for Patients and Care Partners



Thank you to our sponsors!










Thank you to our sponsors!








Voices

tweeters out of ASH






Nearly 30,000 attendees in person in San Diego!
Another 3500 virtual attendees
A record number of Research Abstracts Submitted – over 8500
Approximately 1200 related to Myeloma!!




Meletios A Dimopoulos1, Peter M Voorhees2, Fredrik Schjesvold3, Yael C Cohen4, Vania Hungria5, Irwindeep Sandhu6, Jindriska Lindsay7, Ross I Baker8, Kenshi Suzuki9, Hiroshi Kosugi10, Mark-David Levin11, Meral Beksac12, Keith Stockerl-Goldstein13, Albert Oriol14, Gabor Mikala15, Gonzalo Garate16, Koen Theunissen17, Ivan Spicka18, Anne K Mylin19, Sara Bringhen20, Katarina Uttervall21, Bartosz Pula22, Eva Medvedova23, Andrew J Cowan24, Philippe Moreau25, Maria-Victoria Mateos26, Hartmut Goldschmidt27, Tahamtan Ahmadi28, Linlin Sha29, Els Rousseau30, Liang Li29, Robyn M Dennis31, Robin Carson32, S Vincent Rajkumar33
1National and Kapodistrian University of Athens, Alexandra General Hospital, Athens, Greece; 2Levine Cancer Institute, Atrium Health Wake Forest University School of Medicine, Charlotte, NC, USA; 3Oslo Myeloma Center, Department of Hematology, Oslo University Hospital, Oslo, Norway; 4Tel-Aviv Sourasky (Ichilov) Medical Center and Tel Aviv University, Tel Aviv, Israel; 5Clínica Medica São Germano, São Paulo, Brazil; 6Cross Cancer Institute, University of Alberta, Edmonton, AB, Canada; 7Kent and Canterbury Hospital, Kent, UK; 8Perth Blood Institute, Murdoch University, Perth, Australia; 9Japanese Red Cross Medical Center, Tokyo, Japan; 10Ogaki Municipal Hospital, Ogaki City, Japan; 11Albert Schweitzer Hospital, Dordrecht, The Netherlands; 12Ankara University, Ankara, Turkey; 13Washington University School of Medicine, St. Louis, MO, USA; 14Institut Català d'Oncologia and Institut Josep Carreras, Hospital Germans Trias I Pujol, Barcelona, Spain; 15South-Pest Central Hospital, National Institute for Hematology and Infectious Diseases, Budapest, Hungary; 16Hospital Alemán, Buenos Aires, Argentina; 17Jessa Hospital, Hasselt, Belgium; 18Charles University and General Hospital, Prague, Czech Republic; 19Rigshospitalet, University of Copenhagen, Copenhagen, Denmark; 20SSD Clinical Trials in Oncol-ematologia e Mieloma Multiplo, AOU Città della Salute e della Scienza di Torino, Torino, Italy; 21Medical Unit Hematology, Karolinska University Hospital, Stockholm, Sweden; 22Institute of Hematology and Transfusion Medicine, Warszawa, Poland; 23Knight Cancer Institute, Oregon Health & Science University, Portland, OR, USA; 24University of Washington and Fred Hutchinson Cancer Center, Seattle, WA, USA; 25University Hospital Hôtel-Dieu, Nantes, France; 26University Hospital of Salamanca/IBSAL/Cancer Research Center-IBMCC (USAL-CSIC), Salamanca, Spain; 27GMMG Study Group at University Hospital Heidelberg, Internal Medicine V, Heidelberg, Germany; 28Genmab US Inc., Plainsboro, NJ, USA; 29Janssen Research & Development, LLC, Shanghai, China; 30Janssen Research & Development, Beerse, Belgium; 31Janssen Research & Development, LLC, Raritan, NJ, USA; 32Janssen Research & Development, LLC, Spring House, PA, USA; 33Mayo Clinic, Rochester, MN, USA.
Presented by MA Dimopoulos at the 66th American Society of Hematology (ASH) Annual Meeting & Exposition; December 7-10, 2024; San Diego, CA, USA
https://www.congresshub.com/ASH2024/ Oncology/Daratumumab/Dimopoulos
The QR code is intended to provide scientific information for individual reference, and the information should not be altered or reproduced in any way.

Screening
Key eligibility criteria:
• ≥18 years of age
• Confirmed SMM diagnosis (per IMWG criteria) for ≤5 years
• ECOG PS score of 0 or 1
• Clonal BMPCs ≥10% and ≥1 of the following risk factors:
- Serum M-protein ≥30 g/L
- IgA SMM
- Immunoparesis with reduction of 2 uninvolved Ig isotypes
- Serum involved:uninvolved FLC ratio ≥8 and <100
- Clonal BMPCs >50% to <60%
All patients were required to have CT/PET-CT and MRI imaging during screening
enrollment period: December 2017 to May 2019 at 124 sites in 23 countries
Treatment/active monitoring phase Follow-up phase
1800 mg SCb QW Cycles 1-2, Q2W Cycles 3-6, Q4W thereafter in 28-day cycles until 39 cycles/36 months*
Active monitoring
No disease-specific treatment, with AE monitoring up to 36 months*
• Efficacy follow-up until progression by SLiM-CRAB
• Survival follow-up every 6 months until end of study
Primary endpoint:
• PFS by IRC per IMWG SLiM-CRAB criteriac
Key secondary endpoints:
• ORR
• Time to first-line treatment for MM
• PFS on first-line treatment for MM
• Overall survival
*Or confirmed disease progression (whichever occurred first).
Stratified by number of risk factorsa for progression to MM (<3 vs ≥3)
Disease evaluation schedule
• Laboratory efficacy – Every 12 weeks by central lab until disease progression
• Imaging (CT/PET-CT, MRI) – Yearly (central review)
• Bone marrow – At least every 2 years
IMWG, International Myeloma Working Group; ECOG PS, Eastern Cooperative Oncology Group performance status; BMPC, bone marrow plasma cell; FLC, free light chain; CT, computed tomography; MRI, magnetic resonance imaging; QW, weekly; Q2W, every 2 weeks; Q4W, every 4 weeks; AE, adverse event; IRC, independent review committee; ORR, overall response rate. aRisk factors included involved:uninvolved FLC ratio ≥8 (yes vs no), serum Mprotein ≥30 g/L (yes vs no), IgA SMM (yes vs no), immunoparesis (reduction of 2 uninvolved immunoglobulins vs other), or clonal BMPCs (>50% to <60% vs ≤50%). bDARA SC (1800 mg co-formulated with recombinant human hyaluronidase PH20 [rHuPH20; 2,000 U/mL; ENHANZE® drug delivery technology; Halozyme, Inc.]). cPFS was defined as duration from randomization to initial documented progression to active MM or death due to any cause, whichever occurred first.


*Deaths due to an event occurring after the AE reporting window (ie, events that happened after patient started subsequent therapy or >30 days after last dose) or deaths with unknown reason.


• Wow this is a VERY important study and may well change the way we think about and treat high risk smoldering myeloma
• The study was critical to really prove we can delay the progression to active myeloma and even improve survival with 3 years of daratumumab
• It underscores the important of a DISCUSSION with the health care team as many options can be offered to patients with high risk smoldering MM
• There will me MANY more trials coming in this area, with even more intense therapies like combinations and even CAR T Cell therapy...




RESULTS OF THE PHASE II RANDOMIZED TRIAL
EMN15/HOVON147: CARFILZOMIB-LENALIDOMIDE-
Annemiek Broijl, MD, PhD (1), Fredrik Schjesvold, MD, PhD (2,3), Abdel el Yaakoubi, MSc (1,4), Djamila Issa, MD, PhD (5), Sonja Zweegman, MD, PhD (6), Kon-Siong Jie, MD, PhD (7), Mario Boccadoro, MD, PhD (8), Roman Hajek, MD, PhD (9), Sonia M. Cunha, PhD (4), Maria Vania Karsomenawi (4),Helma Zanders, MSc (4), Bronno van der Holt, PhD (1,4), Pieter Sonneveld, MD, PhD (1)
1 Department of Hematology, Erasmus MC Cancer Institute, Rotterdam, the Netherlands; 2 KG Jebsen Center for B Cell Malignancies, University of Oslo, Oslo, Norway; 3 Oslo Myeloma Center, Department of Hematology, Oslo University Hospital, Oslo, Norway; 4 HOVON Foundation, Rotterdam, the Netherlands 5 Department of Internal Medicine, Jeroen Bosch Hospital, Den Bosch, the Netherlands; 6 Department of Hematology, Amsterdam UMC, Amsterdam, the Netherlands; 7 Department of Internal Medicine, Zuyderland Medical Centre, Sittard-Geleen, Netherlands; 8 Myeloma Unit, Division of Hematology, University of Torino, Torino, Italy; 9 Department of Haematooncology, University Hospital Ostrava, Ostrava, Czech Republic

Early Safety and Efficacy of CAR T-cell Therapy in Precursor Myeloma: Results of the CAR-PRISM Study Using Ciltacabtagene Autoleucel in
Omar Nadeem, Sarah Nikiforow, Kevin DeBraganca, Anna Bosch-Vilaseca, Elizabeth O’Donnell, Adam Sperling, Yuxin Liu, Fran Arters, Marjorie Marto, Colin O’Donnell, Brendan Kineavy, Sarah Holmes, Hope Wei, Emily Durlacher, Elizabeth Grimm, Amy Goguen, Rocio Montes de Oca, Lorenzo Trippa, Eric Smith, Kenneth Anderson, Nikhil Munshi, Mark Wildgust, Jerome Ritz, Craig Tendler and Irene M. Ghobrial
Center for Early Detection and Interception of Blood Cancers
Immune Effector Cell Therapy Program
Dana-Farber Cancer Institute
Harvard Medical School Boston, MA

Does this set a new standard of care in high risk smoldering myeloma?
Do well agree on how to risk stratify?
What are the next steps to further improve earlier diagnosis and treatment? intervention? What should current patients with Smoldering Myeloma do?



• Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion.
• If you have a question that does not get answered today, you can contact our Infoline at
, or email infoline@myeloma.org.








Isatuximab, Lenalidomide, Bortezomib and Dexamethasone
Induction Therapy for Transplant-Eligible Patients With Newly Diagnosed Multiple Myeloma: Final Progression-Free Survival Analysis of Part 1 of an Open-label, Multicenter, Randomized, Phase 3 Trial (GMMG-HD7)

Hartmut Goldschmidt1,2, Uta Bertsch1,2, Ema Pozek3, Axel Benner3, Roland Fenk4, Britta Besemer5, Christine Hanoun6, Roland Schroers7, Ivana von Metzler8, Mathias Hänel9, Christoph Mann10, Lisa B. Leypoldt11, Bernhard Heilmeier12, Stefanie Huhn1, Sabine K. Vogel1, Michael Hundemer1, Christof Scheid13, Igor W. Blau14, Steffen Luntz15, Tobias A. W. Holderried16, Karolin Trautmann-Grill17, Deniz Gezer18, Maika Klaiber-Hakimi19, Martin Müller20, Evgenii Shumilov21, Wolfgang Knauf22, Christian S. Michel23, Thomas Geer24, Hendrik Riesenberg25, Christoph Lutz26, Marc S. Raab1,2, Martin Hoffmann27, Katja C. Weisel11, Hans J. Salwender28, and Elias K. Mai1 for the German-speaking Myeloma Multicenter Group (GMMG) HD7 investigators
1Internal Medicine V, Hematology, Oncology and Rheumatology, Heidelberg University Hospital, Heidelberg, Germany; 2National Center for Tumor Diseases Heidelberg, Heidelberg, Germany; 3Division of Biostatistics, German Cancer Research Center (DKFZ) Heidelberg, Heidelberg, Germany; 4Department of Hematology, Oncology and Clinical Immunology, University Hospital Düsseldorf, Düsseldorf, Germany; 5Department of Internal Medicine II, University Hospital Tübingen, Tübingen, Germany; 6Department for Hematology and Stem Cell Transplantation, University Hospital Essen, Essen, Germany; 7Medical Clinic II, Ruhr-University Bochum, Bochum, Germany; 8Department of Medicine II – Hematology and Oncology, Goethe-University Frankfurt, University Hospital, Frankfurt am Main, Germany; 9Department of Internal Medicine III, Klinikum Chemnitz, Chemnitz, Germany; 10Department for Hematology, Oncology and Immunology, University Hospital Gießen and Marburg, Marburg, Germany; 11Department of Oncology, Hematology and BMT, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; 12Clinic for Oncology and Hematology, Hospital Barmherzige Brueder Regensburg, Regensburg, Germany; 13Department of Internal Medicine I, University Hospital Cologne, Cologne, Germany; 14Medical Clinic, Charité University Medicine Berlin, Berlin, Germany; 15Coordination Centre for Clinical Trials (KKS) Heidelberg, Heidelberg, Germany; 16Department of Hematology, Oncology, Stem Cell Transplantation, Immune and Cell Therapy, Clinical Immunology and Rheumatology, University Hospital Bonn, Bonn, Germany; 17Department of Internal Medicine I, University Hospital Dresden, Dresden, Germany; 18Department of Hematology, Oncology, Hemostaseology, and Stem Cell Transplantation, Faculty of Medicine, RWTH Aachen University, Aachen, Germany; 19Clinic for Hematology, Oncology and Palliative Care, Marien Hospital Düsseldorf, Düsseldorf, Germany; 20Clinic for Hematology, Oncology and Immunology, Klinikum Siloah Hannover, Hannover, Germany; 21Department of Medicine A, Hematology, Oncology and Pneumology, University Hospital Münster, Münster, Germany; 22Center for Hematology and Oncology Bethanien, Frankfurt am Main, Germany; 23Department of Internal Medicine III, University Hospital Mainz, Mainz, Germany; 24Department of Internal Medicine III, Diakoneo Clinic Schwäbisch-Hall, Schwäbisch-Hall, Germany; 25Hematology/Oncology Center, Bielefeld, Germany; 26Hematology/Oncology Center, Koblenz, Germany; 27Medical Clinic A, Clinic Ludwigshafen, Ludwigshafen, Germany; 28Asklepios Tumorzentrum Hamburg, AK Altona and AK St. Georg, Hamburg, Germany


Stratification for randomization prior to:
1. Induction: R-ISS stage (I/II versus III versus not classified)
Treatment for 3 years or until PD Cycles 4+ Isa: 10 mg/kg IV Cycle 1 Cycles 2-3
1
2-3 R: 10/15/25 mg PO; Days 1-14 and 22-35
V: 1.3 mg/m2 SC; Days 1, 4, 8, 11, 22, 25, 29, and 32
d: 20 mg POb; Days 1-2, 4-5, 8-9, 11-12, 15, 22-23, 25-26, 29-30, and 32-33
R: 10 mg (up to 15 mg, if tolerated) PO; continuously
d: 20 mg PO; Days 1, 8, 15, and 22
2. Maintenance: R-ISS stage at study entry (I/II versus III versus not classified) and MRD– after last HDM (no versus yes versus unknown) Induction (3 x 6-week cycles) Maintenance (4-week cycles) Days 1, 8, 15, and 22 Days 1 and 15 Day 1 Days 1, 8, 15, 22, and 29 Days 1, 15, 29
Primary end pointsc: Post-induction MRD– (NGF, 10–5); PFS after second randomization
Key secondary end points: PFS (whole study); OS (whole study and from second randomization); post-induction CR; CR and MRD– after HDM and during and after maintenance therapy
Selected secondary end point: PFS after first randomization


Compared with RVd alone, Isa-RVd led to deeper MRD– response




A Dexamethasone Sparing-Regimen with Daratumumab and Lenalidomide in Frail Patients with Newly-Diagnosed Multiple Myeloma: the Phase 3 IFM2017-03 Trial
S. Manier, J. Lambert, C. Hulin, M. Macro, K. Laribi, C. Araujo, G.M. Pica, C. Touzeau, P. Godmer, B. Slama, L. Karlin, F. Orsini Piocelle, M. Dib, L. Sanhes, N. Bigot, A. Perrot, J. Corre, J.Y. Mary, H. Avet-Loiseau, P. Moreau, X. Leleu, T. Facon

ASH 2024, San Diego



*IFM frailty socre: Age
ECOG
Charlson
Randomization stratified by ISS (I vs II vs III) and age (<80 vs ≥80)
In Arm B low-dose dex (20mg/week) during Cycle 1 and 2 (with Dara)
Interim analysis : 12-months data cut on response rates and safety
Final analysis with primary endpoint: PFS
• Quadruplets are becoming the new standard of care in MOST patients in first-line therapy
• We now have options with 2 monoclonal antibodies: Daratumumab (Darzalex) and Isatuximab (=Sarclisa)
• We also can choose from 2 proteasome inhibitors: Bortezomib (=Velcade) or Carfilzomib (=Kyprolis)
• The exact dosing, timing and length of therapy is still being determined
• There are still some frail patients who will be treated with fewer drugs


Is it really quadruplets for all?
Will we start thinking Quad Eligible vs Transplant Eligible?
Is there a difference between Dara and Isa?
How should we optimally dose Bortezomib, Lenalidomide and Dexamethasone?



• Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion.
• If you have a question that does not get answered today, you can contact our Infoline at
, or email infoline@myeloma.org.







• Transplanteligible NDMM
• Age 18-70 years
• ECOG PS ≤2
V: 1.3 mg/m 2
Days 1, 4, 8, 11 R: 25 mg PO Days 1-21 d: 40 mg PO/IV Days 1-4, 9-12
DARA: 1,800 mg SCb QW Cycles 1-2 Q2W Cycles 3-4
VRd administered as in the VRd group Induction
V: 1.3 mg/m 2 SC Days 1, 4, 8, 11 R: 25 mg PO Days 1-21
d: 40 mg PO/IV Days 1-4, 9-12
DARA: 1,800 mg SCb Q4W R: 10 mg PO Days 1-28 Key eligibility criteria
DARA: 1,800 mg SCb Q2W
VRd administered as in the VRd group
Primary endpoint: PFSc
Key secondary endpoints: Overall ≥CR rate,c overall MRD-negativity rate,d OS
Discontinue DARA therapy only after ≥24 months of D-R maintenance for patients with ≥CR and 12 months of sustained MRD negativity
Restart DARA therapy upon confirmed loss of CR without PD or recurrence of MRD
ECOG PS, Eastern Cooperative Oncology Group performance status; V, bortezomib; SC, subcutaneous; PO, oral; d, dexamethasone; IV, intravenous; QW, weekly; Q2W, every 2 weeks; PD, progressive disease; Q4W, every 4 weeks; MRD, minimal residual disease; OS, overall survival; ISS, International Staging System; rHuPH20, recombinant human hyaluronidase PH20; IMWG, International Myeloma Working Group; VGPR, very good partial response. aStratified by ISS stage and cytogenetic risk. bDARA 1,800 mg co-formulated with rHuPH20 (2,000 U/mL; ENHANZE® drug delivery technology, Halozyme, Inc., San Diego, CA, USA). cResponse and disease progression were assessed using a computerized algorithm based on IMWG response criteria. dMRD was assessed using the clonoSEQ assay (v.2.0; Adaptive Biotechnologies, Seattle, WA, USA) in patients with ≥VGPR post-consolidation and at the time of suspected ≥CR. Overall, the MRD-negativity rate was defined as the proportion of patients who achieved both MRD negativity (10–5 threshold) and ≥CR at any time.
(ASH)
December 9-12, 2023; San Diego, CA, USA

• Objective: To determine the impact of adding DARA to R maintenance on MRD-negative conversion
Key eligibility criteria
• 18-79 years of age
• NDMM with ≥4 cycles of induction therapy and underwent ASCT within 12 months of the start of induction
• ≥VGPR at screeninga
• MRDb positive (10–5) post-ASCT
• No prior anti-CD38
• Randomization within 6 months of ASCT date
Stratification factor
• Cytogenetic riskc (standard risk/unknown vs high risk)
Maintenance: up to 36 cyclesd (28-day cycles)
1,800 mg SCe QW Cycles 1-2, Q2W Cycles 3-6, Q4W Cycles 7+ R: 10 mg PO daily Days 1-28 (after Cycle 3, 15 mg PO daily if tolerated)
Primary endpoint
• MRD-negative (10–5) conversion rate from baseline to 12 months after maintenance treatment
• N = 214 planned to achieve ≥85% power to detect 20% improvement
Secondary endpoints
10 mg PO daily Days 1-28 (after Cycle 3, 15 mg PO daily if tolerated)
• PFS, overall MRD-negative conversion rate, sustained MRD-negative rate, response rates, duration of ≥CR, OS, safety
VGPR, very good partial response; D, daratumumab; SC, subcutaneous; QW, weekly; Q2W, every 2 weeks; Q4W, every 4 weeks; PO, orally; CR, complete response. aAs assessed by International Myeloma Working Group 2016 criteria. bMRD based upon NGS (clonoSEQ®; Adaptive Biotechnologies). cFor stratification, cytogenetic risk was evaluated per investigator assessment, in which high risk was defined as the presence of ≥1 of the following cytogenetic abnormalities: del[17p], t[4;14], or t[14;16]. dStudy treatment continued for a planned maximum duration of 36 cycles or until progressive disease, unacceptable toxicity, or withdrawal of consent. After the end of the

Key eligibility criteria:
• NDMM
• ECOG PS score of 0-2
• Received 4-6 cycles of 3- or 4-drug induction therapy (PI and/or IMiD ± anti-CD38 antibody) and ASCTa ± consolidation with ≥PR
Cohort 1: Tec-Len
SRI Cohort 2: Tec-Len
Tec Q4W SRI Cohort 3: Tec Tec Q4W
Tec-Len
Tec Q4W
Phase 3, randomized study
Dual primary endpoints:
• PFS
• 12-month MRD-negative CR (by NGF; 10–5)
Select secondary endpoints:
• OS
Tec
Tec Q4W
Len
• ≥CR
• CR conversion
• MRD-negative conversion
• MRD negativity/sustained MRD negativity
• PFS2
• TTNT
• Safety
aSingle or tandem ASCT permitted.
ASCT, autologous stem cell transplantation; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; EMN, Stichting European Myeloma Network; IMiD, immunomodulatory drug; Len, lenalidomide; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; NGF, next-generation flow cytometry; OS, overall survival; PFS, progression-free survival; PFS2, progression-free survival after next line of therapy; PI, proteosome inhibitor; PR, partial response; QW, weekly; Q4W, every 4 weeks; SRI, safety run-in; Tec, teclistamab; TTNT, time to next treatment.

• Lenalidomide maintenance remains the standard of care, but we know we can do better, especially in higher risk patients
• More studies are showing the value of adding another drug to lenalidomide, most recently with Daratumumab and Isatuximab
• It is still unclear if ALL patients should have dual therapy
• The length of maintenance therapy is under investigation – it appears there is continued benefit to at least 4 years but MRD status may change that...


Is dual maintenance now become the standard of care?
What should we add to Lenalidomide (=Revlimid)?
Should we treat differently based on risk status?
Should we treat until progression (or intolerance)?
Does MRD change things?
Can we (please) stop maintenance sooner??



• Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion.
• If you have a question that does not get answered today, you can contact our Infoline at
, or email infoline@myeloma.org.







Belantamab Mafodotin, Bortezomib, and Dexamethasone vs Daratumumab, Bortezomib, and Dexamethasone in
Vania Hungria, MD, PhD,1 Pawel Robak, MD, PhD,2 Marek Hus, MD, PhD, DsC,3 Vera Zherebtsova, MD,4 Christopher Ward, MD, PhD,5 P Joy Ho, MBBS, DPhil,6 Roman Hajek, MD, PhD,7 Kihyun Kim, MD,8 Sebastian Grosicki, MD, PhD,9 Hanlon Sia, MBBS, FRACP, FRCPA,10 Adam Bryant, MBBS (Hon), PhD, FRACP, FRCPA,11 Marcelo Pitombeira de Lacerda, MD, PhD,12 Gracia Aparecida Martinez, PhD,13 Anna Maria Sureda Balarí, MD, PhD,14 Irwindeep Sandhu, MD, FRCPC,15 Claudio Cerchione, MD, PhD,16 Peter Ganly, BMBCh, PhD,17 Meletios Athanasios Dimopoulos, MD,18 Chengcheng Fu, MD, PhD,19 Mamta Garg, MD,20 Al-Ola Abdallah, MD,21 Moshe E. Gatt, MD,22 Albert Oriol, MD,23 Michele Cavo, MD,24 Robert Rifkin, MD, FACP,25 Tomoaki Fujisaki, MD, PhD,26 Michał Mielnik, MD,3 Nick Pirooz, MHA,27 Joe Lee, PhD,28 Astrid McKeown, PhD,29 Rachel Rogers, MS,27 Hena Baig, BS,30 Lydia Eccersley, MBBS, PhD, MRCP, FRCPath,28 Sumita Roy-Ghanta, MD,27 Joanna Opalinska, MD, PhD,27 María Victoria Mateos, MD, PhD31
1Clinica São Germano, São Paulo, Brazil; 2Medical University of Lodz, Łódź, Poland; 3Medical University of Lublin, Lublin, Poland; 4Gorodskaya Klinicheskaya Bol’nitsa Im. S.p. Botkina, Moscow, Russia; 5Royal North Shore Hospital, Sydney, NSW, Australia; 6Royal Prince Alfred Hospital, Camperdown, NSW, Australia; 7University Hospital Ostrava and University of Ostrava, Ostrava, Czech Republic; 8Sungkyunkwan University, Samsung Medical Center, Seoul, Republic of Korea; 9Medical University of Silesia, Katowice, Poland; 10Pindara Private Hospital, Gold Coast, QLD, Australia; 11Liverpool Hospital, Sydney, NSW, Australia; 12Universidade da Região de Joinville and Centro de Hematologia e Oncologia, Joinville, Santa Catarina, Brazil; 13Hospital das Clínicas and Instituto do Câncer do Estado de São Paulo, Universidade de São Paulo, São Paulo, Brazil; 14Institut Català d'Oncologia-L'Hospitalet L.-Barcelona, Barcelona, Spain; 15Cross Cancer Institute, Edmonton, AB, Canada; 16Hematology Unit, Istituto Romagnolo per lo Studio dei Tumori "Dino Amadori" - IRST IRCCS, Meldola, FC, Italy; 17Christchurch Hospital, Christchurch, New Zealand; 18National and Kapodistrian University of Athens, Athens, Greece; 19The First Affiliated Hospital of Soochow University, Jiangsu, China; 20University Hospitals of Leicester NHS Trust, Leicester, UK; 21University of Kansas Cancer Center, Fairway, KS, USA; 22Department of Haematology, Hadassah Medical Center, Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel; 23Institut Català d’Oncologia and Josep Carreras Research Institute, Hospital Germans Trias i Pujol, Badalona, Spain; 24IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia "Seràgnoli", Bologna, Italy; 25Rocky Mountain Cancer Centers - Denver - Midtown, Denver, CO, USA; 26Matsuyama Red Cross Hospital, Matsuyama, Japan; 27GSK, Upper Providence, PA, USA; 28GSK, London, UK; 29GSK, Stevenage, UK; 30GSK, Mississauga, ON, Canada; 31Hospital Universitario de Salamanca/IBSAL/CIC/Ciberonc, Salamanca, Spain
Presented at: 66th American Society of Hematology (ASH) Annual Meeting & Exposition; December 7-10, 2024; San Diego, CA, and virtual
BVd, belantamab mafodotin, bortezomib, and dexamethasone; DVd, daratumumab, bortezomib, and dexamethasone; HR, hazard ratio; ITT, intention to treat; NR, not reached; OS, overall survival; R-ISS, Revised International Staging System. a Two patients in the ITT population were randomized, not treated, rescreened, and rerandomized. They are counted as 4 unique patients in this output. b CIs were estimated using the Brookmeyer-Crowley method. c HRs were estimated using a Cox proportional hazards model stratified by the number of lines of prior therapy (1 vs 2 or 3 vs ≥4), prior bortezomib (no vs yes), and R-ISS stage at screening (I vs II or III), with a covariate of treatment. d P
Results from the randomized phase 3 DREAMM-8 study of belantamab mafodotin plus pomalidomide and dexamethasone vs pomalidomide plus bortezomib and dexamethasone in relapsed/refractory multiple myeloma
Suzanne Trudel,1 Meral Beksac,2 Luděk Pour,3 Sosana Delimpasi,4 Hang Quach,5 Vladimir I. Vorobyev,6 Michele Cavo,7
Kazuhito Suzuki,8 Pawel Robak,9 Kristin Morris,10 Amy Phillips-Jones,11 Xiaoou L. Zhou,12 Giulia Fulci,12 Neal Sule,13 Brandon E. Kremer,13 Joanna Opalinska,13 Maria-Victoria Mateos Manteca,14 Meletios A. Dimopoulos15
1Department of Medical Oncology and Hematology, Princess Margaret Cancer Centre, Toronto, ON, Canada; 2Department of Hematology, Ankara Liv Hospital, Istinye University, Ankara, Turkey; 3Department of Internal Medicine, Hematology and Oncology, University Hospital Brno, Brno, Czech Republic; 4General Hospital Evangelismos, Athens, Greece; 5University of Melbourne, St. Vincent’s Hospital, Melbourne, VIC, Australia; 6Leningrad Regional Clinical Hospital, St Petersburg, Russian Federation; 7IRCCS Azienda Ospedaliero-Universitaria di Bologna, Seràgnoli Institute of Hematology, and Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy; 8Division of Clinical Oncology/Hematology, Department of Internal Medicine, The Jikei University School of Medicine, Tokyo, Japan; 9Medical University of Lodz, Lodz, Poland; 10GSK, Durham, NC, USA; 11GSK, Stevenage, UK; 12GSK, Waltham, MA, USA; 13GSK, Collegeville, PA, USA; 14Hematology Department, University Hospital of Salamanca/IBSAL/Cancer Research Center-IBMCC (USAL-CSIC), Salamanca, Spain; 15Department of Clinical Therapeutics, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Suzanne Trudel, MD
Rakesh Popat1, Albert Oriol2, Michele Cavo3, Lionel Karlin4, Irit Avivi5, Wilfried Roeloffzen6, Seok Jin Kim7, Brea Lipe8, Noffar Bar9, Noemi Horvath10, Andrew Spencer11, Chang-Ki Min12, Diana Chen13, Quanlin Li14, Katherine Li15, Ana Slaughter16, Carolina Lonardi17, Nina Benachour18, Arnab Ghosh19, Martin Vogel20, Nikoletta Lendvai19, Tamar Lengil21, Nitin Patel22, Octavio Costa Filho22, Erika Florendo22, Yi Lin23
1University College London Hospitals, NHS Foundation Trust, London, UK; 2Institut Català d’Oncologia and Institut Josep Carreras, Hospital Germans Trias i Pujol, Badalona, Barcelona, Spain; 3IRCCS Azienda Ospedaliero-Universitaria di Bologna, Seràgnoli Institute of Hematology, Bologna University School of Medicine, Bologna, Italy; 4Centre Hospitalier Lyon Sud, Pierre-Bénite, France; 5Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; 6University Medical Center Groningen, Groningen, Netherlands; 7Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea; 8University of Rochester Medical Center, Rochester, NY, USA; 9Yale Cancer Center, Yale University, New Haven, CT, USA; 10Royal Adelaide Hospital, Central Adelaide Local Health Network, Adelaide, Australia; 11Alfred Health-Monash University, Melbourne, VIC, Australia; 12Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul, South Korea; 13Janssen Research & Development, Shanghai, China; 14Janssen Research & Development, Apex, NC, USA; 15Janssen Research & Development, Spring House, PA, USA; 16Cilag GmbH International, Zug, Switzerland; 17Janssen, Buenos Aires, Argentina; 18Janssen Research & Development, Beerse, Belgium; 19Janssen Research & Development, Raritan, NJ, USA; 20Janssen Research & Development, Neuss, Germany; 21Janssen Global Services, Raritan, NJ, USA; 22Legend Biotech USA Inc., Somerset, NJ, USA; 23Mayo Clinic, Rochester, MN, USA
https://www.congresshub.com/ASH2024/ Oncology/Cilta-cel/Popat
The QR code is intended to provide scientific information for individual reference, and the information should not be altered or reproduced in any way.

Anita D’Souza1, Luciano J Costa2, Jesús San-Miguel3, Jesús G Berdeja4, Daniel Morillo5, Cyrille Touzeau6, John McKay7, Thomas G Martin8, Bhagirathbhai Dholaria9, Aurore Perrot10, Albert Oriol11, Anna Maria Sureda Balari12, Thomas Prior13, Debopriya Ghosh14, Lijuan Kang13, Julie S Larsen15, Hein Ludlage16, Lien Vandenberk17, Lingling Chen13, Bas D Koster18, Weili Sun15, Tobias Kampfenkel19, Emma Searle20, Jeffrey V Matous21, Ajai Chari8
1Medical College of Wisconsin, Milwaukee, WI, USA; 2University of Alabama at Birmingham, Birmingham, AL, USA; 3Cancer Center Clinica Universidad de Navarra, Pamplona, Spain; 4Sarah Cannon Research Institute, Tennessee Oncology, Nashville, TN, USA; 5Fundación Jiménez Díaz University Hospital, START Madrid-FJD Early Phase Unit, Madrid, Spain; 6Centre Hospitalier Universitaire de Nantes, Nantes, France; 7Wake Forest University School of Medicine, Winston-Salem, NC, USA; 8University of California, San Francisco, San Francisco, CA, USA; 9Vanderbilt University Medical Center, Nashville, TN, USA; 10Centre Hospitalier Universitaire de Toulouse, Oncopole, Toulouse, France; 11Institut Català d’Oncologia and Institut Josep Carreras, Hospital Germans Trias i Pujol, Badalona, Barcelona, Spain; 12Institut Català d'Oncologia – Hospitalet, IDIBELL, University of Barcelona, Barcelona, Spain; 13Janssen Research & Development, Spring House, PA, USA; 14Janssen Research & Development, Raritan, NJ, USA; 15Janssen Research & Development, Los Angeles, CA, USA; 16Janssen-Cilag Benelux, Leiden, Netherlands; 17Janssen Research & Development, Antwerp, Belgium; 18Janssen Biologics Europe, Leiden, Netherlands; 19Janssen Research & Development, Neuss, Germany; 20The Christie Hospital NHS Foundation Trust and the University of Manchester, Manchester, UK; 21Colorado Blood Cancer Institute and Sarah Cannon Research Institute, Denver, CO, USA
https://www.congresshub.com/ASH2024/ Oncology/Teclistamab/DSouza
The QR code is intended to provide scientific information for individual reference, and the information should not be altered or reproduced in any way.

• This is a rapidly evolving area of myeloma with MANY choices for patients
• It is very likely that Belantamab mafodotin (=Blenrep) will make its way back to the clinic in 2025 and be used in combination
• We still have to sort out exact dosing to reduce the eye related side effects
• We already can use CAR T cell therapy in early relapse, but its ideal patient selection is still being determined
• The “optimal” sequencing of therapies is still not well known, but it will depend on many individual patient factors...


How will Belantamab be used and who is the “ideal” patient for it??
What is the optimal dosing of Belantamab? every 12 weeks?
When should we use CAR T at first (or second) relapse?
Will bispecific antibodies make their way to early relapse?



• Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion.
• If you have a question that does not get answered today, you can contact our Infoline at
, or email infoline@myeloma.org.







Phase 2 Registrational Study of Anitocabtagene Autoleucel for the Treatment of Patients with Relapsed and/or Refractory Multiple Myeloma: Preliminary Results From the iMMagine-1 Trial
Ciara L. Freeman, MD, PhD1; Binod Dhakal, MD, MS2; Gurbakhash Kaur, MD3; Richard T. Maziarz, MD4; Natalie S. Callander, MD5; Adam S. Sperling, MD, PhD6; Carolina Schinke, MD7; Andrzej J. Jakubowiak, MD, PhD8; Noa Biran, MD9; Douglas W. Sborov, MD, MS10; Cindy Varga, MD11; Abhinav Deol, MD12; Abraham S. Kanate, MD13; Mehmet Hakan Kocoglu, MD14; Melhem Solh, MD15; Kamalika C. Banerjee, MS, MA16; Rebecca Chan, MD, PhD16; Myrna Nahas, MD17; Ana Kostic, MD16; Enrique Granados, MD17; Carolyn C. Jackson, MD, MPH17; Christopher R. Heery, MD16; Tim Welliver, MD, PhD16; Krina Patel, MD, MSc18; and Matthew J. Frigault, MD, MS19
(N=98)
(91%)
9% (9/98) of patients had ICANS of any grade; all cases resolved
No delayed or non-ICANS neurotoxicities were observed, including no incidence of Parkinsonism, no cranial nerve palsies, and no Guillain-Barré syndrome (n=98)
Similarly, no delayed or non-ICANS neurotoxicities have been observed in the Phase 1 study1 (n=38, median follow-up of 38.1 months with minimum follow-up of 25 months)
Supportive Measures
Freeman et al, American Society of Hematology 2024, Abstract 1031
a With the exception of n=1 Grade 1 ICANS (confusion) on day 34 post infusion that rapidly resolved
b With the exception of n=1 max Grade 2 ICANS with 29-day duration to resolution
• Etentamig targets BCMA and CD3 on the surface of MM cells and T cells, respectively, resulting in T-cell activation and selective destruction of BCMA-positive MM cells
• Etentamig monotherapy showed promising results in heavily pretreated patients with RRMM in a phase 1 first-in-human dose-escalation study (NCT03933735)1,2
− ORR of 65% (median follow-up of 12 months)
• Daratumumab may have the potential to eliminate immunosuppressor cells and reduce T-cell exhaustion3
A: Bivalent BCMA binding
Designed for high avidity to target cell-surface BCMA
B: Low-affinity CD3 binding
Binding domain designed to reduce cytokine release, with potential for minimal T-cell exhaustion
C: Silenced-Fc tail, Q4W dosing
Allowing for extended half-life and simplified, convenient dosing (Q4W)
• Here, we evaluate the safety and efficacy of combining etentamig with daratumumab and dexamethasone in patients with RRMM
BCMA, B-cell maturation antigen; CRS, cytokine release syndrome; MM, multiple myeloma; ORR, overall response rate; Q4W, every 4 weeks; RRMM, relapsed/refractory multiple myeloma; VGPR, very good partial response.
1. Rodriguez Valdes C, et al. J Clin Oncol. 2024;42:7531. 2. Weisel K, et al. HemaSphere. 2024;8. Abstract 211. doi:10.1002/hem3.104. 3. Krejcik J, et al. Blood. 2016;128:384-394.
Dose escalation (decisions made on the basis of DLTs in C1)
• IV, no step-up dosing
• Hospitalization Cycle 1 Day 1 (minimum 24 hours)
• Subsequent doses in an outpatient setting
• Premedication with regimen dose of dexamethasone
• Treatment Q4W until progressive disease
• Subcutaneous
• 1800 mg once weekly during cycles 1 and 2
• 1800 mg every 2 weeks for cycles 3–6
• 1800 mg every 4 weeks for cycles ≥7
• Oral or IV
• 40 mg weekly
• May be reduced for tolerability
Pretreated Relapsed/Refractory Multiple Myeloma: Updated Results from an Ongoing Phase I Study Demonstrate Clinically Meaningful Activity and Manageable Safety and Inform the Doses and Regimen for Combination Studies
Joshua Richter, 1 Sheeba K Thomas,2 Amrita Krishnan,3 Jacob P Laubach,4 Adam D Cohen,5
Suzanne Trudel,6 Luciano J Costa,7 Nizar J. Bahlis,8 Peter A Forsberg,9 Rayan Kaedbey,10
Rafael Fonseca,11 Andrew Spencer,12 Paula Rodriguez-Otero,13 Maria-Victoria Mateos,14 Chihunt Wong,15 James Cooper,15 Tulika Tyagi,15 Rin Nakamura,15 Voleak Choeurng,15
Alexander Lesokhin16
1Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA; 2The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 3City of Hope, Duarte, CA, USA; 4Dana-Farber Cancer Institute, Boston, MA, USA; 5Abramson Cancer Center and University of Pennsylvania, Philadelphia, PA, USA; 6Princess Margaret Cancer Centre and University of Toronto, Toronto, ON, Canada; 7O'Neal Comprehensive Cancer Center, University of Alabama at Birmingham, Birmingham, AL, USA; 8Arnie Charbonneau Cancer Institute, University of Calgary, Calgary, AB, Canada; 9University of Colorado School of Medicine, Aurora, CO, USA; 10Jewish General Hospital, McGill University, Montreal, QC, Canada; 11Mayo Clinic in Arizona, Phoenix, AZ, USA; 12Alfred Health-Monash University, Melbourne, VIC, Australia; 13Clínica Universidad de Navarra, Pamplona, Spain; 14Instituto de Investigación Biomédica de Salamanca (IBSAL), Hospital Universitario de Salamanca, Salamanca, Spain; 15Genentech, Inc., South San Francisco, CA, USA; 16Memorial Sloan Kettering Cancer Center, New York, NY, USA
Presented at the 66th ASH Annual Meeting │December 7–10, 2024
• Additional treatments are needed for MM
– emergence of triple-class refractory disease in early lines1
– resistance to BCMA- and GPRC5D-targeted agents2
– morbidity and mortality associated with PD and infection3
• Cevostamab (FcRH5xCD3 bispecific antibody)4
– FcRH5: novel target expressed exclusively in B-cell lineage and ubiquitously on MM cells4,5
• Ongoing Phase I study of cevostamab monotherapy (GO39775)6*
– fixed-duration treatment in patients with heavily pretreated RRMM
– prior BCMA and GPRC5D allowed, with increased exposure during the course of enrollment (2017–2023)
Fab region

Aims: (1) present updated safety and efficacy data at the 160 mg target-dose (TD) level; (2) present CRS data with C1 0.3/1.2/3.6 triple-step (TS) dosing at the 160 mg TD level

Helping hematologists conquer blood diseases worldwide
Tocilizumab Prophylaxis for Patients with Relapsed or Refractory Multiple Myeloma Treated with Teclistamab, Elranatamab or Talquetamab
Andrew Kowalski, Jill Lykon, Benjamin Diamond, David Coffey, Marcella Kaddoura, Francesco Maura, James Hoffman, Dickran Kazandjian, and Ola Landgren
Email: axk1682@miami.edu
• Many new immunotherapy approaches are being developed
• Anito-cel is a very attractive novel CAR T that is highly effective with apparently less neuro-toxicity
• New bispecific antibodies like Etentamig and Cevostamab have features that make them attractive such as fewer doses, outpatient administration and a new target
• We still have more to learn about the best way to prevent and manage side effects like Cytokine Release Syndrome, neurological effects and infections


Will anito-cel be the next CAR T? Will its efficacy and safety profile make it attractive?
What do we think of the newer bispecifics Etentamig and Cevostamab?
Should we be using tocilizumab in all patients to prevent Cytokine Release Syndrome?
What other approaches and molecules are coming?



• Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion.
• If you have a question that does not get answered today, you can contact our Infoline at
, or email infoline@myeloma.org.







of

PanagiotisMalandrakis,MD1*,Ioannis Ntanasis-Stathopoulos,MD,PhD,MSc1*,IoannisVKostopoulos,PhD2*,Vasiliki Spiliopoulou,MD1*,DespinaFotiou,MD1*,FoteiniTheodorakakou, MD1*, MagdaliniMigkou,MD1*,NikolaosKanellias1*,Evangelos Eleutherakis-Papaiakovou, MD1*, EfstathiosKastritis,MD1*,MariaGavriatopoulou1*, Ourania Tsitsilonis,MD,PhD2*, Meletios Dimopoulos,MD1 andEvangelosTerpos,MD,PhD1
1DepartmentofClinicalTherapeutics,NationalandKapodistrianUniversityofAthens,SchoolofMedicine,Athens,Greece 2DepartmentofBiology,SchoolofScience,NationalandKapodistrianUniversityofAthens(NKUA),Athens,Greece

Luciano J. Costa1; Eva Medvedova2; Binod Dhakal3; Bhagirathbhai R. Dholaria4; Smith Giri1; Saurabh Chhabra5; Susan Bal1; Kelly N. Godby1; Gayathri Ravi1; Rebecca W. Silbermann2; Natalie Callander6.

1- Division of Hematology and Oncology, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL. 2- Knight Cancer Institute, Orego Health & Science University, Portland, OR. 3- Division of Hematology and Oncology, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI. 4-Vanderbilt University Medical Center, Nashville, TN. 5- Mayo Clinic, Phoenix, AZ. 6-University of Wisconsin, Carbone Cancer Center, Madison, WI.
• We all want ways to give the right therapy to patients for the right amount of time – and I love giving nada!
• MRD (minimal residual disease) is a very powerful tool that can predict outcomes in most patients and can likely be used to decide on therapies and the length of therapies
• It still may be a little early to make many decisions in most patients based on MRD status – but we are collecting the information needed
• This is a critical component of the research work of the IMF...


How important is MRD in myeloma?
Are we ready to make clinical decisions based on MRD status?
When should clinicians be ordering MRD testing and which modality should they use?
Should we be designing trials with MRD in mind?



• Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion.
• If you have a question that does not get answered today, you can contact our Infoline at
, or email infoline@myeloma.org.









Rahul Banerjee, MD; Amber R. Fritz, PhD; Othman S. Akhtar, MD; Ciara L. Freeman, MD, PhD, MSc; Andrew J. Cowan, MD; Nina Shah, MD; Heather J. Landau, MD; Shaji K. Kumar, MD; Dan T. Vogl, MD, MSCE; Yvonne A. Efebera, MD, MPH; Philip L. McCarthy, MD; David H. Vesole, MD, PhD; Adam Mendizabal, PhD; Amrita Y. Krishnan, MD; George Somlo, MD; Edward A. Stadtmauer, MD; Marcelo C. Pasquini, MD, MS
Fred Hutchinson Cancer Center


Urvi A. Shah, MD, MS
Assistant Attending, Myeloma Service
@UrviShahMD

December 8, 2024
Urvi A. Shah^, Laura Lucia Cogrossi^, Francesca Castro, Andriy Derkach, Anna Policastro, JuanJose Garces, Teng Fei, Susan Dewolf, Matteo Grioni, Sofia Sisti, Jenna Blaslov, Peter Adintori, Kinga K. Hosszu, Devin McAvoy, Mirae Baichoo, Justin R. Cross, Jenny Paredes, Aishwarya Anuraj, Sandeep Raj, Charlotte Pohl, Paola Zordan, Victoria Zinsmeyer, Ruben J. Ramos, Marco Lorenzoni, Brianna Gipson, Kylee Maclachlan, Ana Gradissimo, Leonardo Boiocchi, Nathan Aleynick, Camilla Marchigiani, Erica Salehi, Richard Koche, Ronan Chaligne, Torin Block, Neha Korde, Carlyn R. Tan, Malin Hultcrantz, Hani Hassoun, Gunjan L. Shah, Michael Scordo, Oscar B. Lahoud, David J. Chung, Heather Landau, Jonathan U. Peled, Nicola Clementi, Marta Chesi, P. Leif Bergsagel, Sham Mailankody, Michael Pollak, Anita D’Souza, Ola Landgren, Susan Chimonas, Sergio Giralt, Saad Z. Usmani, Neil M. Iyengar, Alexander M. Lesokhin#, Matteo Bellone#, Marcel R.M. van den Brink# , ^co-first authors; #co-last authors
Ashley Rosko, MD
Oncogeriatrics Medical Director
Professor-Clinical of Medicine
Division of Hematology
Co-Director Cancer and Aging Resiliency Clinic
The Ohio State University

Co-Authors: Don Benson, Francesca Cottini, Abdullah Khan, Srinivas Devarakonda, Elvira Umyarova, Naresh Bumma, Lisa Blackburn, Cathie Atkinson, Juan
Lisa M. Christian
70) treated with Teclistamab: An International Myeloma Foundation (IMF) Immunotherapy Database Real-World Analysis
Hira Mian, Thomas Martin, Gregory Pond, Sireesha Asoori, Rakesh Popat, Efstathios Kastritis, Joaquin Martinez-Lopez, Radhika Bansal, Andre Corraes, Saurabh Chhabra, Ricardo Parrondo, Meletios A. Dimopolous, Kwee Yong, Brain GM Durie, Saad Z. Usmani, Nadine Abdallah, Yi Lin, Carlyn Tan







1. Can we reduce of the use of 24 hour urine testing?
2. Can our diets influence our myeloma?
3. How can we better support our caregivers and care partners?
4. How do we best define frailty and have it influence therapy?
5. Will we embrace the #downwithdex movement??



• Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion.
• If you have a question that does not get answered today, you can contact our Infoline at
, or email infoline@myeloma.org.





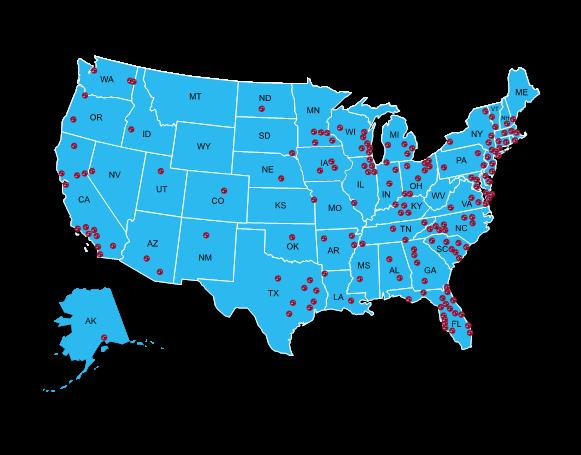
We now that myeloma is the MOST disparate cancer within the African American community – with twice the incidence and twice the mortality
Multiple other groups also experience disparities like Latino Americans, rural dwelling patients and those without insurance...
This is an area of key work at the IMF in the M-Power program
Many abstracts at ASH highlight these disparities and seek methods for health equity...






What about the flu shot for myeloma patients?
What is IVIG and when do we use it?
What is Mass Spectometry and when is it used?
Is there a blood test that can reduce the need of bone marrow tests??






• Open the Q&A window, allowing you to ask questions to the host and panelists. It will be sent to our moderator and panelists for discussion.
• If you have a question that does not get answered today, you can contact our Infoline at
, or email infoline@myeloma.org.









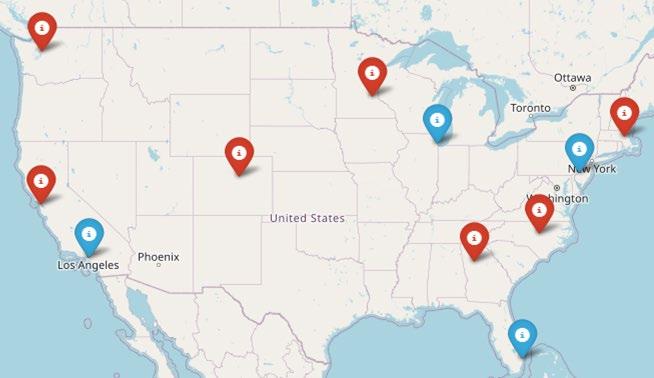
Special interest groups are designed as supplemental support for specific patients & care partners, in addition to local support groups
Las Voces de Mieloma
founded 2022
Virtual group for Spanish speakers
Living Solo & Strong founded 2022
Virtual group for patients without a care partner
High Risk-Myeloma founded 2023
Virtual group for people living with high-risk myeloma
Care Partners Only founded 2024
Virtual group for myeloma care partners
Smolder Bolder founded 2023
Virtual group for people living with smoldering myeloma
MM Families founded 2021
Virtual group for patients & care partners who have young children
Veterans with Myeloma
Starting in 2025
Virtual group for those who serve our Country

Link available here



Assistance with understanding lab results, terminology and disease state
Preparing for medical visits
Access to medical providers
Access to medications
Financial resources
“Thank you so much for the informative conversation and all the time you spent listening and helping me decipher the MM lingo. What an amazing service!”
Thank you for your response and excellent question suggestions for my hematology team.







Myelo:
The first generative AI Assistant designed specifically for multiple myeloma.


Seamlessly matching patients to the latest clinical trials.








































4 PATIENT & FAMILY SEMINARS including world-renowned experts
10 MYELOMA COMMUNITY WORKSHOPS including local myeloma experts








Thank you to our sponsors!









