How does inflammation spread through the body?
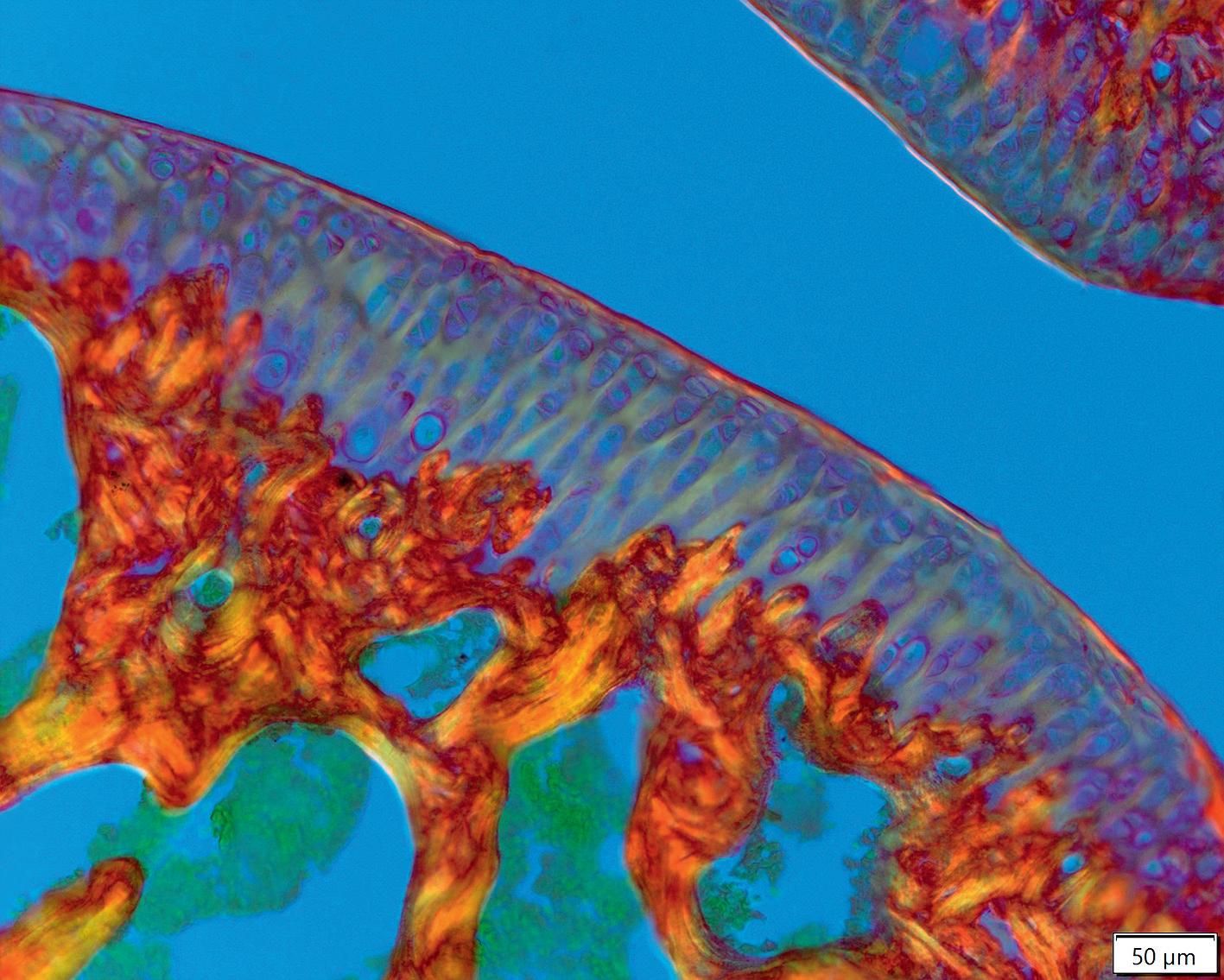
Chronic systemic inflammation (CSI) is associated with the progression of different diseases, including psoriasis, atopic dermatitis and cancer. Researchers in the ERC-funded CSI-FUN project are investigating the underlying molecular mechanisms behind CSI and how it spreads through the body, as explained by Erwin Wagner, the principal investigator of the study.
An inflammatory response may originate in a given organ like the skin, but it can then spread more widely and affect the entire body, causing serious health problems. Based at the Medical University of Vienna (MUV), Erwin Wagner and his group are investigating the underlying mechanisms behind CSI. “We are trying to find the mechanisms by which the original signal – which elicits the inflammatory response – spreads through the body in a systemic way,” he outlines. This research relies to some extent on mouse models for two common inflammatory skin diseases –psoriasis and atopic dermatitis – in which CSI plays a major role in progression. “We aim to describe how inflammation from the skin spreads through the body and affects different sites and organs,” says Erwin Wagner. “Around a third of psoriatic patients develop psoriatic arthritis, a form of joint disease, which often emerges many years after patients are diagnosed with psoriasis.”
Deciphering organ cross-talk
The aim of the study is to build a broader picture of the communication between the skin and the joints, and to identify which molecules, signalling pathways and cells are involved. Researchers in this project are also looking at CSI in the context of cancer associated cachexia (CAC), a metabolic syndrome which can develop in the late stages of cancer and is associated with weight loss. “Many tumours are highly inflamed, and this inflammation can then spread throughout the body, leading to weight loss,” explains Wagner. Rather than looking at the actual tumour itself, the focus of the Wagner group

is the communication between the tumour and the whole organism. “We’re looking at the cross-talk, the communication that takes place between a tumour and the organism. We’re investigating what happens when the tumour sends out signals to start inflammation,” he says. “In each of these cases we’re looking systemically. For instance, in cachexia we look across the different organs in the body, keeping an open mind about what we might find.”
Researchers in the Wagner group ultimately aim to identify the factors behind the initiation of CSI, which then kicks off a cascade leading
to the progression of disease. One important event in the development of CSI is the release of inflammatory mediators, including inflammatory cytokines like IL-6 and IL-17, which activate pro-inflammatory signalling pathways. “These cytokines are important therapeutic targets for both skin diseases and CAC,” outlines Wagner. In his research, Wagner is using mouse models to investigate the molecular mechanisms behind CSI, while he also has access to samples from human patients. “My lab is well-known for generating innovative mouse models of human diseases,

but it was clear from the outset of this project that we would need to have both mouse mechanistic data and human patient samples, in parallel,” he stresses. “One of the attractions of moving to the MUV was to be close to clinicians, so I can now work with them on samples they collect from patients with psoriasis, atopic dermatitis and CAC.”
This research has yielded some important insights. On the basis of their work with mouse models, Wagner and his colleagues have published a paper on CAC demonstrating that after a tumour becomes inflammatory, the patient first loses fat. “The fat burns away, then the muscle becomes affected, and then the other organs,” he explains. A patient who
the first event that happens when disease is induced,” outlines Wagner. In his research, Wagner has explored the impact of removing these proteins on psoriasis and psoriatic arthritis. “Is the disease prevented? Or does the disease have a less severe impact, is it milder?” he asks. “We generated a mouse in which these proteins are absent in all cells, which we call a complete knock-out mouse. In this case the psoriatic disease is very mild. This is positive, but is this because of a systemic effect? Or is it because the S100 proteins have been removed from the skin?”
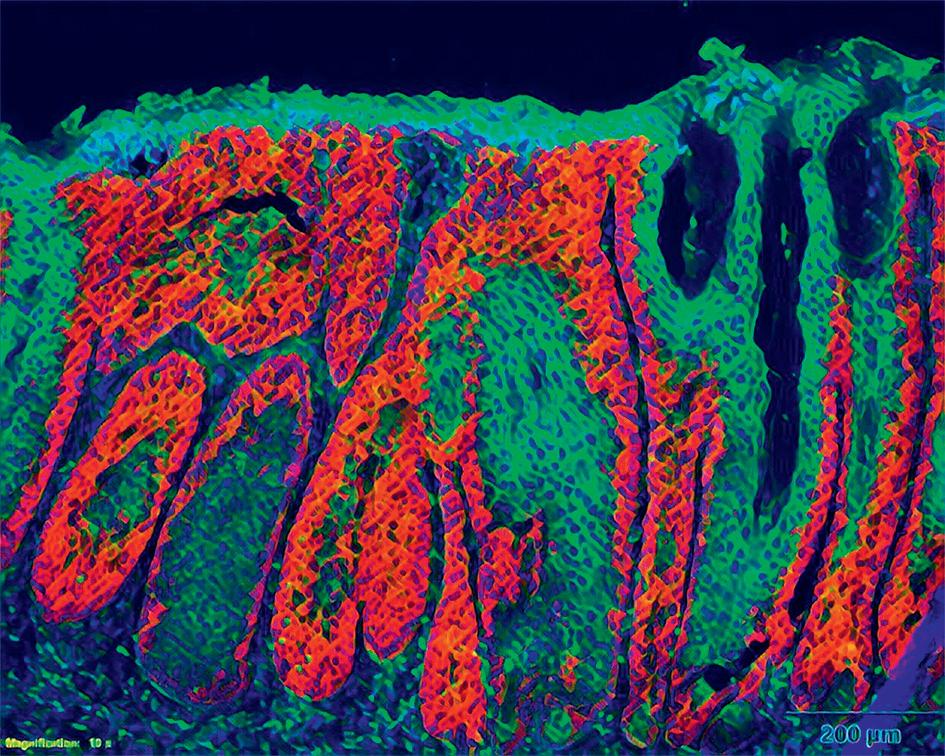
The next step is to remove the S100 proteins only in the skin, and then assess whether the same effect is observed. If it’s an organspecific effect then removing these proteins only in the skin should also lead to a milder disease, but Wagner says this is not what he and his colleagues observe. “We get more disease, so it’s actually worse. Therefore, it’s not the organ-specific effect but rather the systemic effect which needs to be treated,” he says. Researchers have demonstrated that the important factor is the CSI, rather than celltype specific inflammation in the skin, while Wagner is also pursuing further research into the S100 proteins. “We are also trying to understand whether these proteins play a role
in atopic dermatitis. These alarmins are also anti-microbial proteins, which can reduce the microbial load, that might be a factor behind the phenotypes that we see,” he continues. The role of inflammation in osteoarthritis is another interesting avenue of research. “It has long been thought that inflammation has nothing to do with osteoarthritis, but more and more evidence is now emerging that in fact it may play a role. We have good models for osteoarthritis and for psoriatic arthritis and we are studying and comparing the two diseases,” says Wagner. Professor Wagner is also very interested in investigating the importance of inflammation in the context of skin cancer. A number of reports have suggested that fewer cases of specific skin
cancers are found in patients with psoriasis than in the general population. This would suggest that CSI does not always promote cancer, but in some cases can in fact suppress certain types, a topic that the Wagner group is currently exploring. “We have established several mouse models for psoriasis in which we induced a mutation, which normally leads to skin cancer development. On top of this mutation, we also induce CSI and then we ask; does this suppress or promote skin cancer development?” These experiments are ongoing and we are excited to find out whether CSI is tumour-promoting or suppressive in this context. For this challenging project, we received additional EU support from H2020 (MSCA-ITN-CancerPrev-859860).”
CSI-FUN
Chronic Systemic Inflammation: Functional organ cross-talk in inflammatory disease and cancer
Project Objectives
The goal of CSI-Fun is to understand how Chronic Systemic Inflammation (CSI) is involved in Inflammatory Skin Diseases, Arthritis and Cancer. We focus on whole body physiology and perform experiments in model systems, from the petri dish to mice and samples donated by patients, to discover new biomarkers and better preventive and therapeutic strategies.

Project Funding
This project has received additional support from the Medical University of Vienna and the European Union’s Horizon 2020 research and innovation programme (MSCAITN-2019 -859860: CANCERPREV)

Project Partners
This ERC advanced grant has been fully allocated to the host institution (Medical University of Vienna).


Contact Details
Principal Investigator, Erwin Wagner, PhD Univ.-Prof. Dipl.-Ing. Dr. Medical University of Vienna

Department of Dermatology
Department of Laboratory Medicine
Anna Spiegel Research Building Lazarettgasse 14, AKH BT25.2, Level 6 1090 Vienna, Austria T: +43 1 40400 77020 E: erwin.wagner@meduniwien.ac.at W: https://www.meduniwien.ac.at/hp/ dermatologie/wissenschaft-forschung/ genes-and-disease-group-erwin-wagner/
Mouse models
The work with mouse models within the project involves modulating certain genetic events, which affect the severity of a given pathology. For example, in the context of psoriasis, specific proteins are removed from the skin of these mice and within two weeks this genetic alteration will cause a lesion resembling the human disease. “We can then essentially follow the fate of this lesion over time,” outlines Wagner. The development of the lesion can be followed over extended periods, an important consideration in the project given that the systemic manifestations of CSI will not typically be apparent in two weeks. “We can modulate the severity of the lesion, to look at both severe and mild examples. The mice are raised in pretty much standard conditions, so factors like diet and the light/dark cycle aren’t particularly influential. We essentially rely on the genetic manipulation leading either to the induction or removal of a gene - or genes - to cause certain pathologies,” continues Wagner.
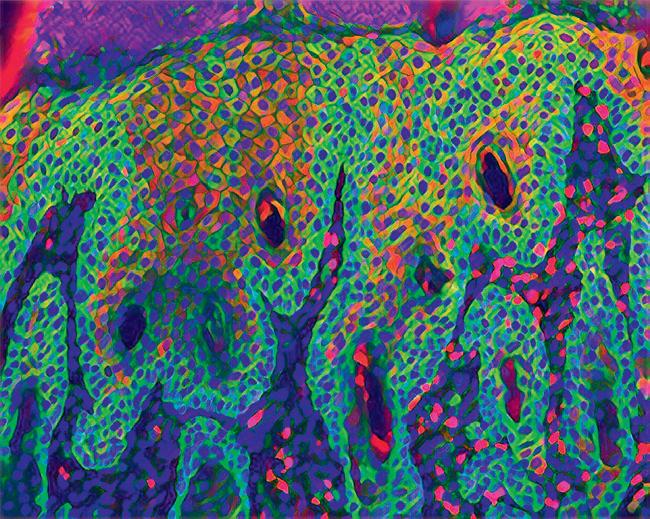
loses a certain amount of weight within six months is categorised as pre-cachexia, which is an important window for treatment, before their condition worsens. “A lot of research focuses on this pre-cachexic stage, where we can study what signals might be important in terms of kicking off the cascade,” says Wagner.
“In the pre-cachexic stage the patient has not yet suffered from any major weight loss, but if it progresses further then they enter cachexia, a deadly condition. New treatments should ideally be targeted at the early changes before the patient comes down with a metabolic impairment.”
Another important paper arising from the group’s research is related to the function of the S100 proteins, a family of molecules that are highly up-regulated when something goes wrong in the body, acting effectively as a danger signal. “We’ve seen that these S100 proteins, which are also called alarmins, are highly up-regulated in our mouse models and also in samples from human patients. In the mice, the up-regulation of these proteins is
Erwin Wagner has pioneered technologies for engineering the mouse genome producing highly instructive mouse models that have revealed mechanisms of development, inflammation, metabolism and cancer. He made ground-breaking discoveries about the roles of AP-1 (Fos/Jun) transcription factors and recently provided key insights to the pathogenesis of cancer cachexia, offering new therapeutic interventions.
Latifa Bakiri joined Erwin Wagner´s team in 2001 as a post-doc and then staff scientist, she focusses on optimising genetically engineered mouse models to study inflammation, metabolism and cancer.

We are trying to find the mechanisms by which the original signal – which elicits the inflammatory response – spreads through the body in a systemic way.The CSI-Fun research team in 2023 with early stage researchers, post-docs and technical assistants from 7 nationalities. Photo credit: Andreas Ebner (MUV). Artistic rendering of triple immune-fluorescent stainings of mouse skin sections with Atopic Dermatitis-like (left) and Psoriasis-like (right) disease. Credit: Andreas Ebner (MUV) ©MedUniWien/feelimage
