10 SURPRISING TRUTHS ABOUT VBDs
Myriah Hinchey, ND, FMAPS


Myriah Hinchey, ND, FMAPS


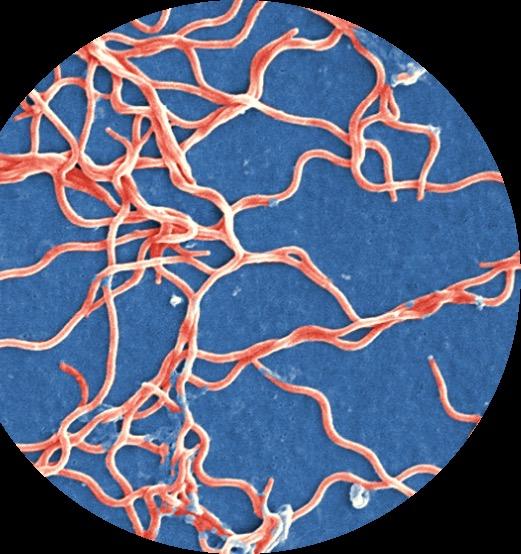
1. Lyme Is a Clinical Diagnosis
2. VBDs May Cause an Abrupt Onset of Neuropsychiatric Symptoms
3. The Borrelia and Cancer Connection
4. EM Rashes are not frequent and Vary in Timing and Appearance
5. High Incidence of VBDs in PANS (Pediatric Acute-onset Neuropsychiatric Syndrome)
6. Pattern Recognition Is Key To Diagnosis of VBDs - Hallmark Symptoms of Key Co-Infections
7. Major overlap of MCAS symptoms with VBD Symptoms
8. Mycotoxin Illness Has a Synergistic Effect with VBDs
9. Reactivation Of VBDs From COVID Happens
10. Mindset Matters - Don’t Give Up Hope!


Evaluating an individual’s health history, exposures, physical exam, and nature, duration, and progression of symptoms is crucial to making a clinical diagnosis as VBDs can present with a wide range of manifestations in multiple body systems.
Testing can be a helpful tool in making a clinical diagnosis, but it is not a determining factor.
1. Symptom evaluation
• Pulmonary
• EM Rash
• GI symptoms
• Musculoskeletal
• Neurological
• Neuropsychiatric
• Reproductive
• Other
• Lyme Transmission History
2. ILADS -- Key considerations in making a clinical diagnosis:
• No single diagnostic element
• Consideration of strengths and weaknesses of lab testing
• Clinical judgment and differential diagnosis
• Comprehensive evaluation
• Balanced approach
3. CDC – Lyme Disease diagnosis:
• Medical history
• Physical examination
• Laboratory testing
• Interpretation of Lab results
• Clinical criteria for diagnosis
4. Functional / Integrative Medicine Other Considerations:
• monitoring immune response
• incorporating other lab markers beyond antibody testing
• conventional labs
• inflammatory and AID markers
• Test for other infections
• Test for tickborne co-infections
• Adrenal, hormone, neurotransmitter, thyroid markers
• Environmental triggers
• Other FM labs
• Temporal Considerations: Understanding the progression and timing of symptom onset and potential exposure to ticks
• Testing Considerations: consideration of specificity, sensitivity, reliability of testing methods, interpretation, recognizing the limitations of each method
• Comprehensive Testing Approach. Integrating both serological and direct detection methods provides a more robust diagnostic framework, reducing the risk of false negatives and enhancing diagnostic accuracy (Brown & White, 2015).

• General Neuropsychiatric Tick-Borne Disease Symptoms -- studies validate the connection to mental health issues: (Maxwell et al., 2022; Horowitz et al., 2018).
•Irritability
•Emotional dysregulation
•Sudden rage/anger
•Nightmares
•Impulse control/hyperactivity
•Conduct problems/oppositional behaviors
•Easy tearfulness
•Withdrawn behaviors
•Confusion
•Obsessive Compulsive Disorder
•Paranoia
•Auditory/visual hallucinations
•Sensory hyperarousal (auditory, visual, and touch)
• Lyme-specific
•Social skill deficits
•PANS
•Autism
•ADHD/Developmental disorders
•Depression
•Anxiety disorders (anxiety disorders (panic disorder, social anxiety disorder, generalized anxiety disorder, posttraumatic stress disorder, intrusive symptoms))
•Panic disorders / panic attacks
•Schizoaffective disorder
•Bipolar disorder / manic episodes
• Psychiatric symptoms are more significant with Babesia and Bartonella co-infections
•Sleep disorders, disturbances, insomnia
•Eating disorders (anorexia, bulimia, weight loss/gain)
•Dementia
•Suicidal tendencies (estimated that 1200 suicides result from Lyme annually)
•Decreased libido
•Addiction
•Anhedonia
•Depersonalization
•Dissociative episodes, derealization
• mood swings, violent outbursts, irritability, depression, disturbed sleep (too much, too little, early awakening), personality changes, obsessive – compulsive disorder (OCD), paranoia, panic/anxiety attacks, hallucinations.
(https://lymediseaseassociation.org/lyme-tbd/medical/lyme-disease-symptoms/)
• Bartonella-specific:
• CNS- irritability and global dysfunction.
• Anxiety, rage attacks, anti-social behavior, panic attacks, insomnia, depression, tremors, seizures, ataxia, hallucinations, schizophrenia, dementia.

“LB causes immune and metabolic effects that result in a gradually developing spectrum of neuropsychiatric symptoms, usually presenting with significant comorbidity which may include developmental disorders, autism spectrum disorders, schizoaffective disorders, bipolar disorder, depression, anxiety disorders (panic disorder, social anxiety disorder, generalized anxiety disorder, posttraumatic stress disorder, intrusive symptoms), eating disorders, decreased libido, sleep disorders, addiction, opioid addiction, cognitive impairments, dementia, seizure disorders, suicide, violence, anhedonia, depersonalization, dissociative episodes, derealization and other impairments”.



“A number of articles have documented an association between LB and seizures [79,153,154,155,156,157,158,159].
Seizures have also been documented associated with Bartonella [155]. Seizure disorders are more common when there is a lengthy delay in diagnosis and effective treatment. Most commonly the seizures are complex partial seizures with significant postictal confusion and are sometimes referred to psychiatrists because they are misdiagnosed as being “psychogenic” or so called “pseudoseizures.” The prevalence of seizures in (LB-H) patients is 20% and they were mostly complex partial seizures [12]” (Bransfield, 2018).



Cancer:
• Shown to be present in various types of breast cancer
• “B. burgdorferi can invade breast cancer tumor cells and it can increase their tumorigenic phenotype, which urges the need for further studies on whether B. burgdorferi could have any role in breast cancer development.” (Gaur et al., 2021)
The Front Lines Of Lyme Disease And Breast Cancer Research:
https://www.newhaven.edu/news/blog/2020/evasapi-research.php
University of New Haven Professor Makes Great Strides in Lyme Disease, Cancer Research
Dr. Sapi - research examining a possible breast cancer/Lyme disease link, Lyme as an epigenetic factor in the development of breast cancer.
“When examining more than 400 invasive breast cancer tissues, they found that a significant number of samples were positive for Borrelia, suggesting that the bacteria may play a role in breast cancer development and metastasis.”
“The normal breast tissues were completely negative for the bacteria, and we have found evidence that they are present in breast cancer tissues. Furthermore, when we introduced Borrelia to cancer cells, we found they start to invade very quickly.”
Borrelia and Lymphomas:

´ A review in the Open Journal of Medical Microbiology found Borrelia in the skin of primary cutaneous B-cell lymphoma patients.
´ Theory: Lyme disease induces systemic inflammation, suppressing the immune system and creating conditions for DNA mutations and cancer.
´ The American Cancer Society notes a few reported cases linking Lyme disease and cancer.


“The hypothesis that an infectious agent may initiate chronic inflammation and facilitate B lymphocyte transformation and lymphogenesis has been raised in recent years. …. The association of … Borrelia burgdorferi with cutaneous MALT lymphoma.”
“Nearly 20% of all cancers are caused by an infection of a microbe, the amount of evidence and information regarding the mechanisms associated with oncogenesis varies dramatically from one organism to the next” (Smith et al., 2014).
Bacterial Infection and Non-Hodgkin B-Cell Lymphoma: Interactions between Pathogen, Host and the Tumor Environment
Monika Maria Biernat, Tomasz Wróbel
•PMID: 34298992
• PMCID: PMC8305669
• DOI: 10.3390/ijms22147372

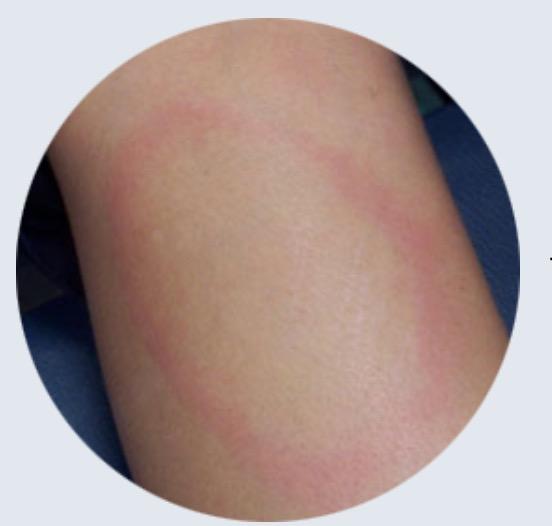
High Variability in the Bull's-eye Rash Occurrence:
• Not universal among those infected with Borrelia
• CDC: 70–80% - disagree
• Present in less than 40% of cases.
• Lyme disease varies in symptoms and presentations
• Not all EM’s are a symmetrical, circular bull's-eye rash.
• Variability of EM rashes reflects complex nature of clinically diagnosing Lyme disease.
Appearance:
• Multiple manifestations.
• Not always a bull’s-eye shape (Lorquet et al., 2023).
• May mimic other rashes, cellulitis or insect bites.
• Resembles conditions like contact dermatitis or tick bite reactions.
• CDC: “solid lesions, blue-purple hues, and crusted or blistering lesions”
Timeframe:
• The rash can appear 3 to 30 days after a tick bite (Wormser et al., 2006).
• Disappears even if untreated.
Classic EM Rash (CDC): “Erythema migrans (EM)— Red annular or homogeneous rash at the site of tick bite; expands gradually over several days to >5 cm in diameter; central clearing may develop as the rash expands, resulting in a “target” or “bull’s-eye” appearance.”
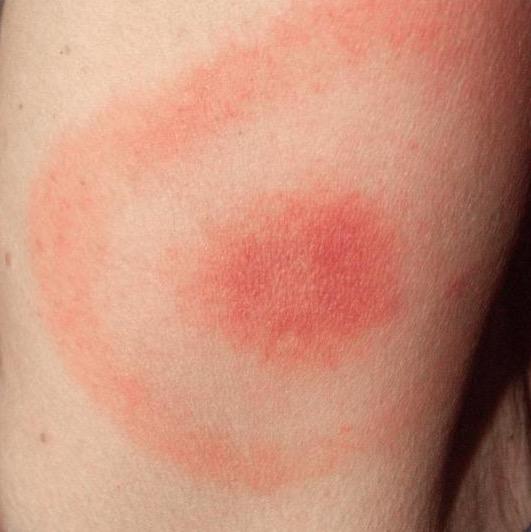

ILADS: “The presence of erythema migrans (EM) rash is a “classic” indicator of Lyme disease, but the appearance of EM rashes is highly variable. Most EM rashes are solid colored, ranging from faint pink to a deep red. Less than 20% of all EMs have the classic “bull’s-eye” appearance. An EM may be missed if not specifically sought on exam, as they are typically asymptomatic and may be unnoticed or unrecognized by the patient”.






PANS / Pediatric Acute-Onset Neuropsychiatric Syndrome
Also referred to as Basal Ganglia Encephalitis / Autoimmune Encephalitis / AE
Characterized by an unusually abrupt onset of cognitive, behavioral, or neurological symptoms.
• Lyme (Borrelia) and Lyme Co-infections (especially Bartonella & Babesia) are a known cause/trigger for PANS (Zuliani et al., 2012).
• Bartonella especially known to be a factor in AE (Breitschwerdt et al., 2019)
• PANS linked with Autism (approx. 25% of those with ASD have PANS/PANDAS (O’Hara, 2014).
• Almost 500,000 cases annually of Lyme Disease
• >40% ticks in northeast carriers of Babesia.
• 42% of AE asscociated w/ Bartonella.
• A case report illustrates successful treatment for a patient with Lyme borreliosis and PANDAS, emphasizing the complexity of these neuropsychiatric disorders (Cross et al., 2021).
• Addressing both infections and autoimmune aspects led to a positive outcome, supported by the resolution of antineuronal antibodies post-treatment
• Another case report of a 14-year-old boy treated for PANS, exhibiting sudden-onset psychotic behavior (hallucinations, delusions, suicidal and homicidal ideation) treated with antimicrobials to resolve Bartonella. Had a resolution of neuropsychiatric and other symptoms (Breitshwerdt et al., 2019).



#6.

Symptoms are chronic but intermittent: affects multiple organ systems simultaneously
Key diagnostic features of symptoms: multisystemic, migratory, cyclic
Hallmark Feature: immune impairment that worsens over time

Common Bartonella Symptoms:
ü CNS irritability – muscle twitching, tremors, insomnia, seizures, agitation
ü Neuropsychiatric – anxiety, mood swings, outbursts, rage, antisocial behavior
ü Headaches – ice pick
ü Tachycardia
ü Photophobia
ü Unilateral symptoms
ü Buzzing
ü Migratory neuropathy / nerve pain
ü Foot/Heel pain
ü Swollen lymph nodes
ü Eyes- uveitis, retinitis, retinal artery and vein thromboses
ü Connective tissues
• tender nodules (skin, along fascia)
• Pain in soles of the feet – esp. when waking in the morning
• tendonitis
• bone pain
• painful joints without synovial swelling
ü Peculiar skin manifestations: Striae or stretch marks

• “Bartonella tracks” (also referred to as BACL- Bartonella Associated Cutaneous Lesions)
• They blanch, do not follow dermal lines
ü GI issues
• Gastritis, mesenteric lymphadenitis, peliosishepatis


ü Night sweats and sometimes day sweats – drenching
ü Shortness of breath, air hunger
ü Chronic cough
ü Headaches–dull,all-over,headinvice
ü Capillary angiomas
ü Flushing
ü Head/tooth/sinus/jaw symptoms
ü Bells Palsy
ü Ear ringing
ü Blurry vision
ü Vivid/violent dreams, nightmares
ü Failure to respond to Lyme treatment
ü • Unrelenting headache
ü (especially head pressure)
ü Paresthesias and dysautonomia
ü Night sweats
ü Rib and bone pain
ü Cough and Air Hunger
ü Encephalopathy, anxiety
ü Myalgias and arthralgias
ü Brain fog, depression, insomnia
ü Gastrointestinal symptoms



Common Lyme Disease Symptoms:
ü Headache (ranging from mild to severe).
ü Cognitive Deficits (including "brain fog," difficulty concentrating, memory issues, and confusion).
ü Mood Changes (such as depression and anxiety).
ü Sleep Disturbances (difficulty falling or staying asleep, and unrefreshing sleep).
ü Erythema Migrans (EM) Rash (a red, expanding bull's-eye rash at the tick bite site).
ü Flu-Like Symptoms (fever, chills, fatigue, muscle / joint aches, headache in early stages).
ü Joint Pain and Swelling (especially in larger joints like knees).
ü Neurological Symptoms (numbness, facial paralysis (Bell's palsy), memory problems
ü Sleep disturbances.
ü Heart Problems (rarely, irregular heartbeat (Lyme carditis), palpitations, rapid pulse.
ü Eye Inflammation (redness and eye discomfort), intermittent blurry/double vision.
ü Fatigue (profound and persistent).
ü Muscle Weakness (decreased muscle tone).
ü Lymph Node Swelling (especially in the neck, occasionally).
ü Arthralgias, myalgias.


ü Others: change in bowel function, pelvic pain, chest/rib pain, twitching, hormone imbalance, irritable bladder, sweats, chills, shortness of breath, cough, dizziness, hair loss, thyroid issues, chronic sore throat, intermittent hearing issues/ringing/buzzing, sensitivity to everything.

Mast Cell Activation Syndrome (MCAS) shares overlapping symptoms with various tick-borne diseases, including Lyme disease, creating a complex clinical scenario (Frieri, 2018).
TBDs have been linked to a dysregulation of mast cells (respond to infections), causing mast cell activation syndrome (MCAS).
• Identifying and treating underlying infections is crucial.
• For those with mast cell activation syndrome, additional measures are needed.
• Treatment should focus on metabolizing histamine and stabilizing mast cells, as this syndrome can persist after infection resolution, leading to chronic symptoms.
• Dealing with infections and the immune system's intricacies may demand multiple strategies for achieving optimal health.
Symptoms of MCAS can overlap with Lyme Symptoms including:
• Lymph node swelling
• Eye pain
• Hot flashes
• Blood pressure dysregulation (POTS)
• Palpitations
• Abdominal pain
• Anxiety
• Fatigue: Both MCAS and Lyme disease can cause significant fatigue, impacting daily functioning.
• Joint Pain: Joint pain is a symptom that can be present in both conditions.
• Muscle Pain: Similar to joint pain, muscle pain is a shared symptom.
• Headaches: Both MCAS and Lyme disease can lead to headaches and migraines.
• Gastrointestinal Issues: Symptoms such as nausea, vomiting, diarrhea, or abdominal pain can occur in both conditions.
• Skin Issues: Rashes, hives, or other skin problems may be present in both MCAS and Lyme disease.
• Neurological Symptoms: Both conditions can manifest with neurological symptoms such as brain fog, difficulty concentrating, or memory issues.

•Immune System Suppression:
• Both mycotoxins and Lyme bacteria can individually compromise the immune system.
• When these conditions co-exist, immune system struggles, leading to reduced efficiency in combating infections and toxins.
• Increased susceptibility to effects of each condition when they co-exist.
•Inflammation:
• Mycotoxins and Lyme bacteria can independently trigger inflammatory responses in the body.
• Mycotoxin illness results in inflammatory symptoms that mimic Lyme.
• The combination of these inflammatory processes amplify and prolong an inflammatory state, contributing to a range of symptom s and potential tissue damage.
•Neurological Impact:
• Both mycotoxins and Lyme bacteria can affect the nervous system.
• The combined impact may lead to more severe neurological symptoms, including cognitive dysfunction, fatigue, and neuropathy.
•Compromised Detoxification:
• Mycotoxins burden the body's detoxification mechanisms, especially the liver.
• Lyme disease can also tax the detoxification pathways.
• When these challenges overlap, the body may struggle to efficiently eliminate toxins, leading to an increased toxic load.
• Carriers of HLA-DR gene
• more susceptible to chronic health issues w/systemic inflammation like mold or Lyme making it difficult to detoxify.
•Chronicity and Persistence:
•Both mycotoxin illness and Lyme disease can become chronic conditions. The synergistic effect may contribute to the persistence of symptoms, making it challenging for individuals to recover fully.
•Exacerbation of Symptoms:
• Mold suppresses immune system, burdens detox pathways, causes inflammation, making Lyme symptoms worse.
• Symptoms of both are vague, wide-reaching, and rarely exist alone.
• Both great imitators, make pre-existing illnesses exacerbated.
• Similar symptoms: fatigue, headaches, cognitive issues like brain fog, memory issues, joint pains.
• Both Lyme and mold diagnosis rely on clinical assessment and history of exposure.

Hypothesis of How VBDs are Reactivated by COVID:
• Resulting inflammation, immune suppression, and immune dysregulation that results from COVID-19 (wild or VAX) creates a hospitable environment for dormant VBD to re-emerge.
⚬ Inflammation in capillaries, microclots, blood coagulation.
• ACE-2 binding activates RAS decreasing all organ function.
• Elevated Gal-3 levels.
• Imbalanced Th1 and Th2 (dominance).
• Elevated IL-6.
• Elevated IL-1beta.
• Elevated TGF-beta.
• Elevated TNF-alpha.
We need to correct the immune system and deal with the cytokine cascade in both.

Prioritizing Mental Well-being in Lyme Disease is Vital, Paralleling Physical Health Management:
• LB causes immune and metabolic effects that result in neuropsychiatric symptoms.
• Detecting and treating mental health issues early enhances prognosis.
• TBDs are a holistic health challenge, impacting cognitive function and emotional stability.
• Integrating mental health care in early treatment stages can mitigate these effects.

• When TBS are treated effectively, neuropsychiatric symptoms can be alleviated, which furthers emotional resiliency.
Mechanisms of Action:
• Mindset significantly influences healing by modulating the body's stress response through the HPA axis.
• A positive mindset favors activation of the Parasympathetic Nervous System (PNS), promoting a restful state conducive to healing,
• Stress-induced responses activate the sympathetic nervous system (SNS) and the release of stress hormones, shifting the body into a fight-or-flight state, diverting resources away from the healing mode of the PNS.
• Enlisting a variety of therapeutic interventions to minimize symptoms and alleviate ongoing stress response.
• Promoting PNS function and healing, while simultaneously uncovering and treating the underlying root causes:
• Belkaid, Y., Harrison, O. J. (2014). Homeostatic Immunity and the Microbiota. Immunity, 41(4), 562–576.
• Branda, J. A., & Strle, F. (2018). Molecular Mimicry in Lyme Borreliosis. Nature Reviews Microbiology, 16(4), 296-307.
• CDC. "Tick Removal and Testing."
• Centers for Disease Control and Prevention (CDC). (2022). "Lyme Disease Diagnosis and Testing."
• Deng, Y., Wu, Y., & Pan, S. (2011). Protective effects of artemisinin against prepatent Schistosoma japonicum infection by Th1 type responses in mice. International Immunopharmacology, 11(12), 1917–1922.
• Efferth, T., Romero, M. R., Wolf, D. G., et al. (2008). The antiviral activities of artemisinin and artesunate. Clinical Infectious Diseases, 47(6), 804–811.
• Eliaz, I. (2017). The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels. Alternative Therapies in Health and Medicine, 23(5), 8–12.
• Eliaz, I., Weil, E., Wilk, B. (2018). Integrative medicine and the role of modified citrus pectin/alginates in heavy metal chelation and detoxification – five case reports. Integrative Medicine: A Clinician's Journal, 17(2), 34–39.
• Farias, I., do Carmo, M. A., Brienze, V. M., Dolder, H. (2016). Cytotoxic effects of Uncaria tomentosa root extracts on primary cultures of human hepatocytes. The Brazilian Journal of Medical and Biological Research, 49(3), e5016.
• Feng, J., Auwaerter, P. G., Zhang, Y. (2019). Drug Combinations against Borrelia burgdorferi Persisters In Vitro: Eradication Achieved by Using Daptomycin, Cefoperazone and Doxycycline. PLoS ONE, 14(3), e0213972.
• Garbisa, S., Sartor, L., Biggin, S., Salvato, B. (2001). Benzo[a]pyrene-diolepoxide involves de novo ceramide biosynthesis in human fibroblasts. FASEB Journal, 15(14), 2529–2531.
• Hoffman, J. D., Yan, D., Hewetson, J., et al. (2015). Treatment of Ixodes scapularis ticks with Metarhizium brunneum F52 (Hypocreales: Clavicipitaceae) to control Ixodes scapularis (Acari: Ixodidae). Journal of Medical Entomology, 52(5), 1052–1056.
• Huebner, J., Shaw, D., & Davis, R. (2015). Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia, 35(5), 381–388.
• Ji, R. R., Xu, Z. Z., Strichartz, G., et al. (2016). Emerging roles of resolvins in the resolution of inflammation and pain. Trends in Neurosciences, 34(11), 599–609.
• Kidd, P. M. (2003). The use of mushroom glucans and proteoglycans in cancer treatment. Alternative Medicine Review, 5(1), 4–27.
• Kohli, D. R., Li, Y., Khasawneh, F. T., et al. (2010). Aspirin and salicylate bind to immunoglobulin heavy chain binding protein (BiP) and inhibit its ATPase activity in human fibroblasts. PLoS ONE, 5(5), e10868.
• Lal, S. M., Hewett, J. I. (2001). The binding of antimalarial drugs to hematin in solution. Journal of Biological Chemistry, 276(44), 40703–40709.
• Lemiale, F., Kong, X. J., & Sorrell, T. C. (2001). Immunological potential of recombinant or soluble cryptococcal mannoprotein. FEMS Microbiology Letters, 203(2), 151–156.
• Li, B., Allendorf, D. J., Hansen, R., et al. (2009). Yeast β-glucan amplifies phagocyte killing of iC3b-opsonized tumor cells via complement receptor 3–sykphosphatidylinositol 3-kinase pathway. The Journal of Immunology, 183(7), 4871–4881.
• Mycology Research Laboratories Ltd. (2007). Beta 1,3 glucan: A protective immune modulator. Alternative Medicine Review, 12(4), 370–378.
• Okeke, M. I., Iroegbu, C. U., Eze, E. N., et al. (2001). Efficacy of nisin in treatment of clinical infections. In: T. J. Montville, K. R. Matthews, A. M. Bonnano (Eds.), Food Microbiology: Fundamentals and Frontiers (2nd ed., pp. 827–837). ASM Press.
• O'Mahony, L., Akdis, M., Akdis, C. A. (2011). Regulation of the immune response and inflammation by histamine and histamine receptors. Journal of Allergy and Clinical Immunology, 128(6), 1153–1162.
• Percival, S. S. (2013). Copper and immunity. American Journal of Clinical Nutrition, 67(5), 1064S–1068S.
• Schaible, U. E., Kaufmann, S. H. E. (2007). Malnutrition and infection: Complex mechanisms and global impacts. PLoS Medicine, 4(5), e115.
• Schepetkin, I. A., Quinn, M. T. (2006). Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. International Immunopharmacology, 6(3), 317–333.
• Zou, J. M., Qin, J., Li, Y. C., et al. (2017). IL-35 induces N2 phenotype of neutrophils to promote tumor growth. Oncotarget, 8(19), 33501–33514.
• Chesney, A. (2017). Integrative Environmental Medicine. Publisher information not available.
• Mead, P. S., Hinckley, A. F., Hook, S. A., Beard, C. B., & Eisen, R. J. (2015). Lyme disease surveillance in the United States: looking for ways to cut the Gordian knot. Zoonoses and Public Health, 62(6), 491-498.
• Ogden, N. H., Lindsay, L. R., Beauchamp, G., Charron, D., Maarouf, A., O'Callaghan, C. J., & Waltner-Toews, D. (2014). Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. Journal of Medical Entomology, 41(4), 622-633.
• Shih, C. M., & Spielman, A. (1993). Accelerated transmission of Lyme disease spirochetes by partially fed vector ticks. Journal of Clinical Microbiology, 31(11), 2878-2881.
• Steere, A. C., et al. (1994). Prevalence and Significance of Asymptomatic Lyme Borreliosis: Review. Infection, 22(Supplement 2), S149-S150.
• Steere, A. C. (2001). Lyme Disease. New England Journal of Medicine, 345(2), 115-125.
• Steere, A. C., Malawista, S. E., Snydman, D. R., Shope, R. E., Andiman, W. A., Ross, M. R., ... & Collins, J. J. (1983). Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis & Rheumatism, 26(12), 1420-1434.
• Wormser, G. P., et al. (2006). Practice Guidelines for the Diagnosis and Treatment of Lyme Disease. Clinical Infectious Diseases, 43(9), 1089-1134.
• Wormser, G. P., Nadelman, R. B., Dattwyler, R. J., Dennis, D. T., Shapiro, E. D., Steere, A. C., ... & Fish, D. (2006). Practice Guidelines for the Treatment of Lyme Disease. Clinical Infectious Diseases, 43(9), 1089-1134.
• Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016 Jun;21(6):738-48. doi: 10.1038/mp.2016.50. Epub 2016 Apr 19. PMID: 27090305; PMCID: PMC4879184.
´ Frieri M. Mast Cell Activation Syndrome. Clin Rev Allergy Immunol. 2018 Jun;54(3):353-365. doi: 10.1007/s12016-015-8487-6. PMID: 25944644.
´ Afrin LB. Mast cell activation disease and the modern epidemic of chronic inflammatory disease. Transl Res. 2016 Aug;174:33-59. doi: 10.1016/j.trsl.2016.01.003. Epub 2016 Jan 18. PMID: 26850903.
´ Talkington, J., & Nickell, S. P. (1999). Borrelia burgdorferi Spirochetes Induce Mast Cell Activation and Cytokine Release. Infection and Immunity, 67(3), 1107-1115. https://doi.org/10.1128/iai.67.3.1107-1115.1999
´ Breitschwerdt EB, Greenberg R, Maggi RG, Mozayeni BR, Lewis A, Bradley JM. Bartonella henselae Bloodstream Infection in a Boy With Pediatric Acute-Onset Neuropsychiatric Syndrome. J Cent Nerv Syst Dis. 2019 Mar 18;11:1179573519832014. doi 10.1177/1179573519832014. PMID: 30911227; PMCID: PMC6423671.
´ Cross, A., Bouboulis, D., Shimasaki C., & Jones, C. R. (2021). Case Report: PANDAS and Persistent Lyme Disease With Neuropsychiatric Symptoms: Treatment, Resolution, and Recovery. Frontiers in Psychiatry, 12. https://doi.org/10.3389/fpsyt.2021.505941
´ Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry. 2012 Jun;83(6):638-45. doi 10.1136/jnnp-2011-301237. Epub 2012 Mar 24. PMID: 22448032; PMCID: PMC3348613.
´ Trevisan G., Ruscio, M., Nan, K., Cinco, M., Trevisini, S., Forgione, P., & Bonin, S. (2022). Case Report: Lyme Borreliosis and Pregnancy - Our Experience. Frontiers in Medicine 9 https://doi.org/10.3389/fmed.2022.816868
´ Sponaugle R. Lyme Study: How Borrelia Bacteria is Transmitted from Mosquitoes to Humans. Sponaugle Wellness Institute. 2016. https://sponauglewellness.com/lyme-study-how-borrelia-bacteria-is-transmitted-from-mosquitoes-to-humans/
´ Smith, Aaron J., et al. “Cancer and Infectious Causes.” Open Journal of Medical Microbiology vol. 04, no. 03, 2014, pp. 161–77. DOI.org (Crossref) https://doi.org/10.4236/ojmm.2014.43019.
´ Kalish, Robert A., et al. “Persistence of Immunoglobulin M or Immunoglobulin G Antibody Responses to Borrelia Burgdorferi 10–20 Years after Active Lyme Disease.” Clinical Infectious Diseases vol. 33, no. 6, Sept. 2001, pp. 780–85. DOI.org (Crossref) https://doi.org/10.1086/322669.
• (Maxwell, Sarah P., et al. “Neurological Pain, Psychological Symptoms, and Diagnostic Struggles among Patients with Tick-Borne Diseases.” Healthcare vol. 10, no. 7, June 2022, p. 1178. DOI.org (Crossref), https://doi.org/10.3390/healthcare10071178.)
• (Horowitz, R.I.; Freeman, P.R. Precision Medicine: The Role of the MSIDS Model in Defining, Diagnosing, and Treating Chronic Lyme Disease/Post Treatment Lyme Disease Syndrome and Other Chronic Illness: Part 2. Healthcare 2018 6 129.)
• Breitschwerdt EB, Greenberg R, Maggi RG, Mozayeni BR, Lewis A, Bradley JM. Bartonella henselae Bloodstream Infection in a Boy With Pediatric Acute-Onset Neuropsychiatric Syndrome. J Cent Nerv Syst Dis. 2019 Mar 18;11:1179573519832014. doi 10.1177/1179573519832014. PMID: 30911227; PMCID: PMC6423671.
• Breitschwerdt E. B., Maggi, R. G., Nicholson, W. L., Cherry, N. A., & Woods, C. W. (2008). Bartonella sp. Bacteremia in Patients with Neurological and Neurocognitive Dysfunction. Journal of Clinical Microbiology, 46(9), 2856-2861. https://doi.org/10.1128/JCM.0083208
• Bransfield RC. Neuropsychiatric Lyme Borreliosis: An Overview with a Focus on a Specialty Psychiatrist's Clinical Practice. Healthcare (Basel). 2018 Aug 25;6(3):104. doi: 10.3390/healthcare6030104. PMID: 30149626; PMCID: PMC6165408.
• Markeljević J, Sarac H, Rados M. Tremor, seizures and psychosis as presenting symptoms in a patient with chronic lyme neuroborreliosis (LNB). Coll Antropol 2011 Jan;35 Suppl 1:313-8. PMID: 21648354.
• Offutt, A., & Breitschwerdt, E. B. (2023). Case report: Substantial improvement of autism spectrum disorder in a child with learning disabilities in conjunction with treatment for poly-microbial vector borne infections. Frontiers in Psychiatry 14 https://doi.org/10.3389/fpsyt.2023.1205545
• Eisen L. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis. 2018 Mar;9(3):535-542. doi 10.1016/j.ttbdis.2018.01.002. Epub 2018 Jan 31. PMID: 29398603; PMCID: PMC5857464.
• Cook MJ. Lyme borreliosis: a review of data on transmission time after tick attachment. Int J Gen Med. 2014 Dec 19;8:1-8. doi 10.2147/IJGM.S73791. PMID: 25565881; PMCID: PMC4278789.
• Morrissette M, Pitt N, González A, Strandwitz P, Caboni M, Rebman AW, Knight R, D'Onofrio A, Aucott JN, Soloski MJ, Lewis K. A Distinct Microbiome Signature in Posttreatment Lyme Disease Patients. mBio. 2020 Sep 29;11(5):e02310-20. doi 10.1128/mBio.02310-20. PMID: 32994327; PMCID: PMC7527730.
• Breitschwerdt EB, Maggi RG, Farmer P, Mascarelli PE. Molecular evidence of perinatal transmission of Bartonella vinsonii subsp. berkhoffii and Bartonella henselae to a child. J Clin Microbiol 2010 Jun;48(6):2289-93. doi: 10.1128/JCM.00326-10. Epub 2010 Apr 14. PMID: 20392912; PMCID: PMC2884525.
• Stricker RB, Middelveen MJ. Sexual transmission of Lyme disease: challenging the tickborne disease paradigm. Expert Rev Anti Infect Ther. 2015;13(11):1303-6. doi: 10.1586/14787210.2015.1081056. Epub 2015 Aug 26. PMID: 26489537.
• (Middelveen M. J., Burke, J., Sapi, E., Bandoski C., Filush K. R., Wang, Y., Franco, A., Timmaraju, A., Schlinger, H. A., Mayne, P. J., & Stricker, R. B. (2014). Culture and identification of Borrelia spirochetes in human vaginal and seminal secretions. F1000Research 3 https://doi.org/10.12688/f1000research.5778.3
• Psarros G, Riddell J 4th, Gandhi T, Kauffman CA, Cinti SK. Bartonella henselae infections in solid organ transplant recipients: report of 5 cases and review of the literature. Medicine (Baltimore). 2012 Mar;91(2):111-121. doi 10.1097/MD.0b013e31824dc07a. PMID: 22391473.
• (Lorquet, J. R., Pell, R., Adams, J., Tak, M., & Ganti L. (2023). A Non-Classical Presentation of Erythema Migrans in a 51-Year-Old Woman With Early Manifestation of Lyme Neuroborreliosis (Bannwarth Syndrome). Cureus 15(6). https://doi.org/10.7759/cureus.39931)
• Gaur, G., Sawant, J. Y., Chavan, A. S., Khatri, V. A., Liu, H., Zhang, M., & Sapi E. (2021). Effect of Invasion of Borrelia burgdorferi in Normal and Neoplastic Mammary Epithelial Cells. Antibiotics 10(11). https://doi.org/10.3390/antibiotics10111295