
4 minute read
Advanced Retinal Implants
Early results promising for next-generation treatments. Cheryl Guttman Krader reports
Retinal prosthesis pioneer Mark Humayun MD, PhD, described two exciting new approaches now in clinical trials during his Charles L Schepens MD Lecture at a recent meeting of the American Academy of Ophthalmology.
Dr Humayun discussed recent results from two projects he leads. One is the development of a 256-channel epiretinal visual prosthesis for patients with end-stage retinitis pigmentosa (RP) known as the Intelligent Micro Implant Eye (IMIE 256, IntelliMicro Medical Co). The other is a regenerative medicine approach using a bioengineered retinal pigment epithelial (RPE) monolayer (California Project to Cure Blindness-Retinal Pigment Epithelium [CPCB-RPE1], Regenerative Patch Technology, LLC) for patients with severe vision loss secondary to advanced dry age-related macular degeneration (AMD).
EPIRETINAL IMPLANT The IMIE 256 was developed to overcome the limitations of existing retinal prosthetics and deliver better efficacy and safety.
“With the original Argus II (Second Sight Medical Product), which is a 60-electrode system, patients with end-stage RP showed improvements from light perception or hand motion vision to 20/1200 visual acuity (VA). Through software enhancements, achieved VA improved to 20/480, but that probably represents the maximum potential given the implant’s low electrode density and high stimulation threshold,” Dr Humayun said. Not only does the IMIE 256 provide the opportunity for better visual function with its greater number of electrodes, but thanks to engineering innovations, it is much smaller in size than the Argus II. Surgical implantation of the IMIE 256 is both easier and safer because the device fits in only one quadrant instead of two.
Initial results from the first five patients implanted with the IMIE 256 were published in August 2021 and showed there were fewer serious adverse events related to the surgery or the device relative to experience with the Argus II.i Outcomes from several functional tests provided evidence the IMIE 256 provides better visual performance.
“The results are limited and early, and there are many challenges ahead. But we are encouraged and having fun. Please stay tuned,” Dr Humayun said.
REGENERATIVE MEDICINE APPROACH Findings from a one-year follow-up in a phase 1/2a clinical trial investigating the CPCB-RPE1 were published simultaneously with the report on the IMIE 256.ii The article describes outcomes for VA and functional tests along with safety data for 16 implanted subjects who were legally blind from advanced dry AMD. Dr Humayun reported that at one year, VA improved in 27% of implanted eyes, whereas control eyes recorded VA losses of 8 to 21 letters.
“There are too few subjects to reach any conclusions about efficacy, but these preliminary findings are exciting considering VA improvement in eyes with GA is unheard of,” he said.
The CPCB-RPE1 cultures a monolayer of hESC-RPE cells on a synthetic parylene membrane—a highly biocompatible, nonerodible substrate. Development of the parylene membrane was done in collaboration with colleagues at the California Institute of Technology and involved micromachining the material to enable surgical implantation and cell retention while allowing for diffusion of macromolecules.
The membrane is foldable to retract into a specialised introducer used to deploy the implant at the target site. Laboratory testing confirmed folding and unfolding the membrane did not result in cell dislodgement.
“Making the membrane foldable allows us to keep the retinotomy size to around 1.5 mm, which is important for minimising the risk of scarring (PVR) and retinal detachment,” Dr Humayun noted.
He added that histological examination was performed on the donated globe from one study subject who passed away from unrelated causes. The evaluations showed that donor cells were present and properly oriented.
Dr Humayun described the implantation surgery as “pretty straightforward”. It involves a small gauge pars plana vitrectomy, posterior hyaloid removal, and retinotomy, followed by implant deployment into the subretinal space. Once the implant unfolds, it is positioned away from the retinotomy. Perfluorocarbon heavy liquid is instilled to flatten the macula, and silicone oil is instilled for long-term tamponade.
Dr Humayun noted the parylene membrane’s design includes a refractive index of 1.5 mm to distinguish it from native tissue on optical coherence tomography.
“Post-implantation imaging shows good coverage of the transplant over the area of GA. At two months post-implant, new formation of external limiting membrane is visible, and it has been seen to persist at follow-up at six months,” he said.
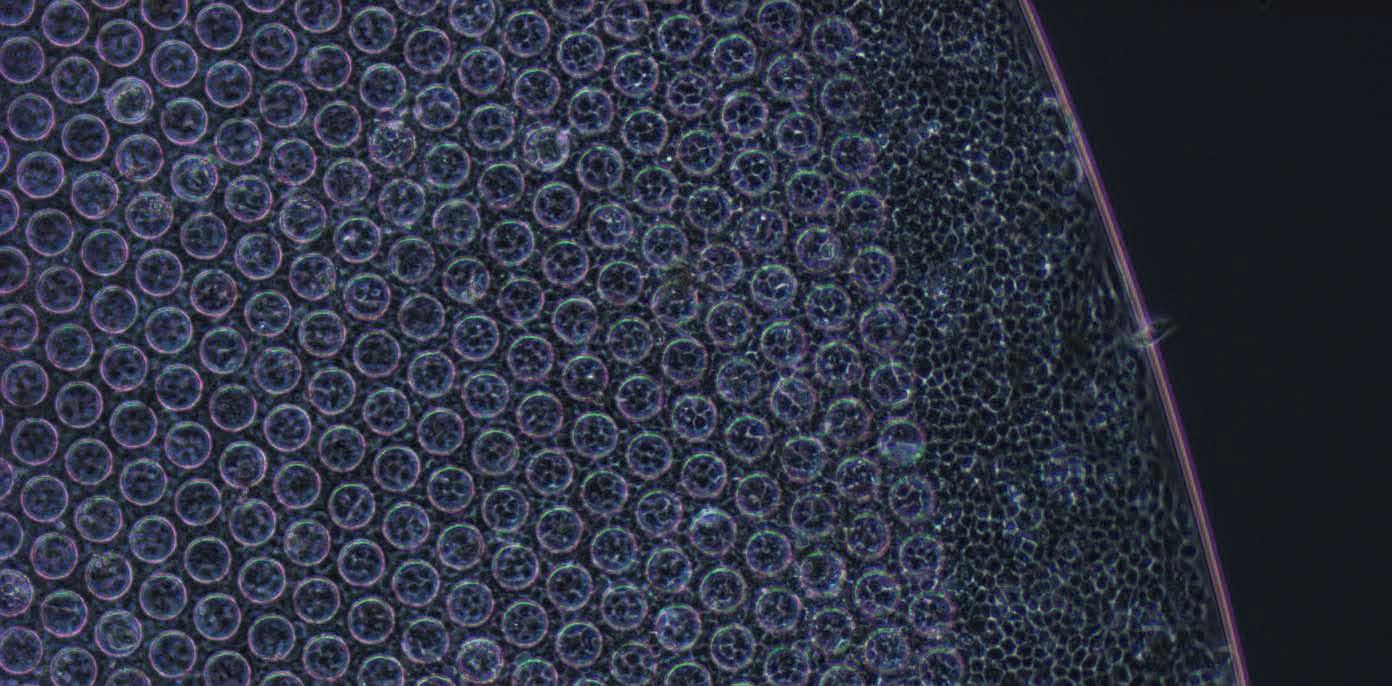
CPBP-RPE1 implant. High magnification view of a portion of the CPBP-RPE1 implant.
This presentation appeared at the Retina Subspecialty Day of the annual meeting of the American Academy of Ophthalmology in New Orleans, Louisiana, USA.
i Xu H, et al. Translational Vision Science & Technology. 2021; 10(10): 14. ii Kashani AH, et al. Translational Vision Science & Technology. 2021; 10(10): 13.
Mark Humayun MD, PhD, is a Professor of Ophthalmology and Biomedical Engineering, University of Southern California, Los Angeles, USA. humayun@med.usc.edu





