SPECIAL REPORT
Improving the Accuracy and Precision of Stereotactic Radiation Therapy Stereotactic Radiosurgery (SRS) for the Treatment of Intra- and Extra-cranial Lesions Motion Management Technologies Advanced Integrated SRS Systems Improve Clinical Outcomes and Workflows Stereotactic Radiosurgery a Precise, Potent Technique to Treat Brain Tumours Future Outlook
Sponsored by
Published by Global Business Media
Stereotactic precision for any anatomy
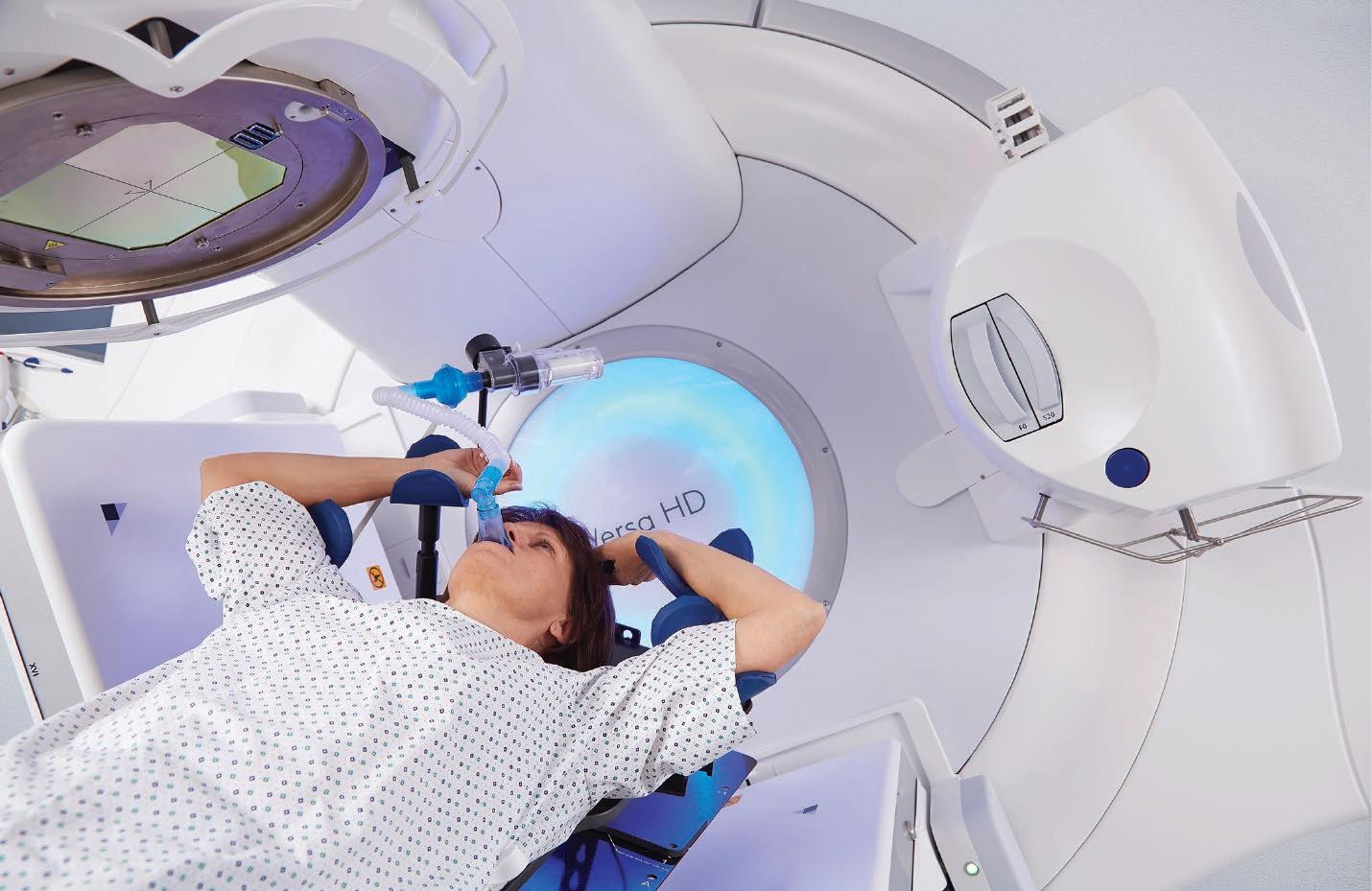
Push the boundaries of your stereotactic practice Stereotactic treatments demand the highest levels of accuracy, precision and efficiency—and we’ve designed a linac that delivers. Versa HD™ enables high definition dynamic radiosurgery (HDRS)— offering absolute SRS and SBRT reliability with anatomically guided accuracy and efficiency. Empower practice growth. Choose Versa HD.
elekta.com/chooseVersaHD
LADVHD200211
SPECIAL REPORT
Improving the Accuracy and Precision of Stereotactic Radiation Therapy Stereotactic Radiosurgery (SRS) for the Treatment of Intra- and Extra-cranial Lesions
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Contents
Motion Management Technologies Advanced Integrated SRS Systems Improve Clinical Outcomes and Workflows Stereotactic Radiosurgery a Precise, Potent Technique to Treat Brain Tumours Future Outlook
Foreword
2
Dr. Sophie Laurenson (BSc Hons., Ph.D. Cantab.), Editor
Stereotactic Radiosurgery (SRS) for the Treatment of Intra- and Extra-cranial Lesions
3
Dr. Sophie Laurenson (BSc Hons., Ph.D. Cantab.)
Sponsored by
Published by Global Business Media
Published by Global Business Media Global Business Media Limited 62 The Street Ashtead Surrey KT21 1AT United Kingdom Switchboard: +44 (0)1737 850 939 Fax: +44 (0)1737 851 952 Email: info@globalbusinessmedia.org Website: www.globalbusinessmedia.org Publisher Kevin Bell Business Development Director Marie-Anne Brooks Editor Dr. Sophie Laurenson (BSc Hons., Ph.D. Cantab.) Senior Project Manager Steve Banks Advertising Executives Michael McCarthy Abigail Coombes Production Manager Paul Davies For further information visit: www.globalbusinessmedia.org The opinions and views expressed in the editorial content in this publication are those of the authors alone and do not necessarily represent the views of any organisation with which they may be associated. Material in advertisements and promotional features may be considered to represent the views of the advertisers and promoters. The views and opinions expressed in this publication do not necessarily express the views of the Publishers or the Editor. While every care has been taken in the preparation of this publication, neither the Publishers nor the Editor are responsible for such opinions and views or for any inaccuracies in the articles.
Š 2020. The entire contents of this publication are protected by copyright. Full details are available from the Publishers. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical photocopying, recording or otherwise, without the prior permission of the copyright owner.
Introduction to Stereotactic Radiosurgery (SRS) The SRS Procedure SRS and SBRT Applications SRS and SBRT Guidelines Conclusion
Motion Management Technologies
6
Dr. Sophie Laurenson (BSc Hons., Ph.D. Cantab.)
Introduction The Principles of Movement Management Movement Management in SBRT Imaging Modalities in SRS and SBRT Procedures Frameless SRS Conclusion
Advanced Integrated SRS Systems 8 Improve Clinical Outcomes and Workflows Dr. Sophie Laurenson (BSc Hons., Ph.D. Cantab.)
Introduction SRS Systems: Integrated Platforms to Improve Patient Outcomes Imaging Technology in SRS Patient Outcomes and Safety Workflows and Cost-effectiveness Conclusion
Stereotactic Radiosurgery a Precise, Potent Technique to Treat Brain Tumours
11
Elekta Ltd
Foreword Precision Tools for SRS Planning for Precision Delivering Hope for a Positive Outcome Challenges Overcome Assuring Quality SRS Looking Ahead
Future Outlook
16
Dr. Sophie Laurenson (BSc Hons., Ph.D. Cantab.)
Introduction Advanced Conformal Arc Therapies Advances in Automation and Workflow Design Combination Therapies Conclusion
References 18 WWW.HOSPITALREPORTS.EU | 1
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Foreword S
TEREOTACTIC RADIOSURGERY (SRS)
radiation, including gamma rays, X-rays and protons.
and stereotactic body radiotherapy (SBRT)
The technology has evolved to enable the treatment
are non-surgical methods of delivering radiation
of a wide range of target sizes and morphologies.
therapy. The techniques were pioneered in the
These advances have been underpinned by technical
1950s to deliver high doses of radiation with
developments in manipulating multiple radiation
sub-millimeter accuracy (1). The fundamental
sources, positioned to cover a range of angles and
mechanism is based on generating free radicals
modulated to tailor radiation exposure levels.
from ionizing radiation sources. Free radicals
This report describes the development and
damage the DNA of tumor cells, inhibiting their
application of modern SRS and SBRT systems
ability to replicate and initiating cell death. The goal
including advances in radiation delivery systems to
is to prevent tumor growth and metastases. In SRS
enable conformal manipulation of beams, patient
procedures, tumor tissue is not surgically resected
motion management solutions and the integration
but diminishes over a period of months following
of these components with imaging modalities and
treatment. Treatment regimens are short relative
sophisticated computer algorithms for treatment
to conventional radiation therapies, consisting of
optimization. The fourth article in this report is provided
a single or few treatment sessions.
by Elekta, a leading manufacturer of SRS and SBRT
The development of SRS is reliant on advances in
systems, highlighting the clinical impact of advanced
radiation technologies that deliver the maximum dose
integrated platforms. The report concludes with an
to the target while minimizing the dose delivered to
overview of the current state-of the-art and outlook
adjacent healthy tissue. The goal is to deliver doses
for SRS technologies.
that will destroy all of the target tumor, permanently controlling tissue growth. SRS systems may be developed based on different forms of ionizing
Dr Sophie Laurenson Editor
Dr. Sophie Laurenson is a scientist and social entrepreneur. She obtained a Ph.D. in Oncology (Biophysics / Biochemistry) from the University of Cambridge in 2007 and has worked in industry and academia for 17 years. Currently, she is the Founder and Managing Director of Limeburners Bay International AG, developing medical technology for resourcelimited settings.
2 | WWW.HOSPITALREPORTS.EU
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Stereotactic Radiosurgery (SRS) for the Treatment of Intra- and Extra-cranial Lesions Dr. Sophie Laurenson (BSc Hons., Ph.D. Cantab.) A wide range of SRS and SBRT systems are available for the treatment of both intra- and extra-cranial lesions.
Introduction to Stereotactic Radiosurgery (SRS) Stereotactic radiosurgery (SRS), also known as stereotactic radiotherapy (SRT), is a nonsurgical method of delivering radiation therapy. The technique was pioneered in the 1950s for the treatment of small tumors and functional abnormalities arising in the brain1. SRS is a high precision radiation treatment that delivers high doses of radiation with sub-millimeter accuracy. Treatment regimens are short relative to conventional radiation therapies, consisting of a single or few treatment sessions. Although SRS was originally developed for the treatment of intracranial lesions, these techniques are now also applied to the treatment of small-to-medium sized extracranial tumors. The extension of SRS to abnormalities occurring throughout the body is termed stereotactic body radiotherapy (SBRT)2. SBRT has been successfully applied to treat tumors of the liver, prostate, lung, abdomen, spine, head and neck. The fundamental mechanism underpinning SRS is consistent with other forms of radiation therapy. The goal is to prevent tumor growth and metastases. Ionizing radiation creates ions by removing an electron from an atom. This generates free radicals which interact with biological molecules to initiate a pathological response. Specifically, radiation damages the DNA of tumor cells, inhibiting their ability to replicate and initiating cell death. In SRS procedures, tumor tissue is not surgically resected but diminishes over a period of months following treatment3. Benign tumors and vascular abnormalities decrease in size throughout a period of several years post-treatment. Malignant and metastatic tumors have been observed to shrink more rapidly. However, many tumors merely become inactive and remain
stable without significant genetic alterations or tissue growth. The development of SRS is reliant on advances in radiation technologies that deliver the maximum dose to the target while minimizing the dose delivered to adjacent healthy tissue4. The goal is to deliver doses that will destroy all of the target tumor, permanently controlling tissue growth. SRS systems may be developed based on different forms of ionizing radiation, including gamma rays, X-rays and protons. SRS can be delivered via gamma ray treatment, medical linear accelerators (linacs), or particle beam accelerators. The technology has evolved to enable the treatment of a wide range of target sizes and morphologies3. These advances have been underpinned by technical developments in manipulating multiple radiation sources, positioned to cover a range of angles and modulated to tailor radiation exposure levels.
Stereotactic radiosurgery (SRS), also known as stereotactic radiotherapy (SRT), is a non-surgical method of delivering radiation therapy.
The SRS Procedure The procedure followed in an SRS treatment regimen is comprised of a series of key stages. The patient is initially immobilized by the application of a stereotactic guiding device. This may be established using either a frame or frameless implementation 5. Procedure preparation and treatment planning is determined based on medical imaging, which is influenced by pathology. It is common for a wide range of two-dimensional (2D) and three-dimensional (3D) imaging modalities to be utilized in SRS procedures. These may include 2D X-ray or fluoroscopic imaging modalities as well as 3D imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), angiograms, and positron emission tomography (PET6). The images are acquired, merged and analyzed to define the stereotactic WWW.HOSPITALREPORTS.EU | 3
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Specifically, radiation damages the DNA of tumor cells, inhibiting their ability to replicate and initiating cell death.
4 | WWW.HOSPITALREPORTS.EU
space, target volume and morphology. This information determines the optimal dose distribution that will affect the target tissue whilst minimizing exposure to normal tissues surrounding the lesion. This process requires the expertise of a multidisciplinary team involving medical physicists, radiation oncologists and technicians, and neurosurgeons. A radiosurgery treatment plan is developed by identifying the dose distribution and effect, as well as any recognized complications. A treatment plan should determine the feasibility of the radiosurgery system to deliver treatment dependably, within an acceptable treatment period7. As a result of this complex treatment process, SRS and SBRT rely on a combination of several technologies to deliver successful treatments including: • systems to immobilize and position the patient during therapy, •h ighly focused radiation beams that converge on a target, • imaging systems to identify the location, size and morphology of lesions. The technical features and integration of these technologies is critical in determining optimal patient outcomes, safety and clinical workflows to ensure successful outcomes. SRS is commonly delivered in outpatient settings, with a single treatment session taking between 1 – 4h. SRS may be delivered in a single treatment, or over multiple sessions lasting between 2 – 5 separate treatments. Multiple session SRS is known as fractionated stereotactic radiotherapy. Fractionating treatment enables high doses to be delivered to the target tissue, while maintaining a tolerable safety profile4. Delivering focused radiation over multiple sessions permits
healthy tissue to heal between treatments. This is important when treating larger tumors, as the volume of normal tissue affected by the applied radiation increases proportionally to tumor size. Fractionating treatment limits the exposure of surrounding normal tissue to individual high radiation doses. SRS and SBRT are challenging to treat targets exceeding 3-4 cm in diameter or targets positioned near to sensitive organs. The effective radiation doses delivered in these situations may surpass tolerance thresholds, posing a disproportionate risk of damage to healthy tissue. In these situations, fractionated radiotherapy delivered by linear accelerator (linac) systems with a non-invasive head frame, make repeated treatments feasible. However, noninvasive frames do not provide rigid fixation. Small deviations in patient positioning can result in temporal geometric target positioning uncertainty, increasing the likelihood of inaccurate beam delivery. To adjust for this uncertainty, a small margin of healthy tissue surrounding the target volume is calculated and treated. As a result, a small volume of normal brain tissue becomes exposed to a high total radiation dose. This exposure can be tolerated by fractionating treatments, resulting in less toxicity than would result from a single fraction dose exposure. Recent advances in the mechanical design and digital automation of radiation delivery systems have enabled improved control of radiation beams. Techniques include intensity-modulated radiosurgery (IMRS), volume modulated arc therapy (VMAT) and dynamic conformal arc therapy (DAC). Each of these methods allows radiation beams to be shaped with high precision, conforming to target morphologies
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
to controlling dose distribution to produce a steep gradient between the target volume and surrounding tissues.
SRS and SBRT Applications SRS and SBRT are alternatives to conventional systemic therapies or invasive surgery. They are options for patients who are unable to undergo surgery or have abnormalities that are difficult to access, located close to vital organs regions or prone to movement within the body8. They are effective at treating both benign and malignant tumors and primary or metastatic tumors. Further, SRS and SBRT are amenable to treating single or multiple targets, which may be intractable using surgical procedures. The use of SRS to treat multiple lesions within the brain is indicated for certain patients9. This is an important aspect to consider as treatment with whole brain radiation therapy (WBRT) is associated with the development of neurocognitive defects.
SRS and SBRT Guidelines As SRS systems gain widespread acceptance and global adoption by the clinical community, many professional and governmental organizations have issued guidance documents on their use. Clinical guidance is important in
ensuring that treatments are informed by the most relevant clinical evidence. The International Stereotactic Radiosurgery Society (ISRS) have commissioned an ongoing project to define guidelines covering a range of SRS and SBRT interventions10. National clinical guidelines ensure that recommendations are modified to suit local variations in resourcing and clinical settings. For example, the DEGRO (Deutschen Gesellschaft fĂźr RadioOnkologie) working group develops practice guidelines on the evidence-based use and practice of SRS and SBRT in Germany11. In the UK SABR/SRS Consensus Guidelines for normal tissue constraints and the SABR/SRS UK Consortium Guidelines are relevant12. Report 101 of the American Association of Physicists in Medicine (AAPM) is referred to in the U.S.A13.
Conclusion A wide range of SRS and SBRT systems are now available for the treatment of both intraand extra-cranial lesions. Advances in radiation beam delivery, imaging technologies and digital automation have underpinned the development of SRS and SBRT platforms. Key technical features enable the precise delivery of high doses of radiation to target tissues, without damaging surrounding healthy tumours.
SRS and SBRT are alternatives to conventional systemic therapies or invasive surgery.
WWW.HOSPITALREPORTS.EU | 5
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Motion Management Technologies Dr. Sophie Laurenson (BSc Hons., Ph.D. Cantab.) Achieving accurate delivery of high radiation doses requires patient immobilization as well as mechanical accuracy. The positioning accuracy includes inter-fraction setup reproducibility (localization) and intra-fraction motion (fixation).
Introduction
The positioning accuracy involves both inter-fraction setup reproducibility (affecting localization) and intra-fraction motion (determining fixation).
Stereotactic radiosurgery (SRS) was pioneered in the 1950s by the neurosurgeon Dr. Lars Leksell1. SRS delivers focused radiation to treat small tumors and other abnormalities in the brain. The technique has subsequently been applied to treat small to medium sized extracranial tumors in a process termed stereotactic body radiotherapy (SBRT). The success of both SRS and SBRT relies on the ability to precisely focus radiation beams on target tissues. In turn, this is reliant on the ability to restrict the motion of patients allowing the exact location of the target tissue to be identified and stabilized. This has been enabled through the development of a series of technologies to control patient movement without compromising patient care experiences.
The Principles of Movement Management SRS systems use a rigid frame to establish a common coordinate system for radiation delivery. Originally, this was performed by fixing an inflexible frame onto the patient’s skull14. Frames are secured using pins, an invasive procedure which may result in potential complications. The frame fixes the patient’s internal anatomy, enabling independent “stereotactic” referencing of the target using a single unified Cartesian coordinates (X, Y, Z) system15. The frame also positions the target site at the isocenter, defined as the mechanical center at which the radiation beams converge. This is often referred to as the Leksell SRS coordinate system and is the basis for high-precision localization and navigation of anatomy16. Integrations of stereo imaging technologies have enabled brain lesions to be accurately mapped in different dimensions and targeted using the Leksell coordinate system. It is critical that the establishment of a coordinate system should consider all aspects of anatomy, including complex vascular structures and functional nerves. 6 | WWW.HOSPITALREPORTS.EU
However, two conditions must be met for this principle to hold true. First, there must be negligible motion between the frame and patient’s skull before and during treatment. This is particularly important when target tissue coordinates are obtained by medical imaging prior to treatment. The positioning accuracy involves both inter-fraction setup reproducibility (affecting localization) and intra-fraction motion (determining fixation). Second, the SRS radiation delivery instrumentation must adhere to a high degree of mechanical accuracy. A total dose positioning accuracy below 1 mm is required for SRS systems12. This ensures that the radiation delivery parameters that are defined during treatment planning, are successfully delivered without compromising patient safety. Historically, the use of fixed frames has made single-session intracranial radiosurgery possible and is often considered the standard of care. However, repeatedly reapplying invasive frames for fractionated SRS is not practical. It increases the chances of complications and decreases the overall patient experience. Alternative frame options have been developed such as a vacuum-assisted dental fixation device attached on to carbon fiber frame17 or frameless SRS procedures.
Movement Management in SBRT Ensuing from the success of frame-based intracranial SRS, the principle was extended to treat extracranial lesions in the 1990s2. In these cases, patient movement was restricted using a stereotactic body frame (SBF). This rigid structure was designed to fixate the patient’s torso, enabling a common coordinate system to be determined for the whole body. The SBF consists of two layers: a rigid outer shell and a malleable inner shell. The outer shell functioned to provide reference coordinates for radiation beam alignment. The inner shell was built from vacuum bags and pillows, allowing greater
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
comfort for the patient whilst ensuring that the body remained fully fixated in reference to the outer shell. The SBF can be easily and accurately positioned in reference to the beam axes of a modern linear accelerator system. This system has been successfully used to treat thousands of patients since early 1990s18. However, the SBF was not effective at controlling internal organ motions resulting from heart beats, breathing or bowel movements19. Abdominal compression devices and heavy strapping have been used to restrict diaphragmatic motions. These accessories are standard in many SBRS settings, although they may cause a degree of patient discomfort during treatments.
Imaging Modalities in SRS and SBRT Procedures Alternative methods of managing patient motions and identifying internal targets have been investigated and implemented. On-line imaging guidance systems represent a major development in localizing internal lesions. They may be installed as a stand-alone device within a treatment area or via an on-board-imager (OBI) device attached to many standard linear accelerators20. Online imaging system functions as a “virtual” body frame. Images acquired prior to treatment are aligned with images acquired during the treatment planning process to generate coordinates using registration algorithms21. This enables the patient setup process and hardware alignment to be integrated within a single procedure. The result is improved accuracy and clinical workflows as well as reduced setup uncertainties for SRS and SBRT treatments.
Frameless SRS More recently, “frameless” SRS has been advocated to improve patient comfort and convenience22. Frameless SRS immobilizes the patient’s head in either a relocatable head frame or a thermoplastic mask. In these settings, the frame is not invasively attached, reducing discomfort and potential complications. These techniques also enable SRS accessibility in healthcare settings where neurosurgical support is not available. Frameless SRS approaches must maintain the millimeter dose delivery precision achieved using conventional framed SRS procedures. This may be achieved using image-guidance technologies such as cone-beam computed tomography (CBCT) to define the brain stereotactic space. Current data suggests that frameless single fraction intracranial SRS may be possible using image guidance23. In-process imaging may reduce the margin required for reliable target coverage to as low as 1.2 mm22. Modern SRS and SBRT systems include adjustable couches, allowing up to six degrees of movement for more precise patient positioning24. These systems may be automated, to integrate patient positioning into the treatment planning stage of complex procedures.
Frameless SRS immobilizes the patient’s head in either a relocatable head frame or a thermoplastic mask. In these settings, the frame is not invasively attached, reducing discomfort and potential complications.
Conclusion Achieving accurate delivery of high radiation doses requires patient immobilization as well as mechanical accuracy. The positioning accuracy includes inter-fraction setup reproducibility (localization) and intra-fraction motion (fixation). Advances in robotics and digital automation are enabling patient position and movement to be controlled with a high degree of accuracy, improving dose delivery and patient experiences. WWW.HOSPITALREPORTS.EU | 7
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Advanced Integrated SRS Systems Improve Clinical Outcomes and Workflows Dr. Sophie Laurenson (BSc Hons., Ph.D. Cantab.) SRS and SBRT are complex, multidisciplinary procedures. Systems integrating and automating radiation delivery, patient positioning, imaging and treatment planning improve patient outcomes, clinical workflows and treatment cost-effectiveness.
Introduction
The complexity of treatment planning means that SRS accuracy must be evaluated end-to-end, taking every stage into account as potential for deviations.
As local therapies against cancerous lesions continue to evolve, management strategies have moved from a “one-treatment-fits-all” approach towards a more tailored and patientcentric design. Stereotactic radiosurgery (SRS) is intrinsically complex, involving multidisciplinary teams managing collections of sophisticated technologies. Regardless of the radiation delivery approach used, the fundamental concepts of radiosurgery remain consistent. They include the precise delivery of high radiation doses, minimal exposure to adjacent tissues, stereotactic localization, and automated dosimetry planning25. SRS is an integrated process, beginning with patient immobilization, target localization and delineation by medical imaging, treatment planning and finally, radiation treatment delivery. High definition dynamic radiosurgery (HDRS) is characterized by the integration of SRS features into a single clinical solution that enables optimization, planning, imaging and dose delivery.
SRS Systems: Integrated Platforms to Improve Patient Outcomes SRS delivery systems can be grouped based on the radiation delivery method employed 16 . Gamma Knife® platforms deliver radiation from a series of Cobalt-60 (60Co) sources, in contrast to systems utilizing a single source linear accelerator (linac). Precision and accuracy in SRS systems stems from the ability to manipulate radiation beams to directly affect only abnormal target tissue, without harming adjacent normal structures. This is achieved through treatment planning, enabling the optimization of dosing and dose distribution suitable for different SRS systems. 8 | WWW.HOSPITALREPORTS.EU
The Gamma Knife®, invented by the Swedish neurosurgeon Lars Leksell1, contains 192-201 cobalt-60 sources, each emitting a beam of gamma ray radiation. The beams converge at a point of intersection, known as the isocenter, to deliver a collimated beam directed towards abnormal target tissues. Mechanical accuracy of SRS radiation units is essential in positioning and aligning external radiation beams toward a target17. Despite weighing over one ton, the Gamma Knife system can achieve a beamfocusing accuracy of less than a millimeter (4). Linac systems generate high-energy x-ray beams that are manipulated with collimators to produce sequential cross-firing (or arcing) beams directed towards the target. Treatment planning involves deploying non-coplanar arcs of circularly collimated radiation beams rotating around a target isocenter26. High output linacs have been developed with improved mechanical accuracy and integrated software for SRS planning. A major advancement in SRS linac was the multileaf collimator (MLC) 27. MLCs are devices consisting of between 80-160 high atomic number “leaves”. Each leaf can independently interact with the radiation field, modulating the beam intensity and dose distribution shape with extreme precision. In this way, the radiation beam may be manipulated to conform to the exact morphology of the abnormal tissue. This ensures that residual disease in not left in situ, whilst limiting damage to adjacent healthy tissues. These advances paved the way towards intensity-modulated radiosurgery (IMRS), now often referred to as volume modulated arc therapy (VMAT). A major concern is the integrity of the imaging spatial accuracy and quality in correlation to the radiation beam isocenter26. Quality assurance checks must be implemented to ensure that the imaging system isocenter correlates with
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
that of the beam delivery system. Irregular gantry rotations or hardware aberrations can result in distortions between systems, which must be calibrated and corrected17-24. Digitally controlled linear accelerators (DC-linac) mitigate against hardware misalignment and miscalibrations. These platforms contain multi-level feedback and sensor systems to control gantry speed, collimator angles, pulse rate and beam energy switches. This level of hardware control enables modulated therapy with a limited number of arcs and has become an essential tool25-29.
Imaging Technology in SRS The complexity of treatment planning means that SRS accuracy must be evaluated endto-end, taking every stage into account as potential for deviations. The accuracy of SRS systems relies heavily on the localization and delineation of target tissues. In this respect, both patient immobilization and imaging of the stereotactic space are critical variables to assess and control. Recent technologic developments have included integrated image guidance systems and robotic couch positioning systems. These advances have enabled enhanced accuracy of SRS systems to within the submillimeter range28. Two-dimensional (2D), three-dimensional (3D) and four-dimensional (4D) imaging and localization techniques are used to determine the position of the target tissue within the body. Integrated imaging systems are designed to correct for setup errors and target motion within patients undergoing SRS treatments. Image-guided radiation therapy (IGRT) uses medical imaging to confirm the target location immediately before and / or during radiation treatment, to improve precision and accuracy. Imaging modalities include 2D radiographic
and fluoroscopic image acquisition, Computed Tomography (CT), Magnetic Resonance Imaging (MRI), and Positron Emission Tomography (PET)/ CT and cone-beam computed tomography (CBCT). Images from multiple modalities may be merged and analyzed to determine the exact spatial coordinates as well as the morphology and size of targets. Fluoroscopic images are used to analyze movements resulting from respiratory motion and radiographic images are used to determine interfractional motion and minimize setup errors. The CBCT images provide soft tissue and skeletal data and are also used to manage interfractional motion and setup errors. When used in combination, image data guide the treatment planning process, to determine radiation delivery, convergence angles and planes and patient positioning during treatments. Sophisticated algorithms and integrated computing capabilities can leverage these large data volumes to provide optimized treatment planning for each patient.
Sophisticated algorithms and integrated computing capabilities can leverage these large data volumes to provide optimized treatment planning for each patient.
Patient Outcomes and Safety The clinical outcomes of SRS treatment take a period of at least several months to become apparent. As a result of this delayed end-point, any errors in targeting or dosing will potentially affect a large number of patients that may be treated during the intervening time29. Further, the high doses and the relatively short course of treatment make SRS and SBRT less tolerant of error than other radiation treatments. SRS systems deliver steep dose gradients with small margins within or adjacent to vulnerable organs. There are fewer opportunities to identify and correct errors in short-course treatments. Other challenges include a necessary reliance on imaging modalities, each of which has its own set of latent errors. Extensive quality assurance WWW.HOSPITALREPORTS.EU | 9
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Integrated Quality Assurance (QA) capabilities are key in ensuring that each component of the SRS system is functioning both in isolation and in combination.
(QA) protocols are necessary to mitigate against these risks30. SRS and SBRT also present challenges in clinical workflow that can manifest in errors. Peer review of treatment planning is strongly recommended by many best practice guidelines. However, peer review may be difficult to implement in clinical settings experiencing resourcing issues. The multidisciplinary nature of SRS clinical implementations means that special resource requirements in staff effort and training may be required. If this requirement is not recognized, inadequate resource allocation may lead to errors. Integrated Quality Assurance (QA) capabilities are key in ensuring that each component of the SRS system is functioning both in isolation and in combination. Further, automated and robotic systems that adjust patient positioning, radiation dosing and delivery and image acquisition and analysis can all contribute to ensuring standardization and quality control in service delivery.
modalities such as CBCT take between 10-12 min to acquire and analyze. This effort is extrapolated if multiple imaging modalities are required to formulate a treatment plan. These considerations should be built into clinical workflow plans and are minimized through integrating imaging, patient positioning and radiation delivery components. Cost-effectiveness analysis studies have demonstrated that both SRS and SBRT are likely to be cost-effective management strategies for various types of cancer. Efficiencies are largely led by absolute cost savings from fewer treatment sessions billed to payers. Despite potential cost savings, both techniques also provide similar or improved cancer control compared to other techniques31. Single dose treatments are more cost-effective that fractionating treatments. Further, they reduce the risk of interfractional variations and improve patient experiences through convenience. For these reasons, integrated SRS systems that enable a high degree of radiation beam modulation are desirable.
Workflows and Cost-effectiveness
The ability to tailor SRS and SBRT technologies to patient requirements will extend the utility of these technologies and expand access to further patient populations. For example, the treatment of low grade gliomas, a heterogeneous group of tumors will be possible through the ability to conform the dose distribution to different tumors sizes and shapes32. Integration with different imaging modalities, robotic patient positioning and automated treatment planning and QA protocols contributes to improved patient outcomes and experiences as well as more efficient and costeffective treatment delivery.
SRS care teams involve radiation oncologists and technicians, neurosurgeons, and medical physicists. Collaborative efforts promote risk minimization and improved patient outcomes. The workflow associated with SRS and SBRT procedures is driven by the degree to which systems are integrated. Patient positioning methods and imaging modalities can both impact on clinical workflows. Using frameless procedures is reliant on high-definition imaging, performed prior to treatment planning5. Advanced imaging
Conclusion
Cost-effectiveness analysis studies have demonstrated that both SRS and SBRT are likely to be cost-effective management strategies for various types of cancer.
10 | WWW.HOSPITALREPORTS.EU
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Stereotactic Radiosurgery a Precise, Potent Technique to Treat Brain Tumours Elekta Ltd Clinicians at the UK’s Nottingham University Hospital use sophisticated radiotherapy technology to lock onto and eradicate the smallest intracranial targets.
Foreword When open surgery, chemotherapy and conventional radiotherapy are poor options for a patient with a brain tumour, physicians turn to stereotactic radiosurgery (SRS). SRS is a technique that harnesses precisely focused photon beams to deliver a highly concentrated radiation dose to abnormal target tissues. Common treatment platforms include linear accelerators such as Versa HD™ (Elekta) and SRS systems such as Leksell Gamma Knife® Icon™ (Elekta). Potent SRS irradiation – often possible with a single treatment session – destroys the DNA within tumour cells, disrupting replication and triggering cell death. While SRS’s high doses spell doom for cancer cells, the technique could cause serious damage to nearby healthy tissues. This may occur as a consequence of imprecise tumour targeting and/or the dose to the lesion permeates into surrounding tissues. Fortunately, modern SRS employs advanced imaging, planning, immobilization, beamshaping, motion management, beam delivery and quality assurance methods to ensure a safe and effective treatment. Nottingham University
Hospitals radiotherapy professionals have been using SRS since 2016 to help patients affected with brain tumours. Over the last two decades, SRS has emerged as an attractive option for individuals with benign and malignant brain tumours, according to Luis Aznar-Garcia, MD, PhD, Consultant Clinical Oncologist and Brain Mets MDT (Multidisciplinary Team) and SRS Lead at the Department of Oncology, Nottingham University Hospitals (NUH), NHS Trust. “SRS in the brain is key,” he says. “For a single brain met (metatstasis), SRS is a patientfriendly alternative to surgery and when there are multiple mets it’s the only treatment. In addition, systemic treatment of brain mets doesn’t work most of the time. SRS can achieve a local control of 80 to 90 percent, which is very similar to surgery outcomes for healthier mets patients. In the case of meningiomas and acoustic neuromas, surgery is an overly aggressive option, whereas SRS provides a minimally invasive alternative with a very good chance of local control.” Dr. Aznar-Garcia adds that he holds a dim view of whole brain radiation therapy (WBRT),
Over the last two decades, SRS has emerged as an attractive option for individuals with benign and malignant brain tumours.
WWW.HOSPITALREPORTS.EU | 11
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Since August 2016, NUH has treated 350 patients, delivering SRS to five patients each week for a range of intracranial indications, including mets, meningioma and postoperative tumour cavity.
12 | WWW.HOSPITALREPORTS.EU
a technique that has been associated with neurocognitive defects. “I try to avoid WBRT, except for patients with leptomeningeal disease or in cases in which there are more than 10 mets,” he says. “At NUH and other centres worldwide, physicians are seeing increasing numbers of patients with mets, a trend due mainly to the variety and effectiveness of systemic treatments to control a patient’s extracranial primary cancer”, says Anna Bangiri, SRS Principal Radiotherapy Physicist at NUH. “People with cancer elsewhere in the body have been living with their primary disease for a long time by virtue of the success of their chemotherapy, long enough for them to develop brain metastases,” she notes. “SRS is giving them an option for a better quality of life and, in certain cases, increased survival.” Since August 2016, NUH has treated 350 patients, delivering SRS to five patients each week for a range of intracranial indications, including mets, meningioma and post-operative tumour cavity. The treatment of metastases is by far the most common SRS target at NUH. This trend inspired the 2018 establishment of the NUH Brain Mets Multidisciplinary Team (MDT) in the UK East Midlands, a joint effort between NUH and Macmillan Cancer Support. “NUH is the only centre in the UK that has a Dedicated Specialist Brain Metastases MDT, which includes neurosurgeons, oncologists, palliative care specialists, physicists and radiographers. This team meets weekly to assess the needs of these patients and develop an
individualized, holistic care plan for them,” Bangiri says. “This allows us to offer unique treatments to our patients, such as a combination of surgery and SRS, in a timely manner.”
Precision Tools for SRS The delivery of safe, effective SRS requires a variety of cutting-edge technologies, the most important of which is the linear accelerator. Before NUH considered adding an SRS service, the department had acquired a pair of Versa HD™ linacs, Elekta’s most advanced and versatile linac platform. This system is designed to give healthcare providers the flexibility to deliver conventional therapies to treat a wide range of tumours throughout the body, while also enabling treatment of highly complex cancers that require extreme targeting precision. “We knew that Versa HD was perfectly suited for providing standard radiotherapy, but we also knew if we wanted to launch an SRS program at some point, then the system would meet all the requirements for SRS by adding some extra supporting technologies,” Dr. AznarGarcia observes. Integral to Versa HD – and a crucial feature for SRS – is the Agility™ 160-leaf multileaf collimator (MLC) for highly conformal beam shaping. Each leaf is just 5 millimetres in width and all 160 leaves are available across the entire 40-centimeter field-of-view. “From an SRS standpoint it’s important that the leaves B can travel quite a long way across the central axis, and the six centimetre per second leaf travel speed of Agility is very helpful,” Bangiri
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
The delivery of safe, effective SRS requires a notes. “Because we treat multiple mets with a single isocentre, having five-millimetre leaves across the whole field-of-view ensures that all the mets in the field will have the same high conformality to the tumour shape.” After committing to establishing an SRS service in 2016, NUH dedicated one of the Versa HD systems as a departmental SRS system. To achieve sub-millimetre accuracy for motion management and positioning correction, they acquired two pieces of supporting technology: Elekta’s HexaPOD™ evo RT robotic positioning system and Brainlab’s ExacTrac® IGRT system for patient position tracking. HexaPOD and ExacTrac work together to ensure extreme positioning precision. These systems ensure that inter- and intra-fractional geometric uncertainties are minimized. According to Bangiri and Dr. Aznar-Garcia, the HexaPOD-ExacTrac combination is a “must-have” for SRS and they use it for every case unless it is physically impractical. “In SRS, patient positioning is paramount, because we’re treating very small volumes with very high doses; a geometric miss could cause damage to normal tissues,” Bangiri explains. “ExacTrac has two x-ray tubes that can very quickly image the patient from two different angles, providing information in six degrees of freedom, the x, y, and z axes and the rotational axes of roll, pitch and yaw at any couch angle. Acquiring images with the linac’s cone beam CT [CBCT] can only be done when the couch is at angle 0°. ExacTrac can also be used to image patients during beam delivery.
“HexaPOD is responsible for interpreting the information that ExacTrac provides,” she continues. “Then, with its own six degrees of positioning freedom, HexaPOD can execute patient position changes down to less than onemillimetre accuracy. “It’s important to note that this degree of accuracy eliminates the need to use a stereotactic frame on the patient’s head, which would mean using fixation pins, making our treatments minimally invasive and comfortable for patients,” Bangiri adds. “For head immobilization, we use a CIVCO head support and an AccuForm™ cushion with Orfit’s 3-point open face hybrid thermoplastic mask. This solution is custom-fit for each patient and not only ensures secure immobilization, but also provides better comfort because it’s softer than a standard plastic headrest.”
variety of cutting-edge technologies, the most important of which is the linear accelerator.
Planning for Precision “Treatment planning for small SRS targets requires a very steep dose fall-off and exceptional target conformality to spare healthy tissue”, Bangiri says. NUH uses Monaco®, an advanced treatment planning solution from Elekta equipped with biological cost functions with multicriterial constrained optimization, a powerful leaf sequence optimizer and the gold-standard Monte Carlo dose calculation algorithm. “The Monte Carlo algorithm is very accurate and helps us produce very complex plans,” she says. “We know from our QA protocols that the plans Monaco produces are precise and from our patient follow-ups we have seen that it delivers the treatment that we intend.” WWW.HOSPITALREPORTS.EU | 13
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Delivering Hope for a Positive Outcome
Integral to Versa HD – and a crucial feature for SRS – is the Agility™ 160-leaf multileaf collimator (MLC) for highly conformal beam shaping.
All intracranial SRS cases are delivered with Volumetric Modulated Arc Therapy (VMAT) beams and with the High Dose Rate Mode (HDRM, i.e., flattening filter-free) of Versa HD. The HDRM provides a rapid fraction and VMAT in combination with the high leaf speeds of Agility, yields exquisite conformance. “Most of the time the treatment happens in a single fraction,” Dr. Aznar-Garcia says. “Depending on the volume of the tumour, we might deliver 22 Gy or 18 Gy if the tumour is larger than two centimetres in diameter. If the tumour is inside the brainstem, we prescribe 15 Gy in one fraction. Occasionally we have to fractionate if the tumour is larger, if the organs-at-risk dose constraints haven’t been met or if we are re-irradiating. Then, the prescription is 27 Gy spread out over three fractions. When we irradiate post-operative cavities – tumours that have been previously excised – we can offer 25 Gy in five fractions if they are very large or if the OAR constraints haven’t been met.”
Challenges Overcome A recent SRS case that exemplified the value of NUH’s MDT was one in which a patient’s surgery left a small portion of residual tumour, something of a rarity at the centre. “We treated the cavity as we would normally do and used an integrated boost for the unresected part of the tumour,” Bangiri recalls. “I don’t think many centres can do that, but since we have the Brain Mets MDT, we were able to collaborate with the neurosurgeons on options. The cavity was large, but the residual that we could see on MRI was quite small. That is something that we had never tried to do before with Monaco, but the planning system managed to do it really well.” A current case that the department is handling is a young patient with a BMI of 46 (356 lbs, 162 kilos) who could not have an MRI planning scan due to his size. “Because of the clear indication for stereotactic radiosurgery for this patient – his young age and a good prognosis with a single metastasis on his CT – the brain mets MDT decided to move forward with SRS,” Dr. Aznar-Garcia says. “We imaged with a CT scan using a one-millimetre slice thickness and contrast and planned with a two-millimetre margin, instead of the usual one millimetre. However, because of the patient’s size we also couldn’t use ExacTrac and had to use the linac’s CBCT. Sometimes we simply have to adapt the treatment pathway and improvise to make things work, which we were able to do in this case.”
Assuring Quality SRS Because sub-millimetre accuracy is essential for SRS, the NUH team meticulously checks its 14 | WWW.HOSPITALREPORTS.EU
treatment tools to ensure they are performing to the required specifications. “QA is crucial to an SRS program because we use very potent doses. High precision and accuracy are paramount, and we want to make sure the patient is getting the treatment that we intend for them to get, and that the treatment is safe for the patient,” Bangiri says. “SRS is a type of treatment where you’re ablating something, we’re aiming for the tumour to not be able to recover from that radiation exposure. If you’re not working with very high accuracy and precision, you might mistreat patients and that can potentially have dire effects for them.” To that end, NUH’s QA protocol calls for daily checks on ExacTrac and HexaPOD and the radiographers check using the MIMI Phantom (Standard Imaging), a QA solution for HexaPOD to confirm that ExacTrac and the linac’s CBCT match and the accuracy of both systems is retained. Physicists use a ball bearing phantom on a monthly basis to recalibrate the systems and check that the coincidences – the CBCT kV, ExacTrac kV and linac isocentres – overlap to established standards. “Until recently, we also checked every PTV for every patient’s plan prior to treatment using CIRS’s Stereotactic End-to-End Verification (STEEV), an anthropomorphic head-and-neck phantom, and EBT XD dosimetry film [GAFChromic],” she says. “We have been able to modify that QA step to check just one tumour per plan because we have gained enough confidence in our practice.” In addition to creating, adhering to and periodically updating its own SRS practice guidelines, the NUH team also follows an exacting set of national and international practice guidelines concerning both extra- and intracranial SRS. These include the UK SABR/ SRS Consensus Guidelines for normal tissue constraints and the SABR/SRS UK Consortium Guidelines; Report 101 of the American Association of Physicists in Medicine (AAPM); International Stereotactic Radiosurgery Society (ISRS) documents and guidelines; as well as National Health Service (NHS) guidelines.
Looking Ahead As SRS technology and clinical research advance, new possibilities are emerging for patients with serious brain disease, according to Dr. Aznar-Garcia. “We would like to not only able to offer SRS to a greater number of patients, but also to apply the technique to patients with larger numbers of metastases and with lesions smaller than 0.1 cc,” he says. “In terms of number of mets per patient, the Yamamoto trial demonstrated that SRS in patients with five to 10 brain mets is non-inferior to SRS in patients with just two to four mets.”
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
The HDRM provides a rapid fraction and VMAT Another promising clinical and research frontier is the increasing use of immunotherapy with SRS, he adds. “The goal of radiation is to achieve local control but combining radiation with immunotherapy has the potential to achieve systemic control as well,” Dr. Aznar-Garcia explains. “Radiation can promote immunogenic cell death and sensitize neoplastic cells to immunotherapy. It does this by encouraging the cells’ expression of tumour antigens that prime immune cells to attack other cells in the body, including those at distant sites that haven’t received radiation. In a sense, radiation transforms the tumour into a vaccine. The synergistic action of SRS combined
with immunotherapy could be a very promising treatment technique to extend overall survival.” To make the brain mets SRS service as accessible as possible, NUH now offers patients the option of virtual clinics via video link. “All patients have to come here for treatment planning and delivery, of course, but the virtual clinics enable video follow-up appointments closer to the patient’s home,” he says. For its exceptional work in making brain mets treatment available to residents of the East Midlands, the Macmillan East Midlands Brain Metastases Service was awarded the 2019 Macmillan Innovation Excellence Award.
in combination with the high leaf speeds of Agility, yields exquisite conformance.
In addition to creating, adhering to and periodically updating its own SRS practice guidelines, the NUH team also follows an exacting set of national and international practice guidelines concerning both extra- and intracranial SRS.
WWW.HOSPITALREPORTS.EU | 15
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Future Outlook Dr. Sophie Laurenson (BSc Hons., Ph.D. Cantab.) The future of SRS will be driven by best practice recommendations to improve patient outcomes, treatment efficiency and cost effectiveness.
Introduction
Currently, systems integration is being leveraged to improve the speed and accuracy of radiosurgery delivery. In parallel, technical advances are focusing on improving patient care experiences.
16 | WWW.HOSPITALREPORTS.EU
Stereotactic SRS and SBRT have become increasingly popular for the treatment of benign and malignant tumors across the world3. The need for devices capable of molding radiation doses to individual lesions, yet preserve healthy tissues was a significant challenge25. Currently, systems integration is being leveraged to improve the speed and accuracy of radiosurgery delivery. In parallel, technical advances are focusing on improving patient care experiences. As the application of these technologies has expanded, technical standards and clinical protocols are developing to match technological advances. Multiple clinical best practice guidelines are now available from professional and governmental organizations. The future of SRS will be driven by best practice recommendations to improve patient outcomes, treatment efficiency and cost effectiveness.
Advanced Conformal Arc Therapies SRS and SBRT for large or irregular targets is dependent upon the ability to conformally shape radiation dose distribution around the target. For treating small targets, a circular tertiary collimator produces a conformal distribution with a steep dose drop-off curve. However, for treating targets in excess of 4cm in diameter, a multileaf collimator (MLC) consisting of between 80 to 160 high atomic number leaves can be used to improve accuracy. Each leaf can independently interact with the radiation field, modulating the beam intensity and dose distribution shape with extreme precision. These advances have paved the way towards beam modulation for intensity-modulated radiation therapy (IMRT), volumetric modulated arc therapy (VMAT), and dynamic conformal arc therapy (DCAT). IIMRT utilizing continuous gantry motion was pioneered in 1995. Unlike conventional arc therapy, the field shape is conformed using an MLC, which shifts during gantry rotation. Twodimensional beams strengthen distributions at various beam angles to deliver multiple
superimposing arcs33. The MLC establishes a single isocenter from multiple off-axis beams and intensity modulation is created by overlapping arcs. This delivers the equivalent effect to that which can be obtained with multiple isocenters34. When planning treatments, the interconnectedness of the beams is critical. Both the leaf motion speed and gantry rotational motion are important variables35. Solutions for planning and delivering dynamic conformal arcs have been commercialized. In some SRS systems, a mechanical isocenter is not required as a spatial reference for treatment beam convergence. Multiple crisscrossing beams can be directed to transverse through the target tissue from different orientations that may not be directed toward a single spatial coordinate. This is often described as the robotic delivery approach and is used to conform dose distribution for complex targets with irregular morphology36. Recently, there has been interest in treating abnormal tissues using dynamic conformal arc therapy (DAC) as an alternative to IMRT or VMAT37-39. This technique shapes the MLC around the tumor on a rotating gantry, improving the resulting dose distributions. Conformal arc therapy generates a radiation field that encompasses the entire projection of the target from every direction. This eliminates the effects of beam interplay. These treatments have a shorter overall treatment time, requiring less treatment planning and dose validation resources39. Delivering the total dose in a shorter period may be more effective, as it minimizes the time available for inter-fractional DNA repair, maximizing treatment effect.
Advances in Automation and Workflow Design Implementing SRS and SBRT programs is dependent on local settings, particularly in available resources and training practices40. Future integration and standardization of SRS platform solutions will benefit clinical settings suffering from local resourcing deficiencies, expanding SRS treatment to more patient populations. In the future, turnkey SRS solutions
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
containing highly integrated systems are likely to develop further. The future of SRS and SBRT technology will make advancements in both treatment quality and in streamlining complex clinical workflows. This would make the total treatment process more labor and cost efficient for healthcare providers. These advances would be underpinned by increased automation in treatment planning and delivery processes such as on-line dose adaption and rapid 3D image acquisition. Current image-guided radiotherapy systems are not optimized for targets that cannot be visualized using cone-beam computed tomography (CBCT). These treatments are prone to both inter- and intra-fractional uncertainties arising from sub-optimal imaging. SRS platforms that integrate magnetic resonance imaging (MRI) with a linac systems allow real-time imaging of a wider range of target locations41. These advances will enable SRS and SBRT to be applied to further targets, enabling this non-invasive therapy to become more widely accessible.
Combination Therapies Radiation therapies have historically been considered as immunosuppressive. This is due to the reductions in white blood cell levels associated with the exposure of bone marrow
and blood pool to radiation42. However, SRS and SBRT procedures result in very limited exposure of these organs to radiation, limiting the immunosuppressive effects. Recent research has revealed that radiation has the potential to augment both local and systemic anti-tumor immune responses. In this context, SRS and SBRT couple be combined with immunotherapy to improve treatment outcomes. There is now a body of clinical evidence supporting this approach in the treatment of intracranial and extracranial tumors43. Future research will focus on the fundamental mechanisms that underpin the induction of immune responses following radiation therapy.
Conclusion In conclusion, advanced technologies that enable volumetric imaging, modulated beam delivery, and real-time motion management and dose adaption will continue to drive SRS and SBRT development. The combination of these technologies will enable procedures to become more automated and accessible to patients. SRS and SBRT also have the potential to be combined with other treatment modes, such as immunotherapies, to improve patient outcomes further.
Recently, there has been interest in treating abnormal tissues using dynamic conformal arc therapy (DCAT) as an alternative to IMRT or VMAT.
Future integration and standardization of SRS platform solutions will benefit clinical settings suffering from local resourcing deficiencies, expanding SRS treatment to more patient populations.
WWW.HOSPITALREPORTS.EU | 17
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
References: 1
Leksell L. The stereotaxic method and radiosurgery of the brain. Acta chirurgica Scandinavica. 1951;102(4):316-9.
Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical
2
experience of the first thirty-one patients. Acta oncologica (Stockholm, Sweden). 1995;34(6):861-70. Fanous AA, Prasad D, Mathieu D, Fabiano AJ. Intracranial stereotactic radiosurgery. Journal of neurosurgical sciences. 2019;63(1):61-82.
3
Wu A, Lindner G, Maitz AH, Kalend AM, Lunsford LD, Flickinger JC, et al. Physics of gamma knife approach on convergent beams in stereotactic
4
radiosurgery. International journal of radiation oncology, biology, physics. 1990;18(4):941-9. Lunsford LD, Niranjan A, Fallon K, Kim JO. Frame versus Frameless Leksell Stereotactic Radiosurgery. Progress in neurological surgery.
5
2019;34:19-27. Gorgulho AA, Ishida W, De Salles AAF. General Imaging Modalities: Basic Principles. In: Lozano AM, Gildenberg PL, Tasker RR, editors. Textbook of
6
Stereotactic and Functional Neurosurgery. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. p. 249-67. De Salles AA, Sedrak M, Lemaire JJ. Future of stereotactic radiosurgery. Shaped beam radiosurgery: state of the art Ist ed. Munich: Springer; 2011.
7
p. 207 -311. Specht HM, Combs SE. Stereotactic radiosurgery of brain metastases. Journal of neurosurgical sciences. 2016;60(3):357-66.
8
Soike MH, Hughes RT, Farris M, McTyre ER, Cramer CK, Bourland JD, et al. Does Stereotactic Radiosurgery Have a Role in the Management of
9
Patients Presenting With 4 or More Brain Metastases? Neurosurgery. 2019;84(3):558-66. International Stereotactic Radiosurgery Society (ISRS) 2020 [Available from: http://www.isrsy.org/public/en/radiosurgery/guidelineproject/.
10
DEGRO. DEGRO Working Group 2020 [Available from:
11
https://www.degro.org/ag-stereotaxie/practice-guidelines-of-the-degro-working-group-stereotactic-radiotherapy-radiosurgery/. Hanna GG, Murray L, Patel R, Jain S, Aitken KL, Franks KN, et al. UK Consensus on Normal Tissue Dose Constraints for Stereotactic Radiotherapy.
12
Clinical oncology (Royal College of Radiologists (Great Britain)). 2018;30(1):5-14. Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: the report of AAPM Task Group
13
101. Medical physics. 2010;37(8):4078-101. Andrews DW, Bednarz G, Evans JJ, Downes B. A review of 3 current radiosurgery systems. Surgical neurology. 2006;66(6):559-64.
14
Kataria T, Gupta D, Karrthick KP, Bisht SS, Goyal S, Abhishek A, et al. Frame-based radiosurgery: Is it relevant in the era of IGRT? Neurology India.
15
2013;61(3):277-81. Sahgal A, Ma L, Chang E, Shiu A, Larson DA, Laperriere N, et al. Advances in technology for intracranial stereotactic radiosurgery. Technology in
16
cancer research & treatment. 2009;8(4):271-80. Kim T, Sheehan J, Schlesinger D. Inter- and intrafractional dose uncertainty in hypofractionated Gamma Knife radiosurgery. J Appl Clin Med Phys.
17
2016;17(2):487-96. Baumann P, Nyman J, Lax I, Friesland S, Hoyer M, Rehn Ericsson S, et al. Factors important for efficacy of stereotactic body radiotherapy of
1
medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta oncologica (Stockholm, Sweden). 2006;45(7):787-95. Hansen AT, Petersen JB, Hoyer M. Internal movement, set-up accuracy and margins for stereotactic body radiotherapy using a stereotactic body
18
frame. Acta oncologica (Stockholm, Sweden). 2006;45(7):948-52. Yoo S, Kim GY, Hammoud R, Elder E, Pawlicki T, Guan H, et al. A quality assurance program for the on-board imagers. Medical physics.
19
2006;33(11):4431-47. Tulik M, Kabat D, Kycia RA, Tabor Z, Woszczyna A, Latala Z. A framework for alignment of on-board imagers of medical linear accelerators. Physica
20
medica : PM : an international journal devoted to the applications of physics to medicine and biology : official journal of the Italian Association of Biomedical Physics (AIFB). 2018;47:80-5. Murphy MJ. Intrafraction geometric uncertainties in frameless image-guided radiosurgery. International journal of radiation oncology, biology,
21
physics. 2009;73(5):1364-8. Soltys SG, Adler JR, Lipani JD, Jackson PS, Choi CY, Puataweepong P, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain
22
metastases. International journal of radiation oncology, biology, physics. 2008;70(1):187-93. Liu RJ, Yang SX, Neylon J, Hall MD, Dandapani S, Vora N, et al. Residual setup errors in cranial stereotactic radiosurgery without six degree of
23
freedom robotic couch: Frameless versus rigid immobilization systems. J Appl Clin Med Phys. 2020. De Salles AA, Gorgulho AA, Pereira JL, McLaughlin N. Intracranial stereotactic radiosurgery: concepts and techniques. Neurosurgery clinics of
24
North America. 2013;24(4):491-8. Liu G, van Doorn T, Bezak E. The linear accelerator mechanical and radiation isocentre assessment with an electronic portal imaging device (EPID).
25
Australasian Physics & Engineering Sciences in Medicine. 2004;27(3):111. Burman CM, Hunt MA, Chui C-S, Losasso TJ, Mageras GS, Zelefsky MJ, et al. Chapter 6 - Three-Dimensional and Conformal Treatment Planning.
26
In: Bragg DG, Rubin P, Hricak H, editors. Oncologic Imaging. Oxford: Elsevier; 2002. p. 81-91. Masi L, Casamassima F, Polli C, Menichelli C, Bonucci I, Cavedon C. Cone beam CT image guidance for intracranial stereotactic treatments:
27
comparison with a frame guided set-up. International journal of radiation oncology, biology, physics. 2008;71(3):926-33. Leksell L. Radiosurgery, an operative system.1978.
28
18 | WWW.HOSPITALREPORTS.EU
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Ford E, Dieterich S. Safety Considerations in Stereotactic Body Radiation Therapy. Seminars in radiation oncology. 2017;27(3):190-6.
29
Lester-Coll NH, Sher DJ. Cost-Effectiveness of Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy: a Critical Review. Current
30
oncology reports. 2017;19(6):41. Niranjan A, Faramand A, Lunsford LD. Stereotactic Radiosurgery for Low-Grade Gliomas. Progress in neurological surgery. 2019;34:184-90.
31
Scaringi C, Agolli L, Minniti G. Technical Advances in Radiation Therapy for Brain Tumors. Anticancer research. 2018;38(11):6041-5.
32
Yu CX. Intensity-modulated arc therapy with dynamic multileaf collimation: an alternative to tomotherapy. Physics in medicine and biology.
33
1995;40(9):1435-49. Jin JY, Wen N, Ren L, Glide-Hurst C, Chetty IJ. Advances in treatment techniques: arc-based and other intensity modulated therapies. Cancer
34
journal (Sudbury, Mass). 2011;17(3):166-76. Hanna SA, Mancini A, Dal Col AH, Asso RN, Neves-Junior WFP. Frameless Image-Guided Radiosurgery for Multiple Brain Metastasis Using VMAT:
35
A Review and an Institutional Experience. Frontiers in Oncology. 2019;9(703). Shi C, Tazi A, Fang DX, Iannuzzi C. Implementation and evaluation of modified dynamic conformal arc (MDCA) technique for lung SBRT patients
36
following RTOG protocols. Medical dosimetry : official journal of the American Association of Medical Dosimetrists. 2013;38(3):287-90. Shi C, Chen Y, Fang DX, Iannuzzi C. Application of modified dynamic conformal arc (MDCA) technique on liver stereotactic body radiation
37
therapy (SBRT) planning following RTOG 0438 guideline. Medical dosimetry : official journal of the American Association of Medical Dosimetrists. 2015;40(1):26-31. Shi C, Chen Y, Fang DX, Iannuzzi C. Application of modified dynamic conformal arc (MDCA) technique on liver stereotactic body radiation
38
therapy (SBRT) planning following RTOG 0438 guideline. Medical dosimetry : official journal of the American Association of Medical Dosimetrists. 2015;40(1):26-31. Morales-Paliza MA, Coffey CW, Ding GX. Evaluation of the dynamic conformal arc therapy in comparison to intensity-modulated radiation therapy in
39
prostate, brain, head-and-neck and spine tumors. J Appl Clin Med Phys. 2010;12(2):3197-. Yang I, Udawatta M, Prashant GN, Lagman C, Bloch O, Jensen R, et al. Stereotactic Radiosurgery for Neurosurgical Patients: A Historical Review
40
and Current Perspectives. World neurosurgery. 2019;122:522-31. Lagendijk JJ, Raaymakers BW, van Vulpen M. The magnetic resonance imaging-linac system. Seminars in radiation oncology. 2014;24(3):207-9.
41
Order SE. The effects of therapeutic irradiation on lymphocytes and immunity. Cancer. 1977;39(2 Suppl):737-43.
42
Sharabi AB, Tran PT, Lim M, Drake CG, Deweese TL. Stereotactic radiation therapy combined with immunotherapy: augmenting the role of radiation
43
in local and systemic treatment. Oncology (Williston Park, NY). 2015;29(5):331-40.
WWW.HOSPITALREPORTS.EU | 19
IMPROVING THE ACCURACY AND PRECISION OF STEREOTACTIC RADIATION THERAPY
Notes:
20 | WWW.HOSPITALREPORTS.EU
Healthcare Professionals Make More Effective Evidence Based Clinical Decisions With Hospital Reports
For the past decade, Hospital Reports has been helping healthcare professionals, within both public and private hospitals, to improve patient outcomes Our Special Reports provide readers with an unparalleled depth of information on specialist subjects, which receive limited coverage in the mainstream medical media. Each report is designed to help healthcare professionals to make more effective treatment decisions, by providing a unique mix of: • Subject specific clinical information • Insight and knowledge from internationally recognised key opinion leaders • Clinical guidelines • Independent data and analysis • Unbiased editorial content
subscriptions@globalbusinessmedia.org www.globalbusinessmedia.org