Welcome!
Thank you for joining us today for the February 1, 2023, International Myeloma Foundation’s Regional Community Workshop –Northeast & Southern states.


Thank you for joining us today for the February 1, 2023, International Myeloma Foundation’s Regional Community Workshop –Northeast & Southern states.




As follow up to today's workshop, we will have the speaker slides and a video replay available.
These will be provided to you shortly after the workshop concludes.











At the close of the meeting a feedback survey will pop up.

This will also be emailed to you shortly after the workshop.

Please take a moment to complete this survey.

February 1, 2023, Agenda
5:30 – 5:35 PM Welcome and Announcements
Kelly Cox, Senior Director Regional Community Workshops

5:35 – 6:10 PM Myeloma 101 & Frontline Therapy

Craig Cole, MD - Michigan State University
6:10 –

6:25 PM Q&A with Panel
6:25 –
6:35 PM Guided Meditation & Stretch Break
6:35 –
7:15 PM Relapsed Therapy & Clinical Trials
David Vesole, MD, PhD, FACP - The John Theurer Cancer Center at Hackensack University Medical Center
7:15 – 7:35 PM Life is a Canvas, You are the Artist
Kimberly Noonan, DNP, ANP-BC, AOCN - IMF Nurse Leadership Board, Dana-Farber Cancer Institute
7:35 – 8:00 PM Q&A with Panel



Regional Community Workshop
Wednesday, February 1st, 2023
 Craig Emmitt Cole, M.D. Assistant Professor Director of
Craig Emmitt Cole, M.D. Assistant Professor Director of
Clinical Research
Department of Internal Medicine






Division of Hematology/Oncology; Hematology Section
Michigan State University College of Human Medicine

Karmanos Cancer Institute

How common is multiple myeloma
Spectrum of plasma cell disorders
Diagnosis of myeloma and labs
Staging and risk stratification
The Science behind the treatments!
Up front therapy strategies: induction, transplant, and maintenance
Bone support
“New Stuff” 4 drug induction therapy
Perspectives in the advancement of myeloma science and survival

BLOOD
• Myeloma is a cancer of the blood

• Myeloma crowds out normal blood forming cells, causing anemia
Normal plasma cells
Antibodies
Calcium high
Renal (kidney) failure
Anemia
Bone destruction
BONES
• Surrounding bone where Myeloma cells grow is damaged/ weakened

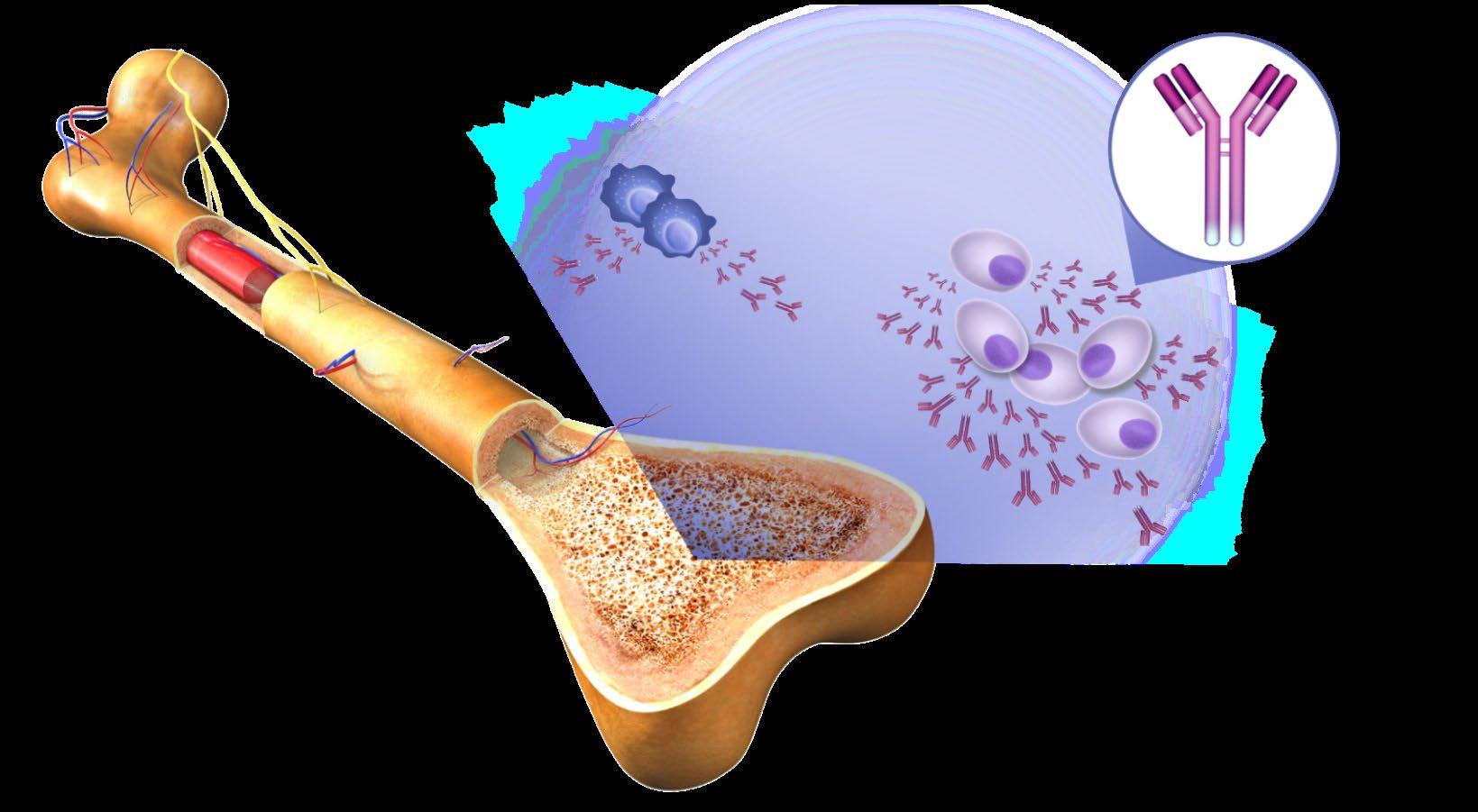
• Myeloma cells activate bone destruction blood calcium levels
Bone
Mutated Cancer Cell
Monoclonal (M) proteins






Bone marrow
Multiple Myeloma cells
Large amounts of M proteins

Low Blood Counts
• Anemia is present in 60% at diagnosis
• May lead to anemia and infection
Decreased Kidney Function

• Occurs in over half of myeloma patients
Bone Damage
• Affects 85% of patients
• Leads to fractures
Bone Turnover
• Leads to high levels of calcium in blood (hypercalcemia)

About 10% to 20% of patients with newly diagnosed myeloma will not have any symptoms.
C: Calcium elevation (>11 mg/dL)



Weakness
Fatigue
Infection
Weakness
Bone pain
Loss of appetite
Weight loss
R: Renal- low kidney function; (serum creatinine >2 mg/dL)
A: Anemia –low red blood count (Hb <10 g/dL)
B: Bone disease (≥1 lytic lesions on skeletal radiography, CT, or PET-CT)
































Monoclonal Gammopathy of Uncertain Significance
M protein under 3 g/dL AND Plasma cells in Bone Marrow <10% AND No CRAB or “SLiM” high risk features
M protein over 3 g/dL (serum) or over 500 mg/24 hrs (urine) AND Plasma cells in Bone Marrow 10%–60% AND No CRAB or “SLiM” high risk features
M protein over 2 g/dL AND Plasma cells in Bone Marrow 20%–60% AND Free Lt Chain Ratio >20
“Evolving type”SMM Increase >10% protein w/in 6mo AND No CRAB or “SLiM” high risk features
Malignant Plasma cells seen on any biopsy (usually bone marrow) AND ≥1 “CRAB” feature
C: Calcium elevation (>11 mg/dL)















R: Renal- low kidney function; (serum creatinine >2 mg/dL)
A: Anemia –low red blood count (Hb <10 g/dL)
B: Bone disease (≥1 lytic lesions on skeletal radiography, CT, or PET-CT)
OR have >1 SLiM ‘high risk” features:
S: >60% Plasma Cells on Bone Marrow biopsy
1% risk of progression/year to multiple myeloma or related conditions
10% risk of progression/year to active myeloma
>46% risk of progression in 2 yr to active myeloma
Li: Serum light chain ratio >100
M: >1 lytic lesions on MRI (or PET/ CT scan)
CBC • Number of red blood cells, white blood cells, and platelets



CoMP
• Measure levels of albumin, calcium, and creatinine. Assess function of kidney, liver, and bone status (alkaline phosphatase) and the extent of disease.
Beta2 MicroG






LDH Lactate Dehydrogenase




















Serum Protein EP
Immuno Fixation
Serum FreeLight Chain
Urine Protein EP
• Determine the level of a protein that indicates the presence/extent of MM and kidney function: USED FOR STAGE
• Determine the level of myeloma cell production and extent of MM : USED FOR STAGE
• Detect the presence and level of M protein = how much myeloma
• Identify the type of abnormal antibody proteins: IgG, IgA, κ,or λ
• Freelite test measures free light chains (kappa or lambda) in blood = how much myeloma
• Detect Bence-Jones proteins (otherwise known as myeloma light chains) in urine (present or not present)
24 hr Urine Analysis
• Determine the presence and levels of M protein and Bence Jones protein in the urine = how much myeloma
Serum Protein EP


Monoclonal protein










• Detect the presence and level of M protein = how much myeloma
Treatment
Serum FreeLight Chain

Treatment
• Freelite test measures free light chains (kappa or lambda) in blood = how much myeloma





Positive Kappa
Monoclonal Serum


Kappa Lt. Chain MM
Kappa Lt. Chain
Lambda Lt. Chain


Light Chains
Ratio: 1




• IgG+kappa
• IgG+lambda
• IgA+kappa
• IgA+lambda
• etc…
• 80% of myeloma cases
Jones protein

• 18% of all myeloma cases
• Renal failure more common in light chain multiple myeloma; creatinine >2 mg/dL in 1/3 of cases
protein present
• Less than 3% of cases of multiple myeloma

• Conventional X-rays reveal punched-out lytic lesions, osteoporosis, or fractures in 75% of patients.
• FDG PET/CT appears to be more sensitive (85%) than skeletal survey for the detection of small lytic bone lesions.
• Diagnosis is confirmed with bone marrow demonstrating greater than 10% involvement by malignant plasma cells with either CRAB or SLiM
Malignant Plasma cells seen on biopsy
AND ≥1 “CRAB” feature
C: Calcium elevation (>11 mg/dL)
R: Renal- low kidney function; (serum creatinine >2 mg/dL)
A: Anemia –low red blood count (Hb <10 g/dL)



B: Bone disease (≥1 lytic lesions on skeletal radiography, CT, or PET-CT)
OR have >1 SLiM ‘high risk” features:
S: >60% Plasma Cells on Bone Marrow biopsy
Li: Serum light chain ratio >100
M: >1 lytic lesions on MRI (or PET/ CT scan)

DNA
Conventional cytogenetic analysis (karyotyping)





FISH
(fluorescence in situ hybridization)

Advances
• Genetic expression profiling [GEP]
• Whole-genome/ whole-exome sequencing
• Plasma cell next generation sequencing

Staging Myeloma: FISH helps to Assign Risk in Myeloma
Risk Category
Findings on Chromosome (FISH) Analysis Results in the Bone marrow
High Risk
FISH:
• Deletion 17th chromosome
• Gain of chromosome 1q
• Translocation 4 and 14
FISH:
Standard Risk
• Hyperdiploid: More than 1 pair of chromosomes (Trisomies)
• Translocation 11 and 14
• Translocation 6 and 14
• Translocation 14 and 16
• Translocation 14 and 20
NGS: p53 mutation (on chrom 17)


• Double Hit Myeloma: 2 high risk
genetic abnormalities
• Triple Hit Myeloma: 3 or more high risk genetic abnormalities
• Others
• Normal
Revised International Staging System for Multiple Myeloma
Stage 1
β2-microglobulin under 3.6 mg/L Normal Lactate
Dehydrogenase (LDH)
AND
Stage 2
Stage 3
β2-microglobulin over 5.5 mg/L
Does not meet Criteria for Stage 1 or 3
HIGH Lactate
Dehydrogenase (LDH)


































AND
High Risk
Cytogenetics
(FISH)
Deletion 17 chromosome
Translocation 4th and 14th
Translocation 14th and 16th
Translocation 14th and 20th
Immunomodulatory Drugs ( Thalomid(Thalidomide), Revlimid(Lenalidomide
Proteasome Inhibitors (Pis):

Velcade(Bortezomib), Ninlaro(Ixazomib), Kyprolis(Carfilzomib)
Antibodies Against Myeloma (Immunotherapy): Darzelex (Daratumumab), Sarclisa(Isatuximab), Empliciti(Elotuzumab)

How does it work?-SCIENCE!
• Direct inhibition of DNA synthesis of myeloma cells.
VEGF bFGF


• Direct inhibition of DNA synthesis of myeloma cells.
• Inhibition of blood vessel synthesis in the bone marrow.
• Direct inhibition of DNA synthesis of myeloma cells.
• Inhibition of blood vessel synthesis in the bone marrow.
• Inhibition of adhesion between the myeloma and bone marrow stromal cells.
IL 6









IL 1β
TNF α
• Direct inhibition of DNA synthesis of myeloma cells.
• Inhibition of blood vessel synthesis in the bone marrow.
• Inhibition of adhesion between the myeloma and bone marrow stromal cells.
• Inhibition of the release of the cytokines IL-6, TNF-α, and IL-1β.
• Direct inhibition of DNA synthesis of myeloma cells.
• Inhibition of blood vessel synthesis in the bone marrow.
• Inhibition of adhesion between the myeloma and bone marrow stromal cells.
• Inhibition of the release of the cytokines IL-6, TNF-α, and IL-1β.
• Activation of the body’s natural killer cells (T-cells) which attack the myeloma cells.
1990s, several thalidomide analogs were synthesized to increase efficacy and minimize toxicity.

2006 FDA approves Lenalidomide (Revlimid).
Revlimid is felt to be 50 to 2000 more potent than thalidomide.
Phase 2 trial 91% new myeloma achieved responses with Lenalidomide plus dexamethasone.
2013 FDA approves Pomalidomide.
Pomalyst and dexamethasone given to multi-refractory myeloma with response rates of 35 to 65%.
Combination of pomalidomide, bortezomib, and dexamethasone in relapsed MM response rates of 72%.
Iberdomide (CC-220) is the newest in the class and is now in clinical trials
CELMODs are the next class of IMiD with CC92480 drug more potent than iberdomide





 Proteasome
Proteasome



• Bortezomib(velcade) approved by the FDA in 2003 in patients with relapsed refractory myeloma.
• Several phase 2 trials in newly diagnosed myeloma with bortezomib-dexamethasone induction.
o Responses 66% to 90%, including 15% to 21% Complete Responses!
• 2012 FDA approves Carfilzomib(Kyprolis); secondgeneration irreversible Proteasome inhibitor
• In refractory myeloma with 48% response rates. Higher in combination!
• Ixazomib (Ninlaro) is new oral boronated reversible proteasome inhibitor currently approved by the FDA in 11/2015.
Bortezomib(Velcade)
Carfilzomib(Kyprolis)


Ixazomib (Ninlaro)

Targets on the Myeloma Cell Surface and Therapeutic Antibodies
Bi-Specific Antibodies
Talquetamab
CAR-T
Antibody Drug
Elotuzumab
Bi-Specific Antibodies
BCMA
Bi-Specific Antibodies
CAR-T
Antibody Drug
Daratumumab and Darzalex Faspro
Isatuximab





























TAK-079
MOR202
Immune Therapies

Ide cel CAR T
Cilta cel CAR T

Teclistamab

Other Bi-Specific Antibodies
Other CAR-Ts
Antibody and Immune System Attack
Increase production of cytoxic macrophages
Complement Protein Attack
Myeloma Cells











Attacking the Myeloma Cell Biology
Inhibition of adhesion between the myeloma and bone marrow stromal cells
Complement Proteins
Natural Killer Cell Good Guys











Manufactured Anti-Myeloma Antibody


Myeloma surface targets
Antibody Receptor







 Macrophages Good Guys
Macrophages Good Guys
*In Clinical Trials/ not FDA approved

Induction
• Velcade/Revlimid/Dex:(VRD)

• Velcade/Thalomid/Dex:(VTD)
• Velcade/Cytoxan/Dex:(CyBorD)
• Darzalex/Revlimid/Dex:(DRD)
• Darzalex/Velcade/Melphalan/Dex
• Darzalex/Velcade/Thalidomide/Dex
• Kyprolis/Revimid/Dex(KRD)
• Darzalex/Velcade/Revlimid/Dex: Dara-RVD
• Ninlaro/Revimid/Dex(IRD)
• Clinical trials
Consolidation
Maintenance
• Stem Cell Transplant
• Continue Induction
• Clinical trial
• Revlimid
• Velcade
• Ninlaro
• Observation
• Thalidomide
• Revlimid/Dara
• Clinical trial
Rescue
Dara+Pomalyst+Dex
Kyprolis+Pomalyst+Dex
Cytoxan+Pomalyst+Dex
Ninlaro+Pomalyst+Dex
Elo+Pomalyst+Dex
Elo+Thaliomide+Dex
Dara+Kyprolis+Dex
Kyprolis+Revlimid+Dex
Elo+Revlimid+Dex
Dara+Revlimid+Dex
Dara+Velcade+Dex
Elo+Velcade+Dex

Cytoxan+Kyprolis+Thalidomide+dex
4 drug therapies of novel agents
Ninlaro+Cytoxan+Dex
Velcade+ Cytoxan+Dex
Velcade+ Pomalyst+Dex
Chemotherapy
Selinexor+Dex
Selinexor+Velcade
Selinexor+ Dara
Isatuximab(Sarclisa)+ Pomalyst+Dex
Darzalex Faspro (under skin)
Ide-cel CAR-T (FDA approved 3/26/2021)
Cilta-cel CAR-T (FDA approved 2/28/2022)




Teclistamab (FDA approved 10/25/2022)


Not Transplant Candidate
High Risk Standard Risk
Revlimid/Velcade/Dex (RVD) or

Dara-Revlimid Dex (Dara-Rd)
9 to 12 cycles
Velcade Based Maintenance of VRD or Continued Dara-Rd Maintenance
Transplant Candidate
Preferred
High Risk
Dara-RVD x 4 cycles
Standard Risk
RVD-light or Dara-RD
Others: D-VMP,D-VTD,RD
9 to 12 cycles
Revlimid Maintenance or Continued DaraRd Maintenance
Other: KRD or Dara-KRD
or Dara-RVD
Early Auto SCT
?Tandem SCT
Velcade (PI) Based Maintenance Continued Dara-Rd Maintenance
https://www.msmart.org/mm-treatment-guidelines









Early Auto SCT
Revlimid Maintenance +/- Dara
Dispenzieri et al. Mayo Clin Proc 2007;82:323-341; Kumar et al. Mayo Clin Proc 2009 84:1095-1110; Mikhael et al. Mayo Clin Proc 2013;88:360-376. v18 //last reviewed June 2020
Delayed Transplant Collect & store Continue Tx for 8m
Revlimid Maintenance +/- Dara


Partial response

50% reduction in M protein
Very good partial response
90% reduction in M protein
immunofixation positive only

Complete remission
No M-protein
immunofixation negative



Minimal Residual Dis
Minimal Residual Dis



Next Generation Molecular testing





-Patients aged 18-65 yrs with symptomatic newly diagnosed MM following 1 cycle of RVD -56 sites within the United States from 2010 to 2018
End Points of Study and Follow -up
• Primary end point: progression-free survival (time to next relapse)
• Secondary end points included:
• Response rates, overall survival, quality of life, and adverse events
• Follow-up on participant status : median of 6 years

















• At a median follow up of 76.0 months, the risk of disease progression or death was 53% higher in the RVD alone group than in the transplantation group (P<0.001)


Revlimid Maintenance

• No difference in time to relapse (PFS) or Overall Survival in standard risk patients who have two transplants, consolidation
Melphalan 200 mg/m² IV Stem Cell Transplant
10 mg/day for 3 cycles, then 15 mg/day* (n = 257) Consolidation

Velcade 1.3 mg/m² IV Days 1, 4, 8, 11


Revlimid 15 mg Days 1-15
Revlimid Maintenance
RVD therapy, or just straight to maintenance after first BMT
Dexamethasone 40 mg IV Days 1, 8, 15 Four cycles (n = 254)
• Straight to maintenance is the easiest!
10 mg/day for 3 cycles, then 15 mg/day
• ? If high risk patients benefit from two transplants
Second (Tandem)
Stem Cell Transplant
Melphalan 200 mg/m² IV














Second ASCT (n = 247) Induction regimens

• Data from 4 randomized trials of Revlimid (lenalidomide) maintenance vs. no maintenance
– Involving a total of almost 2,000 multiple myeloma patients
• The results of the analysis showed that Revlimid maintenance therapy is associated significant improvement in progression-free survival and a modest improvement in overall survival
• Duration of maintenance is unknown

Multiple myeloma can cause weakened areas in the bone called osteolytic lesions which can compress the spinal cord or cause bone destruction.
• Bone strengthening drugs: bisphosphonates (pamidronate & Zometa) or monoclonal antibodies (Xgeva) are given at diagnosis and continued for at least 2 years
•
Vitamin-D and Calcium supplements to help bone healing •


Orthopedic support
– Physical therapy, physical medicine consults, orthopedic/neuro surgery, radiation therapy, etc
•
Minimally invasive procedures: kyphoplasty or vertebroplasty
• Use of medication to control pain
• Anticonvulsants and antidepressants for treat relieve pain from nerve damage or numbness
Randomized 1:1
Transplant-eligible adults with Newly Diagnosed MM, with good performance status and kidney fxn (N = 207)
Induction: Cycles 1-4
Dara-RVD in 21-day cycles (n = 104)
Consolidation: Cycles 5-6†
Maintenance: Cycles 7-32
Dara-RVD in 21-day cycles
D: 16 mg/kg IV D1
VRd: as in induction
Dara-R in 28-day cycles
D: as in consolidation Q4W or Q8W
R: 10 mg PO D1-21 of C7-9 and 15 mg
PO D1-21 of C10
RVD in 21-day cycles (n = 103)
RVD in 21-day cycles
VRd: as in induction
R in 28-day cycles
R: 10 mg PO D1-21 of C7-9 and 15 mg PO D1-21 of C10
Primary endpoint: CR by end of consolidation
reduction in progression or death


















These data support use of D RVd induction/consolidation and D R maintenance as a NEW standard of care in Newly Diagnosed Myeloma

What are YOUR goals of therapy
What is your MM risk/ stage
What are your therapy options
What is your response to tx
Know what side effects to expect so you can report them
Who is on your care team
How to read your M-protein level IgG Kappa M-Protein

Obtain a second opinion

Ask about clinical trials
Be informed and empowered!

• With new biology-based medication 3 and 4 drug regimens the response rates are now >98%
• We have had 32 drugs and tx indications FDA approved for myeloma 2015-2023!




• With novel therapies are used at diagnosis, survival has improved dramatically
— From 3.8 years to >8 years!
— The 10yr relative survival rate has nearly doubled since in the past 20 years
Myeloma is not curable…yet. But is survivable now!









• Open the Q&A window, allowing you to ask questions to the host and panelists. They can either reply to you via text in the Q&A window or answer your question live.



• If the host answers live, you may see a notification in the Q&A window.


• If the host replies via the Q&A box – you will see a reply in the Q&A window.



6:35 – 7:15 PM Relapsed Therapy & Clinical Trials


David Vesole, MD, PhD, FACP - The John Theurer Cancer Center at Hackensack
University Medical Center
7:15 – 7:35 PM Life is a Canvas, You are the Artist
Kimberly Noonan, DNP, ANP-BC, AOCN - IMF Nurse Leadership Board, Dana-Farber Cancer Institute
7:35 – 8:00 PM Q&A with Panel


 David Vesole, MD, PhD, FACP
David Vesole, MD, PhD, FACP

Director, Myeloma Program
MedStar Georgetown University Hospital
Professor of Medicine
Georgetown University School of Medicine
Co-Chief, Myeloma Division
Director, Myeloma Research
John Theurer Cancer Center at Hackensack UMC
Professor of Medicine
Hackensack Meridian School of Medicine
David.Vesole@hmhn.org

• Most patients with MM have multiple distinct subclonal populations as a result of the expansion of genetically different myeloma cells; this causes intratumoral heterogeneity1
• MM is clonally heterogeneous at diagnosis and throughout treament2
• The genomic heterogeneity of MM contributes to treatment resistance and relapse3
• Wide variety of mutations found within a single patient may result in treatment resistance and refractory disease1,3,4
• Furthermore, subclones continually mutate over time, including after treatment, which may contribute to resistance and result in disease progression1,5
Definitions: What is relapsed/refractory disease and a line of therapy?
• Relapsed: recurrence (reappearance of disease) after a response to therapy
• Refractory: progression despite ongoing therapy

• Progression: change in M protein/light chain values
• Line of therapy: change in treatment due to either progression of disease or unmanageable side effects
• Note: initial (or induction) therapy + stem cell transplant + consolidation/ maintenance therapy = 1 line of therapy

Timing of therapy
Biochemical
• Patients with asymptomatic rise in blood or urine M protein, free light chains, or plasma cells
initiation/escalation dependent on many factors
Requires immediate
Clinical
• Based on direct indicators of increasing disease and/or end-organ dysfunction
initiation/escalation of therapy
• At biochemical relapse?
• When myeloma protein starts rising!
• When involved free light chain starts rising!
• At Clinical relapse?
• CRAB criteria (Anemia, kidney failure, high calcium or new bone disease)
• Extramedullary disease (myeloma growing at tumors outsides the bone)
Choices are broadest and guided by
Disease biology
Nature of relapse
Patient preference
Factors to consider
Prior autologous stem cell transplant
Prior therapies
Aggressiveness of relapse
Comorbidities
Psychosocial issues
Access to care
IMiDs Proteasome inhibitors
Thalomid (thalidomide)
Revlimid (lenalidomide)
Pomalyst (pomalidomide)
Kyprolis (carfilzomib)
Ninlaro (ixazomib)
Chemotherapy
anthracyclines Chemotherapy
alkylators Steroids
Novel mechanisms of action
Monoclonal antibodies
Cellular therapy
Velcade (bortezomib) Adriamycin
Cytoxan (cyclophosphamide) Dexamethasone
Doxil (liposomal doxorubicin)
Bendamustine Prednisone
Melphalan
XPOVIO (selinexor)
Empliciti (elotuzumab)
Venclexta (venetoclax)*
Farydak (Panobinostat)†
Pepaxto (melflufen)†
Darzalex (daratumumab)
Sarclisa (isatuximab)
Blenrep (belantamab mafodotin)‡
Tecvayli (teclistamab)§
*Not yet FDA-approved for patients with multiple myeloma; †Withdrawn from the US market in 2021; ‡Antibody-drug conjugate; §Bispecific antibody
Abecma (idecabtagene vicleucel)
Carvykti (ciltacabtagene autoleucel)
How does your oncologist decide what to do?

GREAT NEWS WE HAVE OPTIONS
BAD NEWS YOUR DOCTOR MAY GET CONFUSED
Not Refractory to Lenalidomide* Refractory to Lenalidomide*
Not refractory to CD38 moAB
Dara-refractory or Relapse while on CD38 moAB
Not refractory to CD38 moAB
Dara-refractory or Relapse while on CD38 moAB
KRd (preferred)
DRd
ERd, IRd
(Alternatives)
DKd or Isa-Kd Or
DPd or Isa-Pd
KCd or KPd
(preferred)
VCd or EPd
(Alternatives)
*Consider salvage ASCT in patients eligible for ASCT who have not had transplant before; Consider 2nd auto SCT if eligible and had >36 months response duration with maintenance to first ASCT
Key eligibility criteria
• RRMM
• ≥1 prior line of therapy
• Prior lenalidomide exposure, but not refractory
• Creatinine clearance
≥30 mL/min
DRd (n = 286)


Daratumumab 16 mg/kg IV
• Qw in Cycles 1 to 2, q2w in Cycles 3 to 6, then q4w until PD
R 25 mg PO
• Days 1 to 21 of each cycle until PD
d 40 mg PO
• 40 mg weekly until PD
Rd (n = 283)
R 25 mg PO
• Days 1 to 21 of each cycle until PD
d 40 mg PO
• 40 mg weekly until PD
Primary endpoint
• PFS
Secondary endpoints

• TTP
• OS
• ORR, VGPR, CR
• MRD
• Time to response
• Duration of response
Stratification factors


• No. of prior lines of therapy
• ISS stage at study entry
• Prior lenalidomide
Cycles: 28 days
Statistical analyses
• Primary analysis: ~177 PFS events
Pre-medication for the DRd treatment group consisted of dexamethasone 20 mg,a acetaminophen, and an antihistamine
ISS, international staging system; DRd, daratumumab/lenalidomide/dexamethasone; IV, intravenous; qw, weekly; q2w, every 2 weeks; q4w, every 4 weeks; PD, progressive disease; R, lenalidomide; PO, oral; d, dexamethasone; Rd, lenalidomide/dexamethasone; PFS, progression-free survival; TTP, time to progression; OS, overall survival; ORR, overall response rate; VGPR, very good partial response; CR, complete response; MRD, minimal residual disease. aOn daratumumab dosing days, dexamethasone 20 mg was administered as pre-medication on Day 1 and Day 2.
Multicenter, randomized (1:1), open-label, active-controlled, phase 3 study
not reached
17.5 months
Median follow-up: 32.9 months (range, 0 - 40.0 months)
56% reduction in risk of progression/death for DRd versus Rd
HR, hazard ratio; CI, confidence interval. aExploratory analyses based on clinical cut-off date of October 23, 2017; bKaplan-Meier estimate.
updated analysis: PFS (time without relapse is much better with 3 drugs....)
Drug Formulation Approval
Velcade (bortezomib)
Kyprolis (carfilzomib)

Ninlaro (ixazomib)
Revlimid (lenalidomide)*
Pomalyst (pomalidomide)*





XPOVIO (selinexor)
• IV infusion
• SC injection
• IV infusion
• Weekly dosing
Once-weekly pill
• For relapsed/refractory myeloma
• For relapsed/refractory myeloma as a single agent, as a doublet with dexamethasone, and as a triplet with Revlimid or Darzalex plus dexamethasone
• For relapsed/refractory myeloma as a triplet with Revlimid and dexamethasone
Once-daily pill
• For relapsed/refractory myeloma in combination with dexamethasone
Once-daily pill
• For relapsed/refractory myeloma in combination with dexamethasone
Once-weekly pill
• For relapsed/refractory myeloma as a triplet with Velcade and dexamethasone
*Black box warnings: embryo-fetal toxicity; hematologic toxicity (Revlimid); venous and arterial thromboembolism
IV, intravenous; SC, subcutaneous
OPTIMISMM
• Velcade-Pomalystdex (VPd) vs Vd Regimens compared
• Kyprolis-Revlimiddex (KRd) vs Rd





• Ninlaro-Rd (IRd) vs Rd
• XPOVIO-Velcade-dex (XPO-Vd) vs Vd





Median progression-free survival favored
• VPd: 11 vs 7 months
• KRd: 26 vs 17 months
• IRd: 21 vs 15 months
• XPO-Vd: 14 vs 9 months
Clinical considerations
• Consider for relapse on Revlimid
• VPd associated with more low blood counts, infections, and neuropathy than Pd

• KRd associated with more upper respiratory infections and high blood pressure than Rd
• IRd an oral regimen
• Gastrointestinal toxicities and rashes
• Lower incidence of peripheral neuropathy



• XPO-Vd associated with low platelet counts and fatigue with triplet, but less neuropathy than the Vd

Velcade
• Risk of peripheral neuropathy (PN; numbness, tingling, burning sensations and/or pain due to nerve damage)

Avoid in patients with severe existing PN
Reduced with subcutaneous once-weekly dosing
• High risk of shingles
− Use appropriate vaccination
• No dose adjustment for kidney issues; adjust for liver issues

• Less PN than Velcade
• High risk of shingles
Use appropriate vaccination
• Monitor for heart, lung, and kidney side effects
Use with caution in older patients with cardiovascular risk factors
• High blood pressure
• No dose adjustment for kidney issues; adjust for liver issues

Ninlaro
• Less PN than Velcade
• High risk of shingles

Use appropriate vaccination
• Monitor for rashes and gastrointestinal (GI) side effects


GI effects occur early
• Needs to be taken at least 1 hour before or 2 hours after a meal
Revlimid*
• Rash
Consider antihistamines
• Diarrhea
Consider bile acid sequestrants
• Risk of blood clots


• Risk of second primary malignancies
• Dose adjustment based on kidney function
Pomalyst*
• Low blood counts

• Less rash than Revlimid
• Risk of second primary malignancies





Begin prophylactic anti-nausea medications. Consult with your doctor if nausea, vomiting, or diarrhea occur or persist.
Fatigue
Stay hydrated and active.
Report signs of bleeding right away. Report signs of fatigue or shortness of breath.
Drug Formulation
Approval
Darzalex (daratumumab)

SC once a week for first 8 weeks, then every 2 weeks for 4 months, then monthly
• For relapsed/refractory myeloma as a single agent and as a triplet with Revlimid or Velcade or Kyprolis or Pomalyst plus dexamethasone
Empliciti (elotuzumab)


IV once a week for first 8 weeks, then every 2 weeks (or every 4 weeks with pom)
• For relapsed/refractory myeloma as a triplet with Revlimid or Pomalyst and dexamethasone
Sarclisa (isatuximab)
IV once a week for first 4 weeks, then every 2 weeks
• For relapsed/refractory myeloma as a triplet with Pomalyst or Kyprolis and dexamethasone

Regimens compared
• Darzalex-Revlimiddex (DRd) vs Rd
• Darzalex-Velcade-dex (DVd) vs Vd




• Darzalex-Kyprolis-dex (DKd) vs Kd
• Darzalex-Pomalystdex (DPd) vs Pd
Median progressionfree survival favored
• DRd: 45 vs 18 months
• DVd: 17 vs 7 months
• DKd: 29 vs 15 months
• DPd: 12 vs 7 months
• Consider for relapses from Revlimid or Velcade maintenance
Clinical consider-ations
• DRd associated with more upper respiratory infections, low blood white blood cell counts, and diarrhea
• Consider for patients who are Revlimid-refractory without significant neuropathy
• DVd associated with more low blood cell counts
• Consider for younger, fit patients who are doublerefractory to Revlimid and Velcade
• DKd associated with more respiratory infections
• Sever side effects (possibly fatal) in intermediate fit patients 65 and older
• Consider in patients who are double-refractory to Revlimid and a proteasome inhibitor (Velcade, Kyprolis, Ninlaro)
• Severe low white blood cell counts









ELOQUENT-2





ELOQUENT-3

ICARIA-MM IKEMA
Regimens compared
• Empliciti-Revlimiddex vs Rd
• EmplicitiPomalyst-dex vs Pd
Median progressionfree survival favored
• Empliciti-Rd: 19 vs 15 months
• Empliciti-Pd: 10 vs 5 mos
• Sarclisa-Pomalyst-dex vs Pd
• Sarclisa-Kyprolis-dex vs Kd
Clinical considerations
• Consider for non-Revlimid refractory, frailer patients
• Overall survival benefit with Empliciti-Rd

• Empliciti-Rd associated with more infections




• Consider for patients refractory to Revlimid and a proteasome inhibitor (Velcade, Kyprolis, Ninlaro)
• Sarclisa-Pd: 12 vs 7 mos
• Sarclisa-Kd: 41 vs 19 mos
• Consider for patients refractory to Revlimid and a proteasome inhibitor (Velcade, Kyprolis, Ninlaro)
• Sarclisa-Pd associated with severe low white blood cell counts, more dose reductions, upper respiratory infections, and diarrhea




• Consider for patients refractory to Revlimid and Velcade
• Sarclisa-Kd associated with higher MRD negativity rates
• Sarclisa-Kd associated with severe respiratory infections
Refractory to IMiD, PI, Anti-CD38
Refractory to IMiD, PI, Anti-CD38, Alkylators, and Anti-BCMA
Existing drugs:
Combinations with Cyclophosphamide
that do not have IMiD, PI, Anti CD38 (e.g., KCd)
Anti BCMA strategy
Anti-BCMA
Bispecific
BCMA CAR-Ts
Elotuzumab
Selinexor
Venetoclax
Bendamustine
VDT PACE
New Drugs: Iberdomide, Mezigdomide
New bispecifics (Cevostamab, Talquetamab)
New CAR-Ts
New Monoclonals
New ADCs

Class
Drug
Formulation
Approval
Nuclear export inhibitor
XPOVIO (selinexor)
Twice-weekly pill
• For relapsed/refractory myeloma in combinatio dexamethasone (after at least 4 prior therapie disease is refractory to at least 2 PIs, at least 2 IMiDs, and an anti-CD38 mAb
Antibody-drug conjugate
Blenrep (belantamab mafodotin)*
2.5 mg/kg IV over approximately 30 minutes once every 3 weeks
• For relapsed/refractory myeloma (after at least including an anti-CD38 mAb, a PI, and an IMiD
Chimeric antigen receptor (CAR) T cell
Abecma (idecabtagene vicleucel)†
300 to 460 × 106 genetically modified autologous CAR T cells in one or more infusion bags
• For relapsed/refractory myeloma (after 4 or mo prior lines of therapy, including an IMiD, a PI, a anti-CD38 mAb
CAR T cell
Bispecific antibody



Carvykti (ciltacabtagene autoleucel)‡
0.5 to 1.0 × 106 genetically modified autologous CAR T cells/kg of body weight
Tecvayli (Teclistamab) ‡ Step up dosing then weekly SQ
IMiD, immunomodulatory agent; PI, proteasome inhibitor; mAb, monoclonal antibody
• For relapsed/refractory myeloma (after 4 or mo prior lines of therapy, including a PI, an IMiD, a anti-CD38 mAb
For relapsed/refractory myeloma (after 4 or m therapy, including a PI, an IMiD, and an anti-C
†Black box warning: cytokine release syndrome; neurologic toxicities; hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS); prolonged cytopenia
‡Black box warning: cytokine release syndrome; neurologic toxicities; Parkinsonism and Guillain-Barré syndrome; hemophagocytic lymphohistiocytosis/ macrophage activation syndrome (HLH/MAS); prolonged cytopenia
Abecma and Carvykti are available only through a restricted distribution program
Previous therapies to which the disease was refractory, n (%)
Additional analyses showed clinical benefit with XPOVIO regardless of patient age and kidney function.2,3
Bispecific antibodies
• Elranatamab, talquetamab, cevostamab, and others
• Target BCMA, GPRC5D, or FcRH5 on myeloma cells and CD3 on T cells
• Redirects T cells to myeloma cells
Cereblon E3 ligase modulators (CELMoDs)
• Iberdomide
• Targets cereblon
• Enhances tumoricidal and immune-stimulatory effects compared with immunomodulatory agents
Small molecule inhibitors
• Venetoclax
• Targets Bcl-2
• Induces multiple myeloma cell apoptosis
Collect patient’s white blood cells
Isolate and activate T cells Engineer T cells with CAR gene
Tumor cell
Targeting element (eg, CD19, BCMA, CD20)
Spacer
Transmembrane domain
Expand CAR T cells Infuse same patient with CAR T cells
CD19
Viral vector with CAR DNA
CARengineered T cell

Costimulatory domain (eg, CD28 or 4-1BB)
CD3���� (essential signaling domain)
Median manufacturing time: 17-28 days
Patients undergo lymphodepleting (and possibly salvage/bridging) therapy
3
CR or sCR and MRD NE CR or sCR and MRD-
ORR, overall response rate; PR, partial response; VGPR, very good partial response; CR, complete response; sCR, stringent complete response; MRD, minimal residual disease; PFS, progression-free survival
KarMMa Trial. Munshi NC et al. N Engl J Med. 2021;384:705.
CARTITUDE-1 Trial. Berdeja JG et al. Lancet. 2021;398:314; Martin T et al. J Clin Oncol. June 4, 2022 [Epub ahead of print].
Onset 1 9 days after CAR T-cell infusion
2 9 days after CAR T-cell infusion
Duration 5 11 days 3 17 days
Symptoms
• Fever
• Difficulty breathing
• Dizziness
• Nausea
• Headache
• Rapid heartbeat
• Low blood pressure
Management
• Actemra (tocilizumab)

• Corticosteroids
• Supportive care
• Headache
• Confusion
• Language disturbance
• Seizures
• Delirium
• Cerebral edema
• Antiseizure medications
• Corticosteroids
*Based on the ASTCT consensus; †Based on vasopressor; ‡For adults and children >12 years;
§For children ≤12 years; ‖Only when concurrent with CRS
Xiao X et al. J Exp Clin Cancer Res. 2021;40(1):367. Lee DW et al. Biol Blood Marrow Transplant. 2019;25:625; Shah N et al. J Immunother Cancer. 2020;8:e000734.
Survey of 20 centers. Responses from 17 centers.

Cellular therapies CAR T-cell therapy
Patient’s cells collected
Types
Patient given chemotherapy before cells are infused back into patient Yes,
When in the course of myeloma is this usually done?
Side effects of treatment
After multiple relapses As part of initial treatment
Cytokine release syndrome; confusion
*An immune cell that is the “business end” of the system, in charge of maintaining order and removing cells. †Precursor cells that give rise to many types of blood cells. We actually collect CD34+ve cells.
Fatigue, nausea, diarrhea



Bispecific antibodies are also referred to as dual specific antibodies, bifunctional antibodies, or T-cell engaging antibodies
Bispecific antibodies can target two cell surface molecules at the same time (one on the myeloma cell and one on a T cell)


Many different bispecific antibodies are in clinical development; none are approved for use in myeloma
Availability is off-the-shelf, allowing for immediate treatment
Cohen A et al. Clin Cancer Res. 2020;26:1541.
Examples:
• Elranatamab
• Teclistamab
• TNB-303B (ABBV-383)
• REGN5458
• Cevostamab
• Talquetamab
BCMA, GPRC5D, or FcRH5Drug Formulation
Approval
Tecvayli (teclistamab)*
Step-up dosing† the first week then once weekly thereafter by subcutaneous injection

• For relapsed/ refractory myeloma (after 4 or more prior lines of therapy, including an IMiD, a PI, and an anti-CD38 mAb)
IMiD, immunomodulatory agent; PI, proteasome inhibitor; mAb, monoclonal antibody
*Black box warning: cytokine release syndrome; neurologic toxicities
†Patients are hospitalized for 48 hours after administration of all step-up doses.
Tecvayli is available only through a restricted distribution program.
Median duration of response 18.4 months
CR or better median DOR not reached (95% CI: 16.2–NE)
• Overall median DOR of 18.4 months (95% CI: 14.9–NE), and was not yet mature with data from 71 patients (68.3%) censored
• 12-month event-free rate:
• Overall:
Overall median DOR 18.4 months (95% CI: 14.9–NE)
• Patients with CR or better:
68.5% (95% CI: 57.7–77.1)
80.1% (95% CI: 67.6–88.2)
• 2 patients (1.2%) discontinued due to AEs (grade 3 adenoviral pneumonia; grade 4 PML)
• 1 patient had dose reduction at cycle 21
• The most common AEs were CRS and cytopenias
• Infections occurred in 126 (76.4%) patients (grade 3/4: 44.8%)
• 123 patients (74.5%) had evidence of hypogammaglobulinemiaa
• There were 19 deaths due to AEs, including 12 COVID-19 deaths
• 5 deaths due to teclistamab-related AEs:
• COVID-19 (n=2)
• Pneumonia (n=1)
• Hepatic failure (n=1)
• PML (n=1)
*Based on a recent sampling
• Cytokine release syndrome (CRS)
• Neurotoxicity (ICANS)
• Usually occurs within first 1–2 weeks
• Frequency (all grade and grade 3–5) higher with CAR T
• Cytopenias
• Target unique
• For example, rash, taste disturbance seen with GPRC5D, but not with BCMA
• Infections
• Incidence for bispecifics at RP2D not yet known
• Viruses: CMV, EBV
• PCP/PJP
• Ongoing discussions regarding prophylactic measures IVIG
Anti-infectives
CAR T and bispecific antibodies are very active even in heavily pretreated patients.
Side effects of CAR T cells and bispecific antibodies include cytokine release syndrome, confusion, and low blood counts, all of which are treatable.
Abecma and Carvykti are only the first-generation CAR T cells and target the same protein. Different CAR Ts and different targets are on the way. Bispecific antibodies represent an “off-the-shelf” immunotherapy; Tecvayli was approved in October 2022.
Several additional bispecific antibodies are under clinical evaluation.
• Cancer affects all of us
• Each year in the U.S.A:
• More than half a million people are expected to die of cancer—more than 1,500 people a day
• 1 of 4 deaths is from cancer
• More than 1 million new cancer cases are expected to be diagnosed
• Clinical trials translate results of basic scientific research into better ways to prevent, diagnose, or treat cancer
• The more people that take part, the faster we can:
• Answer critical research questions
• Find better treatments and ways to prevent cancer
Do Many People Participate in Cancer Clinical Trials?
Only 3 percent of U.S. adults with cancer participate in clinical trials
• Cancer clinical trials are
– Carefully controlled research studies
– Conducted by doctors to improve the care and treatment of cancer patients
• The aim of a clinical trial is to
– Study a new therapy or a new use for an already approved therapy
– Compare a new treatment with a standard treatment to find out which one works better and/or has fewer side effects
• Each cancer clinical trial has a written detailed study design called a protocol that includes:
• Why the clinical trial is needed
• Purpose of the clinical trial
• What drug or drug(s) are being tested, with a treatment and follow-up schedule
• Safety measures throughout the clinical trial program
• How outcomes will be measured
• Who is eligible for the clinical trial
• How the clinical trial will be organized, one site or multiple sites
• If the clinical trial is a multi-site trial, all participating physicians must follow the same protocol




Initial
of new drug in lab
Drug studied in lab




Food and Drug Administration approves new drug application
The drug can now be studied in people in carefully controlled clinical trials
How How do clinical trials work?
Phase I investigates for safety and side effects, dosage and best way to give treatment–includes 20 or more people



Phase II determines effectiveness and safety–typically includes fewer than 100 (may include up to 300) people

Phase III looks at effectiveness, side effects and safety in comparison with other treatments–includes 100s to 1000s of people
Phase IV gathers more information after FDA approval & drug is on market
Possible benefits:
•Patients will receive, at a minimum, the best standard treatment
•If the new treatment or intervention is proven to work, patients may be among the first to benefit
•Patients have a chance to help others and improve cancer care
Possible risks:
• New treatments or interventions under study are not always better than, or even as good as, standard care
• Even if a new treatment has benefits, it may not work for every patient
• Health insurance and managed care providers do not always cover clinical trials
Patients may:
• Be unaware of clinical trials
• Lack access to trials
• Fear, distrust, or be suspicious of research
• Have practical or personal obstacles
• Face insurance or cost problems
• Be unwilling to go against their physicians’ wishes
Doctors might:
• Lack awareness of appropriate clinical trials
• Be unwilling to “lose control” of a person’s care
• Believe that standard therapy is best
• Be concerned that clinical trials add administrative burdens




February 1, 2023

Nurse Practitioner
Dana-Farber Cancer Institute
Boston, MA
Kimberly Noonan, DNP, RN, ANP, AOCN

Myeloma treatment

Know your care team, Telehealth & Meeting Prep, & Shared Decision Making
Infection and Side Effect Management

• Rapid and effective disease control



• Durable disease control
• Minimize side effects
• Allow for good quality of life
• Improved overall survival
• Prevent disease - and treatmentrelated side effects
• Optimize symptom management

• Allow for good quality of life
DISCUSS GOALS AND PRIORITIES WITH YOUR HEALTHCARE TEAM
Transplant
Eligible Patients
Transplant
Consolidation
Maintenance
Initial Therapy
Transplant
Ineligible patients
Consolidation/ Maintenance/ Continued therapy

Treatment of Relapsed disease
Everyone
Supportive Care
A meta - analysis identified the most common patient- reported symptoms and impact on QOL, and were present at all stages of the disease. Symptoms resulted from both myeloma disease and treatment, including transplant, and were in these categories:
Physical
• Fatigue
• Constipation
• Pain
• Neuropathy

• Impaired Physical Functioning
• Sexual Dysfunction


Psychological

• Depression
• Anxiety
• Sleep Disturbance
• Decreased Cognitive Function
• Decreased Role & Social Function
Financial
• Financial burden (80%)
• Financial toxicity (43%)
• There are no black and white answers to deciding to undergo a transplant
• Undergoing transplant is a commitment for both you and your care partner
• Understanding the process will help bring to focus elements needed to decide if/when to undergo transplant

Clinical Experience Data from Research
Patient Preference
Adapted from Philippe Moreau, ASH 2015



Steroid Side Effects
Steroid Synergy
Steroids are a backbone and work in combination to enhance myeloma therapy
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over- the - counter or prescription medications

• Medications to prevent shingles, thrush, or other infections
Do not stop or adjust steroid doses without discussing it with your health care provider
• Irritability, mood swings, depression
• Difficulty sleeping (insomnia), fatigue

• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Increase in blood pressure, water retention
• Blurred vision, cataracts
• Flushing/sweating
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increase in blood sugar levels, diabetes

Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR, Eastern Cooperative Oncology Group (2010) Lenalidomide plus highdose dexamethasone versus lenalidomide plus low - dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an o pen - label randomised controlled trial. Lancet Oncol 11(1):29 –37.

AND SIDE EFFECT

– Up to a 3rd of patients on clinical trials has serious infections (requiring IV antibodies or hospitalization)
Increased risk of serious COVID complications despite history of vaccination
– Antibody levels

– Tixagevimab co - packaged with cilgavimab ( EVUSHELD )
– Immediate treatment once diagnosed Nirmatrelvir with Ritonavi ( Paxlovid )
• Start as soon as possible; must begin within 5 days of when symptoms start

7 - 10 fold increased risk of bacterial and viral infections for people with myeloma
Report fever of more than 100.4 °F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
Good personal hygiene (skin, oral)
Environmental control (wash hands, avoid crowds and sick people, etc)
Growth factor (Neupogen [filgrastim])


Immunizations (NO live vaccines)
care team
• Medications (antibacterial, antiviral)
Manage stress
• Rest, relaxation, sleep hygiene
• Mental health / social engagement

• Complementary therapy
Maintain a healthy weight
• Nutrition
• Activity / exercise
Preventative health care
• Health screenings, vaccinations
• Prevent falls, injury, infection
• Stop smoking
• Dental care
Maintain renal health
• Myeloma management
• Hydration
• Avoid renally - toxic medications
– Dose adjust to renal function
• Diabetes management
Protect your bones
• Nutrition, Calcium + D supplement
• Weight- bearing activity / walking
• Bone strengthening agents
“ An ounce of prevention is worth a pound of cure .” Benjamin Franklin
Care partner support is essential for the entire transplant and CAR T processes
• Sedated procedures; Education sessions
• Assistance with daily activities, managing medications and alerting the medical team of changes
• Continued support and assistance is often needed in the early days after returning home. Less assistance will be needed as time goes on.


Care partner can be one person or a rotation of many people.


Diarrhea may be caused by medications and supplements
• Laxatives, antacids with magnesium
• Antibiotics, antidepressants, others
• Milk thistle, aloe, cayenne, saw palmetto, ginseng
• Sugar substitutes in sugar free gum
Avoid caffeinated, carbonated, or heavily sugared beverages
Take anti- diarrheal medication
• Imodium ® , Lomotil® , or Colestid if recommended
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
• Welchol® if recommended
Constipation may be caused by
• Opioid pain relievers, antidepressants, heart or blood pressure medications, others
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B - 12 deficiency
Increase fiber
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®

Fluid intake can help with both diarrhea and constipation, and good for kidneys. Discuss GI issues with health care providers to identify causes and make adjustments to medications and supplements.

Pain can significantly compromise quality of life
Sources of pain include bone disease, neuropathy and medical procedures
Management
• Prevent pain when possible
• Bone strengtheners to decrease fracture risk; anti viral to prevent shingles; sedation before procedures
• Interventions depends on source of pain
• Monitor serum calcium levels
• Imaging may be needed depending on type and location of pain ( eg , MRI, PET- CT)
• May include medications ( eg bone modifying agents), activity, surgical intervention, radiation therapy, etc

• Complementary therapies (Mind - body, medication, yoga, supplements, acupuncture, etc )
Tell your health care provider about any new bone pain or chronic pain that is not adequately controlled
Peripheral neuropathy: damage to nerves in extremities (hands, feet, or limbs)
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness
Prevention / management:
• Bortezomib once - weekly or subcutaneous administration
• Massage area with cocoa butter regularly
• Supplements:
• B - complex vitamins (B1, B6, B12)
• Folic acid, and/or amino acids but do not take on day of Velcade® (bortezomib) infusion
• Safe environment: rugs, furnishings, shoes


If PN worsens, your HCP may:
• Change your treatment
• Prescribe oral or topical pain medication
• Suggest physical therapy
Report symptoms of peripheral neuropathy early to your health care provider; nerve damage from PN can be permanent if unaddressed
All can affect quality of life and relationships
• Fatigue is the most common reported symptom (98.8%)
Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression


• Anxiety reported in >35%


• Depression nearly 25% Financial concerns, disease progression, end - of - life, and change in social and sexual function were highlighted sources
Often, people do not share these symptoms with their provider. Talk to your provider about symptoms that are not well controlled or thoughts of self harm. Help is available.
Financial burden comes from
• Medical costs
• Premiums
• Co - payments

• Travel expenses
• Medical supplies

• Prescription costs
• Loss of income
• Time off work or loss of employment
• Caregiver time off work
Contact the Social Services department at your hospital or clinic to talk to a social worker for assistance.
Funding and assistance may be available
• Federal programs
• Pharmaceutical support
• Non - profit organizations
• Websites:
• Medicare.gov
• SSA.gov
• LLS.org
• Rxassist.org
• NeedyMeds.com
• HealthWellFoundation.org
• Company - specific website


You are central to the care team








Be empowered
• Ask questions, learn more
• Participate in decisions
Communicate with your team
• Understand the roles of each team member and who to contact for your needs

• Participate in support network








Come prepared:
• Bring a list of current medications , prescribed and over the counter
• Write down your questions and concerns . Prioritize them including financial issues

• Have there been any medical or life changes since your last visit?
• Current symptoms - how have they changed (improved, worsened, stable)? Keep a symptom diary. Bring it along
• Communicate effectively : your health care team can’t help if they don’t know
• Know the “next steps” , future appointments, medication changes, refills, etc
Check with your healthcare team –Is telemedicine an option?
Similar planning for “in - person” appointment PLUS:
• What is the process and what technology is needed?

• Plan your labs : are they needed in advance? Do you need an order?
• Plan your location: quiet, well- lit location with strong wi- fi is best
• Plan yourself: consider if you may need to show a body part and wear accessible clothing
• Collect recent vital signs (blood pressure, temp, heart rate) self - serve blood pressure cuff is available at many pharmacies and for purchase
Be empowered to be part of the treatment decision - making

• Ask for time to consider options (if needed/appropriate)
• Understand options; consider priorities
• Use reliable sources of information
• Use caution considering stories of personal experiences
• Consider your goals/values/preferences
• Express your goals/values/preferences; create a dialog


• My top priority is [goal/value]; additional [preferences] are also important.
• I think [treatment] may be a good choice given my priorities… What do you think?
• Arrive at a treatment decision together



























Thank you for joining us today for the February 1, 2023, International Myeloma Foundation’s Regional Community Workshop –Northeast & Southern states.




As follow up to today's workshop, we will have the speaker slides and a video replay available.
These will be provided to you shortly after the workshop concludes.
February 15, 2023
IMF Virtual Regional Community Workshop (RCW) - Midwest Region

5:30 PM Central Time
March 4, 2023* RETURN TO IN PERSON MEETINGS
IMF In-Person Regional Community Workshop (RCW) - San Diego
8:00AM Pacific Time
March 17, 2023
IMF Patient & Family Seminar 2023 - Boca Raton
8:00AM Eastern Time
Registration for these events can be found at myeloma.org under the “EVENTS” tab:













At the close of the meeting a feedback survey will pop up.

This will also be emailed to you shortly after the workshop.

Please take a moment to complete this survey.
