
13 minute read
Breaking Bad Bugs with Repurposed Drugs
Breaking Bad Bugs
WITH REPURPOSED DRUGS
Dr. Meghan Blackledge
Assistant Professor of Chemistry
Dr. Heather Miller
Associate Professor of Chemistry

Approximately two out of every 100 people carry MRSA (methicillin-resistant Staphylococcus aureus). These bacterial organisms are becoming more common and are particularly bad for patients with implanted medical devices like catheters. MRSA is a major health concern because it can outsmart most of the drug treatments that humans try to use to fight it. In fact, the Center for Disease Control calls MRSA a “serious threat” due to its ability to grow even in the presence of many antibiotics.


Drs. Meghan Blackledge and Heather Miller, both professors in the department of chemistry, are working together to combat this serious threat. Their collaboration capitalizes on their individual strengths and different scientific subdisciplines to discover novel methods to combat antibiotic resistance in MRSA. In the labs of Drs. Blackledge and Miller, first-year students through seniors are actively conducting this research. Strolling into the new Wanek School of Natural Sciences, one will find these students at the bench each day culturing
different strains of MRSA, synthesizing novel drug compounds and measuring the effectiveness of these compounds at destroying bacteria. Through these mentored research experiences, HPU students are making novel discoveries, publishing the results in peer-reviewed journals and sharing their findings across the United States.
Synergy as the Key to Combating Bacterial Infections
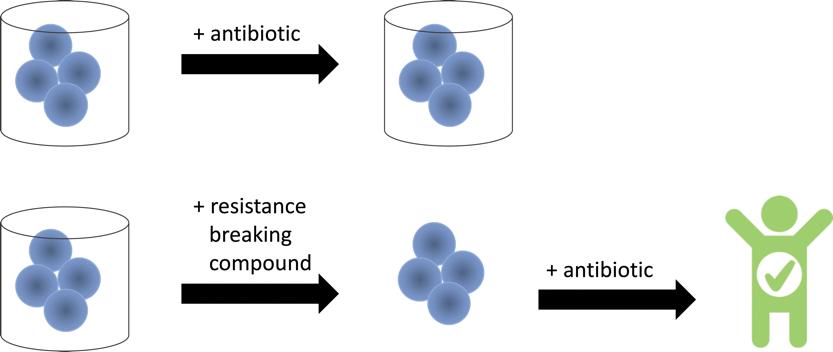
Drs. Blackledge’s and Miller’s collaboration began in 2016. Dr. Blackledge had been approached by a colleague in biology, Patrick Viguiera. He found an antidepressant, amoxapine, that was able to make antibiotics more effective against MRSA. Dr. Blackledge and her student, Kyra Gillard ’18, were searching for FDA-approved compounds that could be effective at reversing antibiotic resistance in MRSA. They hypothesized that this approach, known as drug repurposing, would uncover novel functions for these drugs and could be used as a starting point to develop new therapeutics to target antibiotic-resistant bacterial infections. Drs. Blackledge and Gillard tested amoxapine and structurally related antidepressants to elucidate the mechanism behind the observed effects on antibiotic activity. The antidepressants did not kill the bacteria on their own, but they did restore the activity of antibiotics that the bacteria was normally resistant to. While the findings were interesting on their own, Dr. Blackledge wanted to understand how the antidepressants were working synergistically with the antibiotics in the bacteria. She hypothesized that the antidepressants were interfering with the bacteria’s ability to turn on the genes necessary to evade or break down the antibiotics, the so-called resistance genes. Antibiotic-resistant bacteria are similar to countries defending themselves from missile attacks. Rather than shooting into the sky constantly, countries have monitoring systems that alert them to incoming attacks so that they can mount a defense. Similarly, bacteria have receptors that monitor their surroundings for antibiotics. When an antibiotic is detected, the receptors send signals into the bacterial cell that turn on resistance genes and make proteins that help the bacteria withstand the antibiotic or break down the antibiotic. If the antidepressants were working as Dr. Blackledge hypothesized, then MRSA treated with the antidepressants and antibiotics should not be able to turn on the necessary resistance genes (Figure 1).
Common antibiotics, such as penicillin, are no longer effective treatments against resistant bacteria like MRSA because the bacteria have developed ways to evade the antibiotic or break it down. Antibiotics like penicillin function by inactivating enzymes that are necessary to build the bacteria’s cell wall, which is essential for bacterial growth and survival. Resistance to penicillin and related antibiotics, called β-lactams, is governed primarily by two proteins in MRSA: PBP2a and BlaZ. PBP2a is an enzyme that can still build the bacterial cell wall, albeit less efficiently than the natural enzyme, but cannot be inactivated by β-lactams. BlaZ is a β-lactamase enzyme that, in the presence of β-lactam antibiotics, is released by the bacteria and designed to break apart and inactivate the β-lactams. Together, these two enzymes allow MRSA to breakdown the β-lactams with BlaZ and continue to grow and produce their cell wall in the presence of any remaining antibiotics using PBP2a. Dr. Blackledge hypothesized that when MRSA were co-treated with the antidepressants and a β-lactam antibiotic that levels of BlaZ and PBP2a genes would be reduced compared to treatment with β-lactam alone. This would show that the antidepressants were interfering with MRSA’s resistance mechanisms.
In order to test her hypothesis, Dr. Blackledge enlisted the help of her colleague Dr. Miller, an expert in molecular genetics. Dr. Miller has spent her career studying HIV gene expression and the
human proteins that the virus co-opts to replicate and infect more cells. When Dr. Blackledge first approached her about working together to look at MRSA resistance genes, Dr. Miller was excited, but she had reservations, too, as this joint collaboration would require her to work outside of her familiar field of human and viral gene expression. The collaboration has yielded some outstanding results. After a few tries to optimize conditions, Dr. Miller and her students were able to conduct the necessary experiments that supported Dr. Blackledge’s hypothesis. Dr. Miller and her students found that when MRSA was treated with β-lactam antibiotics, the levels of PBP2a and BlaZ gene expression went up, as expected. When MRSA were co-treated with the antidepressants and β-lactams, however, the gene expression levels of PBP2a and BlaZ were closer to levels seen in the absence of antibiotic. These results were incredibly exciting and the first sign that this project might be the start of a larger collaboration and research focus.
Encouraged by their positive results, Drs. Blackledge and Miller continued screening FDA-approved compounds in an effort to find drugs that would act similarly to the antidepressants but be more potent and have an improved safety profile for use in patients. This led them to find loratadine, the active ingredient in the antihistamine Claritin. Loratadine has an exceptional safety profile in humans and was even more active at suppressing gene expression of PBP2a and BlaZ than the antidepressants had been. However, Drs. Blackledge and Miller had a question that kept nagging them — were these compounds directly affecting the expression of two different genes at once, or was there some master regulator that was the real target of these FDA drugs? They turned to the literature and found evidence that an enzyme, a kinase called Stk1, might be the master regulator that they were looking for.
Stk1 senses changes in the bacterial cell wall through extracellular receptors and phosphorylates substrates that control downstream gene transcription, allowing the bacteria to turn necessary genes on or off in response to changes in the cell wall. In MRSA, Stk1 also controls genes involved in antibiotic resistance, such as BlaZ. Drs. Blackledge and Miller hypothesized that loratadine could be a novel inhibitor of Stk1. With the help of four dedicated undergraduate students,
Figure 1. Antibiotic resistance blockers work with antibiotics to combat antibiotic-resistant bacteria. Antibioticresistant bacteria use enzymes to prevent being killed by antibiotics. Resistance breaking compounds remove the protections of these resistance enzymes so that common antibiotics are effective again.
they showed that loratadine was interacting with Stk1 and that this interaction prevented MRSA from expressing BlaZ and PBP2a in the presence of β-lactam antibiotics (Figure 2). Interestingly, they were also able to use loratadine to show that inhibition of Stk1 could also reduce resistance to vancomycin, a powerful antibiotic that is frequently considered the drug of last resort for patients with serious MRSA infections.
Since this finding, Drs. Blackledge and Miller are continuing their studies into Stk1. Currently, Dr. Blackledge and her group are working to synthesize derivatives of loratadine and other small molecules that inhibit Stk1 to create more potent compounds that they can use in future studies or even be developed into new drugs. The Miller lab is using loratadine and other small molecules to investigate which genes are under the control of Stk1 and whether the genes that Stk1 controls are different across different strains of MRSA. Just as their research focuses on synergistic interactions between these Stk1 inhibitors and antibiotics to combat MRSA, Drs. Blackledge and Miller know that the success of their collaboration comes primarily from their synergy as researchers. They come from different subdisciplines of chemistry and have different specialties, but when taken together they are able to tackle scientific questions that would not be possible to answer if they were working independently.
Growing Resiliance and Life Skills in the Lab
Through the last four years, Drs. Blackledge and Miller have grown their collaboration and taken these research projects in directions that simply would not have been possible if they were working in isolation. To date, they have published two articles with undergraduate students*, filed HPU’s first patent and been awarded a prestigious grant from the National Institute of Health worth nearly $412,000. While hard work, tenacity and a little luck have certainly helped pave the way, the synergy between the two research groups is what truly defines the collaboration and drives the research. Even the physical space in which they work is collaborative, as these researchers have a large, open-concept molecular biology and biochemistry lab in the Wanek School of Natural Sciences. Modeling this collaborative spirit and blurring the lines between disciplines is a role that they do not take lightly.
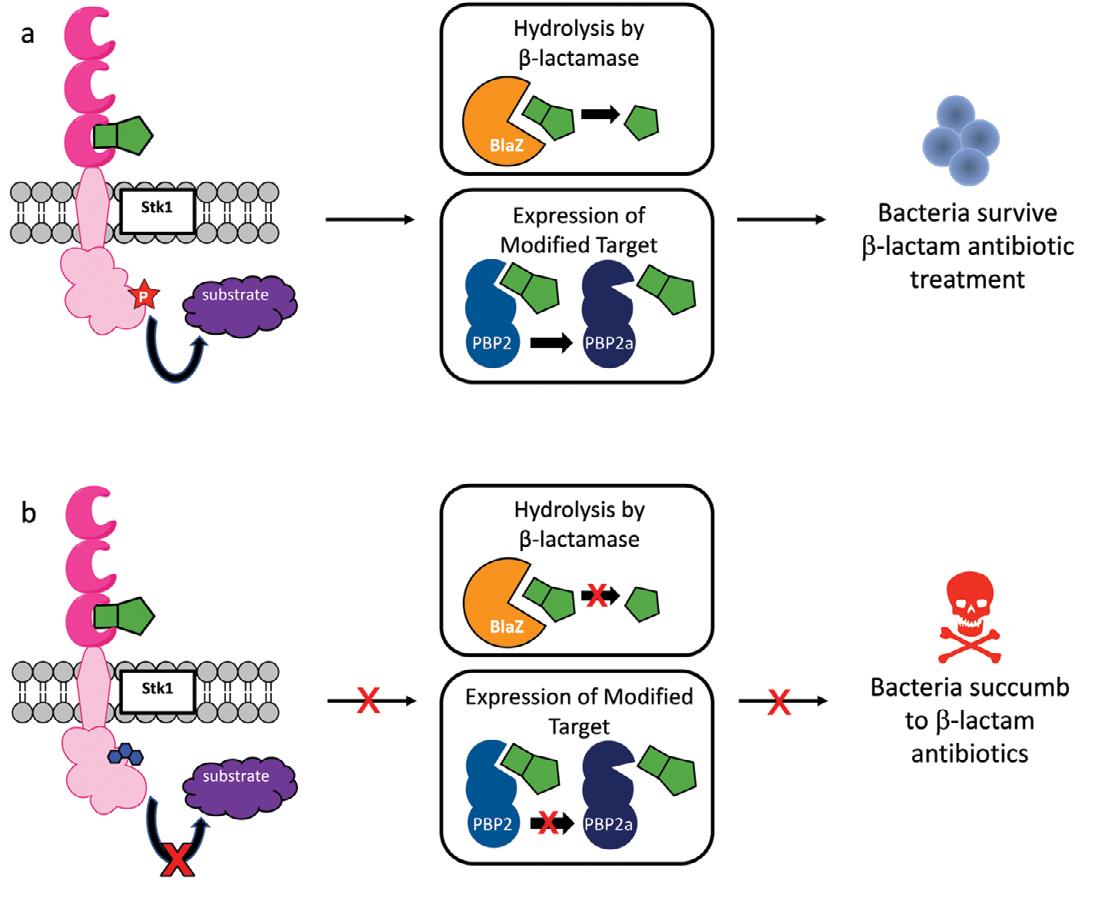
Figure 2. Inhibiting Stk1 breaks β-lactam resistance in MRSA. (a) Stk1 (pink) binds to β-lactam antibiotics (green) and transfers a phosphate group (red star) to its substrates (purple). This leads to production of BlaZ (orange) and PBP2a (dark blue) which allow MRSA to break down and evade β-lactam antibiotics for survival. (b) Inhibition of Stk1 by our molecules (blue) prevents Stk1 from transferring a phosphate group to its substrates. Thus, neither BlaZ nor PBP2a are made by the bacteria and β-lactam antibiotics are able to kill the bacteria.
This synergy has also allowed them to collectively mentor 43 students in their research labs while at HPU. For mentees, they strive to demonstrate that scientists do not work isolated in ivory towers (or dimly lit scary labs late at night, for that matter). Rather, they brainstorm, grab coffee, draw out ideas on whiteboards, email and text each other throughout the day in order to advance their work. Scientists travel to conferences in order to be inspired, present their latest results and gain valuable feedback from a number of diverse perspectives. These experiences allow students at High Point University to gain an authentic view of what a scientist does.
There are also numerous life skills that these undergraduate researchers gain. Ask any scientist about failure rates, and you will likely get a laugh. From the outside, science may look like a polished product: experts in the field carefully perform experiments and make breakthrough discoveries. From the inside, scientists and scientists-intraining face daily failures when experiments do not “work” or when they are confronted with unexpected and seemingly unexplainable results. There are far more failures than successes in the scientific method. For this reason, fostering a growth mindset is critical. Students engaged in research learn firsthand that experiments do not really fail as long as they learn something in the process. They learn new information that they did not have previously, so it is essentially a success. Students engaged in original research approach these frequent challenges, and with the help of a mentor, learn critical thinking skills that allow them to overcome obstacles and forge ahead.
Other benefits from undergraduate research are numerous and include increased interest in the field, increased retention rates, and enhanced creative and independent thinking. Furthermore, involvement in research results in increased graduate school enrollment and career preparedness. In line with the Council for Undergraduate Research’s vision, this scholarly activity not only contributes to the field but also enhances instructional performance. Professors are able to integrate findings from the lab and other related researchers’ labs into course materials to provide cutting-edge instruction and give students a sense of the evolving nature of scientific discovery. This is in contrast to a static, textbookcentered view that students may possess.
Preparing the Next Generation of Women in Science
As two women in science, Drs. Blackledge and Miller find themselves in a position where they can inspire and motivate female students to pursue this type of work. They also recognize that the generation before them had a much different experience in obtaining their degrees. In 1967, less than 20% of U.S. bachelor’s degrees in chemistry were earned by women. This statistic has steadily increased over time. Currently, approximately 50% of bachelor’s degrees in chemistry are earned by women, and this holds true for High Point University. In fact, of the students that have worked in Drs. Blackledge’s and Miller’s groups, 72% have identified as women. Dr. Miller recalls that when she was enrolled in college, there were no female chemistry professors, and her large graduate school department in molecular genetics had only two female professors during her time there. Dr. Blacklege attended a women’s college, but even so, the vast majority of her chemistry professors were male. This was similarly true during her graduate career. In contrast, HPU’s chemistry department has a majority of female faculty. Drs. Blackledge and Miller have been approached by HPU parents at commencement who have thanked them for serving as strong female role models for their children.
Drs. Blackledge and Miller also have frank discussions with students about work-life balance. Each of them must juggle their teaching, research
and service commitments at HPU with having young families. High Point University offers a holistic, liberal arts education to its students and teaches them to balance school work, volunteerism and extracurricular activities. Drs. Blackledge and Miller work to show them that the skills they develop now will help them to lead holistic lives of significance, allowing them to balance commitments to their career with their obligations to family, friends and communities. A recent biochemistry graduate, Molly Hulver ‘19, notes “I learned how to work independently and think critically by drawing conclusions about the data/ results and interpreting what the data meant in a biological context. I also thought it was an incredible experience being able to work alongside such an amazing group of science-driven women!”
Looking Toward the Future
Their recent external funding provides ample financial resources to pursue their collaborative projects. When combined with the state-of-theart laboratory facilities in the Wanek School of Natural Sciences and the acquisition of key instrumentation for research, the sky is the limit. Drs. Blackledge and Miller are now looking toward the next phase of their research and seeking to extend their collaborations with other groups within and outside of HPU. Students will also benefit from these expanded networks. Learning to work with researchers at other universities will grow their scientific knowledge and lead to pipelines for research opportunities and graduate programs across the country.1❧
1 Cutrona, Nicholas, Kyra Renee Gillard, Rebecca Joy Ulrich, Mikaela Seemann, Heather B. Miller, and Meghan S. Blackledge. “From Antihistamine to Anti-Infective: Loratadine Inhibits Regulatory Pasta Kinases in Staphylococci to Reduce Biofilm Formation and Potentiate Β-Lactam Antibiotics and Vancomycin in Resistant Strains of S. Aureus.” ACS Infectious Diseases (2019/05/27 2019).
Gillard, K., H. B. Miller, and M. S. Blackledge. “Tricyclic Amine Antidepressants Suppress Beta-Lactam Resistance in Methicillin-Resistant Staphylococcus Aureus (Mrsa) by Repressing Mrna Levels of Key Resistance Genes.” Chem Biol Drug Des 92, no. 5 (Nov 2018): 1822-29.







