Understanding the New Myeloma Landscape










1
1
IMF Patient and Family Webinar
S. Vincent Rajkumar, MD Mayo Clinic Rochester, MN
Brian G.M. Durie, MD International Myeloma Foundation Studio City, CA
Beth Faiman,PhD, MSN, APN-BC, AOCN®, BMCTN®, FAAN, FAPO IMF Nurse Leadership Board Cleveland Clinic
Taussig Cancer Institute Cleveland, OH
Alfred Garfall, MD Hospital of the University of Pennsylvania Philadelphia, PA
Thank you to our sponsors!















Audience Q&A • Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion. • If you have a question that does not get answered today, you can contact our Infoline at 800-452-CURE (2873) US & Canada, 1-818487-7455, or email infoline@myeloma.org.
Feedback Survey
At the close of the meeting a feedback survey will pop up. Click “continue” to complete the survey.


This will also be emailed to you shortly after the workshop.
Please take a moment to complete this survey.
We Want to Hear From You!
IMF Patient and Family Webinar

10:00 – 10:05 AM Welcome Announcements with Dr. Brian G.M. Durie

10:05 – 10:30 AM Options for Early Disease - S. Vincent Rajkumar
10:30 – 10:55AM Frontline Therapy Options Brian G.M. Durie
5
10:55 – 11:10AM Q&A 11:10 – 11:25AM BREAK 11:25 – 11:50PM Supportive Care - Beth Faiman 11:50 – 12:05 PM Q&A 12:05 – 12:30PM Relapse and Role of New Immune Therapies - Dr. Garfall
12:30 – 12:45PM Q&A 12:45 PM Webinar Survey & Closing Remarks AGENDA *all times listed in Pacific Time Zone


3
➢ Frontline Therapy ➢ Supportive Care
Relapse and Role of New Immune Therapies
Topics for Today’s Webinar ➢ Options for Early Disease
➢
S.
IMF Patient and Family Webinar



Options for Early Disease
7
Dr.
Vincent Rajkumar, Mayo Clinic - Rochester
Options for Early Disease






 S. Vincent Rajkumar Professor of Medicine, Mayo Clinic @VincentRK
Mayo Clinic College of Medicine
Mayo Clinic Comprehensive Cancer Center
Scottsdale, Arizona Rochester, Minnesota Jacksonville, Florida
S. Vincent Rajkumar Professor of Medicine, Mayo Clinic @VincentRK
Mayo Clinic College of Medicine
Mayo Clinic Comprehensive Cancer Center
Scottsdale, Arizona Rochester, Minnesota Jacksonville, Florida
Speaker Name

No conflicts to disclose
Revised IMWG Criteria
MGUS SMM MM

• <10% BMPC AND • <3 gm/dL M protein AND
No MDE
MDE, myeloma-defining events
• ≥10%-60% BMPC OR • ≥3 gm/dL S. M protein OR • ≥500 mg/24h Ur. M protein AND • No MDE
• PCPD, AND • 1 or more MDE • CRAB • ≥60% BMPC • ≥100 FLC ratio • >1 MRI focal lesion
Rajkumar SV, Dimopoulos M, Palumbo A, et al. Lancet Oncol. 2014;15(12):e538-e548.
•

mSMART 3.0: Risk Stratification of Active MM FISH t(4;14) t(14;16) t(14;20) Del 17p 1q gain • Double-Hit Myeloma = Any 2 high risk abnormalities • Triple-Hit Myeloma = 3 or more high risk abnormalities All others including: Trisomies t(11;14) t(6;14) High-Risk Myeloma Standard-Risk Myeloma Rajkumar SV © 2020 msmart.org
INITIAL THERAPY

vs






Six 28-day Cycles of Rd Rd 525 patients Newly diagnosed MM After induction; Both arms recevied Rd Maintenance Until PD, Toxicity or Withdrawal Durie BGM, et al. ASH 2015
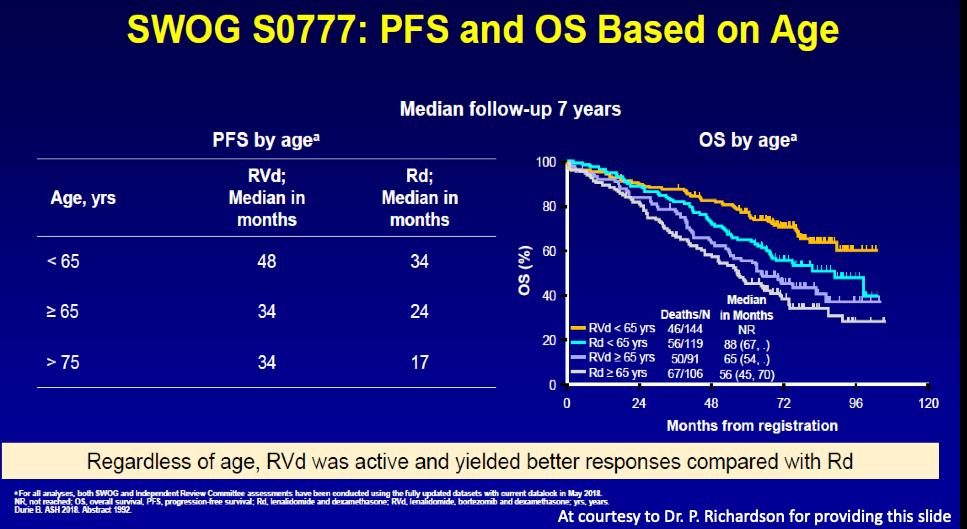
SWOG VRd
Rd Eight 21-day Cycles of VRd VRd


S0777 Trial: VRd
Rd
(10.1016/S0140-6736(16)31594-X) Copyright © 2017 Elsevier Ltd Terms and Conditions PFS: 43 versus 30 months Survival: 72 versus 64 months
vs
Durie et al. The Lancet 2017 389, 519-527DOI:
SWOG VRd vs Rd Updated

VRd Median survival >84 months 5 year survival: 69%
results Durie B. Blood Cancer Journal 2020
Overall Survival
MAIA DRd vs Rd DRd Rd





737 patients Newly diagnosed MM Age >=65

MAIA DRd vs Rd UPDATED RESULTS Facon T, Lancet Oncol 2021 DRd Median survival: >60 months 5-year survival: 66% PFS >65 versus 32 months
KRd vs VRd (ENDURANCE TRIAL)

Kumar S. ASCO 2020
No benefit in PFS or survival with KRd
GRIFFIN TRIAL: Dara-VRd vs VRd Quadruplets as Initial Therapy
3-year PFS rate: 89% vs 81%Sustained
MRD- (12 months): 44% vs 13%
Survival at 3 years: 92%

Laubach J, et al. ASH 2021
ROLE OF TRANSPLANT






IFM 2009: Early vs Delayed Autologous Transplant VRd Transplant Lenalidomide maintenance x 12 months VRd Lenalidomide maintenance x 12 months Delayed transplant at relapse 700 patients Newly diagnosed MM Age <65

47
months 8-year Survival: Identical
IFM 2009 Early vs Delayed Transplant Perrot A. ASH 2020 PFS:
versus 35
(62%)





DETERMINATION: Early vs Delayed Autologous Transplant VRd Transplant Lenalidomide maintenance until progression VRd Lenalidomide maintenance until progression Delayed transplant at relapse 722 patients Newly diagnosed MM Age <65

PFS: 68 versus 46 months 5-year Survival: Identical (80%)
DETERMINATION: Early vs Delayed Transplant Richardson, NEJM 2022
MAINTENANCE

Lenalidomide Maintenance Meta-analysis

PFS: 53 versus 24 months
Survival: >100 versus 86 months
Published in: Philip L. McCarthy; et al. JCO 2017, 35, 3279 3289.
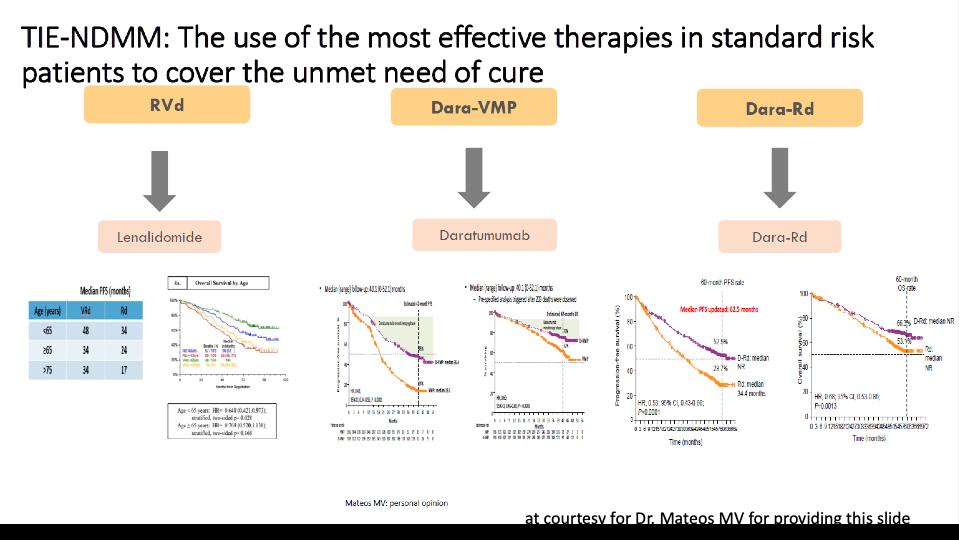
Newly Diagnosed Myeloma: Transplant Eligible

High Risk VRd or Dara-VRd* x 3-4 cycles
maintenance Delayed ASCT at relapse
maintenance
Bortezomib plus Lenalidomide maintenance
Standard Risk VRd x 3-4 cycles Lenalidomide
Lenalidomide
Early ASCT Stem cell collection and cryopreservation; then continue VRd x 5-8 additional cycles Early ASCT
Rajkumar
Blood
*High risk patients
SV.
Cancer J. 2020
High Risk Standard Risk
Newly Diagnosed Myeloma: Transplant Ineligible Rajkumar
SV. Blood Cancer J. 2020

Bortezomib-plus Lenalidomide maintenance
x 8-9 cycles VRd x 8-12 cycles Lenalidomide maintenance DRd until progression
VRd
SMOLDERING MULTIPLE MYELOMA


Factors • M Spike >2g/dL • BMPC >20% • FLC ratio >20 Stratification Low-risk: 0 Intermediate-risk: 1 High-risk: >=2
2018 Risk Stratification of Smoldering Multiple Myeloma (2-20-20)
Lakshman et al, BCJ, 2018 Mayo
IMWG 2019 Risk Stratification of SMM (n=1151)
M Spike: >2g/dL
San Miguel. ASCO 2019. Abstr 8000. Mateos Blood Cancer J. 2020;10:102.

Risk Stratification Groups Number of risk factors Risk of Progression at 2 years Number of patients Low -risk group 0 5% 424 (37%) Intermediate-risk group 1 17% 312 (27%) High-risk group 2-3 46% 415 (36%)
FLC Ratio: > 20 BMPC: > 20%
Len versus Observation in High Risk SMM
90% reduction in risk of end organ damage with lenalidomide or Rd
Mateos M et al. N Engl J Med 2013;369:438 447; Sagar Lonial; Journal of Clinical Oncology 2020 381126 1137


Potential New Myeloma or Smoldering Myeloma Observation Any Myeloma Defining Events? • CRAB, • >60% PC, • FLC >100, • MRI >1 focal No Myeloma Defining Events (SMM) Treat as Myeloma High Risk SMM (Median TTP ~2 years) Intermediate or Low Risk SMM Early Therapy with Len or Rd Clinical Trials Rajkumar SV © 2022
IMWG 2019 Risk Stratification of SMM
Risk Factor Score
FLC Ratio

0-10 (ref) 0 > 10-25 2 > 25-40 3 > 40 5 M protein (g/dL)
0-1.5 (ref) 0 > 1.5-3 3 > 3 4 BMPC%
0-15 (ref) 0 > 15-20 2 > 20-30 3 > 30-40 5 > 40 6 FISH abnormality 2 San Miguel. ASCO 2019. Abstr 8000. Mateos Blood Cancer J. 2020;10:102.












eastern cooperative oncology group Smoldering MM PI: Natalie Callander (Activated May 30, 2019) EAA173: Phase III –Daratumumab to Enhance Therapeutic Effectiveness of Revlimid in Smoldering Myeloma (DETER SMM)(PI: NC) Rd DRd CR/PR/ Stable Prog. anytime Continue therapy For 2 years Off Rx R A N D O M I Z A T I O N N = 288


36
IMF Patient and Family Webinar


 Brian G.M. Durie, MD Cedars-Sinai Outpatient Cancer Center
Brian G.M. Durie, MD Cedars-Sinai Outpatient Cancer Center
Los Angeles, CA
Frontline Therapy Options
37
Frontline Therapy
Brian G.M. Durie, MD


12, 2022

4
November
Focus on Frontline
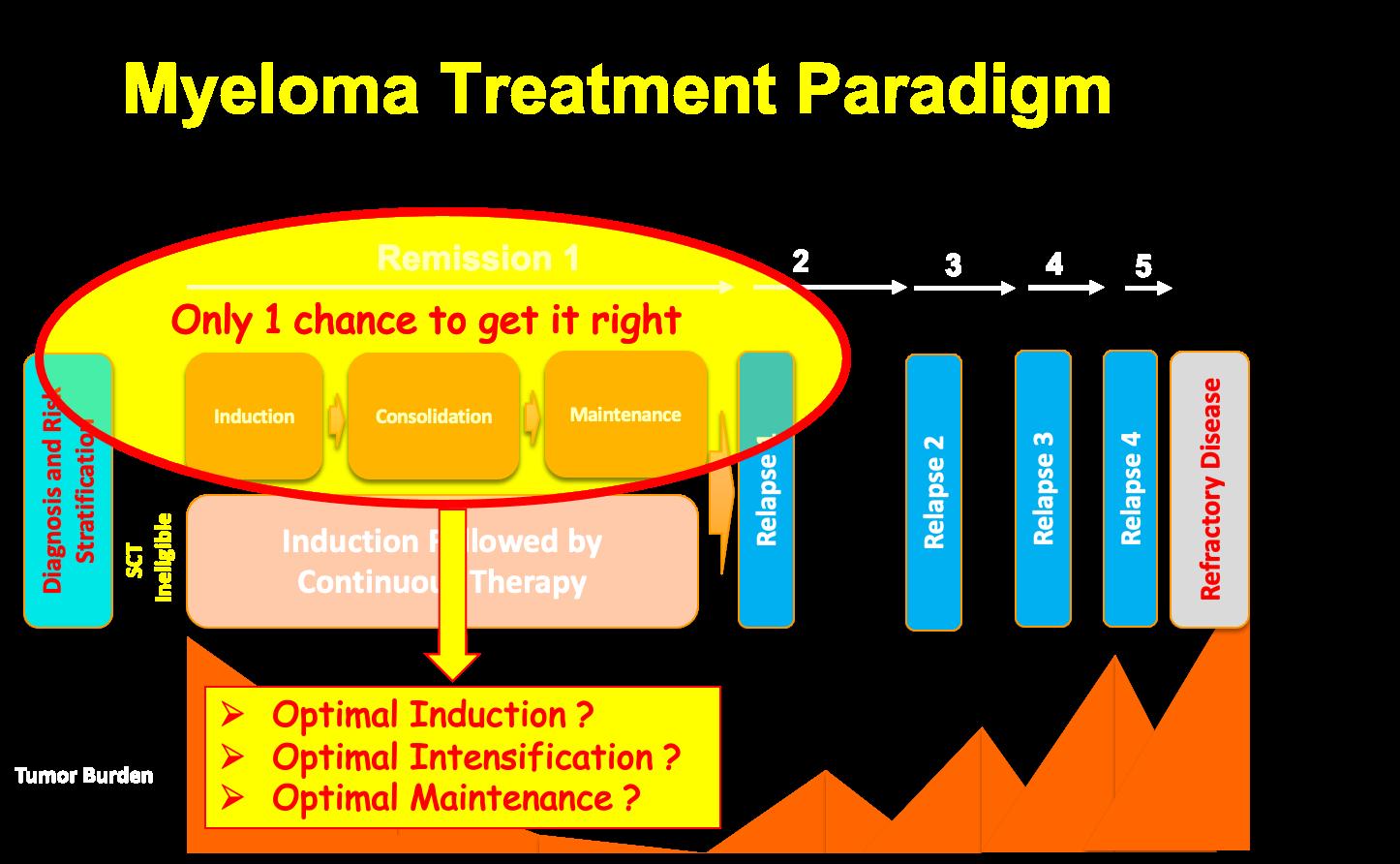

3
5


6 Options for Transplant Eligible ➢ THREE DRUGS : VRd / VTd / VCd ➢ FOUR DRUGS : Dara or Isa [anti CD 38] + TRIPLET
VRd +/- ASCT [IFM 2009]


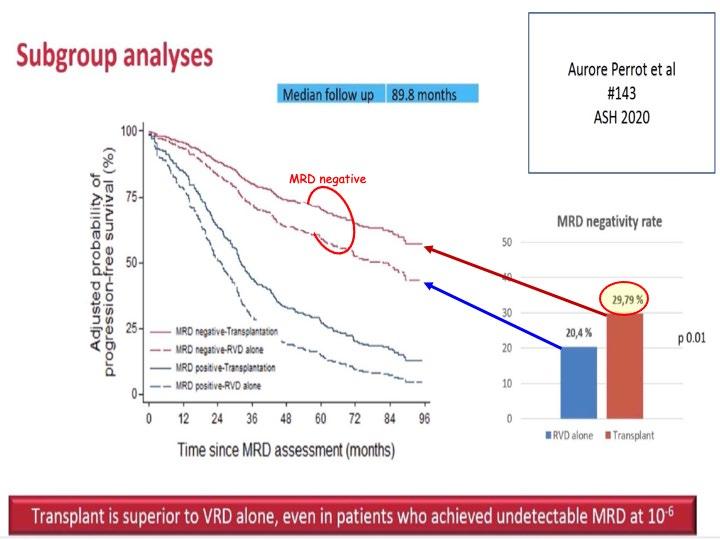
7
Key Secondary Endpoint: Overall Survival (OS)



8
VRd+/- ASCT as SOC

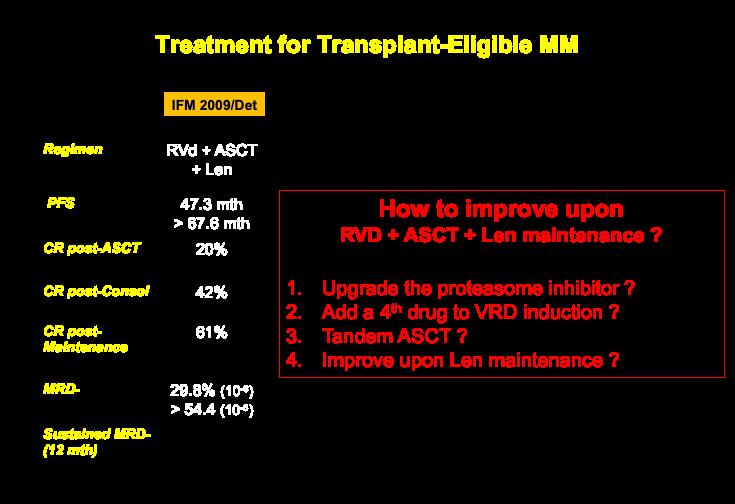
9
NEW TRIALS
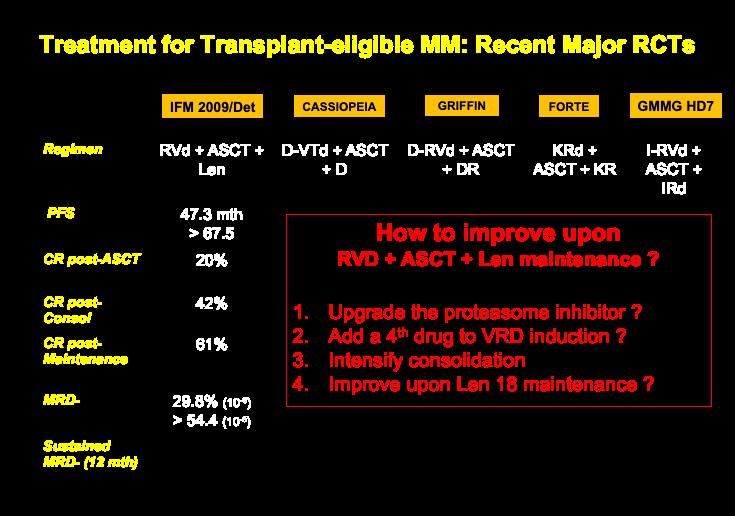

10
Dara-Based FOUR DRUG REGIMENS


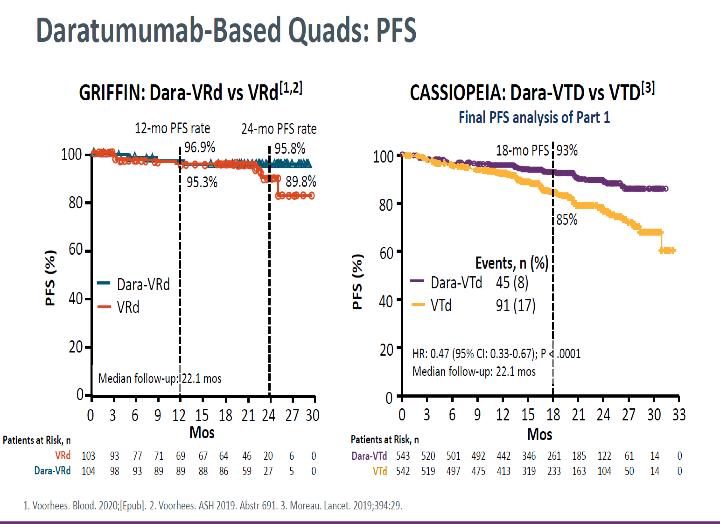
11
MRD with NEW REGIMENS

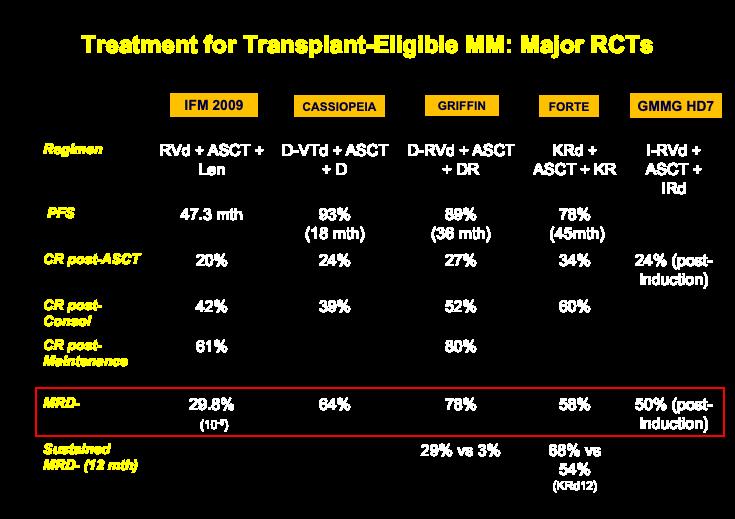
12
MASTER Trial

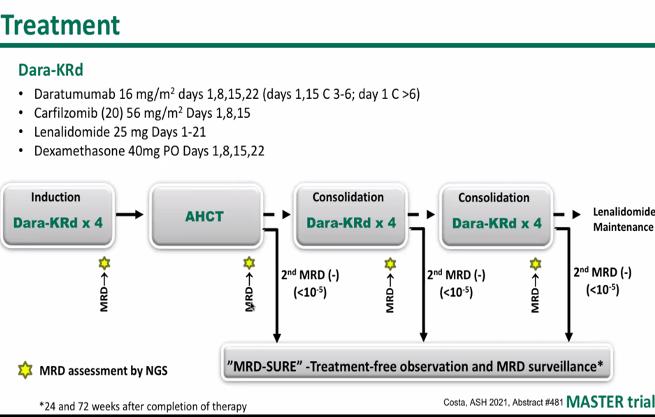

13
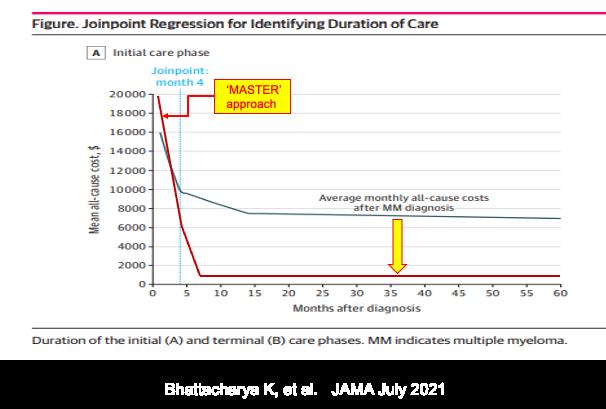
Phase-Specific and Lifetime Costs of Multiple Myeloma


Bhattacharya K, et al. JAMA July 2021
14



15 CURE TRIAL RESULTS for HR SMM ➢CESAR [ KRd + ASCT ] ~ 70% MRD negative ➢ ASCENT [ Dara KRd ] ~ 80+% MRD negative
Considerations for non- transplant patients


16



17


18


19

20


21
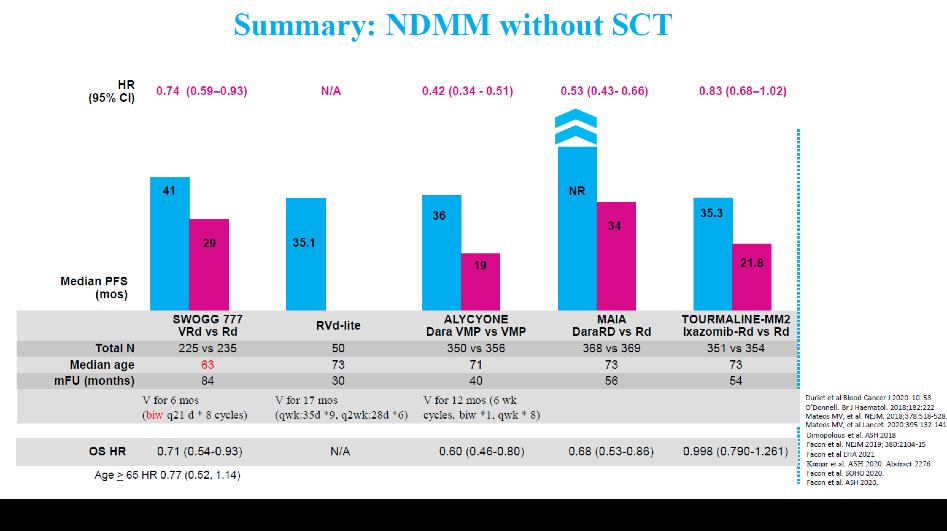
BEST FRONTLINE OPTIONS

3
22
Relapsed/Refractory MM


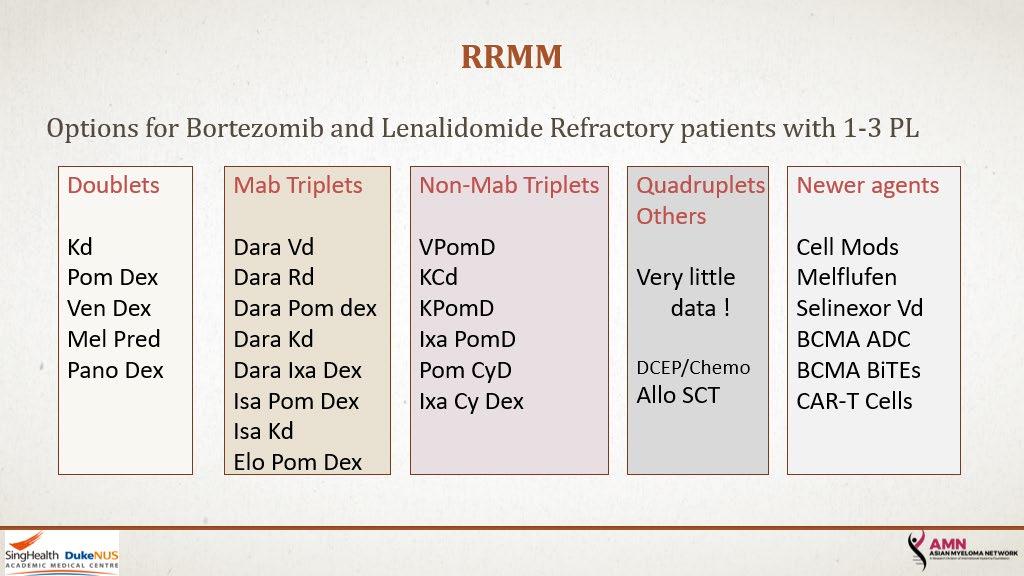
23


24 Options with Prior Dara or Isa ➢ DOUBLETS: Kd or POM d ➢ TRIPLETS: K Pom d or Elo Pom or COMBOS with Dara/Isa ➢ FOUR DRUG COMBOS: DCEP/ Dara KPd /… ➢ New Agents or New Immune Therapies [CAR-T/ Bispecific and more]


25 PLANNING AHEAD
Use what is best for now ...Since so MANY NEW OPTIONS are COMING
➢ Expert consult helps ➢






Audience Q&A • Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion. • If you have a question that does not get answered today, you can contact our Infoline at 800-452-CURE (2873) US & Canada, 1-818487-7455, or email infoline@myeloma.org.


61
Break


62


63
IMF Patient and Family Webinar
11:25 – 11:50PM Supportive Care - Beth Faiman 11:50 – 12:05 PM Q&A 12:05 – 12:30PM Relapse and Role of New Immune Therapies - Dr. Garfall 12:30 – 12:45PM Q&A 12:45 PM Webinar Survey & Closing Remarks AGENDA AFTER BREAK *all times listed in Pacific Time Zone
IMF Patient and Family Webinar



Supportive Care
64
Beth Faiman, PhD, MSN, APNBC, AOCN®, BMTCN®, FAAN, FAPO
Cleveland Clinic Taussig Cancer Institute
LIFE IS A CANVAS, YOU ARE THE ARTIST
 Beth Faiman, PhD, RN, MSN, APN-BC, BMTCN®, AOCN®, FAAN, FAPO
Beth Faiman, PhD, RN, MSN, APN-BC, BMTCN®, AOCN®, FAAN, FAPO
Patient Education Slides 2022 October 29, 2022
Taussig Cancer Institute, Cleveland Clinic Cleveland, OH


GALLERY OF GOALS MYELOMA TREATMENT SUPPORTIVE THERAPIES • Rapid and effective disease control • Durable disease control • Minimize side effects • Allow for good quality of life • Improved overall survival • Prevent disease- and treatmentrelated side effects • Optimize symptom management • Allow for good quality of life DISCUSS GOALS AND PRIORITIES WITH YOUR HEALTHCARE TEAM 66
MANAGING MYELOMA: THE COMPONENTS 67 Supportive Care Initial Therapy Transplant Consolidation Maintenance Treatment of Relapsed disease Transplant Eligible Patients Transplant Ineligible patients Consolidation/ Maintenance/ Continued therapy Everyone




SYMPTOMS Physical • Fatigue • Constipation • Pain • Neuropathy • Impaired Physical Functioning • Sexual Dysfunction Psychological • Depression • Anxiety • Sleep Disturbance • Decreased Cognitive Function • Decreased Role & Social Function Financial • Financial burden (80%) • Financial toxicity (43%) A meta-analysis identified the most common patient-reported symptoms and impact on QOL, and were present at all stages of the disease. Symptoms resulted from both myeloma disease and treatment, including transplant, and were in these categories: Ramsenthaler,
68
PATIENT-REPORTED
et al. 2016. https://doi.org/10.1111/ejh.12790.








• Different CAR-T products use different methods to engineer a patients own T cells to target myeloma cells • BCMA (B-cell maturation antigen) is a protein found on the surface of myeloma cells that is targeted by several CAR-T products CAR T-CELL THERAPY: PATIENT’S OWN T CELLS ENGINEERED BCMA = B cell maturation antigen; CAR T = chimeric antigen receptor T cell; CRS = cytokine release syndrome. Shah UA, Mailankody S. BMJ. 2020;370:bmj.m3176. CAR-T Therapy ide-cel or Abecma cilta-cel or Carvykti Patient T cells CAR-T Therapy Chimeric antigen receptor DNA Grown in lab Reinfused in patient T-cell mediate d death CD269 (BCMA) or other antigen Myeloma cell 69


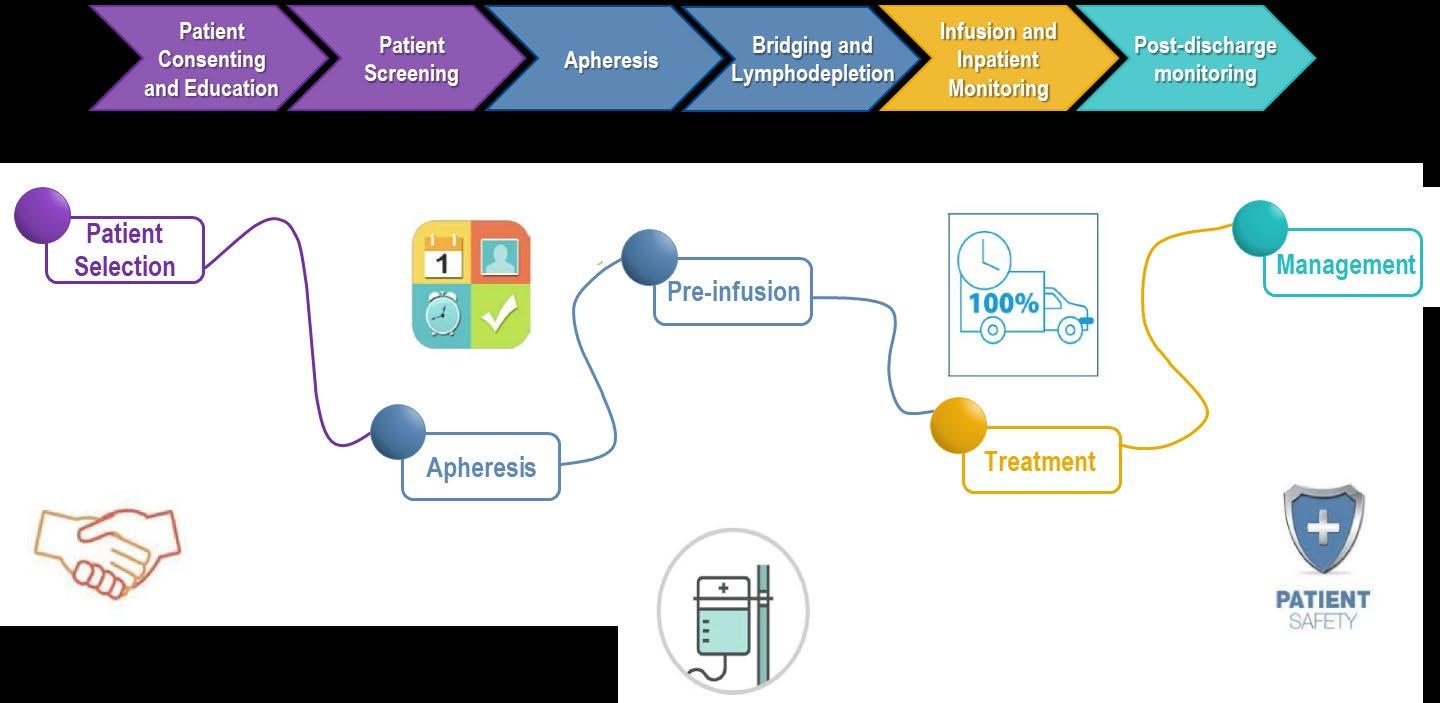
THE PATIENT CAR-T THERAPY JOURNEY Patient with multiple prior therapies Consult with CAR T Center CAR T Treatment Decision Harvest T Cells Lab Engineers T Cells Patient Waits ~4-6 weeks while Lab Grows Engineered T cells Monitor Remain near CAR T center up to 4 weeks May need a bridging therapy 70
A NEW TREATMENT APPROACH

CAR
T:
71
T


BISPECIFIC ANTIBODIES
Bispecific antibody





bispecific antibodies have
MM cell death BCMA CD3 Cytotoxic cytokines
BCMA = B cell maturation antigen; CAR = chimeric antigen receptor; MM = multiple myeloma; scFV = single chain fragment variable. Shah N, et al. Leukemia. 2020;34(4):985 1005. Yu B, et al. J Hematol Oncol. 2020;13:125. cell
MM cell
• Different
differences in
side effects • About
• CRS is
• Some
•
•
• Off-the-shelf
•
BISPECIFIC ANTIBODIES
efficacy,
7 in 10 patients responded
common
had skin/nail disorders
Teclistimab: FDA approved October 25, 2022!
Many other agents currently available in clinical trials (https://clinicaltrials.gov/ )
treatment; no waiting for engineering cells
Infusion (every 1-2 weeks but may vary)
CRS = cytokine release syndrome. Oluwole OO, Davila ML. J Leukoc Biol . 2016;100:1265 1272. June CH, et al. Science. 2018;359:1361 1365. Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321 3330. Brudno JN, Kochenderfer JN. Blood Rev. 2019:34:45 55. Shimabukuro Vornhagen, et al. J Immunother Cancer. 2018;6:56. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625 638.


Diarrhea
CRS Fever Fatigue Headache Nausea / vomiting Shortness of Breath
Weakness Confusion CAR T & BISPECIFICS: UNIQUE SIDE EFFECTS
CRS IS A COMMON BUT USUALLY MILD SIDE EFFECT WITH CAR T & BISPECIFICS 73
Encephalopathy


Headache Confusion Altered wakefulness Hallucinations Ataxia Apraxia Facial nerve palsy Tremors Seizures
Neurotoxicity
cytokine release syndrome; ICANS = immune effector cell associated neurotoxicity syndrome; ICE = Immune Effector Cell Encephalopathy screening tool; MRI
magnetic resonance imaging.
3330. Lee DW,
al. Biol Blood Marrow Transplant.
638.
T & BISPECIFICS: UNIQUE SIDE EFFECTS NEUROTOXICITY IS A RARE BUT SERIOUS SIDE EFFECT OF CAR T AND BISPECIFICS 74
CRS =
=
Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321
et
2019;25:625
CAR
BCMA
▶ Both viral and bacterial – Up to a 3rd of patients on clinical trials has serious infections (requiring IVIg antibodies or hospitalization) ▶ Increased risk of serious COVID complications despite history of vaccination – Antibody levels – Tixagevimab co-packaged with cilgavimab (EVUSHELD- Pre-exposure revention) – Immediate treatment once diagnosed Nirmatrelvir with Ritonavi (Paxlovid) • Start as soon as possible; must begin within 5 days of when symptoms start • Many drug interactions; need good kidney and heart function to take
TARGETED
ARE ASSOCIATED WITH AN INCREASED RISK OF INFECTIONS
THERAPIES
Multiple myeloma
Immune dysfunction
General
Brigle K, et al. Clin J Oncol Nurs. 2017;21(5)suppl:60 76. Faiman B, et al; IMF Nurse Leadership Board. Clin J Oncol Nurs. 2011;15(Suppl):66 76. Miceli TS, et al. Clin J Oncol Nursing. 2011;15(4):9 23. ASH Website. COVID-19 Resources. Accessed January 30, 2022. https://www.hematology.org/covid 19/covid 19 and multiple myeloma
care team
INFECTION CAN BE SERIOUS FOR PEOPLE WITH MYELOMA
Good personal hygiene (skin, oral) Environmental control (wash hands, avoid crowds and sick people, etc) Growth factor (Neupogen [filgrastim]) Immunizations (NO live vaccines) • Medications (antibacterial, antiviral)
fold increased risk of bacterial and viral infections for people with myeloma
76
Infection Prevention Tips
7-10
Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.

Manage stress • Rest, relaxation, sleep hygiene • Mental health / social engagement • Complementary therapy Maintain a healthy weight • Nutrition • Activity / exercise Preventative health care • Health screenings, vaccinations • Prevent falls, injury, infection • Stop smoking • Dental care Maintain renal health • Myeloma management • Hydration • Avoid renally-toxic medications – Dose adjust to renal function • Diabetes management Protect your bones • Nutrition, Calcium + D supplement • Weight-bearing activity / walking • Bone strengthening agents • “Living well” bone health modules
36. Dimopoulous M, et al. Leukemia.
56.
Faiman
“An ounce of prevention is worth a pound of cure.” Benjamin Franklin 77 HEALTHFUL LIVING STRATEGIES: PREVENTION
Faiman B, et al. CJON. 2017;21(5)suppl:19
2009;23(9):1545
Brigle K, et al. CJON. 2017;21(5)suppl:60-76.
B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.
ANIMAL STUDIES
FIRST INTRODUCTION OF AN INVESTIGATIONAL DRUG INTO HUMANS

Determine metabolism and PK/PD actions, MTD, and DLT • Identify AEs • Gain early evidence of effectiveness, studied in many conditions; typically, 20 to 80 patients; everyone gets agent
EFFECTIVENESS IN A CERTAIN TUMOR TYPE • Determine short term AEs and risks; closely monitored • Includes up to 100 patients, typically GATHER
SAFETY INFORMATION COMPARED TO STANDARD OF CARE • Placebo may be involved if no standard of care exists; 100s to several thousand patients • Often multiple institutions; single or double blind APPROVED AGENTS IN NEW POPULATIONS OR NEW DOSE FORMS AE = adverse event; DLT = dose limiting toxicity; MTD = maximum tolerated dose; PD = pharmacodynamics; PK = pharmacokinetics. Faiman B, et al. Adv Pract Oncol. 2016;7:17 29. PHASE 1 PHASE 2 PHASE 3 PHASE 4 Preclinical
•
EVALUATION OF
ADDITIONAL EFFECTIVENESS AND
ACCESS TO PROMISING TREATMENTS 78
CLINICAL TRIALS: EARLY


IMF Infoline US & Canada 800-452 CURE (2873) Worldwide: 1-818-487-7455 infoline@myeloma.org Clinicaltrials.gov https://clinicaltrials.gov/ HOW TO FIND CLINICAL TRIALS 79




















KNOWLEDGE IS POWER USE REPUTABLE SOURCES Download or order at myeloma.org Website: http://myeloma.org IMF InfoLine 1 800-452-CURE 9am to 4pm PST eNewsletter: Myeloma Minute IMF TV Teleconferences 80
YOU ARE NOT ALONE








Audience Q&A • Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion. • If you have a question that does not get answered today, you can contact our Infoline at 800-452-CURE (2873) US & Canada, 1-818487-7455, or email infoline@myeloma.org.


83
IMF Patient and Family Webinar
 Dr. Alfred Garfall Hospital of the University of Pennsylvania
Dr. Alfred Garfall Hospital of the University of Pennsylvania


Relapse and Role of New Immune Therapies
84
IMF Patient & Family Webinar

New Immune Therapies for Relapsed Multiple Myeloma
12
Alfred Garfall, MD
November 2022
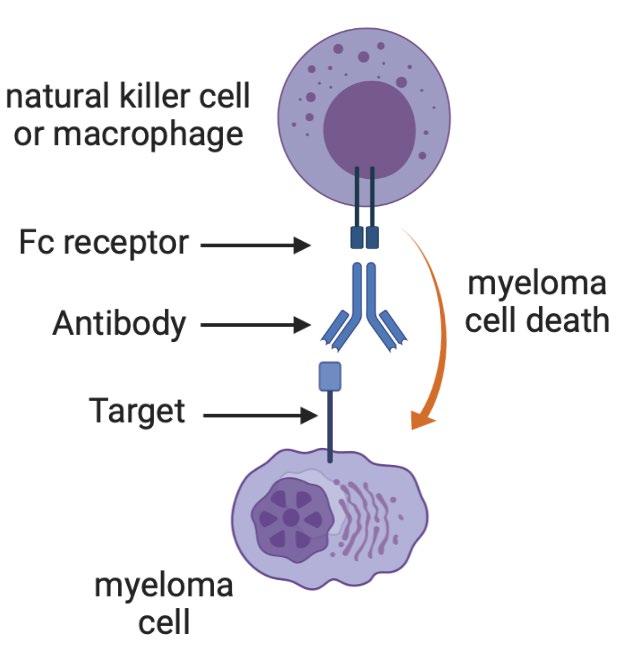
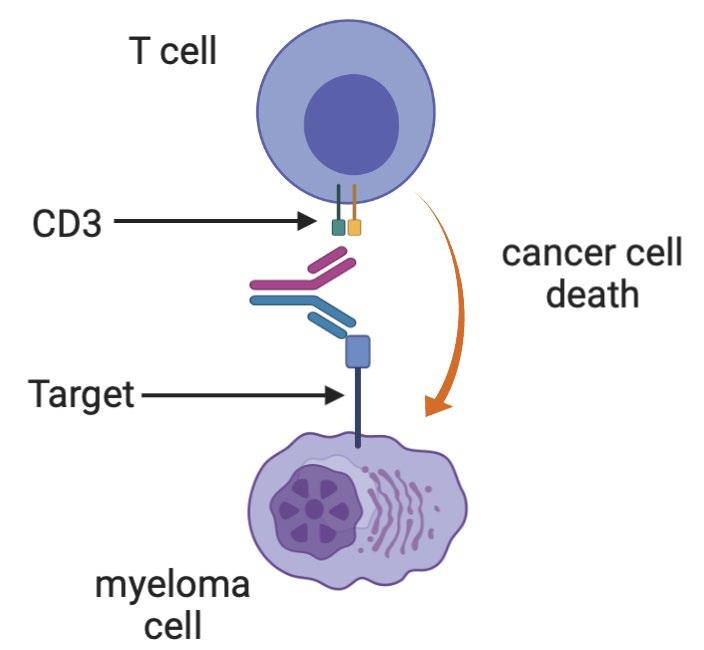
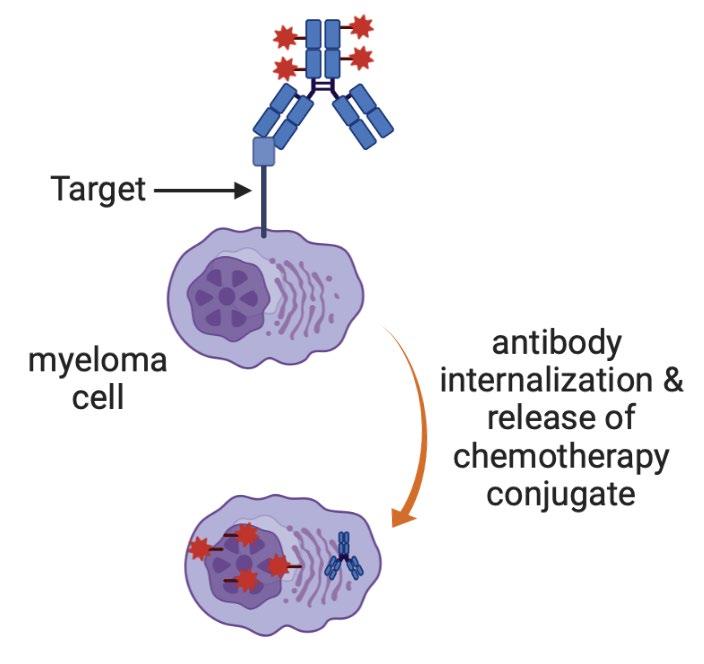
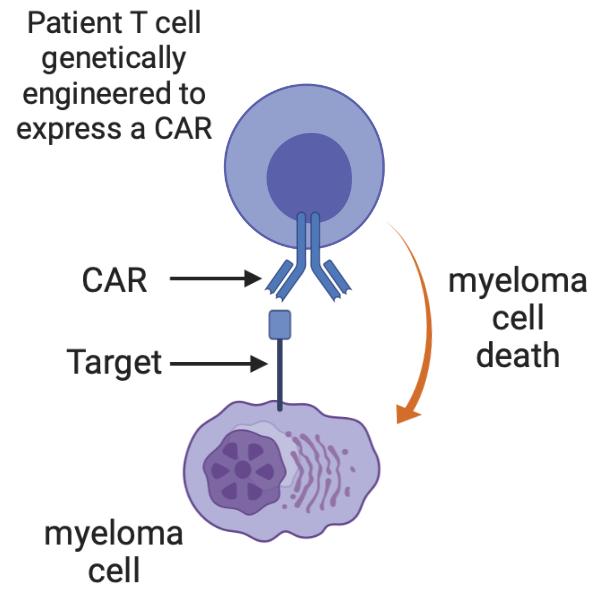
86 Multiple Myeloma Immunotherapies Conventional Monoclonal Antibodies Daratumumab (CD38) Isatuximab (CD38) Elotuzumab (CS1) Bispecific antibodies Teclistamab etc (BCMA) Talquetamab (GPRC5D) Cevostamab (FCRH5) Antibody-drug conjugates Belantamab (BCMA) CAR T cells Ide-cel (BCMA) Cilta-cel (BCMA) Graphics created with BioRender
Some
87
very rough comparisons Conventional Antibodies Antibody-drug conjugates Bispecific antibodies CAR T cells Potency + ++ +++ ++++ Risk + ++ +++ ++++
88
very rough comparisons Conventional Antibodies Antibody-drug conjugates Bispecific antibodies CAR T cells Potency + ++ +++ ++++ Risk + ++ +++ ++++ Side-effects/risks Infection Depends on conjugate (eye irritation for belantamab) Cytokine release syndrome Neurologic toxicity Infection Low blood counts
Some
89 Some very rough comparisons Conventional Antibodies Antibody-drug conjugates Bispecific antibodies CAR T cells Potency + ++ +++ ++++ Risk + ++ +++ ++++ Side-effects/risks Infection Depends on conjugate (eye irritation for belantamab) Cytokine release syndrome Neurologic toxicity Infection Low blood counts Dosing Indefinite treatment Intravenous or subcutaneous Indefinite treatment Intravenous Indefinite treatment Intravenous or subcutaneous One-time therapy T cell collection manufacturing intravenous infusion
90 Some very rough comparisons Conventional Antibodies Antibody-drug conjugates Bispecific antibodies CAR T cells Potency + ++ +++ ++++ Risk + ++ +++ ++++ Side-effects/risks Infection Depends on conjugate (eye irritation for belantamab) Cytokine release syndrome Neurologic toxicity Infection Low blood counts Dosing Indefinite treatment Intravenous or subcutaneous Indefinite treatment Intravenous Indefinite treatment Intravenous or subcutaneous One-time therapy T cell collection manufacturing intravenous infusion Current use All settings Patients who have had ≥4 prior lines of therapy
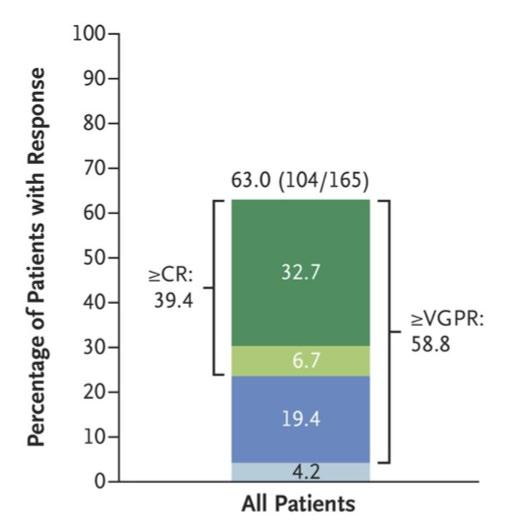
91
BCMA-targeted
‣ MajesTEC-1
patients
at least 3 prior lines of therapy ‣ Most patients responded; responses were durable ‣ Most patients developed low blood counts (improved with
‣ Most patients
low-grade
release syndrome • Fevers • Low blood pressure that
fluids • Half of patients
‣ Neurologic toxicity • 6% “ICANS” • Seizure (1 patient) • Guillain-Barre syndrome (1 patient)
Teclistamab:
bispecific antibody
study of 165
with
time)
developed
cytokine
got better with
with CRS received tocilizumab
After 12 months, teclistamab was still working for 70% of the patients who had responded.
Infections with teclistamab
‣
Teclistamab suppresses the immune system
• Lowers antibody levels
• May impair other parts of the immune system
‣ 76% of patients had infections, 44% had serious infections
• 18% developed pneumonias
• 18% developed COVID19, and some patients died of COVID19
• Some opportunistic infections: fatal brain infection (PML), pneumocystis jirovecii pneumonia
92
A typical patient’s journey with teclistamab
‣ All doses are given as subcutaneous injections
‣ Admit to the hospital for initial “step-up” doses
• Day 1: Dose #1 (0.06 mg/kg)
• At least 48 hours later: Dose #2 (0.3 mg/kg)
• At least 48 hours later: Dose #3 (1.5 mg/kg = full dose)
• At least 48 hours later: Discharge
Cytokine release syndrome or neurologic toxicity can occur after any of these doses and delay the next dose.
‣ Return to clinic around one week after dose #3 to start weekly outpatient doses
• CRS or neurologic toxicity are rare after you leave the hospital
• Blood counts may be low during first couple months of therapy
• Some patients are more tired in the first couple months of therapy ‣ Response is usually clear in the first 1-2 months of therapy (often after first couple doses)
‣ Official dosing is once weekly indefinitely; can consider reducing to every 2-4 weeks if responding well.
‣ Most patients feel very well with long-term dosing
93
Supportive
•
•
•
•
•
•
•
94
care with teclistamab ‣ Measures to prevent infection
Check for hepatitis B exposure
Antiviral medications to prevent shingles and herpes outbreaks (e.g. acyclovir)
Antibiotic to prevent pneumocystis pneumonia (e.g. Bactrim)
COVID19: vaccines, Evusheld, antivirals
Managing low blood counts
Antibody replacement therapy (intravenous immune globulin) ‣
Blood transfusions
Filgrastim (G-CSF) for low neutrophil counts
Bispecific antibodies vs CAR T cells
Bispecifics CAR T cells
Time
Treatment duration Indefinite therapy (for now) One-time therapy
Risk/benefit Perhaps a bit safer but also a bit less potent Perhaps more severe toxicity but maybe more potent
95
to start Can start right away Need to schedule a manufacturing slot and wait for manufacturing.
Availability Hospital requires some training for toxicity management Requires more specialized cellular therapy infrastructure and expertise
Additional promising bispecific antibodies
96
Many additional BCMA-targeted
antibodies are in development
antibody
‣
bispecific
‣ Talquetamab: GPRC5D-targeted bispecific
‣ Cevostamab: FCRH5-targeted bispecific antibody
How to sequence/choose among these advanced therapies
‣ We try to give every patient a try with every promising therapy
‣
We are still learning about patterns of response and resistance; lots of ideas, some interesting trials about to start, but very little good data yet.
Patients who progress after one BCMA-targeted therapy can respond to another ‣ Patients can respond to multiple bispecific antibodies and CAR T cells ‣
‣
Patients with high disease burden may be at higher risk of severe CAR T cell toxicity
97
Some questions
98
‣ Can we start bispecific antibodies outside the hospital? ‣ Is indefinite therapy better than fixed-duration therapy for bispecific antibodies? ‣ Are CAR T cells and bispecific antibodies safer and/or more effective in earlier lines of therapy? ‣ Should we combine CAR T cells and bispecific antibodies with other MM therapies? ‣ Cost, accessibility, and sustainability.
99
‣
Future directions ‣ CAR T cells and bispecific antibodies against non-BCMA targets
CAR NK cells ‣ Trispecific antibodies ‣ NK-cell engaging bispecific antibodies






Audience Q&A • Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion. • If you have a question that does not get answered today, you can contact our Infoline at 800-452-CURE (2873) US & Canada, 1-818487-7455, or email infoline@myeloma.org.


101
Thank you to our sponsors!










Thank you for joining today’s IMF Patient and Family Webinar

Webinar Video Replay and Slides
As follow up to today’s webinar, we will have the speaker slides and a video replay available.

They will be provided shortly after the webinar concludes.

Feedback Survey
Want to Hear From You!
At the close of the meeting a feedback survey will pop up. Click “continue” to complete the survey.


This will also be emailed to you shortly after the workshop.
Please take a moment to complete this survey.
We
IMF Patient and Family Webinar


106





















































 S. Vincent Rajkumar Professor of Medicine, Mayo Clinic @VincentRK
Mayo Clinic College of Medicine
Mayo Clinic Comprehensive Cancer Center
Scottsdale, Arizona Rochester, Minnesota Jacksonville, Florida
S. Vincent Rajkumar Professor of Medicine, Mayo Clinic @VincentRK
Mayo Clinic College of Medicine
Mayo Clinic Comprehensive Cancer Center
Scottsdale, Arizona Rochester, Minnesota Jacksonville, Florida




























































 Brian G.M. Durie, MD Cedars-Sinai Outpatient Cancer Center
Brian G.M. Durie, MD Cedars-Sinai Outpatient Cancer Center








































































 Beth Faiman, PhD, RN, MSN, APN-BC, BMTCN®, AOCN®, FAAN, FAPO
Beth Faiman, PhD, RN, MSN, APN-BC, BMTCN®, AOCN®, FAAN, FAPO








































































 Dr. Alfred Garfall Hospital of the University of Pennsylvania
Dr. Alfred Garfall Hospital of the University of Pennsylvania

































