About InBody
















✓ Unmatched Accuracy
Validated to be 98.6% accurate compared to DEXA scans, ensuring precise body composition measurements
✓ Research & Validation
Backed by over 7,000 studies worldwide, making it one of the most trusted devices in the industry.
✓ Over 140 Million Global Tests
InBody has conducted over 140 million body composition tests worldwide, with real-time counts demonstrating its global trust and usage.
✓ Canadian Medical Device
InBody devices are certified Health Canada Medical Device Type II, meeting strict regulatory standards.
✓ No Monthly Fees
Enjoy full functionality without recurring costs when using the InBody Result Sheets.
✓ On-Site Installation & Training
We offer installation and training across most major cities, ensuring that you and your team are fully supported.
✓ Industry Experience
Established worldwide in 1999 and in Canada since 2005, InBody has a long-standing reputation for quality and innovation.
✓ Trusted by Experts
InBody is used by leading medical facilities, universities, and government organizations across the globe.
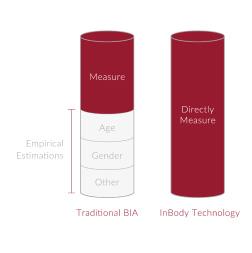
✓ No Empirical Estimation
InBody uses direct measurement without relying on estimations based on age, gender, or ethnicity.
✓ Sarcopenia Analysis on All Models
Every InBody device provides sarcopenia parameters to help identify and manage muscle loss.
✓ Segmental Analysis
Provides a comprehensive breakdown of body composition, including muscle mass and fat in each limb and the trunk, for more targeted insights
✓ LB Web Solution
Easily track and manage data with our LB web solution, simplifying the management of client results.
✓ Free InBody App
Clients can easily track their progress by scanning the QR code on the result sheet and uploading their data to the free InBody app.
✓ Compact Footprint
Our devices are designed to fit seamlessly into your space without compromising functionality.
✓ Tailored Solutions
A wide range of products to meet the specific needs of your business, whether in the medical, fitness, or wellness sectors.
✓ Outstanding Customer Service
InBodyCanada is known for its excellent customer service, providing ongoing support to ensure you get the most out of your InBody device.



InBody Co. Ltd. was founded in Seoul, South Korea, by Dr Kichul Cha.
At Harvard Medical School in the early 1990s, Dr Cha discovered that the results of bioelectrical impedance analysis at that time needed to be more accurate. He recognized that different body parts had different levels of impedance; to accurately analyze body composition, the body would need to be analyzed in segments instead of as a whole.
With the creation of InBody Co. Ltd. in 1996, Dr Cha transformed how body composition would be measured.





InBodyCanada has been operating in Canada since 2004. Headquartered in Ottawa, Ontario, our team members operate within most urban areas across the country, enabling us to provide quick response local support and service.
We have made it our mission to exceed customer expectations and provide our clients\with advanced biomedical technology that combines convenience, accuracy and reproducibility into one easy-to-use device. Whether your area of expertise is fitness, medicine, wellness, or nutrition, we know we can benefit your business or practice.
Our accurate and precise body composition analyzers and fully automated blood pressure monitors have been used in over 5,500 clinical studies worldwide and are trusted by professionals around the globe.




InBody’s medical-grade body composition analyzers rely on four pillars of measurement multi-frequency bioelectrical impedance analysis (DSM-MFBIA)
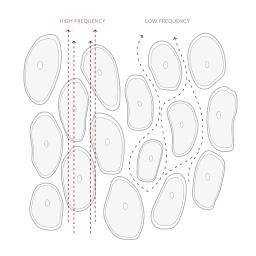
InBody uses multiple currents at varying frequencies to provide precise body water analysis.
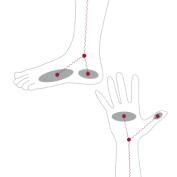
When measuring impedance with electrodes, contact resistance occurs. InBody accounts for contact resistance with strategically placed electrodes to ensure that measurements are accurate and reproducible.



technology to provide clients with accurate and precise direct segmental (DSM-MFBIA) results that have been extensively validated to gold-standard methods.
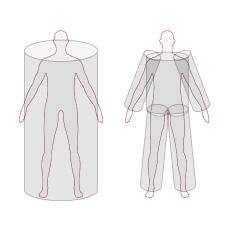
InBody measures each of the body’s five cylinders (left arm, right arm, torso, left leg, and right leg) to deliver accurate and detailed results.
InBody measures your impedance independently, so your results are not affected by your age, sex, ethnicity, athleticism, or body shape.




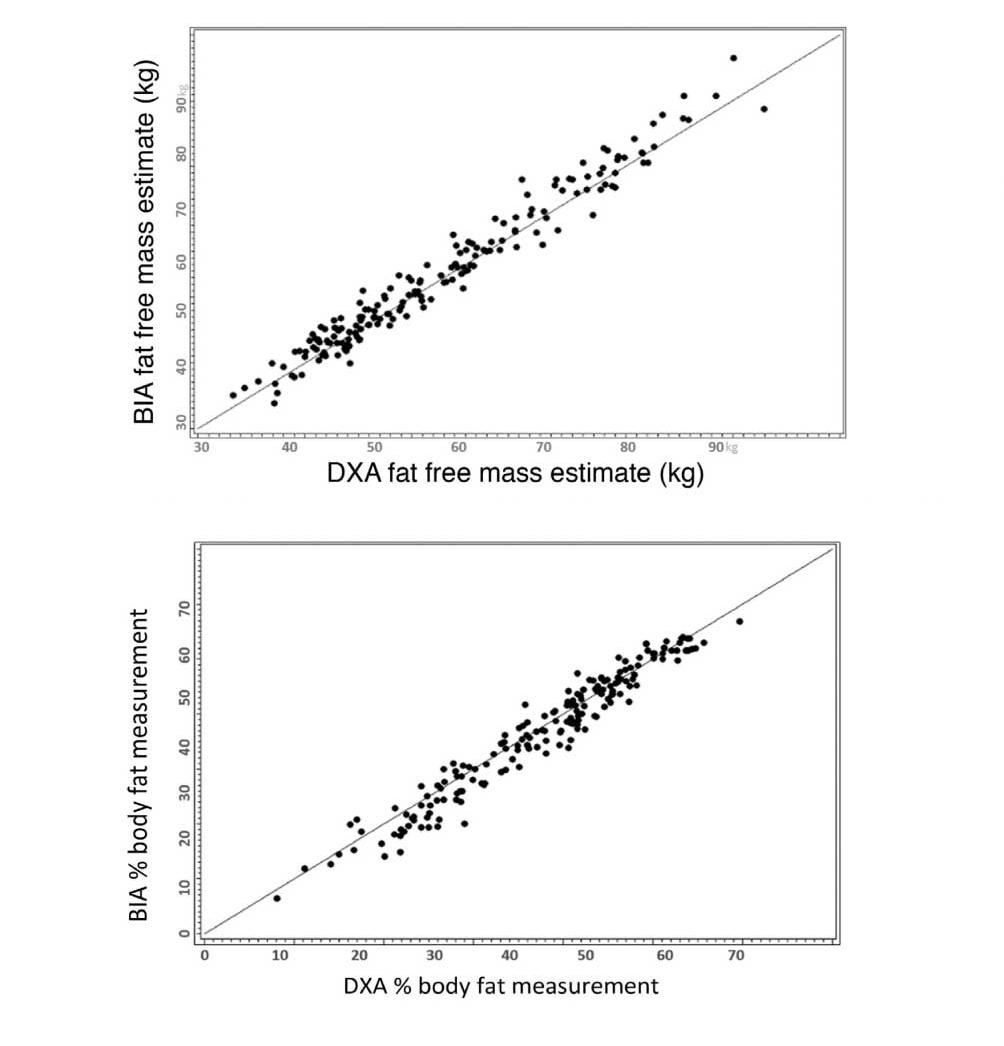
Researchers from the Mayo Clinic compared InBody technology to DXA, a body composition gold standard, for estimating fat-free mass and percent body fat in a 2020 study. They validated InBody as 98% correlated to DXA and overall had good agreement amongst all BMI categories. The authors note that InBody “allows providers to further risk stratify patients and develop treatment plans.” On another note, InBody “takes the same amount of space as a scale and allows our clinical assistants… to obtain body composition measurements in a few minutes while obtaining other vitals (eg, weight, heart rate, and blood pressure).”
Ryan T. Hurt; Jon O. Ebbert; Ivana Croghan; Sanjeev Nanda; Darrell R. Schroeder; Levi M. Teigen; Saketh R. Velapati; and Manpreet S. Mundi. The Comparison of Segmental Multifrequency Bioelectrical Impedance Analysis and Dual-Energy X-ray Absorptiometry for Estimating Fat Free Mass and Percentage Body Fat in an Ambulatory Population. Journal of Parenteral and Enteral Nutrition. 00.0, 1-8 (2020). 10.1002/jpen.1994


A 2022 study published by the British Journal of Nutrition compared the accuracy of DSM-BIA (InBody 770) amongst body composition gold standards (DXA, ADP) and BIS with 110 participants of varying races and ethnicities. The study found that alongside DXA and ADP, InBody provided “excellent to ideal” measures of percent body fat and “ideal” fat free mass measurements compared to the criterion. Moreover, our devices can be used in a multi-ethnic sample “to obtain highly valid results” without estimations based on ethnicity.
Blue, M.; Hirsch, K.; Brewer, G.; Cabre, H.; Gould, L.; Tinsley, G.; Ng, B.; Ryan, E.; Padua, D.; Smith-Ryan, A. (February 2022). “The validation of contemporary body composition methods in various races and ethnicities”. British Journal of Nutrition. 10.1017/ S0007114522000368. PMID: 35109945.*




The Journal of Pediatric Gastroenterology and Nutrition published a 2020 study that evaluated the accuracy of different BIA devices relative to DXA in children with obesity. Amongst other BIA, InBody stood out as it was “relatively accurate for estimating body fat percentage” and was not limited to patients younger than 10 or patients with high body fat percentage (>50%). Beyond being a “reasonably accurate alternative to DXA” in pediatric obesity, InBody is available at a tenth of the cost.
Khan, S., Xanthakos, S. A., Hornung, L., Arce-Clachar, C., Siegel, R., & Kalkwarf, H. J. (2020). Relative Accuracy of Bioelectrical Impedance Analysis for Assessing Body Composition in Children With Severe Obesity. Journal of Pediatric Gastroenterology & Nutrition, 70(6), e129–e135. https://doi.org/10.1097/MPG.0000000000002666



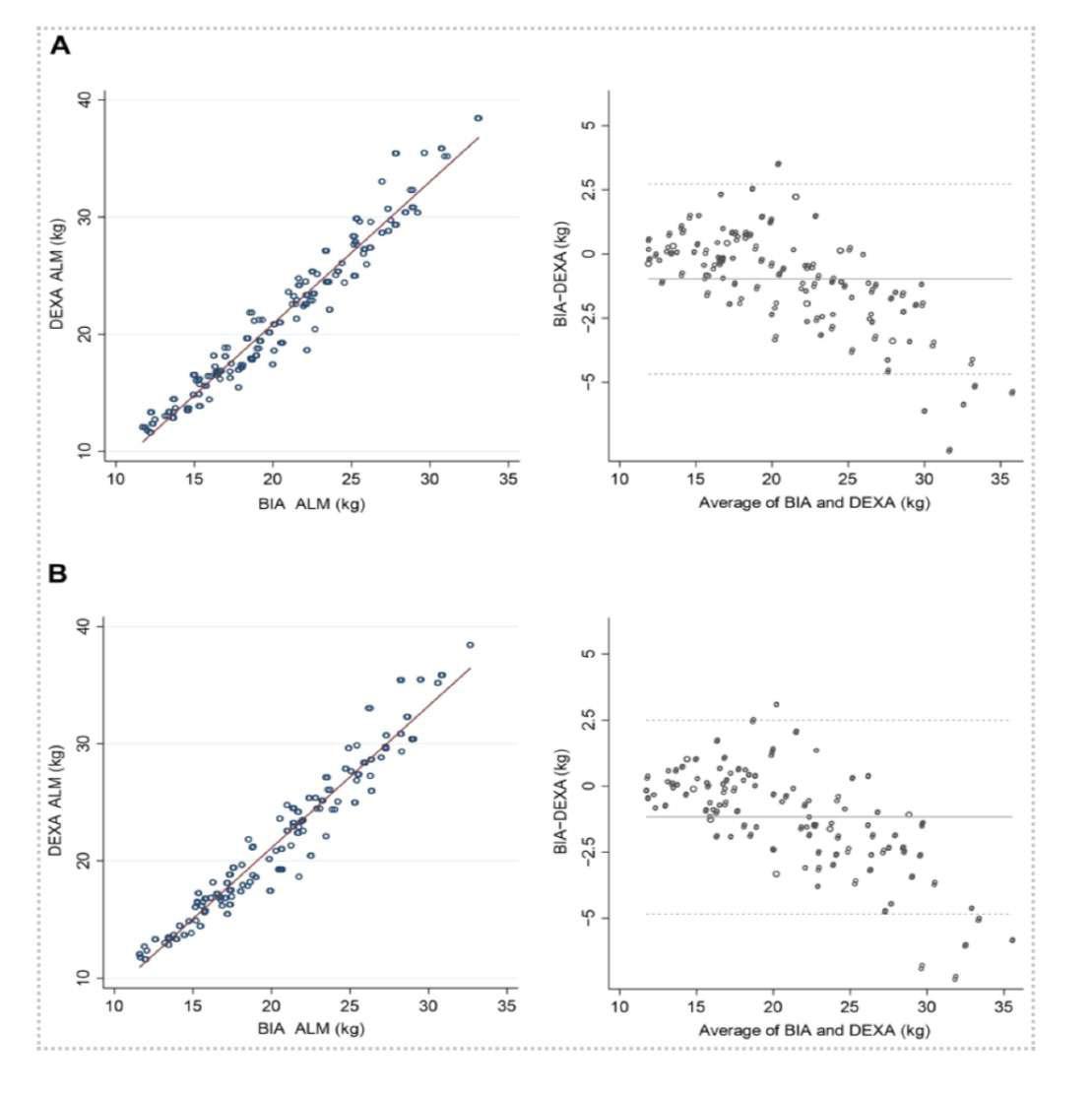
Now with more frequencies, and frequencies up to 3000 kHz, the InBody 970 and the BWA 2.0 devices provide not only more outputs, but greater precision. Life journal in a July 2022 publication evaluated the newest InBody devices compared to dual-energy X-ray absorptiometry (DEXA) in a cohort of 109 individuals. What they found was that high-frequency InBody devices showed a “high correlation with the DEXA results” for assessing appendicular lean mass (β ≥ 0.95), fat-free mass (R2 ≥ 0.95), and percent body fat (R2≥ 0.89).
Yi, Y.; Baek, J.; Lee, E.; Jung, H.; Jang, I. (July 2022). “A Comparative Study of High-Frequency Bioelectrical Impedance Analysis and Dual-Energy X-ray Absorptiometry for Estimating Body Composition”. Life 2022, 12(7), 994; https://doi.org/10.3390/life12070994



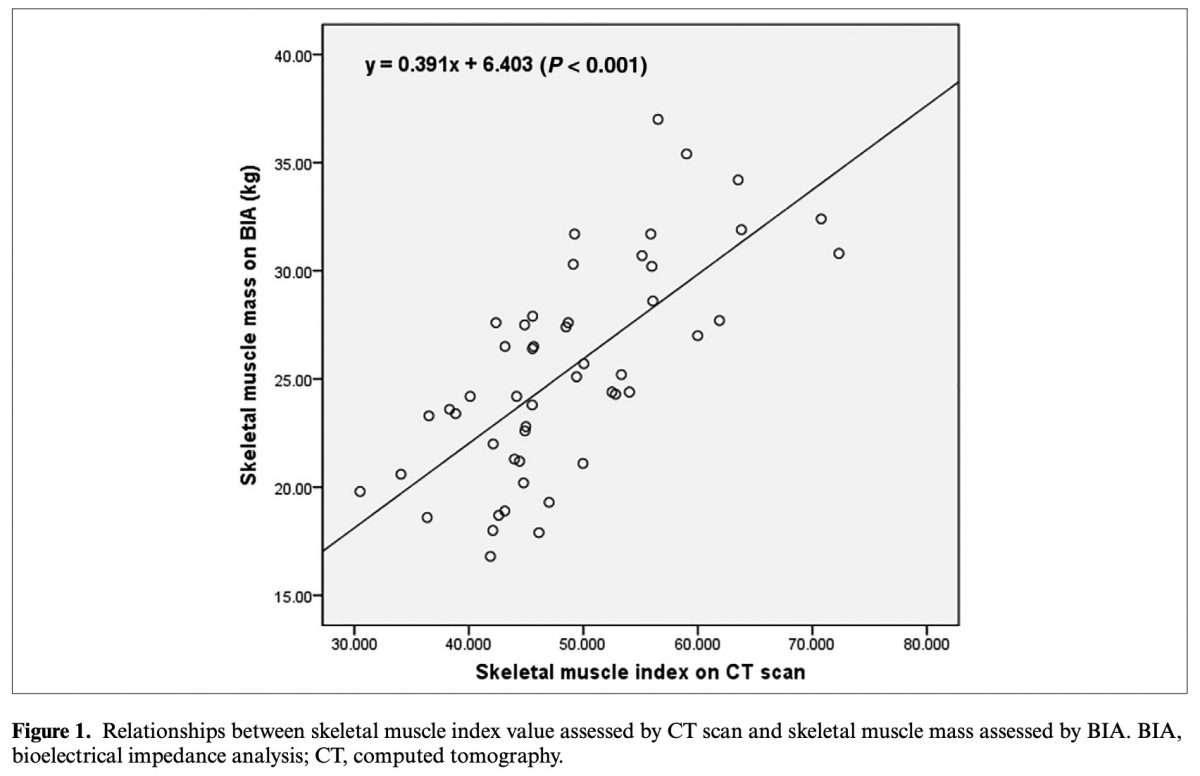
A 2020 publication from Nutrition in Clinical Practice evaluated the accuracy and reliability of InBody compared to computed tomography (CT) to assess skeletal muscle mass (SMM) in colorectal cancer patients. In their results, they found that InBody SMM has “very significant correlation” (p<0.001) to CT even after adjusting for age, weight, and BMI (p<0.001). Overall, the authors suggest that InBody could be “an alternative to CT scan for the assessment of SMM in colorectal cancer patients.”
Eun Young Kim, MD,; Seong Ryong Kim,; Daeyoun David Won;Moon Hyung Choi; and In Kyu Lee. Multifrequency Bioelectrical Impedance Analysis Compared With Computed Tomography for Assessment of Skeletal Muscle Mass in Primary Colorectal Malignancy: A Predictor of Short-Term Outcome After Surgery. Nutrition in Clinical Practice 35.4, 664-674 (2020). https://doi. org/10.1002/ncp.10363




A 2019 study from the Journal of Functional Morphology and Kinesiology compared InBody 770 and DXA for body composition changes in exercise-trained men and women after a 4-week program. The researchers found that the device “can predict changes in body composition (i.e., fat mass, fat-free mass, and percent body fat) similar to a DXA.” InBody’s multiple frequencies “may be less subject to error” and “likely a superior method for the estimation of total body composition.”
Antonio, J.; Kenyon, M.; Ellerbroek, A.; Carson, C.; Burgess, V.; Tyler-Palmer, D.; Mike, J.; Roberts, J.; Angeli, G.; Peacock, C. Comparison of Dual-Energy X-ray Absorptiometry (DXA) Versus a Multi-Frequency Bioelectrical Impedance (InBody 770) Device for Body Composition Assessment after a 4-Week Hypoenergetic Diet. J. Funct. Morphol. Kinesiol. 2019, 4, 23. https://doi.org/10.3390/jfmk4020023



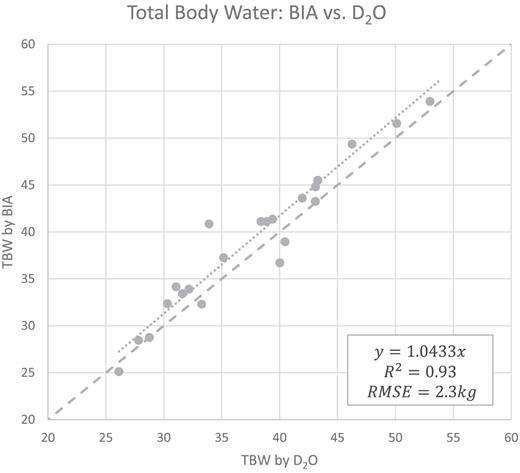
Researchers in the American Journal of Clinical Nutrition assessed the integration of DXA and InBody for a rapid body composition model in a 2018 clinical population study. What they found was that InBody had “high agreement” with D20 TBW measurements, the gold standard for assessing body water. Nonetheless, InBody is “an appealing method for clinical TBW measurement owing to its low cost, rapid results, and amenability to field calibration.”
Ng, B. K., Liu, Y. E., Wang, W., Kelly, T. L., Wilson, K. E., Schoeller, D. A., Heymsfield, S. B., & Shepherd, J. A. (2018). Validation of rapid 4-component body composition assessment with the use of dual-energy X-ray absorptiometry and bioelectrical impedance analysis. American Journal of Clinical Nutrition, 108(4), 708–715. https://doi.org/10.1093/ajcn/nqy158



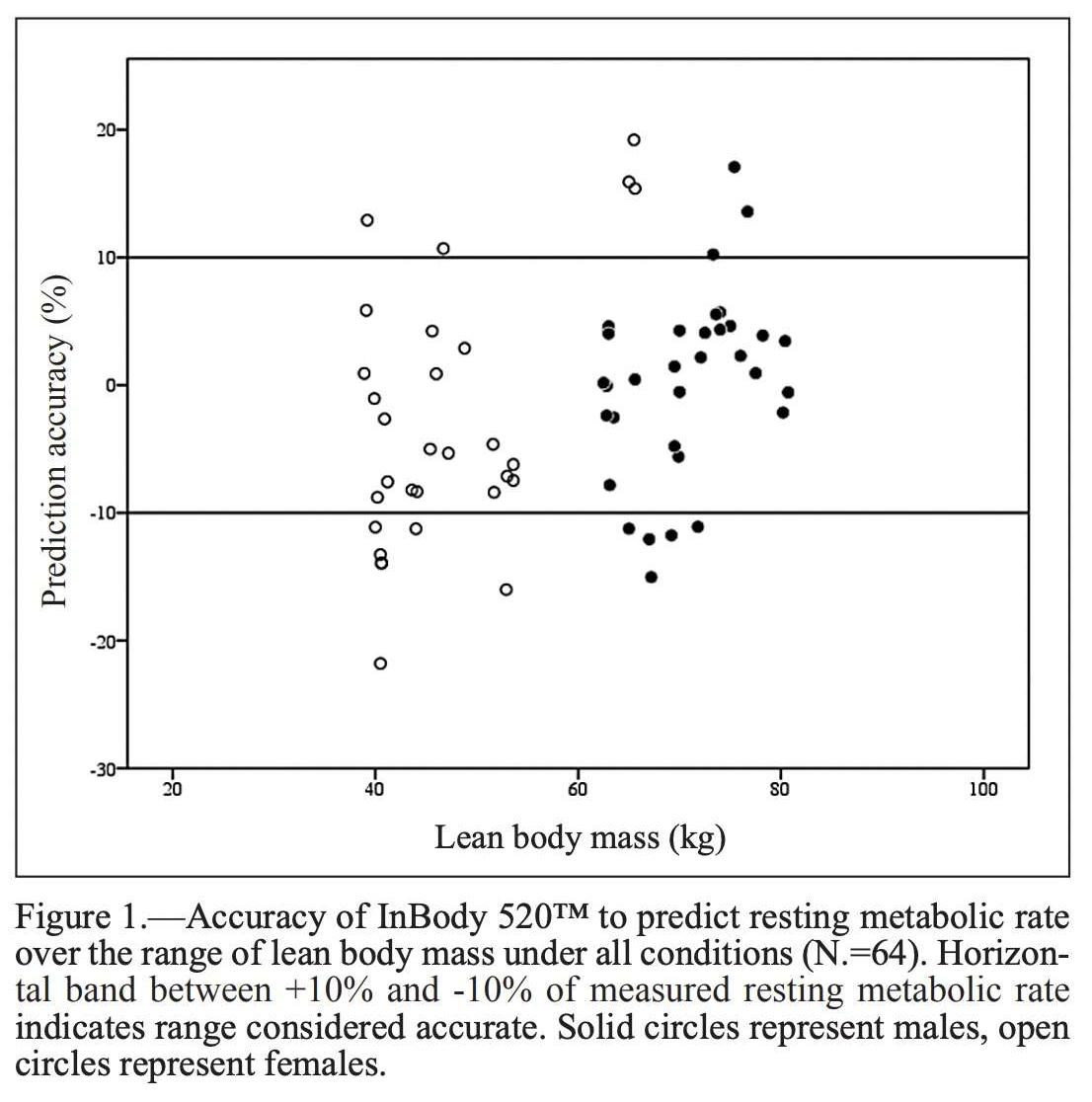
In a 2018 study published by the Journal of Sports Medicine and Physical Fitness, a team investigated the validity in an InBody device for predicting resting metabolic rate (RMR). The team found that InBody “provides valid measurements of RMR” when compared to gas analysis, the current standard. RMR values based on the InBody analysis was “a valid, more practical, and less time-intensive alternative to determine RMR in a clinical setting.”
Salacinski, A. J., Howell, S. M., & Hill, D. L. (2018). Validity of the InBody 520TM to predict metabolic rate in apparently healthy adults. Journal of Sports Medicine and Physical Fitness, 58(9), 1275–1280. https://doi.org/10.23736/S0022-4707.17.06719-6



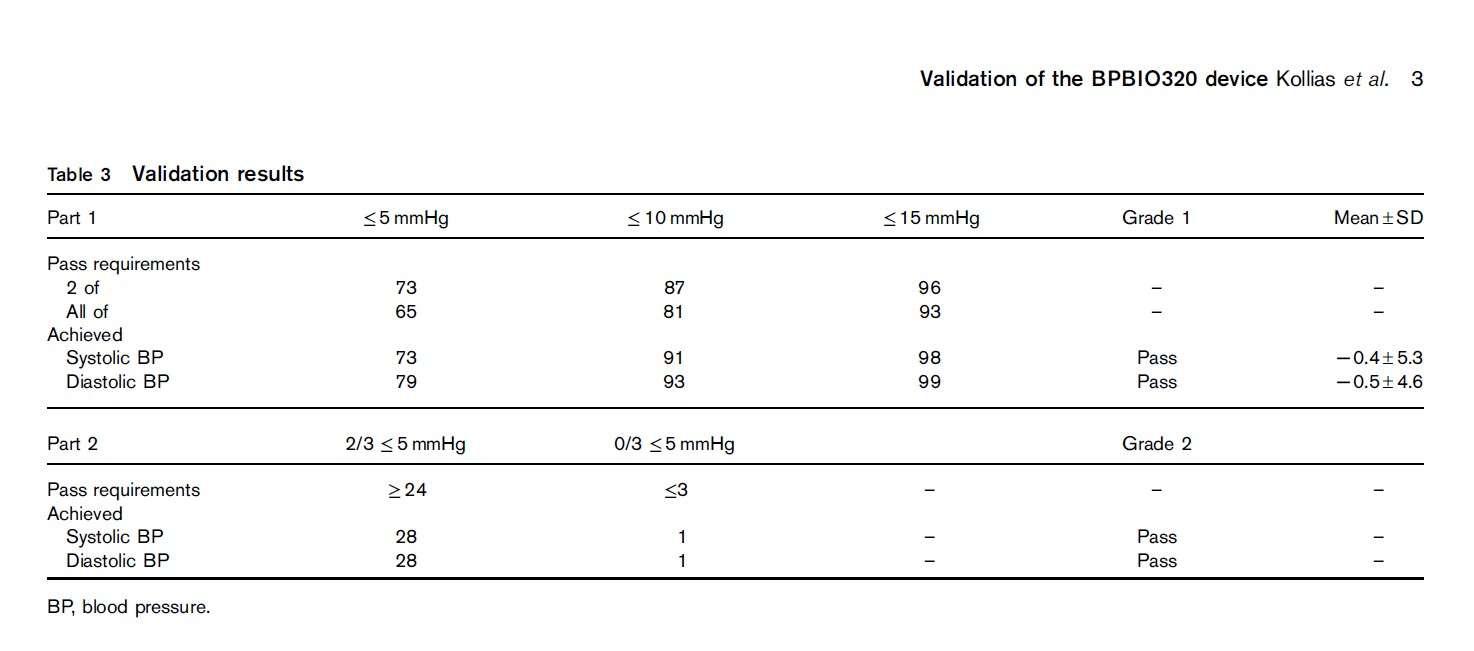
“The single-cuff oscillometric device InBody BPBIO320 developed for self-measurement by adults in public spaces passed all the validation requirements of the 2010 ESH-IP and can be recommended for clinical use.” The test–reference BP difference was 0.5±4.3/−1.1±4.3mmHg (systolic/diastolic) in participants with arm circumference less than 31.4 cm (n=17) and −1.3±6.0/0.2±4.8mmHg in those with an arm circumference of at least 31.4 cm (n=16). This indicates that the margin of error is ~5/5.5 mmHg (systolic/diastolic) in those with smaller arms, and ~7/5 mmHg (systolic/diastolic) in those with higher arm circumference.
Kollias, A., Stambolliu, E., Kyriakoulis, K. G., Papadatos, S. S., & Stergiou, G. S. (2019). Validation of the single-cuff oscillometric blood pressure monitor InBody BPBIO320 for public use according to the 2010 European Society of Hypertension International Protocol. Blood pressure monitoring, 24(1), 30-32.



Derek Shannon, Canada West
derek@inbodycanada.ca | 236-888-5550
Sydney Ireland, Canada Central & East
sydney.ireland@inbodycanada.ca | 416-891-4169
Philippe Miville, Quebec
philippe@inbodycanada.ca | 438-869-6511
Richard Bandura, Atlantic
info@inbodycanada.ca | 613-297-3777
Sebastien Da Silva, Service & Operations Manager
CS@inbodycanada.ca | 613-277-0266
Marketing Team
marketing@inbodycanada.ca









