkurt, MD; Abdullah Soydan Mahmutoğlu, MD
The standard diagnostic tool for laryngeal paraganglioma is generally accepted to be magnetic resonance imaging. However, the role of other imaging modalities has not been evaluated extensively. We describe the case of a 38-year-old man who had a history of voice distortion for several years.
A hypervascular submucosal lesion was detected on maneuvered three-phase contrast-enhanced computed tomography (CT). The CT showed intense contrast enhancement in the first arterial phase (inspiration), a peak level in the....
Histopathologic evaluation of Ecballium elaterium applied to nasal mucosa in a rat rhinosinusitis model Can Mehmet Eti, MD; Yusuf Vayısoğlu, MD; Berkan Kardaş, MD; Rabia Bozdoğan Arpacı, MD; Elif Sahin Horasan, MD; Arzu Kanık, PhD; Neslihan Eti, MD; Serap Yalın, BScPhm;
Derya Ümit Talas, MD
This study aimed to evaluate the antimicrobial effects of the medicinal plant Ecballium elaterium, which is topically applied as a traditional medicine for the treatment of rhinosinusitis. Pure and extract forms of E elaterium were applied to the nasal cavity of rats for the treatment of Streptococcus-pneumoniae–induced rhinosinusitis. The nasal mucosa, soft palate, and trachea of the rats were harvested in
Mastoid obliteration, scutum plasty, and ossiculoplasty without staging after canalwall-up attico-mastoidectomy in adults
Shao-Cheng Liu, MD; Shyi-Gen Chen, MD; Chih-Hung Wang, MD; Bor-Rong Huang, MD
In a retrospective chart review, we evaluated the surgical and hearing results of a single-stage procedure consisting of a canal-wall-up attico-mastoidectomy, mastoid cavity obliteration, scutum plasty, and ossiculoplasty. A total of 77 patients treated between March 2003 and January 2011 with postoperative follow-up of at least 60 months were enrolled. Preoperative and postoperative pure-tone average (PTA) and air–bone gap (ABG) were assessed and compared 1 and 5 years after surgery. At the final follow-up, the tympanic membrane was intact in 71 (92.2%) patients. Retraction pockets were found in 10 (13.0%) patients: with 9 (11.7%) in the pars tensa, 5 (6.5%) in the posterior....
Endoscopic surgery for primary sinonasal malignancies: Treatment outcomes and prognostic factors
Yan Huang, MD; Qian-hui Qiu, MD, PhD;
Shui-xing Zhang, MD, PhD
We retrospectively reviewed the cases of 85 patients with primary sinonasal malignancies who had undergone endoscopic surgery with curative intent achieved by “regional resection.” Our goal was to assess the efficacy of endoscopic surgical treatment vis-à-vis traditional open surgery. Kaplan-Meier data analysis revealed that the 1-, 3-, and 5-year disease-specific survival rates were 82, 60, and 49%, respectively. Multivariate Cox model survival analysis revealed that the 1-, 3-, and 5-year disease-specific survival....
144 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018 www.entjournal.com
JOURNAL ONLINE
Ear, Nose & Throat Journal's website is easy to navigate and provides readers with more editorial content each month than ever before. Access to everything on the site is free of charge to physicians and allied ENT professionals. To take advantage of all our site has to offer, go to www.entjournal. com and click on the “Registration” link. Once you have filled out the brief registration form, you will have full access. Explore and enjoy!
Hemifacial spasm secondary to middle ear cholesteatoma
Maheep Sohal, MD; Nicholas Karter, MD; Marc Eisen, MD, PhD
Hemifacial spasm is a peripheral myoclonus of the VIIth cranial nerve that is characterized by paroxysmal contraction of the muscles of facial expression. It exists in both primary and secondary forms. In rare cases, hemifacial spasm is caused by middle ear pathology. We describe the case of a 90-year-old man with recurrent cholesteatoma and tympanic segment fallopian canal dehiscence manifesting as right-sided hemifacial spasm. His history was significant....
Prevalence of mutations in the GJB2, SLC26A4, GJB3, and MT-RNR1 genes in 103 children with sensorineural hearing loss in Shaoxing, China Hong Yu, MM; Dan Liu, MM;
Jingqun Yang, MM; Zhiqiang Wu, MM
Mutations in the GJB2, SLC26A4, GJB3, and MT-RNR1 genes are known to be a common cause of hearing loss. However, the frequency of hot-spot mutations and genotype-phenotype correlations in patients with sensorineural hearing loss (SNHL) has been less frequently reported. We conducted
a study of 103 children—56 boys and 47 girls, aged 5 months to 9 years (mean: 4.1 yr)—with SNHL who underwent genetic screening for 20 hot-spot mutations of the GJB2, SLC26A4, GJB3, and MT-RNR1 genes. Mutations were detected by multiple-PCR-based MALDI-TOF MS assay. At least one mutated allele was detected in 48 patients (46.6%), and 30 patients (29.1%) carried pathogenic mutations....
ONLINE DEPARTMENTS
Rhinoscopic Clinic: An inverted papilloma arising from the middle turbinate and extending to the maxillary sinus ostium
Jae Hoon Lee, MD
Laryngoscopic Clinic Laryngeal chondrosarcoma
Norman J. Chan, MD; Christopher Fundakowski, MD; Ahmed M.S. Soliman, MD
Geriatic Otolaryngology Clinic: The first cranial nerve: Pathway to the fountain of youth
Denis Lafreniere, MD; Kourosh Parham, MD, PhD, FACS
Reach More Patients.
People come in different shapes and sizes.The same piece of equipment does not fit them all.Make oropharyngeal surgery easier with a simple extension.The CANT Corporation has created the Dedo Extension that fits between the Mayo Stand and the Crowe-Davis mouth gag so that it can be adjusted to fit larger patients.The DE98-A mounts to the square-sided Mayo Stand,while the DE98-B fits the tubular-sided support. The Dedo Extension - a simple but effective solution.


Applicable Procedures
• T&A’s
• Uvuloplasty
• Palatoplasty
• All oropharyngeal procedures using a Crowe-Davis mouth gag
Volume 97, Number 6 www.entjournal.com 145
The Crowe-Davis mouth gag
The Dedo Extension The Mayo Stand
337 • 233 • 2666, ext.9 • www.jrcant.com • PO Box 3522 • Lafa yette,LA 70502 Extension
also available for tubular support Extension
A B Dedo Ext 9 (ENT1/18/05) 1/19/05 1:51 PM Page 1 ENT JOURNAL ONLINE
“DE98-B”
“DE98-A”
GUEST EDITORIAL
Balloon eustachian tuboplasty and the tragedy of the commons
The tragedy of the commons is a well-known economic model that describes what happens when individuals act solely according to their own self-interest in a manner contrary to the common good.1 This problem is not unheard of in our profession, 2 with the tragedy of the medical commons having applicability both to patients and physicians acting solely in their own self-interest. An important lesson is that two responses can occur as the result of the tragedy of the commons: government intervention/regulation and privatization. These two responses are not mutually exclusive.
Many of us are fortunate to have access to innovations that can markedly improve our patients’ lives, and many use these innovations prudently, mindful of our duty to be good stewards of the resources we administer. A few will see newer applications for innovative interventions and will explore those newer applications wisely, in keeping with the good of their patients and profession. As we all know, unfortunately, this is not the conduct of all of our colleagues.
For example, with balloon sinuplasty, an important and valuable technology, self-interest has driven some of our colleagues to push the application of this newly established procedure to achieve economic or financial goals not related to prudent patient care. This problem may occur with any innovative therapy so novel that the limits of its use remain to be understood and defined. As we have seen with balloon sinuplasty, the response of the media, government, and private insurers to this behavior can be devastatingly disproportionate and risk hobbling the appropriate and beneficial use of the new technology.
Through the work of dedicated clinicians and researchers, and with the support of industry, our specialty has another new innovative therapy, balloon eustachian tube (ET) dilatation, or balloon eustachian tuboplasty. Foresight would demand that our specialty make the effort to mitigate the risk of a similar tragedy of the medical commons. The current, published, peer-reviewed literature is the best guide on its application, and could be promulgated as preliminary clinical criteria, to be revised and updated as clinical experience and outcomes dictate.
Based on this literature, reasonable criteria may be laid out along the following lines: Patients likely to be appropriate candidates for eustachian tuboplasty are those with a medical history of unilateral or bilateral
persistent otitis media with effusion or significant nonadherent tympanic membrane atelectasis.3 Examination in all patients should document tympanic membrane morphology and motility on otomicroscopy. Nasal cavity, ET orifice, and nasopharynx should be evaluated by office endoscopy. Audiometry and tympanometry should be obtained for all patients unless contraindicated medically. Audiograms may be normal in patients with a ventilation tube or small perforation.
Conservative measures, including but not limited to intranasal steroids, treatment of laryngopharyngeal reflux, and a trial of tympanostomy tubes, should be considered before eustachian tuboplasty. Those who after conservative measures cannot successfully insufflate with a gentle Valsalva maneuver, preferably after Politzer insufflation, may be considered appropriate candidates for balloon eustachian tuboplasty.
John Stuart Mill, a champion of liberty, warned of the dangers when a minority’s self-interest disproportionately influences how a resource is used.4 Fortunately, we have the ideas of such luminaries as the late Nobel-Prize–winning economist Elinor Ostrom on how to sustainably manage “common-pool resources” through community stewardship.5 For the sake of our patients and colleagues, we have an obligation and the ability to avoid the tragedy of the commons with balloon eustachian tuboplasty and other new technologies.
References
1. Hardin G. The tragedy of the commons. The population problem has no technical solution; it requires a fundamental extension in morality. Science 1968;162(3859):1243-8.
2. Hassanally K. Overgrazing in general practice: The new Tragedy of the Commons. Br J Gen Pract 2015;65(631):81.
3. Silvola J, Kivekäs I, Poe DS. Balloon dilation of the cartilaginous portion of the eustachian tube. Otolaryngol Head Neck Surg 2014;151(1):125-30.
4. Mill JS, Gray J. On liberty. In: On Liberty and Other Essays. NY: Oxford University Press; 2008:5-130.
5. Ostrom E. Governing the Commons: The Evolution of Institutions for Collective Action (Political Economy of Institutions and Decisions). Cambridge, U.K: Cambridge University Press; 1990.
Brian J. McKinnon, MD, MBA, MPH, FACS
Associate Professor and Vice Chair
Department of Otolaryngology–Head and Neck Surgery
Associate Professor
Department of Neurosurgery
Drexel University College of Medicine
Philadelphia
146 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018




















































































IRRIGATING YOUR SINUSES WITH SALINE IS LIKE USING LEECHES TO TREAT PNEUMONIA. IT IS INADEQUATE AND OUTDATED. Xlear is the only scientifically advanced sino-nasal cleansing solution with xylitol. Call 1 877 599 5327 for free samples. • Reduces S.N.O.T. score by 25%. • Improves peak airflow by 36%. • For more studies go to PubMed. #imXlear Located at these and many other fine retailers.
Intradermal nevus of external auditory canal revisited
Pei-Hsuan Wu, MD; Hsin-Chien Chen, MD, PhD
A 61-year-old woman presented to our hospital with an incidental finding of an external ear tumor during a routine physical examination. Otoscopic examination revealed a skin-colored, hair-bearing, dome-shaped mass over the posterior wall of the cartilaginous part of the right external auditory canal (EAC) (figure, A). The mass partially occluded the ear canal with cerumen

impaction medial to the lesion, but the eardrum could be visualized by otoscopy. Audiometry revealed mild symmetric, sloping sensorineural hearing loss without conductive hearing loss.
High-resolution computed tomography (CT) of the temporal bone revealed a soft-tissue lesion located in the posterior wall of the right EAC measuring approximately
Continued on page 152
148 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
From the Department of Otolaryngology–Head and Neck Surgery, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
OTOSCOPIC CLINIC
Figure. A: Otoscopic image shows a dome-shaped lesion in the posterior cartilaginous portion of the EAC. B: High-resolution CT of the temporal bone reveals a soft-tissue lesion in the posterior wall of the right EAC measuring approximately 5.0 × 4.8 mm in axial and coronal views (arrows). C: Histopathologic staining shows that the intradermal nevus is composed of nevus cells (melanocytes) localized only in the dermis (hematoxylin and eosin, original magnification ×200).
Low-grade sinonasal sarcoma with neural and myogenic features
We present the case of a healthy 79-year-old woman who initially presented to the clinic with a midline frontal mass. She reported first noticing the mass 1 year before evaluation, after being struck in the head with a branch while gardening. She presented for evaluation because of progressive enlargement of the mass. She denied headache, pain, nasal congestion, facial pressure, facial numbness, or difficulty breathing.
The patient underwent computed tomography (CT) of the head and sinuses followed by magnetic resonance imaging (MRI) of the brain and sinuses, with and without contrast. Imaging demonstrated a noncystic mass lesion in the region of the frontal sinuses with avid homogenous enhancement. The remainder of the opacified contents in the frontal sinuses, left ethmoid air cells, and left maxillary sinus were clearly separate from

but does not
the
the mass, demonstrating a relatively high T2-weighted signal and lack of enhancement consistent with mucocele formation.
The epicenter of the mass was presumably in the left frontal sinus, although a bulk of the lesion also presented in the midline between the frontal sinuses. No bony separation of the left and right frontal sinus was seen, which could have been secondary to erosion or simply an anatomic variant. The lesion was clearly expansile, enlarging the confines of the left frontal sinus. Additionally, long segments of both the anterior and posterior cortex of the frontal sinus were not present, with other areas thinned.
Surgery was performed using a combined endoscopic approach to the floor of the anterior fossa and a bifrontal craniotomy. Histologically, the specimen revealed a
Volume 97, Number 6 www.entjournal.com 149
From the Department of Surgery (Dr. Hockstein and Dr. Wilhelm) and the Department of Radiology (Dr. Dross and Dr. Farooqui), Christiana Health Care System, Christiana Hospital, Newark, Del.
IMAGING CLINIC A
Neil G. Hockstein, MD; Peter E. Dross, MD; Shoheb Farooqui, MD; Ian N. Wilhelm, MD
B
Figure 1. A: Axial CT image in bone windows demonstrates opacification of the frontal sinus with areas of thinning and frank erosion of the posterior cortex. B: Axial T1-weighted gadolinium-enhanced image at approximately the same level shows an avidly enhancing mass protruding intracranially through the bony defect with mild mass effect on the left frontal lobe. The mass abuts
invade
underlying enhancing dura mater (arrow).
spindle cell neoplasm composed of interlacing fascicles; cellularity was high with only rare mitotic figures. Immunohistochemical staining was focally reactive for both S-100 and SMA. The patient tolerated the procedure well and was discharged home without difficulty. At her follow-up appointment, she was doing well and MRI revealed no evidence of recurrence.
The distinction between bony destruction versus erosion is important because they suggest different pathologic entities. The appearance in our case was more congruent with erosion secondary to an expansile mass with smooth borders and adjacent cortical thinning. Mucocele pressure remodeling might have played a role to some extent, although it was felt the primary
process was due to the mass because the areas of erosion were subjacent to the enhancing mass, which protruded through these regions.
The mass demonstrated intracranial extension, with much of the posterior cortex of the left frontal sinus eroded or thinned. The mass abutted the left frontal dura and created mild mass compression of the underlying brain. There was enhancement of the dura but no evidence of invasion or brain parenchymal involvement (figure 1). The midline component of the mass protruded anteriorly into the adjacent scalp soft tissues, with erosion and thinning of the anterior cortex (figure 2). A small portion of the mass appeared to extend through and widen the frontoethmoidal recess on the left. The inferior portion of the mass also eroded the roof of the left orbit, with extension into the extraconal orbital compartment and compression of the superior rectus muscle (figure 3).
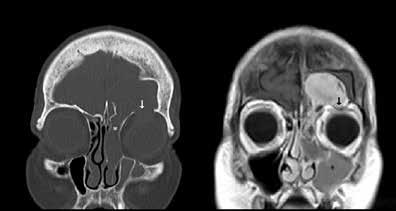
At the time of imaging, undifferentiated sinonasal carcinoma was considered in the differential, although it is typically much more destructive in appearance. Sinonasal lymphoma, melanoma, and metastases were also considered given the strong enhancement of the mass. Sarcomas of the paranasal sinus tract are extremely rare. We present an advanced case of low-grade sinonasal sarcoma with neural and myogenic features (LGSSNFM). To the best of our knowledge, this is the only case involving a patient requiring craniofacial resection and reconstruction at the initial operation. LGSSNMF was first described in 2012 by Lewis et al.1 The tumor presented here differs from what has been previously reported because of its erosive and intracranial nature.
Reference
coronal T1-weighted
image at approximately the same level shows the enhancing mass within the left frontal sinus protruding through the bony defect of the left orbital roof, invading the extraconal space. Notice compression of the superior rectus muscle (black arrow). Also demonstrated is the extension of the enhancing mass through the frontoethmoidal recess into the nasal cavity. There is opacification of the left maxillary sinus (black asterisk) compared to the right; however, it is clearly separable from the mass above, indicative
1. Lewis JT, Oliveira AM, Nascimento AG, et al. Low-grade sinonasal sarcoma with neural and myogenic features: A clinicopathologic analysis of 28 cases. Am J Surg Pathol 2012;36(4):517-25.

150 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018 IMAGING CLINIC
A B
Figure 2. A: Sagittal CT in bone windows demonstrates erosion of both the anterior and posterior cortex of the frontal sinus (arrows). B: Unenhanced T1-weighted sagittal MRI demonstrates the lowsignal mass protruding into the subcutaneous soft tissues, resulting in an obvious cosmetic deformity.
A B
Figure 3. A: Coronal CT in bone windows demonstrates erosion of the left orbital roof (white arrow), with expansion of the left frontoethmoidal recess (white asterisk). B: Corresponding
gadolinium-enhanced
of mucocele formation.
FACIAL PLASTIC SURGERY CLINIC
Autologous lipoinjection in ParryRomberg syndrome

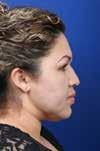
Parry-Romberg syndrome (PRS), or progressive hemifacial atrophy, is characterized by focal and progressive atrophy of facial skin and soft tissue. It usually affects one side and may include medial canthal malposition, enophthalmos, and skin hyperpigmentation.1,2 PRS often presents during the first and second decades of life, affects women more than men, and may continue to worsen for another decade before reaching a stable phase.3 Its etiology remains unknown but may be related to autoimmunity, viral infection, trauma, and/or inherited genetic mutation.1

A 28-year-old woman presented to us with right facial atrophy but with normal movement and sensation. She was diagnosed with PRS and underwent abdominal fat harvest and lipoinjection to the right face. Her preoperative appearance is shown in figure 1.

The procedure was as follows: 2 ml of 1% lidocaine with 1:100,000 epinephrine was injected along the inferior aspect of the umbilicus. Then, 100 ml of 0.25%
lidocaine with 1:400,000 epinephrine was injected along the lower abdomen. After 10 minutes, a 10-ml syringe with a fat harvesting cannula was used to extract fat under low manual negative pressure through a 1-cm incision. The cannula was fanned evenly throughout the lower abdomen in the fat layer. A total of 60 ml of fat was harvested.
The harvested fat was transferred to several 10-ml syringes and centrifuged for 3 minutes. The supernatant and infranatant were discarded, and the bottom 2 ml of fat left in the syringes was transferred to 1-ml syringes. Several stab-incision ports were made in the face with an ophthalmic blade. Using various sized cannulas, the fat was transferred to the right face in multiple layers, including the supraperiosteal, subcutaneous, and subdermal layers (total 12.5 ml).
During a second procedure 4 months later, 20 ml of fat was grafted, and 46 ml was grafted during a third procedure 5 months after the second injection, using
Volume 97, Number 6 www.entjournal.com 151
From Loma Linda University School of Medicine (Ms. Harp), and the Department of Otolaryngology–Head and Neck Surgery, Loma Linda University Medical Center (Dr. Liu, Dr. Inman, and Dr. Ardeshirpour), Loma Linda, Calif.
Alana Harp, BS; Yuan F. Liu, MD; Jared C. Inman, MD; Farhad Ardeshirpour, MD
Figure 1. Photos show the appearance of the patient’s face before lipoinjection.
the same technique. The patient reported great satisfaction (figure 2).
While autologous fat grafting has been used to treat PRS for decades, its outcomes can be unpredictable. Variables affecting outcomes include quality of fat, quantity injected, and distribution of transferred fat. How fat is separated after liposuction also may play a role. Survival is thought to be influenced by how much blood supply is in physical contact with grafted fat. Thus, fat survival is a matter of striking a balance between the number of procedures and fat grafted each time.1
Our technique of slow injection in different layers over a wide region is time-consuming, but it can achieve a smooth contour along with potentially higher fat retention. Our method of increasing injected volumes is opposite that of other reports. One reason we did this was that the patient had greater retention than expected each time, perhaps due to our injection technique or unknown patient factors. It is also possible that fat injection in the face stimulates the development of vasculature, which allows a greater fat-carrying capacity over time.
References
1. Sterodimas A, Huanquipaco JC, de Souza Filho S, et al. Autologous fat transplantation for the treatment of Parry-Romberg syndrome. J Plast Reconstr Aesthet Surg 2009;62(11):e424–6.

2. Slack GC, Tabit CJ, Allam KA, et al. Parry-Romberg Reconstruction: Beneficial results despite poorer fat take. Ann Plast Surg 2014;73(3):307–10.

3. Pagnoni M, Bartoli D, Terenzi V, et al. Lipostructure in ParryRomberg disease. J Craniofac Surg 2012;23(6):e621–3.
Continued from page 148
5.0 × 4.8 mm (figure, B). The tumor was excised with a circumferential incision via an endoscopic, laser-assisted approach under local anesthesia. The wound was packed with Gelfoam for 1 week and left to heal spontaneously. The pathology was proven to be an intradermal nevus (figure, C).
After surgical intervention, the patient experienced an uneventful recovery. There was no evidence of recurrence 6 months postsurgically.

Intradermal nevus is a subtype of melanocytic nevus; its occurrence within the EAC is relatively rare.1-3 We previously reported on 38 cases of intradermal nevus in the EAC.1 A higher incidence was reported in Asian people. The mean age was 40.3 years with a female predominance of approximately 3 to 1. No side predominance was noted. Intradermal nevi were more frequently located posteriorly, followed by the superior, inferior, and anterior quadrants of the EAC.
Most EAC nevi are symptomatic, but some are asymptomatic and are found incidentally. An EAC nevus may cause conductive hearing loss because of its enlarging mass, causing obstruction of the EAC or possibly a keratosis obturans and cholesteatoma.1,3 The differential diagnosis of the EAC nevi should include seborrheic keratosis, senile keratosis, pigmented actinic keratosis, benign pigmented keratosis, common warts, pigmented fibrous histiocytoma, squamous papilloma, blue nevus, atypical nevus, malignant melanoma, and even squamous cell carcinoma.1-3
The preferred treatment is surgical removal; no recurrence with this approach has been reported to date. Most EAC nevi can be excised via a transcanal or transmeatal approach under microscopy, but larger lesions should be excised by endaural incision.3 Surgical skin defects are mostly left to allow granulating and spontaneous healing. Some cases with a larger defect need to be repaired with a free split or full-thickness skin graft or a temporalis fascia graft with Silastic strip.2
In this presented case, we used a laser to excise the nevus via a transcanal approach with endoscopy and allowed the defect to heal spontaneously.
References
1. Lin HC, Wang CH, Su TF, Chen HC. Intradermal nevus of the external auditory canal in a geriatric patient: Case report and literature review. Eur Geriatr Med 2014;5:274-6.
2. Magliulo G, Ciniglio Appiani M, Colicchio MG, Cerbelli B. Melanocytic nevus of the external auditory canal. Otol Neurotol 2012;33(4):e29-30.
3. Fraser L, Smith WK. Excisional technique for intradermal nevi of the external auditory canal. J Otolaryngol Head Neck Surg 2009;38(4):501-3.
152 www.entjournal.com ENT-Ear, Nose & Throat Journal FACIAL PLASTIC SURGERY CLINIC OTOSCOPIC CLINIC
Figure 2. The patient’s appearance is greatly improved, as shown in these photos taken 5 months after the second procedure.



SAVE up to $3870 on a 423 Dual Power Chair & TC100A ENT Cabinet at the AAO-HNSF Conference. This ENT Event will be held on Sept 10-13 at McCormick Place in Chicago. Visit MTI Booth #1425 to explore our prePower/Manual Chairs w/lift, back or tilt LED exam, procedure or back lights ENT Mobile Treatment Cabinets Pneumatic Stools w/Ergo Back Side Chairs w/Twin Arms JOIN MTI AAO-HNSF 3870 877-908-9609
PEDIATRIC OTOLARYNGOLOGY CLINIC
Infant with an unusual pharyngeal mass
Benjamin B. Shields, MD; Erin E. Lampson, MD; Anita L. Sengupta, MD; Tanya C. Watt, MD; Ron B. Mitchell, MD
The differential diagnosis of an infant with a pharyngeal mass includes congenital masses (i.e., dermoid cysts, lymphangiomas, and teratomas), lymphadenitis, hemangiomas, and soft-tissue malignancies. The most common malignancy in the first year of life is neuroblastoma.1 These tumors most frequently present suprarenally2 and rarely in the aerodigestive tract. We report the presentation and management of an infant with an unusual pharyngeal mass diagnosed as a neuroblastoma.
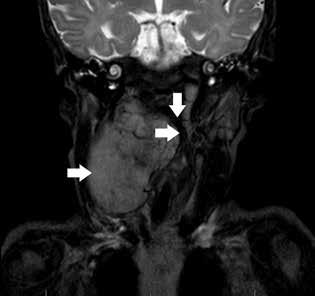
A 19-week-old infant girl presented with noisy breathing of 4 to 6 weeks’ duration. The symptoms worsened when the child cried or was agitated. She had been feeding well without episodes of apnea or cyanosis and was demonstrating appropriate weight gain. The patient was delivered at full term with no complications and had no prior hospitalizations.
Flexible fiberoptic laryngoscopy revealed a bulging right lateral pharyngeal wall and a normal epiglottis, supraglottis, and vocal folds. Magnetic resonance imaging (MRI) confirmed a pharyngeal mass from C1 to C7 measuring 1.6 × 5.1 × 6.4 cm with heterogeneous enhancement and a mass effect with displacement of the oropharyngeal airway (figure 1). The MRI also revealed an enlarged level II right cervical lymph node measuring 12.5 × 8.5 × 3.0 mm. The differential diagnosis included neuroblastoma, rhabdomyosarcoma and, less likely, neurofibroma.
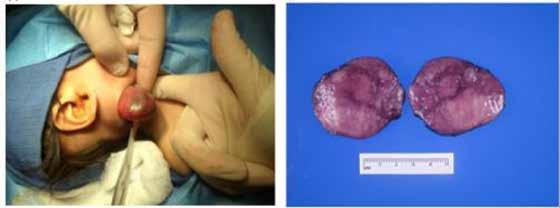
The infant underwent airway evaluation and an excisional biopsy. Direct laryngoscopy revealed a normal supraglottis, vocal folds, and postcricoid space. A posterior bulge of the right hypopharynx was noted. The rest of the airway examination down to the bronchi was normal. The neck was then explored, subplatysmal flaps raised, and the medial border of the right sternocleidomastoid identified. The mass was noted deep to the sternocleidomastoid and posterior to the internal
jugular vein and vagus nerve. It was removed without difficulty (figure 2). Additionally, level II lymph nodes were dissected and removed.
Frozen section of these nodes was consistent with a diagnosis of neuroblastoma. The incision was closed in the standard fashion and a suction drain left in place. The patient had an uneventful recovery, with the drain removed on the fourth postoperative day. A diagnosis of neuroblastoma with metastasis to one of seven resected nodes was confirmed (figure 3).
A complete staging evaluation was then performed by the oncology service. Bilateral bone marrow biopsies and aspirates demonstrated no evidence of neuroblastoma. Cytogenetic analysis of the tumor revealed no amplification of the MYCN oncogene, which occurs
154 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
From the Department of Otolaryngology (Dr. Shields and Dr. Mitchell), the Division of Pediatric Hematology–Oncology, Children’s Medical Center (Dr. Lampson), and the Department of Pathology (Dr. Sengupta), University of Texas Southwestern Medical Center, Dallas.
Figure 1. Coronal section of T2-weighted MRI reveals a large, hyperintense pharyngeal mass compressing the airway.
in approximately 22% of cases and is associated with a poor prognosis.3 A radiolabeled I-131 metaiodobenzylguanidine (MIBG) scan revealed no areas of distant metastasis. Similarly, MRI of the neck, chest, abdomen, and pelvis revealed no suspicious lesions. Urine catecholamines secreted by the tumor were elevated at the time of presentation but normalized after tumor excision.
The patient was classified as having a stage 2B neuroblastoma, given the localized tumor with complete gross resection and ipsilateral lymph node involvement. No chemotherapy was indicated. Recommended follow-up was for MRI scans every 3 months and monthly urine catecholamines to evaluate for disease recurrence.
Neuroblastomas have a highly variable presentation, ranging from aggressive metastasis to spontaneous regression.4 Primary tumors in the neck or upper chest can cause Horner syndrome (ptosis, miosis, anhidrosis) or airway compromise.4 Other presenting symptoms include abdominal discomfort and distension, bone pain, watery diarrhea, periorbital ecchymoses due to orbital metastases, subcutaneous skin nodules, and opsoclonus-myoclonus syndrome.
Treatment of neuroblastoma depends on risk stratification and staging. Adverse prognostic features include patients >12 months of age, MYCN gene amplification, metastatic spread, and a poorly differentiated tumor with
a high mitotic-karyorrhexis index.5 In low-risk patients such as the one described in this report, surgical excision and observation is the mainstay of therapy.
This case highlights the need for a multidisciplinary approach to the treatment of children with a malignant neck mass. While surgical excision was the mainstay of treatment in this infant, staging of the tumor and long-term follow-up is required by pediatric oncology.
References
1. Linet MS, Ries LA, Smith MA, et al. Cancer surveillance series: Recent trends in childhood cancer incidence and mortality in the United States. J Natl Cancer Inst 1999;91(12):1051-8.
2. Orbach D, Sarnacki S, Brisse HJ, et al. Neonatal cancer. Lancet Oncol 2013;14(13):e609-20.
3. Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J Clin Oncol 2009;27(2):289-97.
4. Maris JM. Recent advances in neuroblastoma. New Engl J Med 2010;362(23):2202-11.
5. Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014;64(2):83-103.
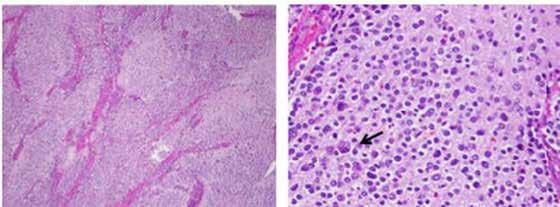
Volume 97, Number 6 www.entjournal.com 155 PEDIATRIC OTOLARYNGOLOGY CLINIC
Figure 2. A: A large pharyngeal mass is removed via excisional biopsy. B: Gross sections of the mass show an encapsulated mass with a vaguely nodular cut surface.
Figure 3. A: Low-power histology of the mass (hematoxylin and eosin stain, original magnification ×4) shows a nodular proliferation of small neuroblasts in a background of fibrillary neuropil. B: High-power histology of the mass (hematoxylin and eosin stain, original magnification ×20) shows small neuroblasts in a background of fibrillary neuropil. One maturing ganglion cell with three nuclei is seen in the lower left of the image (arrow).
Otosclerosis in a nonendemic population: Utility of CT scan and correlation with audiometry and surgical outcome
Lu Hui Png, MBBS, MRCS, MMed(ORL); Jing-Yin Pang, MBBS, MRCS(Edin), MMed(ORL); Amit Karandikar, MBBS, FRCR(UK); Julian Park Nam Goh, MBBS, FRCR(UK); Seng Beng Yeo, MBBS, FRCS(Edin), FAMS(ORL); Heng Wai Yuen, MBBS, MRCS, MMed(ORL)
Abstract
The incidence of otosclerosis in nonendemic patients is low, and preoperative diagnosis can be challenging. The aim of this study was to evaluate computed tomography (CT) findings in patients with otosclerosis and determine their correlation with audiometric findings and surgical outcome in a nonendemic population. We retrospectively reviewed 17 patients from August 2011 to August 2013 with surgically confirmed otosclerosis who underwent preoperative high-resolution CT scans and pre- and postoperative audiometry. Otosclerotic foci were identified on the scans. The density ratio of these foci was calculated and compared with pre- and postoperative audiometric parameters. One patient with Paget disease was excluded from the study. A total of 19 ears were operated on and included in the data analysis. CT scans were normal in 4 ears (21.1%). Hypodense lesions were detected in the remaining 15 (78.9%) ears and the region of interest mapped out. The density ratio was obtained between the hypodense area and adjacent normal labyrinthine bone. No statistically significant correlation was found between the density ratio and any of the audiometric parameters tested (p > 0.05). The diagnosis of otosclerosis in nonendemic areas is challenging. A preoperative CT scan can be useful when otosclerotic foci are present. However, the density ratio of the otosclerotic foci did not correlate with audiometric parameters or surgical outcome.
From the Department of Otorhinolaryngology–Head and Neck Surgery, Changi General Hospital, Singapore (Dr. Png, Dr. Pang, and Dr. Yuen); and the Department of Diagnostic Radiology (Dr. Karandikar and Dr. Goh) and the Department of Otolaryngology, Tan Tock Seng Hospital, Singapore (Dr. Yeo).
Corresponding author: Lu Hui Png, Department of Otorhinolaryngology–Head and Neck Surgery, Changi General Hospital, 2 Simei Street 3, Singapore 529889. Email: Luhpng@gmail.com
Introduction
In 1860, Toynbee first described stapes fixation as a cause of hearing loss in the population.1 This cause of hearing loss was subsequently named otosclerosis by Politzer in 1893.2 As it is the most common cause of progressive conductive hearing loss in adults, reports in the literature on the diagnosis, management, and surgical outcome of this condition are abundant.3
The underlying pathologic processes involved in otosclerosis can affect various parts of the otic capsule, but the fissula ante fenestram is the most commonly involved area. When involved with otosclerosis, this region can appear as a lucency on computed tomography (CT).4 In the clinical setting, high-resolution CT studies by Marx et al demonstrated a relationship between the degree of otosclerosis on CT and pre/ postoperative pure-tone audiometry findings, which suggests that CT is a potential tool for prognostication, as well. 5
The combination of a normal ear examination and air-bone gap on audiogram with a typical history makes the clinical diagnosis of otosclerosis in endemic regions easy and straightforward. Importantly, the incidence of otosclerosis is significantly lower in various nonendemic populations throughout the world. For example, clinical otosclerosis is particularly rare in ethnicities such as Asians and Africans. 6,7 Therefore, in nonendemic areas, the clinical diagnosis of otosclerosis may be more challenging as other entities such as middle ear and ossicular pathologies present with proportionately higher frequency. In these populations, the use of CT scans may be helpful to distinguish between these conditions; typical findings on CT in patients with otosclerosis will help to clinch the diagnosis.
156 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
ORIGINAL ARTICLE
Single. Sterile. Simple.
• 14 single-use vials contain 1 premeasured dose each— dose BID/7 days2

• Every dose is sterile, precise, and preservative free2
• No drop counting. No mixing or shaking required2
IMPORTANT SAFETY INFORMATION
Contraindications
OTOVEL is contraindicated in:
• Patients with known hypersensitivity to fluocinolone acetonide or other corticosteroids, ciprofloxacin or other quinolones, or to any other component of OTOVEL.
• Viral infections of the external ear canal, including varicella and herpes simplex infections and fungal otic infections.
The following Warnings and Precautions have been associated with OTOVEL: Hypersensitivity reactions, potential for microbial overgrowth with prolonged use, and continued or recurrent otorrhea.

The most common adverse reactions are otorrhea, excessive granulation tissue, ear infection, ear pruritis, tympanic membrane disorder, auricular swelling, and balance disorder
For additional Important Safety Information, please see Brief Summary of Prescribing Information on adjacent page and full Prescribing Information available at www.otovel.com.
1. US Food and Drug Administration. Orange Book: Approved drug products with therapeutic equivalence evaluations. https://www.accessdata.fda.gov/scripts/cder/ob/. Accessed February 1, 2017. 2. Otovel [package insert]. Atlanta, GA: Arbor Pharmaceuticals, LLC. Otovel is a registered trademark of Laboratorios Salvat, S.A. with the US Patent and Trademark Office and under license by Arbor Pharmaceuticals, LLC. Trademarks are the property of their respective owners. © 2017 Arbor Pharmaceuticals, LLC. All rights reserved. PP-OTO-US-0135 The first and only combination ear drop for AOMT in single-use vials 1 For treatment of acute otitis media in children with tympanostomy tubes (6 months
older) due to S. aureus, S. pneumoniae, H. influenzae, M. catarrhalis,
aeruginosa.
References:
and
and P.
Drop into www.otovel.com/ENT to learn more about this AOMT treatment.
A previous study looking at otosclerosis in the Japanese population showed good correlation between CT findings and audiometry. 8 Specifically, the authors noted that the incidence of demineralization of the inner ear was significantly less in the Japanese compared with Caucasians, which they attributed to racial differences. This “degree” or “severity” of demineralization has been the subject of recent studies.
Min et al noted that the incidence of positive CT findings in Korean patients with clinical otosclerosis was 73%.9 This percentage is significantly higher than that demonstrated by previous studies of otosclerosis in ethnic Asians. The authors also assessed the relationship between CT findings and audiometric parameters by evaluating the degree of demineralization using a polygonal region of interest (ROI) program and concluded that the density ratio of hypodense lesions influenced surgical outcomes. However, the extent of hypodense lesions did not correlate with pre- or postoperative hearing levels. This lack of correlation was postulated to be due to the rising incidence of otosclerosis in Asians, which they attributed to changes in lifestyle and diet.10
The aim of our study was to examine CT findings in patients with otosclerosis within a nonendemic population and their correlation with audiometric findings and surgical outcomes.
Patients and methods
This was a retrospective study involving patients from a tertiary referral center between August 2011 and August 2013. Our study had Institutional Review Board exemption (SingHealth Centralised Institutional Review Board). Inclusion criteria encompassed consecutive patients during the designated study period with surgically confirmed otosclerosis who underwent preoperative high-resolution CT scans and pre- and postoperative audiometry.
Excluded were patients with concomitant comorbidities that could account for temporal bone findings similar to those of otosclerosis (i.e., Paget disease). One patient was subsequently excluded because of a history of Paget disease on further investigation. Seventeen patients were identified after applying our selection criteria.
All patients underwent stapedotomy using an otologic drill system. Pre- and postoperative puretone audiometry was performed in the same facility with air-conduction (AC) and bone-conduction (BC) thresholds, and the air-bone gap (ABG) was recorded. Audiometric data were calculated using the average of 0.5-, 1-, 2-, and 4-kHz thresholds. All patients un-
derwent audiometry within 6 months before surgery to 3 months after.
Preoperative high-resolution CT scans were reviewed by two head and neck radiologists (JG, AK) for the characteristic otosclerotic foci. CT scans were performed using a Siemens SOMATOM Sensation 64 CT scanner, looking at temporal bones with bony reconstruction on noncontrast axial high-resolution CT. Scanner specifications included slice thickness of 0.6 mm, collimation of 0.6 mm, scan interval of 0.4 mm, Kv of 120, and mAs of 250.
The density ratio of the otosclerotic foci was calculated and correlated to pre- and postoperative audiometric parameters. The presence of otosclerotic foci was determined by the visualization of a hypodense lesion on CT by the radiologist. Subsequently, the size of this hypodense area was evaluated using a polygonal ROI program that allowed assessment of its borders in the fissula ante fenestram within an oval ROI.
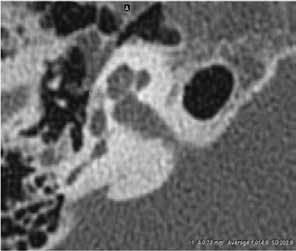
The density ratio was obtained between the hypodense area of otosclerotic foci in the fissula ante fenestram and a consistent area of the adjacent normal labyrinthine bone, which in our case was the inner portion of the lateral semicircular canal (figure). This ratio was calculated using the following formula:
Density ratio = density of the otosclerotic foci in the fissula ante fenestram/density of normal labyrinthine bone
The demographics of patients were recorded and further stratified according to age, sex, and race, as well as age at diagnosis and duration of disease. For the sake of completeness, an established CT grading system was incorporated into our results and compared against corresponding density ratios, where grade 1 represents solely fenestral lesions; grade 2 represents patchy localized cochlear disease to either basal cochlear turn (grade 2A), middle/apical turns (grade 2B), or both basal and middle/apical turns (grade 2C); and grade 3 represents diffuse confluent involvement of the entire cochlea.11
Results
Sixteen patients were included with a mean age of 46.1 years (range: 27 to 67). From this whole population, 19 ears were operated on and included in our data analysis. The ratio of men to women was 7:9, with a racial distribution of 11 Chinese (68.8%), 1 Malay (6.3%), 3 Indian (18.8%) and 1 Eurasian (6.3%). Mean age at diagnosis was 44.9 years. The mean duration of disease was 44.3 months before surgery was performed.
Pre- and postoperative audiometric data appear in table 1. Five ears (26.3%) had a Carhart notch on
158 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
PNG, PANG KARANDIKAR, GOH, YEO,YUEN































































C L I N I C A L LY P R O V E N, TO D I S S O LV E WA X , I N O N E T R E ATM E N T | | | || | | | | | L | | | | T | T H | | | | F | | T | F | L | | | |V| I L | B L | S P | I N G 2017 | | | | || | | ||| | | |N| P|SSIBL| SI|| |FF||T |F |U| | | I|P |TI|N T|| T||NT| S|IL| F||| | | T| | ||
audiometry. Two patients (12.5%) were documented to have a family history of hearing loss.
Seven patients (43.8%) had clinical evidence of otosclerosis unilaterally and 9 patients (56.3%) had both ears affected. Of the 9 patients with bilateral otosclerosis, 3 patients (18.8%) had the contralateral side operated on subsequently.

All patients recovered well after surgery, with no postoperative vertigo. None of the patients required revision surgery.
Review of CT scans showed 4 ears (21.1%) with normal CT scans, and hypodense lesions in the remaining 15 ears (78.9%), with the ROI mapped out. These 15 ears had a reduced density ratio of otosclerotic foci to normal labyrinthine bone. The 4 ears with normal CT scans had normal density ratios. No statistically significant correlation was found between the density ratio and any of the audiometric parameters tested, as well as degree of ABG improvement after stapedotomy ( p > 0.05).
The CT scans were subsequently graded according to the Symons and Fanning CT grading system for otosclerosis11 and compared against corresponding
density ratios (table 2), with no statistically significant correlation noted.
Discussion
Previous otologic studies have shown varying results regarding the correlation between radiologic findings and audiometric thresholds in otosclerosis. However, many of these reports examined a primarily Caucasian population in which the diagnosis of otosclerosis is frequently easily reached. The reason, in part, is related to the low incidence of otosclerosis in other races. To our knowledge, this is the first report examining and correlating CT findings with audiometry as well as surgical outcomes in Southeast Asian patients.
In histologic studies, Schuknecht and Barber reported no correlation between the degree of otosclerosis and magnitude of sensorineural hearing loss.12 They also noted that bone conduction thresholds appeared to be unrelated to the size or activity of the otosclerotic lesion. In contrast, de Groot et al showed a positive correlation between otic capsule density and bone conduction thresholds,13 which was subsequently confirmed by Marx et al. 5
160 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018 PNG, PANG KARANDIKAR, GOH, YEO,YUEN
A B C
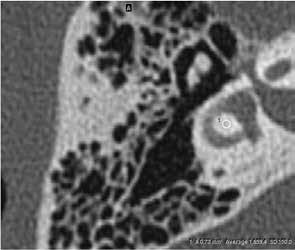
Figure. A: In this CT image of temporal bone, the arrow points to the hypodense otosclerotic focus at the right fissula ante fenestram. B: Oval region of interest measures the density of the otosclerotic focus (size is 0.73 mm2, CT value: 1,014.9 HU). C: Oval region of interest measures the density of the normal temporal bone (size is 0.73 mm2, CT value: 1,659.4 HU), with a density ratio of 0.611.
CT scans positive for hypodense lesions (n = 15) CT scans negative for hypodense lesions (n = 4) Total (n = 19) Mean preoperative BC, dB 36.89 32.43 36.15 Mean preoperative AC, dB 70.30 64.90 69.40 Mean preoperative ABG, dB 33.41 32.47 33.25 Mean postoperative BC, dB 29.66 27.10 29.09 Mean postoperative AC. dB 38.81 36.58 38.31 Mean postoperative ABG. dB 9.15 9.48 9.22 Key: BC = bone conduction; AC = air conduction; ABG = air-bone gap
Table 1. Audiometric data according to CT temporal bone findings
In our study, positive CT findings of hypodense otosclerotic foci were noted in 78.9% of ears compared with 73% noted in Korean otosclerosis and 53.7% noted in Japanese otosclerosis. 8,9 Our figure is closer to that in studies of Caucasians, likely due to the heterogenicity of an Asian population that included patients of multiple races and ethnicities. Interestingly, of those patients with normal CT findings, a Carhart notch was noted on audiometry in 2 of 4 ears (50%). This finding corroborates the point that early otosclerosis may present solely with audiologic findings.
Furthermore, our results show no relationship between otosclerotic density ratios and audiometric findings and surgical outcomes. The lack of relationship is partially in keeping with Min et al’s results, showing no correlation between density ratios and pre- and postoperative hearing thresholds, although they did show an inverse relationship when density ratios were compared with postoperative ABGs.9 In contrast, Kiyomizu et al showed a good correlation between preoperative CT findings and audiometry. 8 The lack of a relationship in our study can potentially be attributed to the relatively few patients included; however, the pathophysiology of the otosclerotic foci
on CT scans has not been fully elucidated and may be a contributing factor.
In a recent retrospective literature review, Wegner et al evaluated the diagnostic value of CT scans in adult patients with a clinical suspicion of otosclerosis, specifically looking at positive and negative post-test probabilities.14 The positive post-test probability was defined as the probability of the presence of otosclerosis in case of a positive CT scan, whereas the negative post-test probability represented the probability of otosclerosis in case of a negative or normal CT scan.
In most of the studies reviewed by Wegner et al, especially in patient populations with a high prevalence of otosclerosis, the positive and negative post-test probabilities were relatively high, with positive post-test probability being 99% in one of the studies and negative post-test probabilities ranging from 51% to 67%.14 The subsequent conclusion was that preoperative CT imaging appears to be unnecessary in the diagnosis of otosclerosis, as the prevalence of otosclerosis in patients with a clinical suspicion of the disease generally seems to be high. Furthermore, a normal CT finding may not fully exclude otosclerosis as seen in the high negative post-test probabilities. Nevertheless, the confidence in a diagnosis of otosclerosis is less in a patient from a nonendemic compared with an endemic area.
Interestingly, in the Wegner review, the positive post-test probability for populations with a low prevalence of disease was only 23%. In our study, the rate of positive CT findings was much higher at 78.9%. In addition, the Wegner review showed that patients from nonendemic areas had a much lower negative post-test probability (3%) than populations with a high prevalence of disease.
Apparently, in nonendemic areas, patients with a clinical suspicion of otosclerosis and a negative CT scan will be unlikely to have the disease. However, 21% of patients with negative CT scan features in the current study were found to have otosclerosis. When coupled with our findings of high rates of otosclerosis
Volume 97, Number 6 www.entjournal.com 161 OTOSCLEROSIS IN A NONENDEMIC POPULATION: UTILITY OF CT SCAN AND CORRELATION WITH AUDIOMETRY AND SURGICAL OUTCOME
CT grade Density ratio Ear 1 1 0.6525 Ear 2 Normal 1 Ear 3 1 0.2505 Ear 4 2B 0.3289 Ear 5 1 0.6097 Ear 6 1 0.6075 Ear 7 1 0.5929 Ear 8 Normal 1 Ear 9 2B 0.959 Ear 10 Normal 1 Ear 11 2B 0.5845 Ear 12 2B 0.6682 Ear 13 2B 0.6817 Ear 14 2B 0.7148 Ear 15 1 0.7393 Ear 16 2B 0.5218 Ear 17 2B 0.7170 Ear 18 Normal 1 Ear 19 2C 0.5063
Table 2. CT grade (Symmons and Fanning) versus CT density ratio
in nonendemic patients, we can postulate that even though a positive CT scan may not indicate a high probability of a nonendemic patient having otosclerosis, it will likely capture the majority of patients who do have the disease, which can be further evaluated with exploratory tympanotomy. Hence, even though CT imaging may not have a large value in populations endemic for otosclerosis, preoperative CT scans are useful for preoperative diagnosis and planning in nonendemic areas.
Conclusion

The diagnosis of otosclerosis in nonendemic areas can be challenging. Preoperative CT scans are useful when otosclerotic foci are present. In this study, the density ratio of otosclerotic foci did not correlate with audiometric parameters or surgical outcomes. A follow-up study involving a larger patient base from multiple centers within nonendemic areas may be able to further elucidate this relationship.
References

1. Toynbee J. Diseases of the Ear. Philadelphia: Blanchard and Lea; 1860.
2. Politzer A. Uber primäre erkrankung der knöchernen labyrinthkapsel. Z Ohrenheilkd Kr Luftwege [in German]. 1893;25:309.
3. House JW, Cunningham CD. Otosclerosis. In: Flint PW, ed. Cummings Otolaryngology: Head and Neck Surgery. 6th ed. Philadelphia: Elsevier; 2015:2211.

4. Lee TC, Aviv RI, Chen JM, et al. CT grading of otosclerosis. AJNR Am J Neuroradiol 2009;30(7):1435-9.
5. Marx M, Lagleyre S, Escudé B, et al. Correlations between CT scan findings and hearing thresholds in otosclerosis. Acta Otolaryngol 2011;131(4):351–7.
6. Altmann FN, Glasgold A, MacDuff JP. The incidence of otosclerosis as related to race and sex. Ann Otol Rhinol Laryngol 1967;76(2):377-92.
7. Levin G, Fabian P, Stahle J. Incidence of otosclerosis. Am J Otol 1988;9(4):299-301.
8. Kiyomizu K, Tono T, Yang D, et al. Correlation of CT analysis and audiometry in Japanese otosclerosis. Auris Nasus Larynx 2004;31(2):125–9.
9. Min JY, Chung WH, Lee WY, et al. Otosclerosis: Incidence of positive findings on temporal bone computed tomography (TBCT) and audiometric correlation in Korean patients. Auris Nasus Larynx 2010;37(1):23–8.
10. Yagi T. Incidence and characteristics of otosclerosis in the Japanese population. Auris Nasus Larynx 2002;29(3):257–60.
11. Marshall AH, Fanning N, Symons S, et al. Cochlear implantation in cochlear otosclerosis. Laryngoscope 2005;115(10):1728-33.
12. Schuknecht HF, Barber W. Histologic variants in otosclerosis. Laryngoscope 1985;95(11):1307–17.
13. de Groot JA, Huizing EH, Damsma H, et al. Labyrinthine otosclerosis studied with a new computed tomography technique. Ann Otol Rhinol Laryngol 1985;94(3):223–5.
14. Wegner I, van Waes AM, Bittermann AJ, et al. A systematic review of the diagnostic value of CT imaging in diagnosing otosclerosis. Otol Neurotol 2016;37(1):9–15.
162 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
PNG, PANG KARANDIKAR, GOH, YEO,YUEN
Practice Analysis Human Resources Equipment Solutions Insurance & Billing Financial Reporting Buying Group Pricing Recruiting Discover the Spectrum Difference Learn how you can increase profits for your audiology and hearing aid services. Spectrum Services: Spectrum Audiology is an ENT physician-owned consulting and buying group exclusive to the ENT community, specializing in quality diagnostic audiology and hearing aid services with a proven track record of success with ENT practices across the U.S. Learn more about our services and request a complimentary practice analysis to determine the potential of your audiology and hearing aid business. The ENT community’s simple solution to professional and profitable audiology. “All of our re s ources, energies and in novation are focused to w ard s t he success of our ENT p artners and gro w ing t heir practices ” William McCrae CEO/President SpectrumAudiology.com • Contact Daniel Shafer Today: (210) 479-1874
Study of steroid effects on graft and inner ear outcomes in tympanoplasty: Randomized controlled trial
Ali Kouhi, MD; Sasan Dabiri, MD; Amin Amali, MD; Nasrin Yazdani, MD; Mahboubeh Baroodabi, MD; Taha Kouchakinejad, MD; Alireza Mohseni, MD
Abstract
More studies are needed to investigate the side effects of steroids in tympanoplasty, owing to the paucity of such studies in the literature. This randomized, controlled clinical trial included 59 patients with chronic otitis media who underwent tympanoplasty and were randomized after surgery to a systemic steroid or no steroid treatment. Patients were randomized into two groups. Perforation size, graft outcome, and complications such as tinnitus and hearing loss were compared between the two groups. Postsurgical steroid injection had no effect on graft outcome (p = 0.927) or tinnitus (p = 0.478). Tympanic membrane perforation (p = 0.92), plaque size (p = 0.94), bleeding amount (p = 0.38), and mucosal status (p = 0.96) during surgery had no effect on graft outcome after the tympanoplasty. In conclusion, administration of steroids after tympanoplasty failed to improve outcome and may put the patient at risk of side effects.
Introduction
Chronic otitis media is characterized by an irreversible tympanic perforation associated with chronic inflammation of the middle ear, and it is the most important cause of hearing loss in developing countries.1,2 Tympanoplasty is indicated to rehabilitate hearing and to treat chronic infection.3 The various modern surgical approaches to tympanoplasty include endomeatal, endaural, and postauricular routes.3-5 Inner ear complications such as sensorineural hearing loss, trauma to the inner ear, and
tinnitus have been reported with these surgeries.6-9 Surgical technique is considered the main factor in determining success, but the influence of steroid-containing agents is unknown.10
Steroids have been effective in the treatment of some inner ear pathologies, such as noise-induced and sudden sensorineural hearing loss.11,12 Animal studies suggest possible inhibition of neovascularization by steroids that can result in delayed wound re-epithelialization;13,14 however, the clinical information on the effects of shortterm corticosteroid administration on the healing of differentiated tissue like tympanic membrane is limited. Due to the possible complications of steroid agents and the absence of clear evidence of their efficacy in tympanoplasty, we performed this study to assess the influence of corticosteroids on tympanoplasty outcomes.
Patients and methods
This randomized, controlled clinical trial included patients who underwent tympanoplasty at the Otolaryngology Clinic of Amir-A’lam Hospital in 2013 and 2014. All the surgeons in the study were board members and were experienced in tympanoplasty. Examiners and audiologists were blinded to the randomization.
After taking histories, doing physical examinations, and performing additive tests such as audiometry, we enrolled patients with chronic otitis media (COM) who were candidates for tympanoplasty. We excluded patients with cholesteatoma, severe damages to ossicles, history of ear surgery, active infection, total tympanic membrane loss, and age >75 years.
From the Otorhinolaryngology Research Center, Department of Otolaryngology, Head and Neck Surgery, Amir-A’lam Hospital, Tehran University of Medical Sciences, Iran.
Corresponding author: Ali Kouhi, MD, Otorhinolaryngology Research Center, Amir-A’lam Hospital, North Sa’adi Ave., Tehran, Iran. Email: a-kouhi@tums.ac.ir
Funding/support: This study was supported by a grant from the Tehran University of Medical Sciences (grant number: 92-03-48-23363).
The final dataset included 59 patients who were randomly divided into two groups: one group of 29 patients who received dexamethasone (8 mg twice in a 12-hour interval) after surgery and a second group of 30 patients who did not receive dexamethasone. Other interventions (i.e., surgical techniques, use of a prophylactic antibiotic, and administration of preoperative steroid injection) were the same in both groups.
Volume 97, Number 6 www.entjournal.com 163 ORIGINAL ARTICLE
Patients’ demographics (age, medical and surgical history, medication history, and disease duration) were obtained through questionnaires. Data on complications such as tinnitus, hearing loss, and graft condition were collected.
Patients were examined twice before surgery and once at month 3 after surgery. Perforation and plaque size were documented before the surgery as the number of quadrants involved (table 1). Hearing was assessed via audiometry (pure-tone audiometry, speech audiometry, and tympanometry), and the graft condition was assessed by microscopic otoscopy.
Data were analyzed using SPSS (v. 20) software. For the quantitative and qualitative comparison between the two groups, the t test and chi-square test, respectively, were used. The confidence interval was set at 95%, and p values <0.05 were considered statistically significant.
Results
Of the 59 total patients, 31 (52.5%) were female and 28 (47.5%) were male. Patients’ ages ranged from 10 to 77 years (average: 40.78) (table 2). No significant difference existed between the two groups in the find-
ings on physical examination before surgery or in the patients’ demographics.
Bleeding volume and mucosal status during the operation were also investigated. Patients were divided into three subgroups based on bleeding volume: 47.5% had no bleeding, 27.1% had moderate bleeding, and 25.4% had severe bleeding. The extent of bleeding was defined according to the surgeons’ judgment: bleeding was considered moderate when it was not problematic for the procedure and severe when it was obscuring surgeons’ vision and some measures needed to be taken. Regarding mucosal status, 74.6% had normal mucosal status, 23.7% had edematous mucus, and 1.7% had polypoid mucosal status.
The graft was intact in 55 (93.2%) patients after tympanoplasty. The graft failed in 4 (6.8%) patients, 2 of whom had received a steroid injection before the surgery and 2 of whom did not receive a steroid. Steroid injection had no effect on the graft result after the tympanoplasty in this study (p = 0.927).
A total of 33 (55.9%) patients had tinnitus before the surgery; the tinnitus continued in 5 patients after tympanoplasty and was absent in 28 patients after surgery. In addition, 4 patients who did not have tinnitus
164 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
KOUHI, DABIRI, AMALI, YAZDANI, BAROODABI, KOUCHAKINEJAD, MOHSENI
No. Patients Graft outcome p Value* Intact (n, %) Failure (n, %) Perforation size 0.92 1 of 4 TM quadrants 17 16 (94.1%) 1 (5.9%) 2 of 4 TM quadrants 28 27 (96.4%) 1 (3.6%) 3 of 4 TM quadrants 12 11 (91.7%) 1 (8.3%) 4 of 4 TM quadrants 2 1 (50%) 1 (50%) Plaque Size 0.94 No plaque 41 38 (92.7%) 3 (7.3%) 1 of 4 TM quadrants 10 9 (90%) 1 (10%) 2 of 4 TM quadrants 6 6 (100%) 0 (0%) 3 of 4 TM quadrants 1 1 (100%) 0 (0%) 4 of 4 TM quadrants 1 1 (100%) 0 (0%) Bleeding volume 0.38 No bleeding 28 26 (92.9%) 2 (7.1%) Moderate 16 14 (87.5%) 2 (12.5%) Massive 15 15 (100%) 0 (0%) Mucosal status 0.96 Normal 44 41 (93.2%) 3 (6.8%) Edematous 14 13 (92.9%) 1 (7.1%) Polypoid 1 1 (100%) 0 (0%)
on graft outcome. Key: TM = tympanic membrane.
Table 1. Comparison of preoperative and intraoperative physical findings with graft outcome
*Effect
Table 2. Patient demographics
before surgery had this complaint after the surgery. No significant effect of steroid injection on tinnitus was observed (p = 0.478) (table 3).
Postsurgical vertigo was also evaluated: 47 patients (79.7%) had no vertigo, 11 (18.6%) had dizziness, and 1 (1.7%) had true vertigo postsurgically. No significant relationship between steroid use and postsurgery vertigo was present ( p = 0.531). A post hoc evaluation
found no significant effect of perforation and plaque size on graft outcome ( p = 0.92 and p = 0.94, respectively) (table 1).
As seen in table 1, 50% of the lost grafts belonged to the patients in the subgroup with no bleeding and 50% to the group with moderate bleeding during the operation. No patients with severe bleeding had graft failure. The volume of bleeding had no effect on the tympanoplasty graft outcome (p = 0.38). Mucosal status during the surgery had no significant effect on graft outcome (p = 0.96) (table 1).
Discussion
The complications of tympanoplasty include graft failure and conductive hearing loss. Postoperative steroids have been used to reduce complications to the inner ear. Con-
ABG = air-bone gap; BC = bone conduction; SD = standard deviation.
Volume 97, Number 6 www.entjournal.com 165 STUDY OF STEROID EFFECTS ON GRAFT AND INNER EAR OUTCOMES
IN TYMPANOPLASTY: RANDOMIZED CONTROLLED TRIAL
No. patients With steroid Without steroid p Value Graft outcome 59 29 (49.2%) 30 (50.8%) Fail 4 2 (50%) 2 (50%) 0.927 Intact 55 27 (49.1%) 28 (50.9%) BC changes Mean (± SD) 29 –0.12 (0.26) –1.12 (0.31) 0.02 ABG changes Mean (± SD) 29 8.53 (2.18) 13.08 (1.97) > 0.05 BC Changes 250 Hz (Mean ± SD) 0.00 (0.24) 0.33 (0.41) 0.494 500 Hz (Mean ± SD) 0.00 (0.24) –1.0 (0.44) 0.056 1,000 Hz (Mean ± SD) –0.17 (0.30) –0.50 (0.27) 0.428 2,000 Hz (Mean ± SD) –0.34 (0.23) –1.8 (0.69) 0.052 4,000 Hz (Mean ± SD) –0.51 (0.37) –0.16 (0.61) 0.630 Tinnitus 0.478 Had tinnitus before surgery, healed after surgery 28 12 (42.9%) 16 (57.1%) No tinnitus before and after surgery 22 13 (59.1%) 9 (40.9%) Had tinnitus before surgery, did not heal after surgery 5 3 (60%) 2 (40%) No tinnitus before surgery, complained of tinnitus after surgery 4 1 (25%) 3 (75%) Vertigo 0.531 No vertigo 47 22 (46.8%) 25 (53.2%) Dizziness 11 6 (54.5%) 5 (45.5%) True vertigo 1 1 (100%) 0 (0%) Key:
Table 3. Comparison of outcome parameters in patients with steroid and without steroid injection
Steroid injection Yes (n = 29) No (n = 30) Total p Value Mean age (yr) 38.61 42.80 40.47 0.274 Sex (M:F) 13:16 15:15 28:31 0.446
sidering the various side effects associated with steroids and the paucity of data in the literature on the effects of steroids on tympanoplasty outcomes, we assessed the effects of steroid use on graft outcome and hearing in 59 patients with COM.
In 2007, Starkweather and Friedman conducted a study to evaluate the effect of ciprofloxacin 0.3% and dexamethasone 0.1% on graft healing during tympanoplasty.14 According to their study, healing of the tympanic membrane occurred in 95.3%, and the mean time to healing was 49 days. Sobol et al found an overall rate of healing of 95% and a time to healing of 30 to 60 days with the use of dexamethasone, indicating no effect on graft outcome or healing time.15
In 2007, Hebda et al evaluated the effects of the ciprofloxacin-dexamethasone (CDX) combination in 30 rats after myringotomy.16 After 14 days, the healing rates were 100% in the non-CDX group and 89% in the CDX group, which improved to 100% at day 28. This result indicated no difference in overall healing between the two groups, but that healing was slower in the CDX group.
In our study, the graft failed in 4 patients, 2 of whom had received a steroid injection before surgery and 2 of whom had not. Hence, our study also shows no differences in graft outcome. However, we cannot draw conclusions about the time to healing with the use of postoperative steroid injection because we evaluated graft outcome after 3 months.
House et al conducted a study to determine the safety of using a suspension of polymyxin B, neomycin, and hydrocortisone (PNH) in tympanoplasty.17 In each surgery, the gelatin sponge saturated with PNH suspension was placed in the middle ear, and bone-conduction thresholds were measured at 500, 1,000, 2,000, and 4,000 Hz before and after the surgery. A slight improvement (average: –0.545 dB) in all frequencies except 4,000 Hz (+0.560 dB) was observed. In our study, the average bone-conduction changes were significantly different between the two groups (p = 0.02). Evaluation of the bone-conduction changes at each frequency separately showed a meaningful difference at 500 and 2,000 Hz, (p = 0.056 and p = 0.052, respectively) but not at the other frequencies.
Riechelmann et al designed a study to evaluate the effects of perioperative glucocorticoid during stapes surgery on postoperative complications.18 In 95 patients who underwent stapedotomy, postoperative bone-conduction thresholds, sensorineural hearing loss, vertigo, and tinnitus were compared between patients who received prednisolone and controls. Prednisolone recipients showed no improvement in hearing and had vertigo at more frequencies.18 This finding supports the lack of efficacy of steroids on vertigo and tinnitus found in our study.
Conclusion
The present study showed no beneficial effect of steroid administration in patients undergoing tympanoplasty. Routine steroid administration in these patients appears to have no benefit while exposing patients to the risk of the side effects of steroids.
References
1. Maharjan M, Kafle P, Bista M, et al. Observation of hearing loss in patients with chronic suppurative otitis media tubotympanic type. Kathmandu Univ Med J (KUMJ) 2009;7(28):397-401.
2. Roland PS. Chronic suppurative otitis media: A clinical overview. Ear Nose Throat J 2002;81(8 Suppl 1):8-10.
3. Sergi B, Galli J, De Corso E, et al. Overlay versus underlay myringoplasty: Report of outcomes considering closure of perforation and hearing function. Acta Otorhinolaryngol Ital 2011;31(6):366-71.
4. Olusesi AD, Opaluwah E, Hassan SB. Subjective and objective outcomes of tympanoplasty surgery at National Hospital Abuja, Nigeria 2005-2009. Eur Arch Otorhinolaryngol 2011;268(3):36772.
5. Zollner F. The principles of plastic surgery of the soundconducting apparatus. J Laryngol Otol 1955;69(10):637-52.
6. Briggs RJ, Luxford WM. Chronic ear surgery: A historical review. Am J Otol 1994;15(4):558-67.
7. Indorewala S, Pagare R, Aboojiwala S, Barpande S. Dimensional stability of the free fascia grafts: A human study. Laryngoscope 2004;114(3):543-7.
8. Kazikdas KC, Onal K, Boyraz I, Karabulut E. Palisade cartilage tympanoplasty for management of subtotal perforations: A comparison with the temporalis fascia technique. Eur Arch Otorhinolaryngol 2007;264(9):985-9.
9. Mauri M, Lubianca Neto JF, Fuchs SC. Evaluation of inlay butterfly cartilage tympanoplasty: A randomized clinical trial. Laryngoscope 2001;111(8):1479-85.
10. Sheehy JL, Glasscock ME 3rd. Tympanic membrane grafting with temporalis fascia. Arch Otolaryngol 1967;86(4):391-402.
11. Oishi N, Schacht J. Emerging treatments for noise-induced hearing loss. Expert Opin Emerg Drugs 2011;16(2):235-45.
12. Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol 1980;106(12):772-6.
13. Brauchle M, Fässler R, Werner S. Suppression of keratinocyte growth factor expression by glucocorticoids in vitro and during wound healing. J Invest Dermatol 1995;105(4):579-84.
14. Starkweather A, Friedman RA. Effect of ciprodex on graft healing in tympanoplasty. Adv Ther 2007;24(2):427-35.
15. Sobol SE, Keswani S, Parvadia JK, et al. Effect of corticosteroidantibiotic agents on granulation tissue in a murine model. Arch Otolaryngol Head Neck Surg 2005;131(4):330-5.
16. Hebda PA, Yuksel S, Dohar JE. Effects of ciprofloxacindexamethasone on myringotomy wound healing. Laryngoscope 2007;117(3):522-8.
17. House JR 3rd, House LK. Ototoxicity of polymyxin B, neomycin, and hydrocortisone suspension in tympanoplasty surgery. Otolaryngol Head Neck Surg 2014;150(2):282-4.
18. Riechelmann H, Tholen M, Keck T, Rettinger G. Perioperative glucocorticoid treatment does not influence early post-laser stapedotomy hearing thresholds. Am J Otol 2000;21(6):809–12.
166 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
KOUHI, DABIRI, AMALI, YAZDANI, BAROODABI, KOUCHAKINEJAD, MOHSENI
Antibiotics, steroids, and combination therapy in chronic rhinosinusitis without nasal polyps in adults
Yuan F. Liu, MD; Clare M. Richardson, MD; Stewart H. Bernard, MD; Christopher A. Church, MD; Kristin A. Seiberling, MD
Abstract
Despite a lack of robust data regarding their efficacy, oral antibiotics and steroids remain two of the most common treatments for chronic rhinosinusitis without nasal polyps (CRSsNP). We sought to objectively compare the efficacy of antibiotics and steroids, independently and in combination, for the initial treatment of CRSsNP. To that end, we conducted a retrospective chart review of 100 patients—51 men and 49 women, age 20 to 85 years (mean: 50)—who were treated for CRSsNP from January 2010 through January 2015. Of this group, 17 patients were treated with an antibiotic only, 28 with a steroid only, and 55 with both agents. All patients underwent computed tomography (CT) before and after treatment, and we compared the three groups’ pre- and post-treatment Lund-Mackay CT scores, symptom scores, and rates of surgery. The average time between the pre- and post-treatment visits was 4.4 weeks. The mean Lund-Mackay CT score for the entire study population was significantly lower after treatment than at baseline (6.3 vs. 9.1; p < 0.001); however, there were no significant differences among the three groups in either pre- or post-treatment scores. Symptom scores were significantly better in the com-
From the Department of Otolaryngology–Head and Neck Surgery, Loma Linda University Medical Center, Loma Linda, Calif. (Dr. Liu, Dr. Church, and Dr. Seiberling); the Department of Otolaryngology, Case Western Reserve University School of Medicine, Cleveland (Dr. Richardson); and the Department of Otolaryngology, University of Florida College of Medicine, Gainesville (Dr. Bernard). The study described in this article was conducted at Loma Linda University Medical Center.
Previous presentation: The information in this article has been edited for publication and updated from its original presentation as a poster at The Triological Society’s Combined Otolaryngology Spring Meetings; April 22-26, 2015; Boston.
Corresponding author: Yuan Liu, MD, Department of Otolaryngology–Head and Neck Surgery, Loma Linda University Medical Center, 11234 Anderson St., Room 2586A, Loma Linda, CA 92354. Email: yuliu@llu.edu
bination therapy group than in the two monotherapy groups (p < 0.001). In all, 40 of the 100 patients underwent surgery; the difference in surgery rates among the three groups was not statistically significant (p = 0.884). Surgery was performed on 9 of the 52 (17.3%) patients who either were followed for at least 1 year or who had had surgery within the first year postoperatively; again, there were no significant differences among the three groups (p = 0.578). We conclude that although the Lund-Mackay CT scores decreased significantly in the antibiotic, steroid, and combination therapy groups, no one regimen was superior to any other for treating CRSsNP in our study.
Introduction
Chronic rhinosinusitis (CRS) can be defined as a group of disorders characterized by inflammation of the nose and paranasal sinus mucosa for at least 12 consecutive weeks.1 The range of symptoms of CRS is broad; they include facial pain/pressure, congestion, nasal discharge, headache, fever, and hyposmia.1,2
A unified theory to explain the pathophysiology of these symptoms remains elusive despite multiple attempts to identify an underlying etiology. 3 However, a common thread among all patients with CRS is a persistent inflammation of the nasal and paranasal sinus mucosa. The mechanism that triggers this inflammation has yet to be determined, but it appears to be multifactorial. Several theories have surfaced over time, including those that implicate bacterial and/or fungal infection, biofilms, superantigens, allergy, dysfunction of the innate immune system, and anatomic defects.1,3,4
Because of the lack of a confirmed cause, treatment options for CRS are varied; it includes topical and oral antibiotics, topical and oral steroids, nasal irrigation, antifungals, decongestants, mucolytics, antihista-
Volume 97, Number 6 www.entjournal.com 167 ORIGINAL ARTICLE
mines, leukotriene inhibitors, and surgery. 3,5,6 Therapy for CRS differs for patients with and without nasal polyps, the latter of whom are the focus of this study.
Traditionally, standard treatments were formulated on the presumption of an infectious cause, which led to the widespread use of antibiotics for the treatment of CRS without nasal polyps (CRSsNP).7 However, multiple attempts to define the pathogenesis and microbiology of this entity have left us with conflicting data, providing little direction for treatment.1
Despite the lack of compelling evidence regarding their efficacy, two of the most common treatments for CRSsNP continue to be antibiotics and steroids.6-8 A survey of members of the American Rhinologic Society published in 2007 revealed that oral antibiotics were “almost always” prescribed (>90% of respondents) and that oral steroids were prescribed by 50 to 90% of respondents.7 Both treatment modalities are recommended only as “options” in recently published reviews because of the lack of high-quality studies of their efficacy. 3,8
Considering the paucity of robust data, we sought to compare the efficacy of an antibiotic alone, a steroid alone, and both together as the initial treatment for CRSsNP.
Patients and methods
We conducted a retrospective study of all patients who had been treated for sinusitis at the Loma Linda University Medical Center in California from January 2010 through January 2015. We reviewed the electronic medical records system in our Department of Otolaryngology–Head and Neck Surgery to identify diagnoses of chronic maxillary, frontal, ethmoid, sphenoid, and unspecified chronic sinusitis (ICD-9 codes 473.0, 473.1, 437.2, 437.3, and 437.9, respectively).
Our exclusion criteria were an age of less than 18 years, the presence of nasal polyps, a history of previous sinonasal surgery, and the use of an antibiotic or steroid within 4 weeks of a patient’s initial visit. We also disqualified patients who had received an antibiotic prescription for less than 10 days at their initial visit and those who had less than 4 weeks of total follow-up. Finally, we denied eligibility to patients who had had a sinonasal or cranial neoplasm, cerebrospinal fluid rhinorrhea, cystic fibrosis, a bone or cartilage disorder, or a history of sinonasal or cranial trauma, vasculitides, or a connective tissue or autoimmune disorder.
After these exclusions, we identified 284 patients who had had at least one pretreatment computed tomography (CT) scan of the sinuses. Finally, we identified 100 patients—51 men and 49 women, age 20 to 85 years (mean: 50)—who had had both a pre- and post-treatment CT scan for inclusion in the full analysis.
In addition to demographic data, we compiled information on symptoms at the initial visit, the details of treatment, and changes in symptoms at the first follow-up. We reviewed the pre- and post-treatment sinus CT scans and generated a Lund-Mackay CT score9 for each scan. Finally, we assessed differences in the prevalence of various symptoms before and after treatment and calculated the number of patients who required surgery.
Of the 100 patients, 17 had been treated with an antibiotic only, 28 with a steroid only, and 55 with both agents.
Statistical analysis. Analysis of variance (ANOVA) was used to test hypotheses involving three groups, and the two-tailed Student t test was used for two groups. Matched-pair t tests were used for comparing data within the same group. Differences in the prevalence of symptoms were analyzed with an extended version of the Fisher exact test. For all tests, a p value of less than 0.05 was considered to represent statistical significance.
Ethical considerations. This study was approved by the Loma Linda University Medical Center’s Institutional Review Board.
Results
Concomitant conditions. Asthma and smoking were not common in the study population; allergic rhinitis was much more prevalent (table 1).
Antibiotics. The antibiotics used included amoxicillin/clavulanic acid, trimethoprim/sulfamethoxazole, ciprofloxacin, clarithromycin, and moxifloxacin.
Overall, the mean duration of antibiotic therapy was 19.0 days (median: 21; range: 10 to 21) in the antibiotic group and 14.2 days (median: 14; range: 10 to 21) in the combination group. The difference was statistically significant (p < 0.001) (table 1).
Steroids. Two steroids were used in our study: methylprednisolone and prednisone. The two therapies were about evenly split in the steroid group, while methylprednisolone use was significantly more common in the combination group (p = 0.001) (table 1).
The duration of steroid therapy was 6 days for those taking methylprednisolone and 20 days for those on prednisone.
Follow-up. Overall, the mean interval between the pre- and first post-treatment visits was 4.4 weeks (median: 4; range: 2 to 10). There were no statistically significant differences in the length of follow-up among the three groups (table 1).
Lund-Mackay CT scores. In the study population as a whole, the mean pretreatment Lund-Mackay CT score was 9.1 (median: 9; range: 0 to 20). After treatment, the overall score improved significantly (mean 6.3; median: 6; range: 0 to 17) (p < 0.001). There were
168 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
LIU, RICHARDSON, BERNARD, CHURCH, SEIBERLING
Table 1. Baseline characteristics
* The p values represent comparisons among the three groups or, when appropriate, between two variables.
† Statistically significant difference.
Key: SD = standard deviation; N/A = not applicable; Tx = treatment.
no statistically significant differences among the three groups in either pre- or post-treatment scores (table 2). Significant post-treatment improvement in Lund-Mackay scores was seen in each of the three groups (p ≤ 0.002) (figure 1).
In addition, 53% of the antibiotic group, 46% of the steroid group, and 40% of the combination group exhibited improvements on CT that resulted in Lund-Mackay CT scores of 4 or less post-treatment.
Symptoms. We recorded the pretreatment presence of nasal obstruction, postnasal drip, rhinorrhea, facial pain/pressure, and loss of smell for each patient (table 3). There was no significant difference in baseline symptoms among the three groups (table 3).
After treatment, improvement among the three treatment groups differed significantly (p < 0.001).
Post hoc pairwise comparisons of the prevalence of each symptom among the three groups were not performed because the data were incomplete, and therefore a statistical analysis of these data would have been misleading. However, general trends were apparent, with the combination group experiencing the greatest improvement (figure 2).
Surgery. In all, 40 of the 100 patients underwent surgery; the difference in surgery rates among the three groups was not statistically significant (p = 0.884). Surgery was performed on 9 of the 52 (17.3%) patients who either were followed for at least 1 year or who had had surgery within the first year postoperatively; again, there were no significant differences among the three groups (p = 0.578).
Volume 97, Number 6 www.entjournal.com 169 ANTIBIOTICS,
STEROIDS, AND COMBINATION THERAPY IN CHRONIC RHINOSINUSITIS WITHOUT NASAL POLYPS IN ADULTS
Variable Antibiotic group (n = 17) Steroid group (n = 28) Combination group (n = 55) All patients (N = 100) p Value* Demographic data Sex, n (%) 0.587 Male 10 (59) 16 (57) 25 (45) 51 Female 7 (41) 12 (43) 30 (55) 49 Age, yr. mean ± SD 56 ± 17.9 48 ± 19.0 50 ± 16.0 50 ± 17.3 0.236 (median, range) (63, 26 to 75) (42.5, 20 to 83) (52, 22 to 85) (49.5, 20 to 85) Concomitant conditions, n (%) Asthma 2 (12) 5 (18) 6 (11) 13 0.685 Smoking 2 (12) 1 (4) 7 (13) 10 0.397 Allergic rhinitis 10 (59) 9 (32) 29 (53) 48 0.126 Antibiotics duration, days, 19.0 ± 3.6 N/A 14.2 ± 4.2 15.4 ± 3.8 <0.001† mean ± SD (median, range) (21, 10 to 21) (14, 10 to 21) (14, 10 to 21) Distribution of steroid Tx, n (%) Methylprednisolone N/A 13 (46) 46 (84) 59 0.001† Prednisone N/A 15 (54) 9 (16) 24 Follow-up, wk, mean ± SD (median range) Time to first follow-up 4.8 ± 1.7 4.5 ±2.0 4.3 ± 1.6 4.4 ± 1.7 0.839 (4, 1.9 to 10) (4.4, 2 to 9.4) (4, 2 to 8.9) (4, 2 to 10) Total follow-up time 45.3 ± 59.3 29.7 ± 34.7 48.0 ± 57.5 42.4 ± 52.6 0.152 (19.9, 4 to 225.7) (15.5, 4 to 137.7) (24.0, 4 to 216.1) (20.4, 4 to 226)
Table 2. Lund-Mackay CT scores
that roxithromycin daily for 3 months significantly improved SNOT-20 (Sinonasal Outcome Test-20) scores after 12 weeks, although not after 6 or 24 weeks. Nasal endoscopy scores and saccharine transit times also improved.
* Column p values represent comparisons among the three groups, and row p values represent comparisons between the pre- and post-treatment assessments.
† Statistically significant difference. Key: CT = computed tomography; SD = standard deviation.
Of those patients who had a post-treatment Lund-Mackay of 4 or less, 33% of the antibiotic group, 46% of the steroid group, and 23% of the combination group eventually underwent surgery.
The mean time to surgery was 12.0 weeks (median 9.7; range: 3 to 49), which did not differ significantly among the three groups (p = 0.122).
Discussion
The role that bacteria play in the chronic inflammatory state seen in CRS is ambiguous and controversial. Also, it is unclear which bacteria clinicians should target with antibiotic treatments. Some authors have suggested that antibiotics merely change the bacterial milieu of CRS rather than eradicating the underlying inflammatory process.4,8 Despite the widespread use of antibiotics, the data supporting it in CRS are limited. Wallwork et al conducted a double-blind, randomized, controlled trial of 64 patients who were treated with systemic antibiotics for CRSsNP.10 They found
The most current update on the treatment of CRSsNP with antibiotics was reported in a consensus statement by Orlandi et al.11 They found insufficient evidence to recommend against or for nonmacrolide antibiotics, and macrolides were offered as an optional treatment.
Antibiotics and steroids have been paired for decades for the treatment of CRS, and the role of steroids in the treatment of CRS with nasal polyps is well established.12 As a result, steroids are becoming more frequently prescribed for CRSsNP to combat mucosal inflammation, which is the common thread in patients with CRS. In support of the anti-inflammation theory, studies have demonstrated a reduction in inflammatory markers (e.g., IL-8, IL-6, TNF-α), increased ciliary beat frequency, and decreased sinonasal secretion viscosity in CRS patients with macrolide use.13,14
Some authors have suggested that controlling the mucosal inflammation, whether it is with steroid or macrolide therapy, will provide relief to patients.12 A strong recommendation was given to the use of steroids in CRS with nasal polyps due to a reduction in polyp size and consistently significant clinical improvement. 3,5 However, to the best of our knowledge, no study to date has examined the effect of oral steroids alone for the treatment of CRSsNP. Poetker et al reviewed four case series in which steroids were used along with other therapies, and they concluded that they could not make a recommendation on the use of systemic steroids for treating CRSsNP.12
The primary outcomes measures in our study were the decrease in Lund-Mackay CT scores, reductions in symptom scores, and the need for surgery. All three treatment groups exhibited a significant decrease in Lund-Mackay CT scores by

170 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
Mean ± SD (median, range) Score Antibiotic group (n = 17) Steroid group (n = 28) Combination group (n = 55) All patients (N = 100) p Value* Pretreatment 8.3 ± 5.0 9.6 ± 4.7 9.0 ± 4.2 9.1 ± 4.4 0.631 (8, 1 to 20) (9.5, 2 to 20) (9, 0 to 17) (9, 0 to 20) Post-treatment 5.9 ± 5.0 6.1 ± 4.5 6.5 ± 4.8 6.3 ± 4.7 0.876 (4, 0 to 17) (4.5, 0 to 14) (6, 0 to 15) (6, 0 to 17) Decrease in score 2.4 ± 2.6 3.5 ± 4.6 2.5 ± 4.2 2.8 ± 4.1 0.515 (3, –4 to 7) (2, –6 to 14) (2, –9 to 11) (2, –9 to 14) p Value 0.002† <0.001† <0.001† <0.001†
LIU, RICHARDSON, BERNARD, CHURCH, SEIBERLING
Figure 1. Matched-pair mean Lund-Mackay CT scores reflect significant improvement in all three groups after therapy.
2.4 to 3.5 points on average, but how does this translate to clinical improvement? In a study by Ashraf and Bhattacharyya, the average score for patients who underwent imaging for nonrhinologic symptoms was 4.3.15 In our study, the mean pretreatment score was 9.1 and the post-treatment score was 6.3.
In our study, 53% of the antibiotic group, 46% of the steroid group, and 40% of the combination group exhibited improvements on CT that resulted in Lund-Mackay CT scores of 4 or less post-treatment. However, 33, 46, and 23% of these patients, respectively, eventually underwent surgery. One interpretation of this finding is that our CRS “cure” was only temporary and that patients relapsed, which led to the need for surgery.

Lund-Mackay CT scores have been shown to be positively associated with polyp grades, but the association between changes in scores and improvements in clinical symptoms is equivocal. Hopkins et al found that Lund-Mackay CT scores did not correlate strongly with symptom severity represented by SNOT-22 results.16 However, studies exist that both support and
contest these findings, with the consensus leaning more toward the former.17-20 Unfortunately, we could not find any literature describing the relationship between Lund-Mackay CT scores and disease severity in CRSsNP. Therefore, we hesitate to speculate on what our finding of significantly decreased Lund-Mackay CT scores after treatment really means in terms of clinical improvement.
In our study, surgery was performed on 40% of all patients and on 77% of those who were followed for more than 1 year or who had surgery within 1 year, with each of the three treatment groups having similar rates. Young et al reported a surgery rate of 52.5% in 80 CRS patients who were treated with 3 weeks of an oral antibiotic, an oral prednisone taper, an intranasal steroid, and saline lavage. 21 McNally et al reported a surgery rate of only 6% in 200 CRS patients who were treated with 4 weeks of an oral antibiotic, nasal corticosteroid, nasal lavage, and topical decongestant. 22 Lal et al 23 reported a 31% surgery rate, while Subramanian et al 24 reported a 10% rate.
The rates in these studies vary widely, as do the exact treatment regimens, the duration of treatment, and the presence of nasal polyps. Although our surgery rate was within the range reported in the literature, it is difficult to make comparisons, since our population included only CRSsNP patients and our treatment modalities were different. However, this wide range of surgical rates may also illustrate the great variability in decisions to pursue surgery, which are based on patient preference, surgeon experience, and different treatment philosophies.
Volume 97, Number 6 www.entjournal.com 171
n (%) Group Nasal obstruction Postnasal drip Rhinorrhea Facial pain/ pressure Loss of smell p Value Antibiotic (n = 17) 7 (41) 12 (71) 10 (59) 9 (53) 3 (18) Steroid (n = 28) 14 (50) 20 (71) 15 (54) 25 (89) 14 (50) 0.670 Combination (n = 55) 27 (49) 42 (76) 42 (76) 47 (85) 38 (69)
Table 3. Pretreatment symptoms in the three treatment groups
ANTIBIOTICS, STEROIDS, AND COMBINATION THERAPY IN CHRONIC RHINOSINUSITIS WITHOUT NASAL POLYPS IN ADULTS
Figure 2. Chart shows the percentages of patients in each treatment group who experienced improvement in their symptoms after treatment (NO = nasal obstruction; PND = post-nasal drip; RHI = rhinorrhea; FP/P = facial pain/pressure; LOS = loss of smell).
The various controversies over antibiotic use, steroid use, and outcomes measures in the literature were inspirations for our study. Our finding that there was no difference in treatment efficacy among the antibiotic, steroid, and combination groups as represented by changes in Lund-Mackay CT scores and need for surgery indicate a need for further research.
Shortcomings of our study include the use of different types of antibiotic, different durations of antibiotic therapy, the use of two different steroids, and the lack of randomization into treatment groups. However, the effect of using different antibiotics may be insignificant given that studies suggest no difference in efficacy between broad-spectrum antibiotics such as extended-spectrum penicillins (e.g., amoxicillin/clavulanic acid) and fluoroquinolones (e.g., ciprofloxacin). 25,26 Also, our study did not include the use of a validated symptoms survey, which would have helped to better correlate Lund-Mackay CT scores with clinical symptoms. Finally, there is an intrinsic bias in using CT scans as an outcomes measure. For instance, patients with significant improvement might not have undergone post-treatment CT, thereby leading to a possible underestimation of the effect of treatment. Future prospective studies may also benefit from standardized follow-up for gauging the maintenance of symptom relief and disease recurrence, which have been shown to be common. 8
In conclusion, we found that Lund-Mackay CT scores decreased significantly in patients with CRSsNP after treatment with an antibiotic, a steroid, or combination therapy. However, we found no evidence that any of these treatment modalities was superior to any other in improving CT findings.
References
1. Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg 2003;129(3 Suppl):S1-32.
2. Dietz de Loos DA, Hopkins C, Fokkens WJ. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope 2013;123(1):57-63.
3. Orlandi RR, Smith TL, Marple BF, et al. Update on evidence-based reviews with recommendations in adult chronic rhinosinusitis. Int Forum Allergy Rhinol 2014;4(Suppl 1):S1-15.
4. Brook I, Frazier HE, Foote PA. Microbiology of the transition from acute to chronic maxillary sinusitis. J Med Microbiol 1996;45(5):372-5.
5. Hamilos DL. Chronic rhinosinusitis: Epidemiology and medical management. J Allergy Clin Immunol 2011;128(4):693-707; quiz 708-9.
6. Suh JD, Kennedy DW. Treatment options for chronic rhinosinusitis. Proc Am Thorac Soc 2011;8(1):132-40.
7. Dubin MG, Liu C, Lin SY, Senior BA. American Rhinologic Society member survey on “maximal medical therapy” for chronic rhinosinusitis. Am J Rhinol 2007;21(4):483-8.
8. Russell PT, Bekeny JR. Oral antibiotics and the management of chronic sinusitis: What do we know? Curr Opin Otolaryngol Head Neck Surg 2014;22(1):22-6.
9. Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S35-40.
10. Wallwork B, Coman W, Mackay-Sim A, et al. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope 2006;116(2):189-93.
11. Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol 2016;6(Suppl 1):S22-209.
12. Poetker DM, Jakubowski LA, Lal D, et al. Oral corticosteroids in the management of adult chronic rhinosinusitis with and without nasal polyps: An evidence-based review with recommendations. Int Forum Allergy Rhinol 2013;3(2):104-20.
13. Scadding GK, Lund VJ, Darby YC. The effect of long-term antibiotic therapy upon ciliary beat frequency in chronic rhinosinusitis. J Laryngol Otol 1995;109(1):24-6.
14. Suzuki H, Asada Y, Ikeda K, et al. Inhibitory effect of erythromycin on interleukin-8 secretion from exudative cells in the nasal discharge of patients with chronic sinusitis. Laryngoscope 1999;109(3):407-10.
15. Ashraf N, Bhattacharyya N. Determination of the “incidental” Lund score for the staging of chronic rhinosinusitis. Otolaryngol Head Neck Surg 2001;125(5):483-6.
16. Hopkins C, Browne JP, Slack R, et al. The Lund-Mackay staging system for chronic rhinosinusitis: How is it used and what does it predict? Otolaryngol Head Neck Surg 2007;137(4):555-61.
17. Bhattacharyya N. Radiographic stage fails to predict symptom outcomes after endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope 2006;116(1):18-22.
18. Bradley DT, Kountakis SE. Correlation between computed tomography scores and symptomatic improvement after endoscopic sinus surgery. Laryngoscope 2005;115(3):466-9.
19. Stewart MG, Donovan DT, Parke RB Jr., Bautista MH. Does the severity of sinus computed tomography findings predict outcome in chronic sinusitis? Otolaryngol Head Neck Surg 2000;123(1 Pt 1):81-4.
20. Stewart MG, Johnson RF. Chronic sinusitis: Symptoms versus CT scan findings. Curr Opin Otolaryngol Head Neck Surg 2004;12(1):27-9.
21. Young LC, Stow NW, Zhou L, Douglas RG. Efficacy of medical therapy in treatment of chronic rhinosinusitis. Allergy Rhinol (Providence) 2012;3(1):e8-e12.
22. McNally PA, White MV, Kaliner MA. Sinusitis in an allergist’s office: Analysis of 200 consecutive cases. Allergy Asthma Proc 1997;18(3):169-75.
23. Lal D, Scianna JM, Stankiewicz JA. Efficacy of targeted medical therapy in chronic rhinosinusitis, and predictors of failure. Am J Rhinol Allergy 2009;23(4):396-400.
24. Subramanian HN, Schechtman KB, Hamilos DL. A retrospective analysis of treatment outcomes and time to relapse after intensive medical treatment for chronic sinusitis. Am J Rhinol 2002;16(6):303-12.
25. Legent F, Bordure P, Beauvillain C, Berche P. A double-blind comparison of ciprofloxacin and amoxycillin/clavulanic acid in the treatment of chronic sinusitis. Chemotherapy 1994;40(Suppl 1):8-15.
26. Namyslowski G, Misiolek M, Czecior E, et al. Comparison of the efficacy and tolerability of amoxycillin/clavulanic acid 875 mg b.i.d. with cefuroxime 500 mg b.i.d. in the treatment of chronic and acute exacerbation of chronic sinusitis in adults. J Chemother 2002;14(5):508-17.
172 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018 LIU,
RICHARDSON, BERNARD, CHURCH, SEIBERLING
Nasal airway obstruction: Prevalence and anatomic contributors
David W. Clark, MD; Anthony G. Del Signore, MD, PharmD; Roheen Raithatha, MD; Brent A. Senior, MD
Abstract
Surgical treatments for nasal airway obstruction (NAO) are commonly offered as part of otolaryngology practice. Anatomic causes include septal deviation, inferior turbinate hypertrophy, and nasal valve collapse (NVC). This study was performed to determine the prevalence of anatomic contributors to NAO. A total of 1,906 patients with sinonasal complaints were surveyed by 50 otolaryngologists in varying U.S. geographic regions. Patients were first evaluated using the Nasal Obstruction Symptom Evaluation (NOSE) instrument to assess the NAO symptoms and their severity. Physicians then examined patients for the presence of the three anatomic contributors. Presence of septal deviation and turbinate hypertrophy was assessed through an internal nasal exam with direct or endoscopic visualization based on the physician’s standard methodology for diagnosis. Presence of NVC was determined by the modified Cottle maneuver. Among all patients surveyed, prevalence was 67% for NVC, 76% for septal deviation, and 72% for inferior turbinate hypertrophy. We found that 64% of the patients (n = 1,211) had severe/extreme NOSE scores (≥55), representing the most likely nasal obstruction candidates for intervention. In these patients, the prevalence of NVC, septal deviation, and inferior turbinate hypertrophy was 73, 80, and 77%, respectively. Eighty-two percent of the 236 patients with severe/extreme NOSE scores who reported prior septoplasty and/or inferior turbinate reduction
From the Department of Otolaryngology–Head and Neck Surgery, Baylor Scott & White Health, and Texas A&M Health Science Center College of Medicine, Temple, Texas (Dr. Clark); the Department of Otolaryngology–Head and Neck Surgery, Mount Sinai Beth Israel, New York, N.Y. (Dr. Del Signore); ENT & Allergy Associates, New York, N.Y. (Dr. Raithatha); and the Department of Otolaryngology–Head and Neck Surgery, University of North Carolina at Chapel Hill, Chapel Hill, N.C. (Dr. Senior).
Corresponding author: Brent A. Senior, MD, Department of Otolaryngology–Head and Neck Surgery, University of North Carolina at Chapel Hill, 170 Manning Dr., Campus Box #7070, Chapel Hill, NC 27599. Email: bsenior@med.unc.edu
Financial support: This study was supported with funding by Spirox, Inc., Redwood City, Calif. Dr. Clark., Dr. Del Signore, and Dr. Senior are consultants for Spirox, Inc.
had NVC. Our study revealed a comparable prevalence of all three anatomic contributors across all patients and the subset with severe/extreme NOSE scores, highlighting the importance of evaluating the lateral nasal wall as a component of NAO treatment strategy.
Introduction
Nasal airway obstruction (NAO) is a common presenting symptom in otolaryngology practices and has been described as a source of significant patient discomfort and financial burden.1,2 Diagnosis of nasal obstruction includes symptom assessment via the Nasal Obstruction Symptom Evaluation (NOSE) instrument1 and physical exam of the nasal valve, including lateral nasal wall, septum, and inferior turbinates.
Etiologies of nasal obstruction consist of inflammatory and anatomic contributors. 3 Anatomic causes include inferior turbinate hypertrophy, septal deviation, and nasal valve dysfunction.4 Nasal valve dysfunction can have static and dynamic components, where dynamic nasal valve dysfunction (hereby defined as nasal valve collapse [NVC]) is caused by lateral wall insufficiency. Many patients have more than one anatomic cause for their nasal obstruction; however, the prevalence of these anatomic causes has not been reported. A survey of patients complaining of nasal obstruction was conducted to establish the prevalence of these anatomic causes.
Patients and methods
Patients’ identifying information was not collected; therefore, Institutional Review Board approval was not sought for this survey.
Fifty physicians, including general otolaryngologists, rhinologists, and facial plastic surgeons in various U.S. geographic regions surveyed patients with symptoms of nasal obstruction and/or sinonasal complaints. Data collected included (1) the severity of nasal obstruction, (2) anatomic contributors to nasal obstruction, and (3) history of nasal surgery.
The NOSE instrument was used to assess the severity of nasal obstruction, using five questions, each scored
Volume 97, Number 6 www.entjournal.com 173 ORIGINAL ARTICLE
on a scale of 0 to 4.1 The total NOSE score was converted to a 100-point scale, which defines nasal obstruction severity using a classification system: mild (5 to 25), moderate (30 to 50), severe (55 to 75), or extreme (80 to 100). 5 The patient group with severe or extreme scores was of interest as these patients are potential candidates for intervention.
Three components of the nasal valve anatomy, including the lateral nasal wall, septum, and inferior turbinates, were evaluated by physicians during office visits. The evaluation included an internal nasal exam with direct or endoscopic visualization, based on the physician’s standard methodology for diagnosis, and a modified Cottle maneuver. The modified Cottle maneuver (intranasal stabilization of the lateral nasal wall) was performed to gently support the lateral nasal wall cartilage on each side of the nose while the patient was asked to inspire.6,7 A modified Cottle maneuver was considered positive if the patient reported improvement in breathing.
Results
A total of 1,906 patients were surveyed by 50 physicians from nine U.S. states (Calif., Fla., Ga., Ill., In., N.C., N.Y., Pa., and Texas). Based on the NOSE scores, most (63%) patients had severe (37%) or extreme (26%) nasal obstruction, 24% reported moderate nasal obstruction, and 11% had mild nasal obstruction. A small number of patients (2%) were asymptomatic. Most patients had multiple anatomic contributors (table 1). Among all patients, the prevalence was 67% for NVC, 76% for septal deviation, and 72% for inferior turbinate hypertrophy. The prevalence in the subgroup of patients with severe/extreme nasal obstruction for NVC, septal deviation, and inferior turbinate hypertrophy was 73, 80, and 77%, respectively. Among the patients considered to be symptomatic (severe/extreme nasal obstruction, n = 1,211), 46% presented with NVC, 51% had septal deviation, and 49% had inferior turbinate
hypertrophy. Most patients with NVC and inferior turbinate hypertrophy had bilateral contribution (84 and 60%, respectively).
Among all patients, septoplasty and/or turbinate reduction procedure history was available for 331 patients. Seventy-one percent (n = 236) of these patients presented with severe/extreme nasal obstruction. The prevalence of NVC in these patients was 82% (table 2). Eleven patients reported previous lateral nasal wall surgery. Other surgeries reported included functional endoscopic sinus surgery and cosmetic rhinoplasty; these were not analyzed as they are not intended to address anatomic contributors to nasal obstruction.
NVC = nasal valve collapse.
174 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
CLARK, DEL SIGNORE, RAITHATHA, SENIOR
Anatomic contributor combination prevalence, % (n) All patients N = 1,906 Severe/ extreme n = 1,211 Nasal valve collapse NVC only 6% (121) 6% (72) NVC + septal deviation 13% (252) 14% (166) NVC + inferior turbinate hypertrophy 7% (138) 7% (85) NVC + septal deviation + inferior turbinate hypertrophy 40% (762) 46% (558) Total NVC 67% (1,273) 73% (881) Septal deviation Septal deviation only 5% (102) 2% (28) Septal deviation + inferior turbinate hypertrophy 18% (337) 18% (213) NVC + septal deviation 13% (252) 14% (166) NVC + septal deviation + turbinate hypertrophy 40% (762) 46% (558) Total septal deviation 76% (1,453) 80% (965)
turbinate hypertrophy Inferior turbinate hypertrophy only 7% (131) 6% (72) Inferior turbinate hypertrophy + septal deviation 18% (337) 18% (213) NVC + inferior turbinate hypertrophy 7% (138) 7% (85) NVC + septal deviation + inferior turbinate hypertrophy 40% (762) 46% (558) Total inferior turbinate hypertrophy 72% (1,368) 77% (927) Asymptomatic 3% (63) 1% (17)
Table 1. Prevalence of anatomic contributors in overall cohort of patients with nasal obstruction and those with severe/extreme obstruction
Inferior
Key:
Table 2. Prevalence of NVC among patients with severe/extreme nasal obstruction who reported prior septoplasty and/or inferior turbinate reduction
*Anatomic contributors are not mutually exclusive.
Discussion
To the best of our knowledge, this is the largest assessment of the prevalence of anatomic contributors to nasal obstruction that has been conducted. The results showed that NVC (73%) was as common as septal deviation (80%) and inferior turbinate hypertrophy (77%) in patients with severe/extreme nasal obstruction. Furthermore, in a subset of patients with severe/extreme nasal obstruction despite prior septoplasty and/or inferior turbinate reduction, NVC prevalence was high (82%), suggesting that missed or untreated NVC may be associated with symptom persistence.
Nasal obstruction is associated with a reduction in quality of life and is a common surgically treated problem. 8,9 Identification of anatomic contributor(s) is critical for selection of an appropriate treatment strategy. The finding that septal deviation, inferior turbinate hypertrophy, and NVC are common anatomic contributors to nasal obstruction is consistent with the frequency of septoplasty and inferior turbinate reduction procedures performed by otolaryngologists.10,11
Unfortunately, surgery is associated with diminished satisfaction and persistent nasal obstruction over time.12,13 Furthermore, a fraction of the patients with NAO who were ideal candidates for septoplasty did not achieve significant improvement after the procedure.1,14 Although these results show that NVC is an equally prevalent anatomic contributor, analysis of commercial payer and Medicare fee-for-service data indicates that its surgical correction is less frequent than septoplasty and inferior turbinate reduction. A potential explanation for this divergence in treatment is infrequent screening for NVC.
Most patients with nasal obstruction have multiple anatomic contributors, and diagnoses of septal deviation and inferior turbinate hypertrophy are commonly achieved; however, surgical techniques to address these anatomic contributors do not directly address weakness in the lateral nasal wall.15 The NVC clinical consensus statement published in 2010 concluded that
NVC is a distinct etiology for nasal obstruction and that the validity of the modified Cottle maneuver for identifying NVC suggests suitability for inclusion in examinations.6
Previous reports of patients who underwent revision septoplasty and who suffered from persistent nasal obstruction after their primary septoplasty showed that NVC was highly prevalent and frequently required correction during the revision surgery.16 This finding, coupled with high prevalence rates in patients with severe/extreme nasal obstruction, suggests that more routine diagnosis of NVC may inform optimal treatment for patients.
Treatment options for NVC include invasive cartilaginous grafts, such as batten grafts and lateral crural strut grafts, and less invasive options, such as absorbable nasal implants. Temporary, noninvasive options include nasal strips and dilators. This survey did not examine the outcomes of the treatment options.
This survey showed that the prevalence of NVC was as common as the prevalence of septal deviation and inferior turbinate hypertrophy in patients with nasal obstruction. Therefore, assessment of all anatomic contributors, including NVC, is critical to ensure adequate symptom reduction.
Acknowledgments
The authors thank the following physicians for participating in this survey: Ford Albritton, MD (Texas); Steven Bomeli, MD (Ga.); Russell Briggs, MD (Texas); Robert Butler, MD (Texas); Jeffrey Campbell, MD (N.C.); Ajaz Chaudhry, MD (Ga.); David Clark, MD (Texas); Michael Cohen, MD (N.Y.); William Cohen, DO (Calif.); Chris Dehan, MD (Texas); Anthony Del Signore, MD (N.Y.); Jeff Feinfield, MD (Calif.); Alexis Furze, MD (Calif.); David Godin, MD (N.Y.); Barbara Goheen, MD (N.C.); Alexander Goldin, MD (Ill.); Douglas Holmes, MD (N.C.); Raymond Howard, MD (Ga.); Thomas Hung, MD (Texas); Bennie Jarvis, MD
Volume 97, Number 6 www.entjournal.com 175 NASAL AIRWAY OBSTRUCTION: PREVALENCE AND ANATOMIC CONTRIBUTORS
Septoplasty and inferior turbinate reduction (n = 157) Septoplasty only (n = 54) Inferior turbinate reduction only (n = 25) Anatomic contributors* NVC 82% (128) 83% (45) 80% (20) Septal deviation 52% (82) 52% (28) 72% (18) Inferior turbinate hypertrophy 46% (73) 72% (39) 48% (12)
(N.C.); Richard Jones, MD (N.C.); Paul Juengel, MD (N.C.); Alex Kim, MD (Ill.); Daniel Kurtzman, MD (Ill.); Charles Lano, MD (Texas); Michael Layland, MD (Ill.); Gregory Levitin, MD (N.Y.); Frederic Levy, MD (N.C.); Lisa Liberatore, MD (N.Y.); Douglas Liepert, MD (In.); Neal Lofchy, MD (Ill.); Ram Madasu, MD (Fla.); Neelesh Mehendale, MD (Texas); Rob Nason, MD (Texas); Anand Ponnappan, MD (Ill.); Roheen Raithatha, MD (N.Y.); Suresh Raja, MD (Fla.); Angelo Reppucci, MD (N.Y.); Waldemar Riefkohl, MD (N.C.); Aaron Rogers, MD (Ga.); Cooper Scurry, MD (N.C.); Luke Shellenberger, MD (Texas); Farhad Sigari, MD (Calif.); Richard Thrasher III, MD (Texas) Satish Vadapalli, MD (Calif.); Glenn Waldman, MD (Calif.); Richard Weinstock, DO (Fla.); Allison Wyll, MD (Texas); David Yen, MD (Pa.); Jay Youngerman, MD (N.Y.).
References
1. Stewart MG, Smith TL, Weaver EM, et al. Outcomes after nasal septoplasty: Results from the Nasal Obstruction Septoplasty Effectiveness (NOSE) study. Otolaryngol Head Neck Surg 2004;130(3):283-90.
2. Chandra RK, Patadia MO, Raviv J. Diagnosis of nasal airway obstruction. Otolaryngol Clin North Am 2009;42(2):207-25.
3. Moche JA, Palmer O. Surgical management of nasal obstruction. Oral Maxillofac Surg Clin North Am 2012;24(2):229-37.
4. Barrett DM, Casanueva FJ, Cook TA. Management of the nasal valve. Facial Plast Surg Clin North Am 2016;24(3):219-34.
5. Lipan MJ, Most SP. Development of a severity classification system for subjective nasal obstruction. JAMA Facial Plast Surg 2013;15(5):358-61.
6. Rhee JS, Weaver EM, Park SS, et al. Clinical consensus statement: Diagnosis and management of nasal valve compromise. Otolaryngol Head Neck Surg 2010;143(1):48-59.
7. Fung E, Hong P, Moore C, Taylor SM. The effectiveness of modified cottle maneuver in predicting outcomes in functional rhinoplasty. Plast Surg Int 2014;2014:618313.
8. Rhee JS, Book DT, Burzynski M, Smith TL. Quality of life assessment in nasal airway obstruction. Laryngoscope 2003;113(7):1118-22.

9. Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: Demographics and perioperative outcomes. Laryngoscope 2010;120(3):635-8.
10. Manoukian PD, Wyatt JR, Leopold DA, Bass EB. Recent trends in utilization of procedures in otolaryngology–head and neck surgery. Laryngoscope 1997;107(4):472-7.
11. Brunworth J, Holmes J, Sindwani R. Inferior turbinate hypertrophy: Review and graduated approach to surgical management. Am J Rhinol Allergy 2013;27(5):411-15.
12. Illum P. Septoplasty and compensatory inferior turbinate hypertrophy: Long-term results after randomized turbinoplasty. Eur Arch Otorhinolaryngol 1997;254 Suppl 1:S89-92.
13. Roblin DG, Eccles R. What, if any, is the value of septal surgery? Clin Otolaryngol Allied Sci 2002;27(2):77-80.
14. Siegel NS, Gliklich RE, Taghizadeh F, Chang Y. Outcomes of septoplasty. Otolaryngol Head Neck Surg 2000;122(2):228-32.
15. Matthias C. Surgery of the nasal septum and turbinates. GMS Curr Top Otorhinolaryngol Head Neck Surg 2007;6:Doc10.
16. Becker SS, Dobratz EJ, Stowell N, et al. Revision septoplasty: Review of sources of persistent nasal obstruction. Am J Rhinol 2008;22(4):440-4.
176 www.entjournal.com ENT-Ear, Nose & Throat Journal June 2018
CLARK, DEL SIGNORE, RAITHATHA, SENIOR




www.compulinkadvantage.com/entehr 800.456.4522 Request a Demo Today ENT’s All-in-One EHR Solution “ I strongly recommend Compulink to any otolaryngology practice because of its ENT specificity, ease of use, cost, confidentiality, and friendly, personal customer support. ” Romeo C. Agbayani, Jr, MD, Marin ENT EHR PM RCM ASC ANALYTICS PATIENT ENGAGEMENT TELEHEALTH AUDIT PROTECTION ENT Specific AI-Driven EHR Designed For Speed Chart From A Single Screen 2015 ONC Certified Money-Back Satisfaction Guarantee EHR & PM Leader for 33 Years!