26 minute read
Understanding the Role of Mesothelioma UK Palliative Care Needs of
Mesothelioma Patient Research
from page 9
Advertisement
team of mesothelioma clinical nurse specialists (CNSs).
Mesothelioma UK is committed to ensuring that those diagnosed with mesothelioma have support opportunities equal to that of all patients with cancer through National Health Service (NHS) access to a nurse specialist who is an expert in all aspects of mesothelioma. Currently, the charity provides funding for 25 mesothelioma CNSs based in NHS hospitals across the United Kingdom.
Creating a national expert team of specialist nurses has enabled nationwide insight and experience to be generated and collated regarding mesothelioma. Linking with academic colleagues and institutions, the following studies have been developed with and fully supported by the Mesothelioma UK CNS team.
Mesothelioma and Radical Surgery (MARS STUDY 2): Patient Experience 1 Arm of a Feasibility Trial
MARS 2 5,6 aimed to evaluate the benefi ts of surgery by comparing chemotherapy and surgery against chemotherapy alone. Semi-structured ing the incidence of mesothelioma telephone interviews were conducted among UK military personnel post-randomization, post-surgery (surand veterans, although it is susgery arm), and at 6 and 12 months. pected that this group may encounter particular chalReceiving a Diagnosis of lenges, including securing Mesothelioma (RADIO timely diagnosis, accessing STUDY Meso): Improving the care and support, and obtain2 Patient Experience ing fi nancial help. MiMES aims RADIO Meso 5,7 aimed to address these issues. to understand the experiData collection is ence of communicating a now complete, analysis is diagnosis of malignant ongoing, and fi ndings pleural mesothelioma are being tested among (MPM) from the perstakeholders. We are spective of patients, developing some edufamily caregivers, and cational materials for health professionals. health professionals Key findings include and other support staff the importance of providMs. Liz Darlison that will help inform the ing the diagnosis as an ongoprovision of services to ing process and the need to individuals with an armed provide continuity and consistency. forces background who have been diagnosed with mesothelioma. The expected Military Mesothelioma date of completion is June 2020.
Experience Study STUDY (MiMES): Understanding MiMES PhD Studentship
3 the Impact of As part of the MiMES Mesothelioma for STUDY project, Mesothelioma UK Military Personnel and Veterans 4 has funded a PhD student, Th ere is currently no evidence reportwho has completed her
confi rmation review and a literature review exploring the existing research about the psychological effects of mesothelioma on patients and Dr. Clare Gardiner caregivers. Once ethics clearance has been obtained, approximately 10 participants are expected to be interviewed, beginning in the spring of 2020. Expected date of completion is summer 2022.

Broad Molecular Testing
from page 8
genomic alterations that open the door to clinical trials of investigational therapies.
“NGS should absolutely be the standard of care at this point, especially now that payers are starting to cover it,” said Nathan A. Pennell, MD, PhD, of the Taussig Cancer Institute, Cleveland Clinic Foundation. Th ree tissue-based NGS tests have been approved by the U.S. Food and Drug Administration for patients with lung cancer: FoundationOne CDx, the Oncomine Dx Target Test, and the MSKIMPACT assay. Moreover, in 2017, the U.S. Centers for Medicare and Medicaid Services (CMS) approved coverage of NGS testing under the Parallel Review Program, and private payers have started to follow suit.
“Th ere are too many biomarkers to do individual gene tests anymore; you just can’t get them all done. And it costs a lot more to do multiple tests and bill for each individually than it does to do one NGS test,” Dr. Pennell said. Dr. Pennell and his colleagues recently devised a mathematical model that showed that upfront tissue-based NGS testing was both faster—with a 2-week turnaround time—and less costly than testing sequentially for alterations in EGFR, ALK, ROS1, BRAF, MET, HER2, RET, and NTRK1 using single-gene tests or testing simultaneously for alterations in EGFR, ALK, ROS1, and BRAF using hotspot panels followed by single-gene tests for the remaining biomarkers. 7
5 A qualitative study using documentary analysis, interviews, and consultation meetings with stakeholders. Th e recommendations developed suggest that all patients with mesothelioma have access to a specialist mesothelioma team within a streamlined mesothelioma care pathway and that patients are equipped with the necessary information to guide their decision continued on page 13
Although NGS off ers an obvious solution for hospitals that lack testing capabilities, NGS uptake in community settings remains anemic due to many of the same clinical and logistical problems that plague single-gene tests. Obtaining adequate tissue still tops the list of challenges. “Frequently in the community, tissue is obtained by bronchoscopy, so bronchoalveolar lavage or fi ne needle aspiraone must take negative liquid biopsy results with a grain of salt due to variable amounts of tumor DNA shed into circulation and lower sensitivity levels compared with tissue-based testing, positive results are clinically actionable. Th us, plasma NGS off ers a means to enhance testing rates and improve widespread delivery of molecularly guided therapy.
Mesothelioma Patients’ Experiences of Follow-Up STUDY Across Th ree NHS Trusts
tion cytology is all that is available,” Dr. Gutierrez said. “Th e lack of tissue when you want to do tissue-based NGS can be a nightmare,” which has prompted Dr. Gutierrez to order plasma-based cell-free circulating tumor DNA NGS testing up front in appropriate settings—for example, if a patient is referred to him with only a fi ne needle aspiration specimen.
Accumulating data support the use of plasma NGS as a worthy tool for genomic biomarker testing, especially in situations where tissue is scarce. 8,9 Although “Have a conversation with all the stakeholders—the pathologists, the interventional radiologists, the pulmonologists, the surgeons—to make sure everyone knows the importance of molecular testing and ensures that it’s a priority.”
Aside from leveraging tissue or plasma NGS for newly diagnosed patients with lung cancer, Dr. Pennell believes that one of the simplest ways to improve broad molecular testing within institutions is to promote internal communication.
Dr. Pennell hopes that these conversations will boost lung cancer to the same level as breast cancer in terms of molecular testing. “You’d be shocked if you walked into any oncology offi ce in America to see a woman with breast cancer who did not have a pathology report listing estrogen-receptor, progesterone-receptor, and HER2 FISH status along with the diagnosis and grade,” he explained. “Molecular testing has become a standard component of breast cancer care. And yet when we talk about lung cancer, molecular testing doesn’t get that same gut-level understanding of how important it is. It almost seems more optional, and it’s not,” Dr. Pennell said. ✦
Please note that the full reference list for this article appears online at lungcancernews.org.
References:
1. Jordan EJ, Kim HR, Arcila ME, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for effi cient patient matching to approved and emerging therapies. Cancer
Discov. 2017;7(6):596-609. 2. Nadler E, Espirito JL, Pavilack M, Boyd M,
Vergara-Silva A, Fernandes A. Treatment Patterns and Clinical Outcomes Among Metastatic NonSmall-Cell Lung Cancer Patients Treated in the Community Practice Setting. Clin Lung Cancer. 2018;19(4):360-370. 3. Gutierrez ME, Choi K, Lanman RG, et al.
Genomic profi ling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18(6):651- 659. 4. Lindeman NI, Cagle PT, Aisner DL, et al.
Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Th orac Oncol. 2018;13(3):323-358.
Mesothelioma Patient Research conducted to examine similaripatients with palliafrom page 12 ties and diff erences by gender. tive care needs and Consultations on the fi ndings their families. making with regard to standard treatare currently being conWe hope this ment pathways. ducted with mesothelioma research will
The findings are being written up nurse specialists, asbescontribute to for publication in a peer-reviewed acatos support group forums, better underdemic journal. We are working with solicitors, and patient groups. standing of the Mesothelioma UK to disseminate our Expected date of completion is care and support recommendations with the aim of using August 2020. Prof. Angela M. Tod individuals diagthese to infl uence policy and practice to nosed with mesotheimprove the quality of care received by Mesothelioma and lioma need toward the end patients with mesothelioma. Asbestos Guidelines of life, and will establish the important STUDY Study (MAGS) role of Mesothelioma UK CNSs in meetExploring Clinical 9 MAGS aims to explore the ing these needs. Decision Making in experiences of presentaAn experienced research associate STUDY Mesothelioma Treatment tion, diagnosis, treatment, and care for has recently been appointed to manage 6 Pathways Across Th ree healthcare workers with mesothelioma. the study and will begin her role in NHS Trusts: A MixedTh e study will develop a critical account April 2020. Expected date of compleMethods Study of that experience, which will aid in the tion is June 2021. Although a mixed-methods study development of recommendations to using database analysis, interincrease awareness about the In Summary views, and consultation dangers of asbestos among In just a few years, the collaboration meetings with stakehealthcare workers. between an expert team of nurses, holders was expected Malignant mesoa charity committed to patients, the to commence in thelioma (MM) is an NHS, and successful health academics March 2020, it is aggressive, rare cancer has resulted in an excellent, clinically now on hold due to caused by exposure relevant portfolio of research. Th e colCOVID-19 restricto and inhalation of laboration supports the identifi cation of tions. asbestos. Incidence is relevant studies, the securing of research
NHS Health Research Authority approvals for this Dr. Catherine Henshall higher in certain occupational groups, although grants, and the dissemination of fi ndings. Tools and resources have been study have been obtained. healthcare is not considered developed on the basis of fi ndings from a high-risk industry for MM. Despite the research, ensuring timely and maxiLiving Well With this, many healthcare workers work in mum impact. Malignant Pleural old buildings in which asbestos is likely
Mesothelioma (MPM): Improving the Patient and Family Caregiver Experience: A Qualitative Study
Th is study seeks to identify core themes and recommendations for “living well”
STUDY 7
to be present. Th ere is a concern that cases of MM due to such exposure could increase over time. Raised awareness and action taken by individual healthcare workers and employers could help avoid this.
for individuals with MPM and their A rapid review of published and family members and caregivers and to unpublished research examining the identify recommendations to help them experience of healthcare workers Th is approach is replicable in other achieve meaningful and fulfi lling lives. who have developed mesothelioma is countries. Th e hub-and-spoke national
Individual interviews are completed complete. A Freedom of Information approach to specialist nursing, appropriand transcribed, and analysis of tranrequest has been submitted to identify ate funds or grants, and a close workscription is currently being conducted. how many cases have been submitted ing relationship with committed health Expected date of completion is August against the NHS for mesotheliomaacademics are all equally essential. 2020. related compensation. This is partially Opportunities to forge global partnercomplete and reveals higher levels of ships would be welcome. ✦ Gendered Experience mesothelioma in the population of of Mesothelioma Study healthcare workers than shown by the About the Authors: Ms. Darlison is head of STUDY (GEMS) Office for National Statistics mesotheServices, Mesothelioma UK, and Consultant 8 GEMS aims to explore the lioma mortality figures. Nurse, University Hospitals Leicester, United experiences of men and Kingdom. Dr. Gardiner is senior research women with mesothelioma, their family Understanding the Role fellow, Health Sciences School, The University caregivers, and the various staff they meet of Mesothelioma UK of Sheffield, United Kingdom. Dr. Henshall is and to understand the reason for any difSTUDY CNSs in Meeting the National Institute for Health Research 70@70 ferences that are identifi ed between the 10 Palliative Care Needs of senior nurse research leader, and senior experiences of men and women. Our Patients and Families: nursing research fellow, Oxford Brookes goal is to establish with participants how A Mixed-Methods Study University, and head of Research Delivery, services should best be delivered to be Th e aim of this study is to explore the Oxford Health NHS Foundation Trust, accessible and acceptable to both men palliative care needs of patients with United Kingdom. Prof. Tod is professor of and women. mesothelioma and to assess the diverse Older People and Care, Division of Nursing
Secondary analysis of data from ways in which Mesothelioma UK CNSs and Midwifery, The University of Sheffield, an asbestos support group has been are helping support and empower United Kingdom.
The hub-and-spoke national approach to specialist nursing, appropriate funds or grants, and a close working relationship with committed health academics are all equally essential.


References: 1. Cancer Research UK. Mesothelioma statistics.
Accessed March 5, 2020. https://www. cancerresearchuk.org/health-professional/cancerstatistics/statistics-by-cancer-type/mesothelioma. 2. Peto J, Matthews FE, Hodgson JT, Jones JR.
Continuing increase in mesothelioma mortality in Britain. Lancet. 1995;345(8949):535-539. 3. Hodgson JT, McElvenny DM, Darnton AJ, Price
MJ, Pete J. Th e expected burden of mesothelioma mortality in Great Britain from 2002-2050. Br J
Cancer. 2005;92(3):587-593. 4. Royal College of Physicians. National
Mesothelioma Audit. Accessed April 5, 2020. https://www.rcplondon.ac.uk/projects/nationalmesothelioma-audit. 5. Th e University of Sheffi eld. Mesothelioma Patient
Experience Research Group. Accessed April 5, 2020. https://www.sheffi eld.ac.uk/health-sciences/ our-research/nursing-themes/enhancing-lives/ mesothelioma-patient-experience-researchgroup. 6. Warnock C, Lord K, Taylor B, Tod A. Patient experiences of participation in a radical thoracic surgical trial: fi ndings from the Mesothelioma and Radical Surgery Trial 2 (MARS 2). Trials. 2019;20:598. 7. Taylor B, Warnock C, Tod AM. Communication of a mesothelioma diagnosis: developing recommendations to improve the patient experience. BMJ Open Respir Res. 2019;6:e000413.
INDUSTRY AND REGULATORY NEWS


FDA Approves Durvalumab for Extensive-Stage SCLC
March 27, 2020—The U.S. Food and Drug Administration approved durvalumab in combination with etoposide and either carboplatin or cisplatin for the fi rst-line treatment of patients with extensive-stage SCLC. Th e approval was based on the randomized, multicenter, openlabel, active-controlled CASPIAN trial, which compared durvalumab plus chemotherapy vs chemotherapy alone for previously untreated patients with extensive-stage SCLC.
In CASPIAN, median OS was 13.0 months (95% CI: 11.5, 14.8) for the combination compared with 10.3 months (95% CI: 9.3, 11.2) for chemotherapy alone (HR 0.73; 95% CI: 0.59, 0.91; p = 0.0047). Median PFS was 5.1 months (95% CI: 4.7, 6.2) and 5.4 months (95% CI: 4.8, 6.2), respectively. ORR was 68% (95% CI: 62, 73) and 58% (95% CI: 52, 63), respectively.
With this approval, durvalumab joins atezolizumab in combination with standard platinum and etoposide as options for front-line treatment in the management of chemotherapy-naive extensivestage SCLC. ✦
GLOBAL INITIATIVES
Lung Cancer in the Brazilian Health System: Screening, Drug Approvals, Barriers to Care, and Success Stories
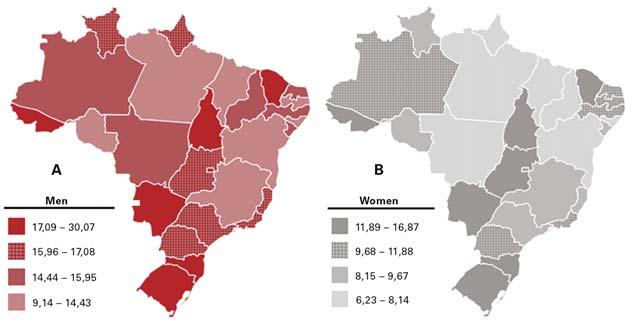


By Ricardo Sales dos Santos, MD, PhD, and According to a large Fig. Geographic Representation of Lung Cancer Adjusted Incidence Rates Per A) 100,000 Men and Juliana Franceschini, PhD cancer registry database B) 100,000 Women, Estimated for 2020 in Brazil 25 study, which included a Th e reality of lung cancer in Brazil is as total of 35,018 patients alarming as it is in the rest of the world. diagnosed with NSCLC, However, problems related to the diagnoand data from 258 hospisis and treatment of lung cancer in Brazil tals in 25 states of Brazil are aggravated by intrinsic characteristics and the Federal District of the national health system. Th e lack (Brasília) between 2000 of access to healthcare in certain regions and 2011, a progresinterferes with the analysis of lung cancer sive increase occurred in incidence and mortality in the country. the percentage of cases Each year, approximately 30,000 new of advanced stages of cases of lung cancer are diagnosed in NSCLC. 8 Efforts have Brazil. 1,2 Th e southern and southeastern been made to promote regions have higher lung cancer prevaexpansion of cancer care lence rates (between 20 and 55 cases per to the Brazilian popu100,000 inhabitants) than the northern lation in the Brazilian and northeastern states (less than 10 Public Health System (SUS). The in diff erent regions of the country have therapy equipment. However, it is notecases per 100,000 inhabitants; Brazilian Ministry of Health started isolated screening programs. At worthy that the availability of appliances Fig.). 3 A possible explanaimplemented in 2000 the the moment the data from these proand especially disposable equipment is tion for this fact lies in “Expande” Project, with grams are being consolidated for a more almost exclusive to the private healtha broader coverage of the coordination of detailed discussion, in partnership with care system. 18 By 2020, fewer than fi ve cancer services in the the National Cancer the “Propulmão” Program, an initiative public hospitals could be cited as one of South and Southeast Institute of Brazil. of medical practitioners and healthcare the state-of-the-art lung cancer care proregions of Brazil, The principal goals professionals whose mission is to previders nationwide. which have more than of this project were to vent lung cancer through education, Brazil is a country of continentwice as many speexpand access to cancer awareness, and discussion on lung tal dimensions which, despite having cialized centers when compared to the other Dr. Ricardo Sales dos Santos treatment in the country and also to reduce regional care. 14
Fortunately, there has Dr. Juliana Franceschini Brazil. Between 2013 and Brazil has just over 700 thoracic surwider availability to the population. 20 In been a major drop-off in 2016, results of the fi rst geons and approximately 600 radiation many cases, patients need to go to court smoking rates in Brazil since the 1980s, Brazilian national screening study oncologists, for an estimated population to access high-cost medications in the reducing the number of smokers by more (BRELT1) were published. 11,12 BRELT1 of more than 200 million. (In contrast to public and private system. Access is furthan 50%, thanks to adherence to public validated low-dose CT imaging as a the United States, only general thoracic ther aff ected by the rapid advancement policies on cigarette control with tax screening method in our region, which surgeons and not cardiothoracic surand specifi cities of the new technologies, increases, a smoking ban in public places, has a high rate of granulomatous disgeons are included in this specialty in which are oft en inaccessible either due and limitations on cigarette advertising. 4-6 ease. We noticed that this rate did not Brazil.) Despite the reasonable number to the clinical condition of the patients, increase the number of biopsies nor did of professionals, there is a higher conmany of whom are too ill to benefi t, or Diagnosis it aff ect the prevalence of lung cancer centration of these individual in large due to competing priorities in the case Unfortunately, most cases of lung cancer compared to other studies published in centers, in cities with more than one milof public health managers, who are comin Brazil are diagnosed at an advanced the northern hemisphere: biopsies were lion inhabitants (15 cities in the country, pelled to cover high costs without the stage; fewer than 10% of cases are diagperformed in approximately 3% of the three of them in the state of São Paulo). 16 certainty of therapeutic effi cacy. nosed at an early stage (stages I and II). 7 790 study participants, and diagnosis Furthermore, there is a shortage of radioHowever, headway is being made. Symptomatic patients have great diffi culty of early stage lung cancer occurred in therapy devices, with fewer than 250 Th ere are promising initiatives involving in securing a diagnosis in both the public 1.5% of participants. Despite these data, units nationwide, and this greatly slows research groups allied with the pharmaand private healthcare systems. Various to date, there are no national public access to radiotherapy treatment. 17 ceutical industry to provide large-scale analyses of patients diagnosed with lung policies on the subject. Nor are there Since 2010, new technologies for the molecular testing for patients diagnosed cancer show that most require multiple defi nitive statements from specialized diagnosis and treatment of lung cancer with lung cancer. Some pharmaceutical medical appointments and face diffi culnational medical entities supporting have been established in diff erent states, companies off er molecular testing with ties in screening or work-up, delaying screening as it should be supported. 13 In such as endobronchial ultrasound, free access to doctors and no charge for diagnosis by many weeks or months. 4 this scenario, some medical institutions robotic surgery systems, and ablative continued on page 15
One of the basic and important barriers among the 10 largest economies in the world, still faces structural problems in mentioned regions. The inequalities. 9 to lung cancer screening is the number sanitation, outbreaks of infectious dislow supply of cancer serof CT scanners in the public system, eases, and epidemics of communicable vices yields fewer diagScreening which is responsible for the healthcare diseases. In addition, violence accounts noses and possibly Since the results of of approximately 70% of the Brazilian for thousands of deaths each year. 19 In many deaths without the National Lung population. Th e private system has six this context, discussion about the treata defi ned cause; this Screening Trial were times more CT scanners, with numbers ment of lung cancer in advanced stages, may be related to published in 2011, 10 similar to those found in high-income with appropriate access to molecular tests the diagnosis of lung efforts have been countries. 15 and coverage of the costs of new drugs cancer or other types made to implement in precision oncology, confronts several of cancer. screening programs in Overcoming Obstacles practical and theoretical obstacles for
David LeDuc
has been named Associate Director, Oncology Patient Affairs at AstraZeneca. In this role, Mr. LeDuc will be responsible for building and strengthening relationships with key stakeholders and advocates and incorporating the patient voice in all aspects of the company’s work. Mr. LeDuc came to AstraZeneca from the Go2 Foundation for Lung Cancer, where he was Executive Director and then Chief Growth Offi cer.
Shirish Gadgeel,
MD, is now the Chief of Division of Hematolog y and O n c ol o g y an d As s o c i ate Director of Patient Experience and
Names and News
Clinical Care at Henry Ford Cancer Institute/Henry Ford Hospital, Detroit, Michigan. He is also a Professor at Wayne State University, where he previously served as leader of the Th oracic Oncology Multidisciplinary Team and Associate Professor of Internal Medicine. He also served as the coleader of the Molecular Th erapeutics Research Program of the Core Cancer Center Grant of Karmanos Cancer Institute in Detroit. Dr Gadgeel has also been a Principal Investigator (PI) of a Southwest Oncology Group trial, S0528. Dr. Gadgeel is an extremely active member of IASLC. He is a member of the editorial board for the Journal of Th oracic Oncology, the editorial group for ILCN, and of the IASLC Communications Committee and the ILCN Editorial Group.
Gideon Blumenthal, MD, has transitioned from Deputy Director of the Oncology Center of Excellence (OCE) at the U.S. Food and Drug Administration (FDA) to Vice President of Global Regulatory Affairs f o r O n c o l o g y at Merck. Dr. Blumenthal was with the FDA for more than a decade, aft er more than 12 years of service at the National Cancer Institute.
Paul G. Kluetz, MD, has been named the FDA’s new Deputy Director of the OCE. During his 10 years with the FDA, Dr. Kluetz founded the OCE’s PatientFocused Drug Development program. Th e IASLC looks forward to our continued collaboration and partnership with the FDA with regard to lung cancer therapies, diagnostics, and policies, such as major pathologic response and smoking cessation.
Lung Cancer in Brazil
from page 14
patients 21-24 ; such partnerships should allow information on the occurrence of mutations and molecular biomarkers in our population to be reported, with better delineation of public policy in the acquisition of medicines.
In our opinion, one of the greatest challenges of the Brazilian health system is the need to integrate the activities of the private system with the public system, generating demand and access in an organized and structured way to various technologies already available in some regions. New value-based compensation models need to be quickly tested and made viable, at the risk of further disruption of the provision of medical services. ✦
In addition to her roles of Associate D i r e c t o r f o r Clinical Research at Cedars-Sinai Cancer and Medical Oncology Director of Women’s Guild Lung Institute in the Department of Medicine at Cedars-Sinai Cancer, Karen L. Reckamp, MD, MS, has been named the Director of the Division of Medical Oncology there. Most recently, Dr. Reckamp served as Co-Director of the Lung Cancer and Th oracic Oncology Programs and Medical Director of the Clinical Research Operations and Clinical Trials Offi ce at City of Hope. Dr. Reckamp is an active IASLC volunteer, serving as a member of both the Communications Committee and the ILCN Editorial Group. ✦
About the Authors: Dr. Sales dos Santos is the head of Respiratory Medicine at Hospital Cárdio Pulmonar, Salvador/Bahia, Brazil. He works as staff thoracic surgeon at Hospital Israelita Albert Einstein, São Paulo, and CLION, Einstein’s Oncology Network, Salvador/Bahia, Brazil. He contributes as member of IASLC´s Screening & Early Detection Committee, ProAr Foundation, and Propulmão Program. Dr. Franceschini is a physical therapist and research coordinator at Propulmão Program, project manager at ProAr Foundation, and a postdoctoral researcher in the Respiratory Division, Universidade Federal de São Paulo/ UNIFESP, Brazil.
Visit JTO.org to read a more extensive article published in February 2020 about lung cancer prevention, diagnosis, and treatment in Brazil.
Regional perspectives also are available at JTO.org for: • China, • Italy, • Canada, • Saudi Arabia, • and 14 other countries.
References:
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. 2. Ferlay J, Colombet M, Soerjomataram I, et al.
Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953. 3. INCA. Estimativa 2018: incidência de câncer no
Brasil. Rio de Janeiro: INCA; 2017:128. 4. de Sa VK, Coelho JC, Capelozzi VL, de Azevedo
SJ. Lung cancer in Brazil: epidemiology and treatment challenges. Lung Cancer (Auckl). 2016;7:141-148. 5. Monteiro CA, Cavalcante TM, Moura EC, Claro
RM, Szwarcwald CL. Population-based evidence of a strong decline in the prevalence of smokers in Brazil (1989-2003). Bull World Health Organ. 2007;85(7):527-534. 6. Ministério da Saúde. Vigitel Brazil 2018: surveillance of risk and protective factors for chronic diseases by telephone survey: estimates of frequency and sociodemographic distribution of risk and protective factors for chronic diseases in the capitals of the 26 Brazilian states and the Federal District in 2018. Brasília: Ministério da
Saúde; 2019:132. 7. Araujo LH, Baldotto C, Castro G, Jr., et al. Lung cancer in Brazil. J Bras Pneumol. 2018;44(1):55-64. 8. Costa G, Th uler LC, Ferreira CG. Epidemiological changes in the histological subtypes of 35,018 non-small-cell lung cancer cases in Brazil. Lung
Cancer. 2016;97:66-72. 9. INCA. Expansão da Assistência Oncológica (Projeto EXPANDE) Rio de Janeiro: INCA. https://www.inca.gov.br/acesso-a-informacao/ acoes-e-programas/projeto-expande. Published 2018 [updated July 17, 2018]. Accessed January 9, 2020. 10. Kramer BS, Berg CD, Aberle DR, Prorok PC.
Lung cancer screening with low-dose helical CT: results from the National Lung Screening Trial (NLST). J Med Screen. 2011;18(3):109-11. 11. Santos RS, Franceschini JP, Chate RC, et al. Do
Current Lung Cancer Screening Guidelines Apply for Populations with High Prevalence of Granulomatous Disease? Results from the First Brazilian Lung Cancer Screening Trial (BRELT1).
Ann Th orac Surg. 2016;101(2):481-488. 12. Santos RS, Franceschini JP, Kay FU, et al. Lowdose ct screening for lung cancer in brazil: A study protocol | Rastreamento de cancer de pulmão por meio de TC de baixa dosagem no Brasil: Protocolo de pesquisa. J Bras Pneumol. 2014;40(2):196-199. 13. Mathias C, Prado GF, Mascarenhas E, et al. Lung
Cancer in Brazil. J Th orac Oncol. 2020;15(2): 170-175. 14. Propulmão Program. http://www.propulmao. com.br/. Accessed January 9, 2020. 15. FIOCRUZ. A saúde no Brasil em 2030 - prospecção estratégica do sistema de saúde brasileiro: estrutura do fi nanciamento e do gasto setorial. 22 ed. Rio de Janeiro: Fiocruz/Ipea/ Ministério da Saúde/Secretaria de Assuntos Estratégicos da Presidência da República; 2013:168. 16. Tedde ML, Petrere Jr. O, Pinto Filho DR, et al.
General thoracic surgery workforce: training, migration and practice profi le in Brazil. Eur J
Cardiothorac Surg. 2015;47(1):e19-24. 17. Ministério da Saúde. Censo Radioterapia.
Brasilia; 2019:10. 18. Interfarma. Câncer no Brasil - A jornada do paciente no sistema de saúde e seus impactos sociais e fi nanceiros. Associação da Indústria
Farmacêutica de Pesquisa (Interfarma); 2019:88. 19. Reis C, Barbosa L, Pimentel VP. O desafi o do envelhecimento populacional na perspectiva sistêmica da saúde. BNDES Setorial. 2016;44:37. 20. Kaliks RA, Matos TF, Silva VA, Barros LHC.
Diff erences in systemic cancer treatment in Brazil: my Public Health System is diff erent from your Public Health System. Braz J Oncol. 2017;13(44):12. 21. Bristol-Myers Squibb. IODetect. https://www. iodetect.com.br. Accessed January 9, 2020. 22. MSD. PD-Point – Programa de Biomarcador
MSD. https://www.pdpoint.com.br. Accessed
January 9, 2020. 23. Pfi zer. Pfi zerAlvo. http://alkalvo.com.br. Accessed
January 9, 2020. 24. AstraZeneca. ID. http://www.programaid.com.br.
Accessed January 9, 2020. 25. INCA. Neoplasia maligna da traqueia, dos brônquios e dos pulmões (taxas ajustadas). Rio de Janeiro: INCA. https://www.inca.gov.br/ estimativa/taxas-ajustadas/neoplasia-malignada-traqueia-dos-bronquios-e-dos-pulmoes Accessed May 20, 2020.
SHARE YOUR
COVID-19 EXPERIENCES


The IASLC is collecting member and patient experiences with
COVID-19 as a way to pool knowledge and connect our members during this public health crisis. Visit LungCancerNews.org and click the COVID-19 banner to access the latest research and IASLC statements. To submit your story, please send via email to
COVID19@iaslc.org.
Help us create hope and fund research!elp us create hope and fund research! DONATE TODAY


WHY SUPPORT THE ILC FOUNDATION? The answer is simple. We fund fellowships to support innovative research for conquering lung cancer. Every dollar you give to the ILC Foundation goes straight to research.





IASLC.org/ILC-Foundation