
June 20, 2024
Thank you to our sponsors!







June 20, 2024
Thank you to our sponsors!





MGUS, SMM, & DYNAMIC MODELING OF PROGRESSION ICELAND Updates
FRONTLINE THERAPY
MRD Update & MRD2STOP Trial BELANTAMAB: DREAMM-7 and DREAMM-8 Trials CAR T Updates: Fast CAR BISPECIFICS: Teclistamab Real-World Data
VENETOCLAX: CANOVA Update


• 50% risk of progression at 2 years is a reasonable definition of high-risk group to target
– Depend on the risk of intervention
• Multiple risk assessment models have been developed
– Original and or updated Mayo system (20/2/20)
– Spanish method
– IMWG risk score
– Dynamic models





















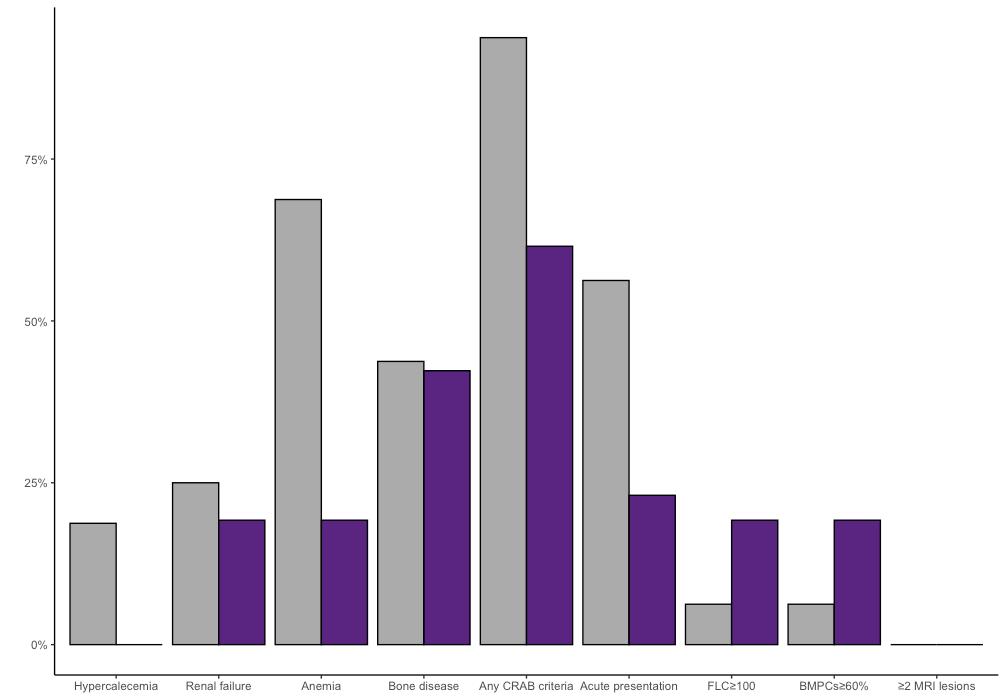
Any CRAB criteria present at diagnosis
OR=0.11 95%CI: 0.002-0.96; p=0.03
Acute presentation
OR: 0.24 95%CI: 0.05-0.98; p=0.047
In Manuscript, S. Rögnvaldsson et al
Control Arm
Intervention Arm

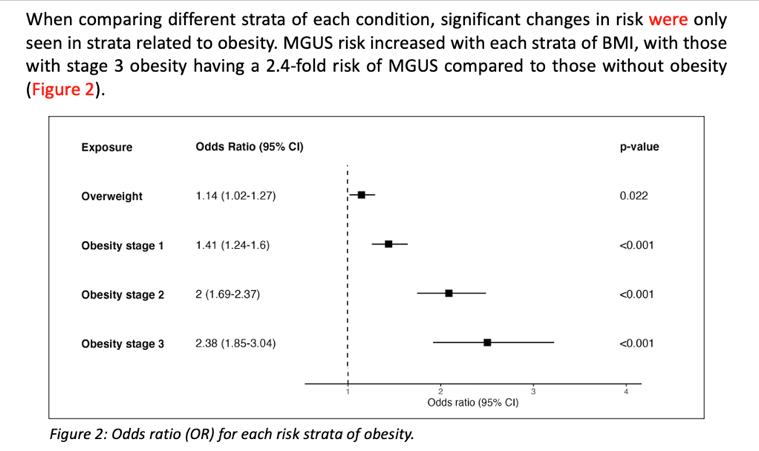
Obesity Linked to MGUS
Screened MGUS NOT LINKED to Autoimmunity
Prediction of Need for Bone Marrow






Sonneveld P, Moreau P, Dimopoulos MA, et al. Daratumumab + bortezomib/lenalidomide/dexamethasone in transplant-eligible patients with newly diagnosed multiple myeloma: analysis of minimal residual disease in the PERSEUS trial. Presented at: EHA 2024 Congress; June 15, 2024; Madrid, Spain





IMF
Dr.


Dr.





































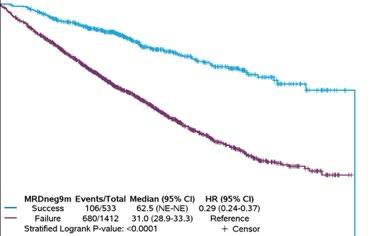
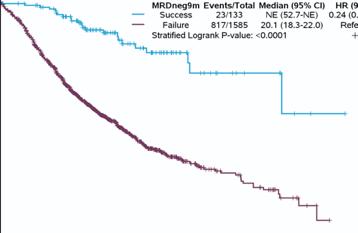
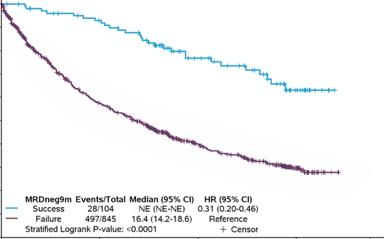
• Consistent high individual-patient-level correlations provide strong evidence that 9 months MRDneg-CR rate at 10-5 threshold reasonably likely predicts clinical benefit of PFS in NDTE, NDTinE and RR MM populations
‒ Promising trial-level correlations pooling 3 populations provide supportive evidence
‒ Similar results were seen for 12 months MRDneg-CR rate at 10-5 threshold
‒ Similar results were seen for OS, except in the scenarios with low events
MRDneg-CR rate classified at 10-5 threshold at 9 and 12 months IS reasonably likely to predict clinical benefit in NDTE, NDTinE, and RR MM settings


9 months MRDneg-CR Status, Classified at 10-5 Threshold
Clinical Endpoint: Progression-Free Survival


47/83 able to discontinue maintenance
40 MRD negative at 10-7: 3-year PFS was 85%
5 [11%] disease progression; 6 [13%] became MRD positive at 10-6
















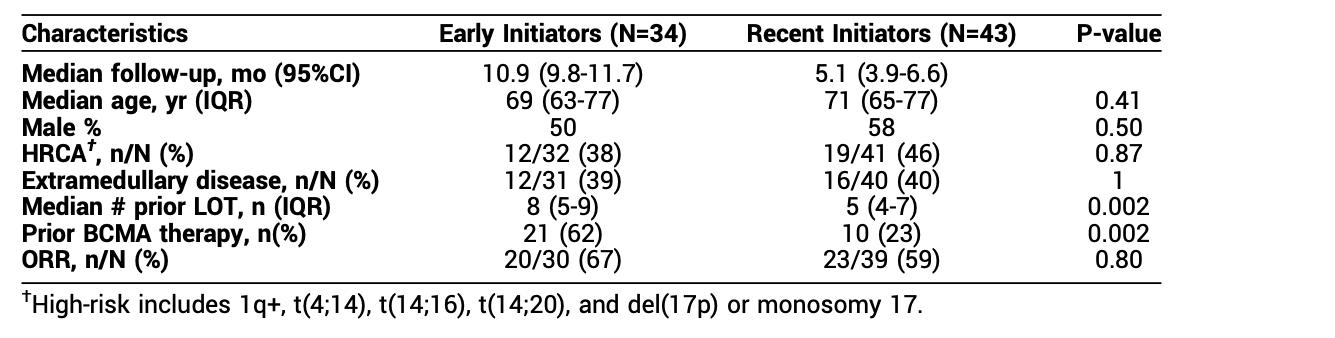
FRONTLINE GCO12F .. FasT CAR T : Newly diagnosed high risk patients
novel manufacturing gives * young phenotype * T cells
VRd followed by CAR T as induction
ORR 100 % and MRD negative @ 10-6 also 100%
Well-tolerated : all CRS grade 1 or 2 within 4 days EXCELLENT RESULTS : PFS and OS AWAITED





for t[11;14];
HIGH or 1q+




- Testing/Biology
- The Future for Myeloma Care ???
… Prospects for Cure?





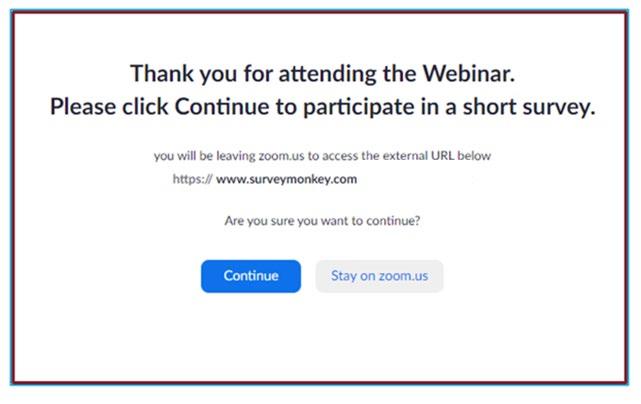
We Want to Hear From You!
At the close of the meeting a feedback survey will pop up. Click “continue” to complete the survey. This will also be emailed to you shortly after the workshop.

Please take a moment to complete this survey.
Thank you to our sponsors!


