
August 2024
Sheraton Times Square New York































August 2024
Sheraton Times Square New York































Significantly higher incidence rates of plasma cell disorders (PCDs) and T-cell lymphoma (TCL) were reported in blacks compared to whites in the United States. Survival differences by race among both PCD and TCL highlight the need for improved access to care for minorities. More recently, two B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T-cell therapies were approved for treating myeloma, a PCD - Carvykti (ciltacabtagene autoleucel) for patients with relapsed myeloma that have been treated with ≥1 line of therapy and Abecma (idecabtagene vicleucel) for patients treated with ≥2 lines of therapy. While these are lifesaving immunotherapies, limited access due to higher costs is a reality.
Adding to this, the Food and Drug Administration (FDA) has mandated black box warnings for BCMA-CAR-T cell therapies following the reports of increased risk of TCL following CAR-T administration. This warning is based on the reported incidence of approximately 22 cases of TCLs among the 27,000 patients who received CAR-T cell therapies commercially (0.081%).
Treatment with CAR-T cell therapies theoretically could lead to TCLs as a result of CAR vector insertion into a cancer gene, leading to deregulated clonal proliferation of mature T cells. While the true nature and frequency of the TCLs following BCMA-CART cell therapy are yet to be fully discerned, it also raises an important epidemiological question of the incidence of the concomitant diagnosis of PCD and TCL, with a concern of increased risk of TCL among myeloma patients following BCMA-CART. Given the higher incidence of both these malignancies in black patients, we evaluated the racial differences in the incidence of concomitant malignancies.

Ferdinand Anokwuru, Monica Hartley Brown, MD, MMSC


Background: Multiple myeloma, an incurable blood cancer with high morbidity, remains a significant healthcare challenge despite advancements in therapy. Racial disparities, especially between black and white patients, affect clinical outcomes, influenced by factors such as socioeconomic status and access to quality healthcare. This study aims to explore how these health-related social determinants impact treatment outcomes and potentially affect quality of life in black and white patients receiving bispecific therapies for multiple myeloma.
Methods: This is a retrospective study, wherein between January 2023 and January 2024, relevant patients’ electronic medical records and healthcare databases were reviewed to obtain social determinants of health information, including health insurance, proximity to the healthcare facility, and zip code. We compared this information between black and white patients, including demographics for each group. Clinical outcomes were measured in terms of time to next treatment (TTNT), progression-free survival, and overall survival. Statistical analyses were conducted to identify any significant differences in treatment outcomes between the two racial groups.
Results: The study included patients with multiple myeloma treated with FDA-approved bispecific therapies, namely teclistamab, talquetamab and elranatamab. Differences were observed in all the SDOH parameters evaluated, namely health insurance, proximity to the healthcare facility and zip code. Findings also showed differences in clinical outcomes for black and white patients. These disparities were associated with variations in treatment response, with black patients generally experiencing less favorable outcomes.
Conclusions: This study demonstrates that social determinants of health, including health insurance, proximity to the healthcare facility, and zip code, may be associated with adverse treatment outcomes in black patients receiving bispecific therapies for multiple myeloma compared to their white counterparts. The findings underscore the need for targeted interventions to address these disparities, such as improving access to quality healthcare facilities and improving health insurance and support services for disadvantaged populations. Efforts to reduce these disparities could potentially enhance clinical outcomes for black patients with multiple myeloma.

Diandra Adu-Kyei, BA1, Tondre Buck, MD


Multiple myeloma (MM) is the second-most common hematologic malignancy in the United States and Europe1. It occurs two to three times more frequently in Black people, thus making it the most common hematologic malignancy among said patient population. Current guidelines recommend proteasome inhibitors, immunomodulatory agents, and corticosteroids, with or without monoclonal antibodies, followed by stemcell transplantation as initial treatment for transplant-eligible patients. Additionally, the use of bortezomib, lenalidomide, and dexamethasone in combination (RVd) has become the standard induction regimen1. Bortezomib, a selective inhibitor of the 26S proteasome, disrupts the major intracellular protein degradation pathway, leading to cell cycle arrest and apoptosis. It is not only an important agent in induction, but also an option for maintenance therapy in cytogenetically defined high-risk patients.
However, bortezomib-induced peripheral neuropathy (BIPN) can arise as a result of its use. BIPN often leads to dose reduction, therapy interruption, supportive medication initiation, and/or discontinuation. Incidences of grades 1 and 2 BIPN range from 40% to 70% across studies1,2. Low-grade BIPN typically presents as mild to moderate distal sensory loss, pain at fingertips and toes, and mild motor weakness. All grade peripheral neuropathy (PN) was reported in 72% of patients receiving subcutaneous bortezomib as part of the RVd induction regimen in the GRIFFIN trial1. A study found that overall, 112 (40%) patients experienced new or worsening PN after initiating bortezomib-containing therapy. The incidence of BIPN was higher in the Black group than in the non-Black group, and Black race was associated with an increased risk of BIPN. A significant independent association between Black race and the incidence of BIPN was observed1. It is thus important to screen patients for preexisting neuropathy, especially Black patients among patients of color, who are more predisposed to PN.
Research recently found genetic markers that may need to be added to the screening process in order to more effectively identify patients who may be at risk3. For example, single-nucleotide polymorphisms (SNPs) associated with immune function (CTLA4

rs4553808, CTSS rs12568757), reflexive coupling within Schwann cells (GJE1 rs11974610), drug binding (PSMB1 rs1474642), and neuron function (TCF4 rs1261134, DYNC1I1 rs916758) associated with BIPN4. Patients studied were significantly older, had more comorbid conditions (such as diabetes and baseline PN, which is predictive for development of BIPN), had longer treatment duration and higher cumulative dose of bortezomib, and developed more PN.
Unfortunately, analyses of DNA samples (from whole blood) were limited to those from Caucasian patients as they formed the largest homogenous population in the Favis et al. paper. In the Corthals et al. study, DNA samples (from peripheral blood nucleated cells or CD138 negative bone marrow cells) similarly excluded 15 patients of non-European descent to ensure homogeneity of allelic frequencies. The studies specifically did not include non-European subjects, which means that these genes may not even manifest in minority populations. Patients of color need to be more intentionally researched in order to adequately assess their needs. Additional genetic testing during routine MM blood analysis would help further identify a significant variable in BIPN development. Ultimately, after testing has proactively identified comorbidities, relevant SNPs, and overall risk of PN, alternative prescriptions and courses may need to be considered to bridge the new treatment gap. In addressing MM among Black patients, the increased incidence rate of BIPN demonstrates that it is critical to avoid the onset of secondary issues to which they also remain vulnerable, through thorough and holistic screening.
References:
1. Sun LF, Maples KT, Hall KH, et al. Impact of Black Race on Peripheral Neuropathy in Patients With Newly Diagnosed Multiple Myeloma Receiving Bortezomib Induction. JCO Oncology Practice. 2023;19(9):793-798. doi:10.1200/op.22.00781
2. Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood. 2008;112(5):15931599. doi:10.1182/blood-2008-04-149385
3. Corthals SL, Kuiper R, Johnson DC, et al. Genetic factors underlying the risk of bortezomib induced peripheral neuropathy in multiple myeloma patients. Haematologica. Nov 2011;96(11):1728-32. doi:10.3324/haematol.2011.041434
4. Favis R, Sun Y, van de Velde H, et al. Genetic variation associated with bortezomibinduced peripheral neuropathy. Pharmacogenet Genomics. Mar 2011;21(3):121-9. doi:10.1097/FPC.0b013e3283436b45



Abstract:
Multiple myeloma is a hematologic malignancy characterized by the abnormal plasma cell proliferation in the bone marrow. Management involves a complex treatment regimen with various medications and often lifelong therapy. While advancements in multiple myeloma treatment have improved patient outcomes, disparities continue to persist in treatment utilization and adherence, particularly with minority populations experiencing lower survival rates and limited care access. This retrospective comparative analysis seeks to identify and address such disparities in medication adherence and treatment access among multiple myeloma patients. Using data from the All of Us Research Program under the National Institute of Health, 1,414 patients diagnosed with multiple myeloma were studied. The factors investigated include pharmacy drug access, treatment modalities, and qualitative survey responses on healthcare access. The pharmacy drugs assessed include Lenalidomide, Dexamethasone, Melphalan, Bortezomib, and Daratumumab, while treatment modalities encompass chemotherapy, immunotherapy, and stem cell transplantation. Survey responses measure medication adherence and access. The findings of this study reveal that there are significant disparities in healthcare access, medication adherence, and affordability among racial groups, with minorities exhibiting lower rates. Moreover, disparities in drug utilization may stem from differences in access to healthcare services, prescription practices, or patient preferences among different racial groups. Further investigation is needed to understand the underlying factors driving these disparities. Addressing these disparities is crucial for achieving equitable access to healthcare for all individuals, irrespective of race or ethnicity
Erneisha



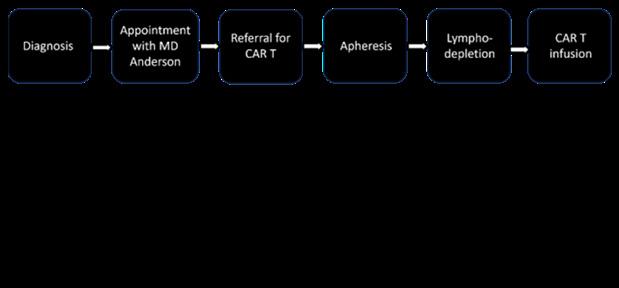
Background: Chimeric antigen receptor T-Cell (CAR T) therapy is one of the most efficacious novel therapies for relapsed refractory multiple myeloma (RRMM), offering a one-and-done treatment option that significantly improves quality of life for patients who have endured prolonged continuous therapy. However, due to complex logistics and potential complications associated with CAR T, access is restricted to specialized centers, leading to disparities in access in both the clinical trial and SOC setting, and particularly affecting Black patients. For patients who can undergo CAR T therapy, disparities in treatment based on various demographic and clinical variables remain underexplored.
Objective: This study aims to identify disparities in time to treatment for patients with RRMM undergoing CAR T therapy, based on measurable variables including sex, primary language, comorbidity burden, neighborhood-level social determinants of health, race/ethnicity, insurance types, and prior lines of therapy.
Methods: This retrospective single center study included RRMM patients who underwent apheresis for idecabtagene vicleucel (Ide-Cel) between 11/2018 and 02/2023 as standard of care (SOC) or on clinical trials (KarMMa 2, KarMMa 3). Data on demographics, disease features, prior treatment, comorbidities via a revised HCT-CI score, time to treatment, and follow-up were extracted from medical records. Area Deprivation Index (ADI) was measured based on zip code at time of CAR- T referral. Comparisons across racial/ethnic groups utilized chi-squared tests and ANOVA. Four time to treatment durations were calculated (Figure 1). Time to treatment comparisons were conducted using two-sample t-tests and ANOVA with Tukey post-hoc analysis.
Results: Our analysis included 84 patients (24 trial, 60 SOC), 45 of whom self-identified as White, 21 as Black and 13 as Hispanic/Latino. The median age was 64.4y (range 53.5-75). Black and Hispanic/Latino patients had longer times from successful apheresis to CAR T infusion (6.74y and 8.08y, respectively compared to 5.24y). Patients with five or more prior lines of therapy and those with Medicare insurance experienced significantly longer times from diagnosis to infusion (7.70y vs 4.08y and 7.09y vs 4.96y respectively). Variables such as ADI, high-risk disease features, and HCT-CI comorbidity score did not correlate to difference in time to treatment categories.
Conclusion: While the existence of inequities in medical treatment is well-documented, this study highlights specific areas where disparities in time to treatment occur for patients with RRMM undergoing CAR T therapy. Further research is required to understand the drivers of the delays in the treatment process and why they occur. This will allow for the development of strategies to mitigate these disparities.
Tyra Grischke, Benjamin Derman, MD

Tyra Grischke Indiana University School of Medicine


Introduction:
Chimeric antigen receptor (CAR) T-cell therapy is a novel immunotherapy for advanced multiple myeloma (MM) involving genetic modification of a patient’s T-cells to generate the CAR product. CAR T-cell products idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) demonstrated superior outcomes over standard care, leading to FDA approval. However, there can be delays from insurance approval to T-cell collection (brain-to-vein time, B2V) and from T-cell harvest to infusion (vein-to-vein time, V2V).
This study analyzed B2V and V2V times for CAR T-cell therapy in MM, their interaction with insurance status and disease characteristics, and their impact on outcomes.
Methods:
We conducted a retrospective study of 64 consecutive insured patients at the University of Chicago collected for commercial CAR T-cell therapy between April 2021 and June 2024. Cox proportional hazard models estimated hazard ratios (HRs) for progression-free survival (PFS) and overall survival (OS), indexed from the time of T-cell collection.
Results:
There were 40 patients with Medicare and 24 patients with private insurance. Baseline characteristics were comparable across insurance types, with private group being younger in age (median 60 vs 71 years), receiving a higher proportion of cilta-cel infusions (96% vs 70%), and receiving more bridging therapy (100% vs 75%).
Median B2V times were 29.5 days (Medicare) and 39.5 days (private) (p=0.02), and a B2V time <15 days in 23% (Medicare) vs 4% (private) (p=0.049). Median V2V times were 62 days (Medicare) and 59 days (private) (p=0.78).
With a median follow-up time from T-cell collection of 9.5 months for the entire cohort, there were a total of 25 progression events and 21 deaths: 5 patients died prior to receipt of CAR T-cell therapy, 8 died directly from complications of CAR T-cell therapy, and 8 died following disease progression. 1/10 patients with a B2V <15 days died before receiving CAR T-cell therapy vs 9/54 (17%) with B2V >15 days.
On univariate analysis, the following variables were significantly associated (p<0.05) with inferior PFS and OS: receipt of ide-cel, ECOG performance status, aggressive disease features, prior BCMA directed therapy (PFS only), bone marrow plasmacytosis >60%, and ferritin > median (285). Insurance type and age were not significantly associated with PFS or OS.
Age-adjusted multivariate analysis revealed that younger age (HR 1.05, p=0.03), ide-cel vs cilta-cel (HR 3.9, p=0.007), worse ECOG PS (HR 1.9, p=0.02), prior BCMA-directed therapy (HR 5.9, p<0.001), and bone marrow plasmacytosis >60% (HR 3.3, p=0.02) were associated with worse PFS. Only worse ECOG PS (HR 2.8, p=0.002) was associated with worse OS.
Conclusions:
Patients with Medicare had numerically shorter B2V time, and a higher proportion had a B2V <15 days. While a shorter B2V time may be associated with a lower risk of dying before receiving CAR T-cells, neither insurance type, B2V time, nor V2V time were associated with outcomes overall. Multivariate analysis showed that younger age, receipt of ide-cel vs cilta-cel, worse ECOG PS, receipt of BCMA-directed therapy, and BMPC >60% were associated with worse PFS.
Brittany Grossi, Nina Kim, Monal Kohli, Bingcao Wu, Emeka Umeh, Dee Lin, Gideon Aweh, Emily Achter, Laura Hester, Sian Walker, Christina Hearty, Peter M Voorhees6



Background
Multiple myeloma (MM) is twice as prevalent among Black Americans as in White Americans.1 Prior evidence demonstrated disparities in access to innovative therapies and underrepresentation in clinical trials of Black patients (pts) with MM.2 Teclistamab, a first-in-class B-cell maturation antigen (BCMA) x CD3 bispecific antibody, was approved to treat pts with relapsed/refractory MM through the pivotal MajesTEC-1 trial, which included 13% Black pts.3 In the real-world, 15% of newly diagnosed MM pts were observed to be Black, as reported in a cross-sectional analysis of the All-payer Real-world Multiple Myeloma Research-ready Data (ARMMRD) registry.4 To understand access and outcomes for Black pts with MM, we sought to describe patient characteristics, treatment patterns, and safety outcomes in Black pts with MM receiving teclistamab in the real-world.
Methods
This was a retrospective observational cohort study using the ARMMRD registry. Pts (≥18 years) with MM who received teclistamab between 10/26/22 and 1/31/24 were included. Pts were indexed on the earliest outpatient claim of a teclistamab 30 mg/3 mL vial or admission date of the earliest hospitalization encounter containing teclistamab. Pts with race data available were stratified into two cohorts: Black pts and pts of other races. Pts with a complete step-up dosing (SUD) period were identified using a claims-based algorithm. Demographic and clinical characteristics were captured 6 months prior to or on the index date. Treatment history was captured using all available pre-index data. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were identified via ICD-10-CM codes and symptom-based codes (‘Keating algorithm’5 for CRS) in pts with a complete SUD period. Variables were summarized descriptively; time to less frequent dosing (LFD) schedules (e.g., bi-weekly dosing) was evaluated using Kaplan-Meier analysis.
Results
Among 578 teclistamab-treated pts with race information available, 86 (15%) were Black and 492 (85%) were other races (White: 471 [96%]; Asian: 21 [4%]). Compared to pts of other races, higher proportions of Black pts were <65 years (38% vs 24%) and had Medicaid (22% vs 8%); a lower proportion had Medicare Fee-For-Service (FFS) (45% vs 68%). Black pts had a higher mean QuanCharlson Comorbidity Index (QCCI) score (4.9 vs 3.9) and higher prevalence of baseline comorbidities than pts of other races. Fewer Black pts received prior stem cell transplantation (SCT) compared to

other races (36% vs. 46%), but a similar proportion had prior exposure to BCMA-targeted therapies (16% for both).
Among 44 Black pts and 257 pts of other races with an identified complete SUD period, a higher proportion of Black pts had ≥1 CRS event (per ICD-10-CM codes: 41% vs 37%; per the Keating algorithm: 41% vs 29%). Most of the CRS events were grade 1 or 2 among both Black pts (89%) and pts of other races (85%). The ICANS rates were similar in Black pts (11%) and pts of other races (11%), and most of the ICANS events were grade 1 or 2.
At a median (range) follow-up of 3.7 months (0.3-11.2) for Black pts and 3.2 months (0.1-13.7) for pts of other races, LFD schedules were observed in 15 Black pts and 87 pts of other races. The probability of switching to LFD was similar between Black pts and pts of other races, with a 6-month switch probability of 53% and 54%, respectively, after teclistamab initiation.
In this first real-world study in the US using a nationally representative, all-payer claims data to evaluate Black pts receiving teclistamab, we observed that more Black pts with MM were younger, had Medicaid insurance, and a higher comorbidity burden than pts of other races. Numerically higher CRS rates during SUD were also observed in Black pts vs. pts of other races. However, except for lower rates of SCT in the Black cohort, prior treatment and teclistamab treatment patterns, including the probability of switch to LFD schedules, appeared similar between Black pts and pts of other races. While access barriers remain to be further understood, our results found similar proportions of Black pts treated with teclistamab as benchmarked to the general MM population in the registry, suggesting equitable access to teclistamab across races. Further research with a larger sample size and longer follow-up to assess longitudinal outcomes are warranted to confirm and expand these findings.
Disclosures
MK, EU, GA, and EA are employees of STATinMED LLC and have received consulting fees from Janssen Scientific Affairs, LLC. NK, BW, DL, LH, and CH are employees of Janssen Scientific Affairs, LLC and stockholders of Johnson & Johnson. SW is a former employee of Janssen and current contractor and stockholder of Johnson & Johnson. BG is a medical student at the Howard University College of Medicine and has no disclosures. PV has received honoraria for consulting or advisory boards from Abbvie, AstraZeneca, BMS, GSK, Johnson and Johnson, Karyopharm, Lava Therapeutics, Regeneron, Sanofi; research funding from Abbvie, GSK, Johnson and Johnson, Regeneron.
References
1. Cancer Stat Facts: Myeloma. Accessed June 17, 2024. https://seer.cancer.gov/statfacts/html/ mulmy.html
2. Bhutani M, Blue BJ, Cole C, et al. Addressing the disparities: the approach to the African American patient with multiple myeloma. Blood Cancer J. Dec 18 2023;13(1):189. doi:10.1038/s41408-023-009610
3. Moreau P, Garfall AL, van de Donk N, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. Aug 11 2022;387(6):495-505. doi:10.1056/NEJMoa2203478
4. Lin D, Liu Y, Kim N, et al. A US national view of prevalent and newly diagnosed multiple myeloma in 2021: a retrospective analysis of national all-payer claims database. Presented at: 64th American Society of Hematology and Exposition; December 10-13, 2022; New Orleans, LA.
5. Keating SJ, Gu T, Jun MP, McBride A. Health care resource utilization and total costs of care among patients with diffuse large B cell lymphoma treated with chimeric antigen receptor t cell therapy in the United States. Transplant Cell Ther. Jul 2022;28(7):404 e1-404 e6. doi:10.1016/j.jtct.2022.03.021

Jayla Mondy, Saurav Das, Dhaani Ailawadhi, Yaw Adu, Sikander Ailawadhi MD


Introduction: Therapeutic advancements for MM have led to improved patient survival, but notable disparities exist Factors that impact patient decisions regarding healthcare choices have not been studied enough. Additionally, with improved survivorship, the medical, psychosocial, and financial burden on patients should be analyzed.
Methods: An anonymous, online, 45-question English survey exploring demographics, social determinants of health (SDOH), selected clinical characteristics, treatment preferences, and survivorship burden was distributed to MM patients within the Mayo Clinic system and to all International Myeloma Foundation (IMF) support groups. Multiple model logistic regression was done for multivariate analysis of patient responses, using R language (Bluesky Statistics, V7.40).
Results: The analysis included 2239 participants with 53.7% males, median age 68 years (range 2195) and median age at MM diagnosis of 67 years. Non-Hispanic Whites were the largest (89.3%) racial-ethnic group. Most participants (70.8%) had a college degree and approximately two-thirds had Medicare as primary insurance. MM was diagnosed within 5 years for 58.4% and 73.5% were currently on MM treatment. Majority (76.1%) had never been on a clinical trial, 59.1% had received stem cell transplant, 7.8% had undergone CAR-T therapy and 8% had received bispecific antibodies. Majority (65.9%) preferred oral drugs while 19.9% preferred injection and 14.1% infusion therapy. Top factors for treatment decisions were physician recommendations (92.2%), effectiveness (88.5%), treatment understanding (74.4%), side effects (57.1%), and family/caregiver input (37.8%). Median time to treatment facility was 30 minutes. Increase in healthcare needs since MM diagnosis were reported by 47.8% and majority reported ongoing symptom burden with fatigue (78.3%), neuropathy (60%), and pain (53.2%). Nearly half (48.7%) of participants reported anhedonia since MM diagnosis, however majority (55.6%) still reported “good” quality of life. 15% had missed >10 days of work and 15.4% had suffered job loss. Table 1 shows significant findings for MM survivorship burden by sociodemographic groups.
Conclusions: Decision-making for MM treatment is complex and there are several factors that affect patients behavior. Improved survival in MM is associated with significant survivorship burden which is associated with sociodemographic disparities. These must be addressed systematically to achieve equitable healthcare utilization and optimal clinical, psychosocial and financial wellbeing.
Griffiths Y, Shekarkhand T, Derkach A, Rajeeve S, Tan C, Mailankody S, Firestone R, Jurgens E, Miller K, Usmani SZ.



BACKGROUND:
Multiple myeloma (MM) is a plasma cell neoplasm that accounts for 10% of hematologic cancers overall and is the most common blood cancer in Black Americans1. The median age of diagnosis is 69 years and the etiology of disparities in incidence rates is largely unknown. However, race, age, gender, family history, and exposure to toxins are considered known risk factors2–4. The survival of MM has more than quadrupled in the past two decades. More recently, T-cell redirecting therapies including bispecific antibodies (BsAb) and chimeric antigen receptor (CAR) T-cell therapies have shown particular promise for the treatment of MM5–8. While the emergence of BsAbs and CAR T-cell therapies appear to be revolutionizing the MM treatment landscape in the US, little is known about access, utilization, and responses to these treatments across racial and ethnic groups9. Few studies have thoroughly explored racial and ethnic disparities in outcomes associated with bispecific and CAR T-cell therapies. In this single-center study, we compared outcomes of patients treated with CAR T-cell therapy and commercial bispecific antibody therapy based on race and ethnicity.
METHODS:
We performed an IRB-approved retrospective analysis of clinical outcomes for 254 patients with relapsed/refractory multiple myeloma who received CAR T-cell therapy or commercial bispecific antibody therapy at Memorial Sloan Kettering Cancer Center (MSKCC) between March 22, 2017, to May 17, 2024. All analysis was performed using the CRAN R version 3.3.0 (The R Foundation for Statistical Computing, Vienna, Austria). Disease characteristics analyzed include time since diagnosis, revised International Staging System (R-ISS) stage, cytogenetic risk, presence of extramedullary disease (EMD) and heavy and light chain isotypes. Outcomes assessed include overall therapy response rate and progression-free survival (PFS) and overall survival (OS) as per IMWG guidelines10.
RESULTS:
The patient characteristics are summarized in Table 1. In this study population, 254 participants were included with a median age of 66.4 years old. 14% of patients included were Black, while 75% of patients were White, and 5% of patients were Asian. 6% of patients included were unidentified or identified as other. 4% of patients included in this study were Hispanic while 94% of patients were non-Hispanic and 2% were missing ethnicity data. 140 (55%) patients received CAR T-cell therapy, 20 (8%) received talquetamab, and 94 (37%) received teclistamab or elranatamab. Median time from diagnosis to start of T-cell redirecting therapy was 5.7 (interquartile range [IQR], 3.7-8.5) years. Patients in our study were heavily pretreated having received a median of 6 (IQR, 4-8) prior lines of therapy. We saw a statistically significant relationship between mean age of patient during treatment and race, with both Black and White populations having an older mean age compared to Asians (P<0.05). However, there was no statistically significant difference in mean age between Hispanic and non-Hispanic groups. There was no significant difference in the number of prior lines of therapy used among racial or ethnic groups. Presence of extramedullary disease showed no statistically significant difference between racial groups with 58% of Asian patients, 34% of unidentified or missing patients, 50% of Black patients and 47% of White patients reporting positive EMD (p=0.2964).
Of the patients who received CAR T-cell therapy, 16 (11.4%) were Black, while 110 (78.6%) were White, 7 (5%) were Asian, and 7 (5%) identified as other or were unidentified. Among patients treated with talquetamab, 4 were Black, 10 were White, 3 were Asian, and 3 identified as other/unidentified. Of the patients who received Teclistamab/Elranatamab therapy, 16 were Black, while 71 were White, 2 were Asian, and 5 identified as other or were unidentified (p=0. 0475).
36% of Hispanic patients received CAR T therapy, 9% received Talquetamab therapy, and 55% received Teclistamab/ Elranatamab. 57% of non-Hispanic patients received CAR T-cell therapy, 8% received Talquetamab therapy, and 35%

received Teclistamab/Elranatamab (p= 0.25987).
The overall response rates with all T-cell redirecting therapies were 56% in Black, 65% in White, 75% in Asian, 60% in pts who identified as other or were unidentified (p=0.3848). Overall response rate in Hispanic patients was 55% and 69% of non-Hispanic patients (0.3286). The VGPR or better rates with T-cell redirecting therapies were 42% in Black, 50% in White, 67% in Asian, and 40% in pts who identified as other or were unidentified (p=0.3103).
The 6-month and 12-month PFS rates with T-cell engaging therapies were 48.4% (95%CI, 33.9%-69.1%) and 29.9% (16.2%-55.0%) in Black patients, 55.4% (95% Cl 48.5%-63.3%) and 34.7% (95% CI 27.6%-43.8%) in Whites, 66.7% (95% CI 41.5%-100%) and 66.7% (95% CI 41.5%-100%) in Asian patients, and 42% (95% CI 21.8%-81.0%) and 42% (95% CI 21.8%-81.0%) in patients who identified as other or were unidentified, respectively (P=0.23). Progression-free survival rates in Hispanic patients were 27.3% (95% CI 10.39%-71.6%) at 6-months and 18.2% (95% CI 5.19%-63.7%) at 12-months and 55.6% (95% CI 49.3%-62.7%) in non-Hispanic at 6-months and 36.0% (95% CI 29.3%-44.2%) at 12-months (p=0.29).
The overall survival rates for Black patients were 76.1% (95% CI 62.8%-92.2%) at 6 months and 71.6% (95% CI 57.1%-89.7%) at 12 months. For Asian patients, overall survival was 100% (95% CI 100%-100%) at 6 months and 52.5% (95% CI 22.1%100%) at 12 months. In White patients, overall survival at 6 months was 80.9% (95% CI 75.3%-86.9%) and 70.3% (95% CI 63.4%-78.0%) at 12 months. In patients who identified as other or were unidentifiable, overall survival was 69.5% (95% CI 48.0%-100%) at 6 months and 57.9% (95% CI 34.6%-96.9%) at 12 months (p=0.95). Overall survival in Hispanic patients at 6 months was 72.7% (95% CI 50.6%-100%), and 60.6% (95% CI 36.4%-100%) at 12 months, while in non-Hispanic patients’ overall survival at 6 months was 80.9% (95% CI 75.9%-86.3%) and was 69.5% (95% CI 63.2%-76.6%) at 12 months (p=0.78). We did not observe a statistically significant difference in PFS and OS rates among different racial or ethnic groups.
DISCUSSION:
Overall, we saw no statistically significant difference in overall response rates to T-cell redirecting therapies when stratified by patient race. Neither bispecific antibody therapies nor CAR T-cell therapies showed disparate response rates in Black populations when compared to their White counterparts. This demonstrates a promising future for bispecific and CAR T therapies. However, only 14% of this overall cohort was comprised of Black or African American patients. A more robust study across multiple centers with increased diversity may provide further insight into racial disparities in care and outcomes. This study highlights the urgent necessity for equitable treatment options and access to care across all populations. Historically, Black populations in the US have been marginalized and faced difficulties receiving the newest and highest quality treatments. As such, Black individuals have experienced increased burden and severity of many diseases, including multiple myeloma. This single center study shows no statistically significant difference in therapy response or outcomes. Thus, we must continue to invest resources into equitable health care along the multiple myeloma care continuum including access to novel treatment options, additional supportive care, and improved responses to treatment.
1. Alexanian Raymond, Dimopoulos Meletios. The Treatment of Multiple Myeloma. N Engl J Med. 1994;330(7):484-489. doi:10.1056/NEJM199402173300709
2. Smith CJ, Ambs S, Landgren O. Biological determinants of health disparities in multiple myeloma. Blood Cancer J. 2018;8(9):1-7. doi:10.1038/s41408-018-0118-z
3. Marinac CR, Ghobrial IM, Birmann BM, Soiffer J, Rebbeck TR. Dissecting racial disparities in multiple myeloma. Blood Cancer J. 2020;10(2):1-8. doi:10.1038/s41408-020-0284-7
4. Padala SA, Barsouk A, Barsouk A, et al. Epidemiology, Staging, and Management of Multiple Myeloma. Med Sci. 2021;9(1):3. doi:10.3390/medsci9010003
5. Caraccio C, Krishna S, Phillips DJ, Schürch CM. Bispecific Antibodies for Multiple Myeloma: A Review of Targets, Drugs, Clinical Trials, and Future Directions. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.00501
6. Nath K, Mailankody S, Usmani SZ. The Role of Chimeric Antigen Receptor T-Cell Therapy in the Era of Bispecific Antibodies. Hematol Oncol Clin North Am. 2023;37(6):1201-1214. doi:10.1016/j.hoc.2023.05.011
7. Firestone RS, Mailankody S. Current use of CAR T cells to treat multiple myeloma. Hematol Am Soc Hematol Educ Program. 2023;2023(1):340-347. doi:10.1182/hematology.2023000434
8. Firestone R, Lesokhin AM, Usmani SZ. An Embarrassment of Riches: Three FDA-Approved Bispecific Antibodies for Relapsed Refractory Multiple Myeloma. Blood Cancer Discov. 2023;4(6):433-436. doi:10.1158/2643-3230.BCD-23-0176
9. Gasoyan H, Fiala MA, Doering M, Vij R, Halpern M, Colditz GA. Disparities in Multiple Myeloma Treatment Patterns in the United States: A Systematic Review. Clin Lymphoma Myeloma Leuk. 2023;23(11):e420-e427. doi:10.1016/j. clml.2023.08.008
10. Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-e346. doi:10.1016/S14702045(16)30206-6



Background: Multiple myeloma (MM) is the most common hematologic cancer in Black people, driven by the higher prevalence of asymptomatic precursor condition Monoclonal gammopathy of Undetermined Significance (MGUS). The prevalence of MM and MGUS is 2 to 3 times more common than in White individuals, affects Black people at a younger age, family members have twice the risk of developing the condition and Black people typically have worse outcomes due to myriad reasons. Despite these compelling facts, Black people anecdotally have little awareness of the condition.
Aim: To conduct a qualitative survey assessment in the community to determine the existing knowledge and awareness of MM and elicit their specific health literacy and educational needs.
Methods: Adults over 18 years of age were approached in community settings (barbershops, churches, community events) in Louisville Kentucky, USA. Electronic surveys were distributed to those who agreed to participate in this assessment via QR code. Paper surveys were administered to those who were not able to complete electronically. The survey gathered non identifying demographic information, included graded responses via Likert scale, structured “yes or no” questions and multiple select options to gauge awareness of MM and the educational needs of the community. Descriptive statistics described the results and chi square test was used to determine associations, p value <0.05 was considered statistically significant.
Results: Two hundred and twenty five hours (225 hrs) were logged in outreach and community contact from March 2024 to July 2024. 150 people were approached and all completed surveys; 118 completed electronically and 32 required paper surveys. Median (range) age of participants was 33 (19-79) years; 63% (n=95) were female. Most respondents were selfidentified Black/African American (76%; n=114). Fifty-three percent (54%) were college/university educated, 39% had a further graduate or professional degree. The majority of the cohort, 67%, were “not very familiar or “not familiar at all” with MM. There was no difference in awareness of MM between Black and Non-Black individuals (chi square p=0.26). Most were not aware that MM is the most common blood cancer among Black individuals 89% (n=134), of which 75% were Black respondents. 89% of the respondents ( which included 78% Black individuals) wanted to learn more and thought learning about MM and related conditions was important. Most preferred to get more information from their health care team (63%); particularly so for Black people (p=0.04) social media was not a preferred source, selected by only 7%. Most (81%) felt comfortable discussing their health conditions including screening for cancer with their medical team, with no difference in this by self-identified race (Black vs Non-Black) p= 0.45. Overwhelmingly, people pointed to “lack of information” as the main barrier to awareness of MM (95%), followed by “limited access to health care resources” (58%) and “cultural stigma” (25.8%). Half of the respondents (50%) thought that these barriers could be addressed through education by their Health care team followed by Community Organizations/ Outreach Effort (37%). Again, social media was not favored as a mode of information (13%). Important questions the community wanted answered were “what are the symptoms of multiple myeloma” (80%), “should they or family members be screened” 79% and “who gets affected” (78%) among others.
Discussion: For the first time, we have gained unique insight into the health awareness and health education needs regarding Multiple Myeloma from a community perspective. Significant time was required to elicit community knowledge and their concerns with respect to MM. Most of these individuals had at least college level education or obtained a professional degree, yet 67% had little to no awareness with the condition. For a condition disproportionately impacting Black people, just over 1/3 of Black people expressed any familiarity with MM. However, that MM is still a relatively rare malignancy could explain some of the lack of familiarity. Despite reports of lack of engagement with and mistrust towards the health care system by Black people, this data demonstrates the contrary, that Black individuals desire more information on MM, do indeed trust and rely on their physicians/ health team for their health education. Interestingly, we found that social media was not a favored resource for information on MM, though the median age of the cohort was 33 years. The respondents in this study show keen interest in increasing their knowledge of MM and MGUS, not only for themselves but with concern for their family members. Improving health literacy of MM at a community level will be key to encouraging self-advocacy and reducing the delay in presentation for to ultimately improve the outcomes for MM in the Black community.

Divya Rath, Aishwarya Anuraj, Andriy Derkach, Urvi A. Shah *Contributed equally


Introduction: Multiple Myeloma (MM) is a blood cancer that disproportionately affects Black populations. Diabetes mellitus (DM) and elevated body mass index (BMI) are modifiable risk factors linked to increased risk of MM. Furthermore, these risk factors also disproportionately affect Black populations compared to White populations. In this study, we aim to calculate the population attributable fraction (PAF) - MM incidence attributable to elevated BMI and DM among adults ≥18 years and compare this between non-Hispanic Black (NHB) and non-Hispanic White (NHW). We further estimate the degree to which these risk factors contribute to the excess MM risk in Black populations compared to White populations.
Methods: To calculate the estimated of PAF of incident MM attributable to BMI and DM, relative risks (RRs) were obtained from estimates of hazard ratios reported in two large epidemiologic populationbased studies evaluating MM risk with DM (Gong et al. Diabetologia 2021) and with elevated BMI (Hofmann et al AJE 2013). Based on this paper estimate of MM risk for overweight category (hazard ratio [HR] 1.09) and obese category range (HR 1.26 to 1.55) was utilized. Furthermore, the prevalence of overweight (BMI: 25-30) and obese (BMI:>30) BMI categories for the US were obtained from 2016-2021 from the Behavioral Risk Factor Surveillance System (BRFSS), a nationwide telephone survey of adults 18 years or older designed to provide reliable estimates of health-related behavioral risk factors; this data was stratified by race or age. National level prevalence of diabetes data for age groups 18-44, 45-64, 65-74, and 75+ was obtained from the Centers for Disease Control and Prevention through their US Diabetes Surveillance System from the Division of Diabetes Translation for 2000-2022; this data was stratified by race.
Results: For NHW populations, the prevalence of obesity was 31.3%, and overweight was 36.8%. For NHB populations the prevalence of obesity was 43.2%, and overweight was 33.6%. The PAF due to elevated BMI ranged between 11-19.5% for NHW and 13.5-22.5% for NHB. The excess in MM cases due to elevated BMI in NHB compared to NHW ranges between 2.5-3%. Results for DM will be presented at the meeting.
Conclusions: The proportion of MM attributable to modifiable risk factors such as diabetes and high BMI (both of which affect black populations disproportionately compared to white populations) is significant. Up to approximately 1 in 5 MM cases in NHW and 1 in 4 MM cases in NHB is estimated by our analysis to be attributable to elevated BMI as a risk factor. Thus, this suggests a need for comprehensive intervention strategies to lower BMI and reduce DM prevalence among communities at risk of developing MM, as well as implementing preventive measures to reduce the long-term impact of these chronic conditions.

Primary therapy outcomes in relation to primary cytogenetic subtypes in a large cohort of Black patients with multiple myeloma at a single center
Semegne Hiruy, Myra Robinson, Peter Voorhees, and Manisha Bhutani


Background: Multiple myeloma (MM) occurs 2-3 times more frequently in Black individuals, who also experience higher mortality compared to White patients. The role of cytogenetics in contributing to these racial disparities remains unclear as data on Black patients are limited. Here, we evaluated cytogenetic subtypes and their impact on disease characteristics, treatment response, and outcomes in Black patients with MM.
Methods: Data were gathered retrospectively from the Levine Cancer Institute MM database spanning January 2012 to March 2022. We focused on 467 eligible Black patients who underwent FISH cytogenetic testing at diagnosis, and compared outcomes across three cytogenetic groups: standard-risk (SR) (t(11;14), t(6;14), other IgH translocations); high-risk (HR) (t(14;16), t(14;20), t(4;14), del17p, del1p, 1q gain/amp); and trisomy without translocations
Results: The cohort, with an average age of 62.6 years, exhibited a diverse range of cytogenetic abnormalities. Approximately 24% had normal FISH results, while 28% had IgH translocations (with or without trisomies) and 33% displayed trisomies without IgH translocation. Notably, t(11;14) was the most common IgH translocation at 16%, followed by t(4;14) at 5.4% and t(14;16) at 2.6%. Patient demographics and CRAB features were generally consistent across cytogenetic groups. However, anemia was more frequent in certain subgroups, such as patients with t(4;14), t(14;20), and 1q gain/amplification. Among 456 treated patients, 45.8% received a PI+IMiD regimen predominantly consisting of RVD as first-line therapy, and 39.9% underwent upfront autologous stem cell transplantation. Overall, the initial therapy response rate was promising, with 91.7% achieving a response, and 63.4% achieving a very good partial response or better. Significant differences emerged in overall survival (OS) across cytogenetic groups, with median OS of 5.5 years in HR, 6.8 years in SR, and 8.3 years in trisomy groups. Progression-free survival did not differ significantly.
Conclusion: To our knowledge, this is the largest single institution report on cytogenetics in Black patients with MM. Our findings underscore the impact of cytogenetic subtypes on MM outcomes in Black patients, highlighting the need for tailored therapeutic approaches based on genetic risk stratification.


