Thank you for joining us today for the June 22nd, 2024, International
Welcome!
Myeloma Foundation’s Regional Community Workshop –Charlotte
Thank you to our sponsors!








IMF Regional Community Workshop June 22nd, 2024 - Agenda
9:00 – 9:05 AM Welcome & Introductions, Yelak Biru
9:05 – 9:20 AM IMF’s Vision, Yelak Biru
9:20 – 9:50 AM Myeloma 101, Pete Voorhees, MD
9:50 – 10:00 AM Q&A
10:00 – 10:35 AM Life is a Canvas, You are the Artist – Amy Pierre, RN, MSN, ANP-

10:35 – 10:45 AM Q&A
10:45 – 11:00 AM Coffee Break
11:00 – 11:35 AM Frontline Therapy, Cindy Vargas, MD
11:35 – 11:45 AM Q&A
11:45 AM – 12:00 PM Engaging & Partnering with the IMF Sylvia Dsouza
11:45 AM – 12:40 PM LUNCH
BC
12:40 – 1:10 PM

IMF Regional Community Workshop
June 22nd, 2024 – Agenda after lunch
Local Patient & Care Partner Panel
Patients – Ellen Herman & Ivy Walker
Care Partners – Bruce Herman & Ron Walker
1:10 – 1:30 PM Maintenance Therapy, Cindy Varga, MD
1:30 – 1:40 PM Q&A
1:40 – 2:25 PM Relapsed Therapies & Clinical Trials, Pete Voorhees, MD
Closing Remarks/Coffee & Networking
Q&A
2:25 – 2:35 PM
2:35 – 3:00 PM
Multiple Myeloma affects patients and families.
The IMF provides FREE resources to help both patients and families.
Established in 1990, the IMF’s InfoLine assists over 4600 callers annually and answers questions across a wide variety of topics including:

Frequent topics:
Treatment questions along the spectrum of care
Clinical Trial access and understanding
Side effect management and health issues
Financial resources for myeloma-related expenses
Myeloma Specialist Referral contact information
Support group information
Caregiver Support




6 Contact the InfoLine: 800-452-CURE (2873) InfoLine@myeloma.org
Paul Hewitt, Missy Klepetar, & Teresa Miceli
Educational Publications

A core mission of the IMF is to provide thorough and cutting-edge education to the















New publications




The IMF Support Group Team is Here For You! 8 Shared Experiences Help to Better Understand the Myeloma Journey • Support Groups Empower Patients & Care Partners with information, insight, & hope • The IMF provides educational support to a network of over 150 myeloma specific groups We are happy to help connect you with an existing support group or help form a new one! We assist with virtual, in-person, and hybrid options for meetings. Reach out to us at SGTeam@myeloma.org Support.myeloma.org
Local Support Groups: You Are Not Alone!

The Charlotte Area Multiple Myeloma Support Group
Meets in-person on the 3rd Saturday of every other month at 10am Eastern
The Asheville Multiple Myeloma Support Group
Meets in-person on the 1st Tuesday of each month at 10am Eastern

The Historic West
Charlotte Multiple Myeloma Support Group
Meets virtually on the 3rd Saturday of each month at 11am Eastern
9
Local Support Groups: You Are Not Alone!
The Lake Norman Area Multiple Myeloma Support Group
Meets in-person on the 3rd Thursday of each month at 6:30pm Eastern
The Triangle Area Multiple Myeloma Support Group
Meets in-person on the 4th Saturday of each month at 10am Eastern


The Western Wake Multiple Myeloma Support Group
Meets in a hybrid fashion on the 2nd Saturday of each month at 10am Eastern

The Winston-Salem Multiple Myeloma Support Group
Meets in a hybrid fashion on the 4th Wednesday of each month at 11:30am Eastern
10
IMF – Special Interest Virtual Groups

Special interest groups are designed as a supplemental support for specific populations of patients, in addition to their local Support Groups
Las Voces de Mieloma
Designed for Spanish speaking patients only
Living Solo & Strong with Myeloma
Designed for patients without a care partner


New!
Care Partners Only
Designed to address the needs of care partners only

Smolder Bolder
Created for people living with Smoldering Multiple Myeloma
MM Families
High Risk Multiple Myeloma
Designed to address the needs of the high-risk MM population
MGUS 4 Us
Created for people living with MGUS
For patients/care partners with young children

11
IMF’s Vision
 Yelak Biru President & CEO, 28
Yelak Biru President & CEO, 28
year Myeloma Patient


Vision

A world where every myeloma patient can live life to the fullest, unburdened by the disease.

Our Four Pillars

The potential of coordinated, consolidated and global SUPPORT to patients and their partners is unparalleled

The need for informed and global ADVOCACY is critical to both improving lives and finding the cure

Raise the Bar
Examine the why of all our actions to ensure they are purpose-driven, meaningful, and effective.
Mission

Improving the quality of life of myeloma patients while working toward prevention and a cure!

Myeloma specific EDUCATION historically was not patient directed nor of high caliber – the right knowledge is power

The medical and RESEARCH world can often exist in silos due to institutional demands and limitations
Strategy

Broaden our Reach
Address unmet patient needs by expanding our reach to diverse & underserved populations in everything we do.

Innovate Every Step of The Way
Provide those who need it most with what they need the most, throughout their myeloma journeys.
13 8

Myeloma 101
Pete Voorhees, MD
Atrium Health, Levine Cancer Institute Charlotte,
NC
Peter Voorhees, M.D. Chief, Plasma Cell Disorders Division, Atrium Health / Levine Cancer Institute
Clinical Professor of Medicine, Wake Forest University School of Medicine
 Myeloma 101
Myeloma 101
• Multiple myeloma is a cancer of plasma cells
• Plasma cells are a type of white blood cell that reside in the bone marrow and produce antibodies to fight infection
• When a plasma cell acquires the right set of gene mutations and chromosome changes, they can turn cancerous and accumulate in the bone marrow, leading to multiple myeloma




What is Multiple Myeloma?
Bone marrow
• The 2nd most common blood cancer in the US
• The most common blood cancer in Black patients in the US
• Represents 1.8% of all new cancer diagnoses in the US
• 34,920 newly diagnosed patients in the US in 2021
• 138,415 patients living with myeloma in 2021
SEER Cancer Stat Facts: Myeloma. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/mulmy.html

Myeloma Statistics
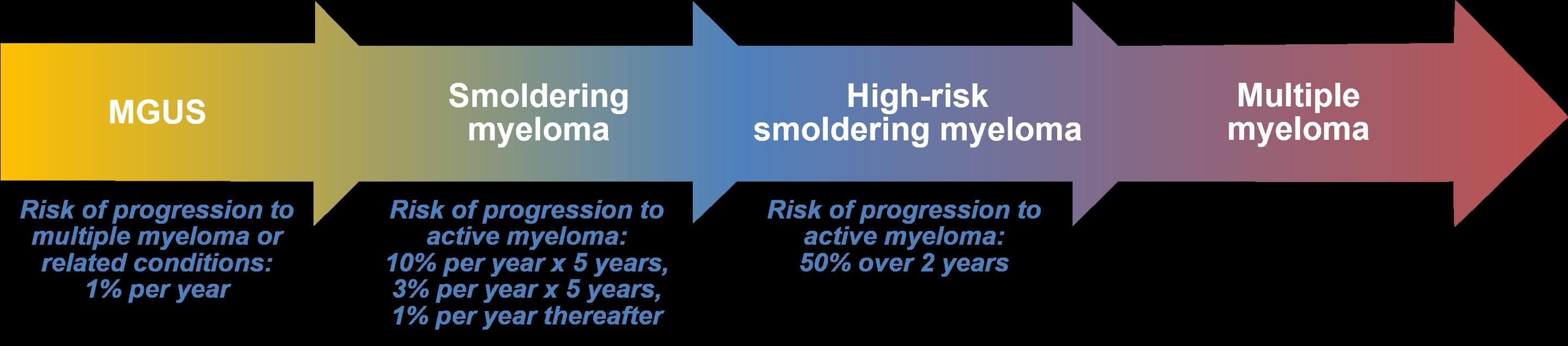
From Precursor Myeloma, or MGUS, to Multiple Myeloma

Multiple
International Myeloma Working Group Diagnostic Criteria for Plasma Cell Dyscrasias Diagnosis
myeloma
Multiple myeloma Not required >10% (or <10% if plasmacytoma + CRAB or biomarker of malignancy or >1 plasmacytoma)
One of the following:
1. CRAB criterion present*
2. Clonal bone marrow PCs ≥60%
3. Involved:uninvolved SFLC ratio ≥100 AND affected light chain 100 mg/L or higher
4. >1 focal lesion on MRI studies

Updated IMWG Diagnostic Criteria
M-protein Bone marrow PCs Myeloma-defining events*
No
MGUS < 3g/dL < 10%
OR
No
Smoldering
≥ 3g/dL in serum OR >500 mg/24hr in urine
> 10% - 60% PCs
*CRAB
1. HyperCalcemia. Serum calcium >1mg/dL higher than the ULN or >11mg/dL 2. Renal insufficiency. CrCl <40ml/min or SCr >2mg/dL 3. Anemia. Hgb <10g/dL or >2g/dL below the LLN 4. Bone lesions. >1 osteolytic lesion on skeletal radiography, CT, or PET-CT
criteria for myeloma-defining events
• 77,469 healthy adults 55 to 74 years of age were enrolled in the prospective prostate, lung, colorectal and ovarian cancer screening trial
• 71 developed MM over the study
* Presence of M-protein or abnormal serum FLC ratio Landgren, O. et al. Blood 2009;113:5412-5417

MGUS precedes Myeloma
Blood draw prior to MM diagnosis, years MGUS* n/N % (95% CI) 2 27/27 100 (87.2 – 100) 3 57/58 98.3 (90.8 – 100) 4 47/48 97.9 (88.9 – 100) 5 35/37 94.6 (81.8 – 99.3) 6 25/25 100 (86.3 – 100) 7 14/15 93.3 (68.1 – 99.8) 8 14/17 82.4 (56.6 – 96.2)
MGUS Prevalence: Olmsted
County, Minnesota
21,463 of 28,038 residents of Olmstead County ≥ 50 evaluated
• Serum samples obtained through Mayo Clinic or its affiliates
• SPEP performed. Abnormal results followed up with serum IFE
• Largely Caucasian population

Age Men Women Total 50-59 82/4038 (2.0) 59/4335 (1.4) 141/8373 (1.7) 60-69 105/2864 (3.7) 73/3155 (2.3) 178/6019 (3.0) 70-79 104/1858 (5.6) 101/2650 (3.8) 205/4508 (4.6) ≥80 59/709 (8.3) 111/1854 (6.0) 170/2563 (6.6) Total 350/9469 (3.7) 344/11,994 (2.9) 694/21,463 (3.2)
Kyle et al. N Engl J Med. 2006 Mar 30;354(13):1362-9
Defined as: 1) No heavy chain M spike; 2) abnormal serum FLC ratio; 3) elevation of affected free light chain
• 19% of MGUS is light chain MGUS
• 23% of light chain MGUS pts had renal disease

Men Women Total 50-59 22/3450 (0.6%) 14/3717 (0.4%) 36/7167 (0.5%) 60-69 25/2554 (1.0%) 15/2776 (0.5%) 40/5330 (0.8%) 70-79 28/1608 (1.7%) 15/2242 (0.7%) 43/3850 (1.1%) 80-89 6/577 (1.0%) 21/1433 (1.5%) 27/2010 (1.3%) Total 81/8189 (1.0%) 65/10,168 (0.6%) 146/18,357 (0.8%)
Light Chain MGUS Prevalence: Olmstead County, Minnesota
Dispenzieri et al. Lancet.
2010 May 15;375(9727):1721-8
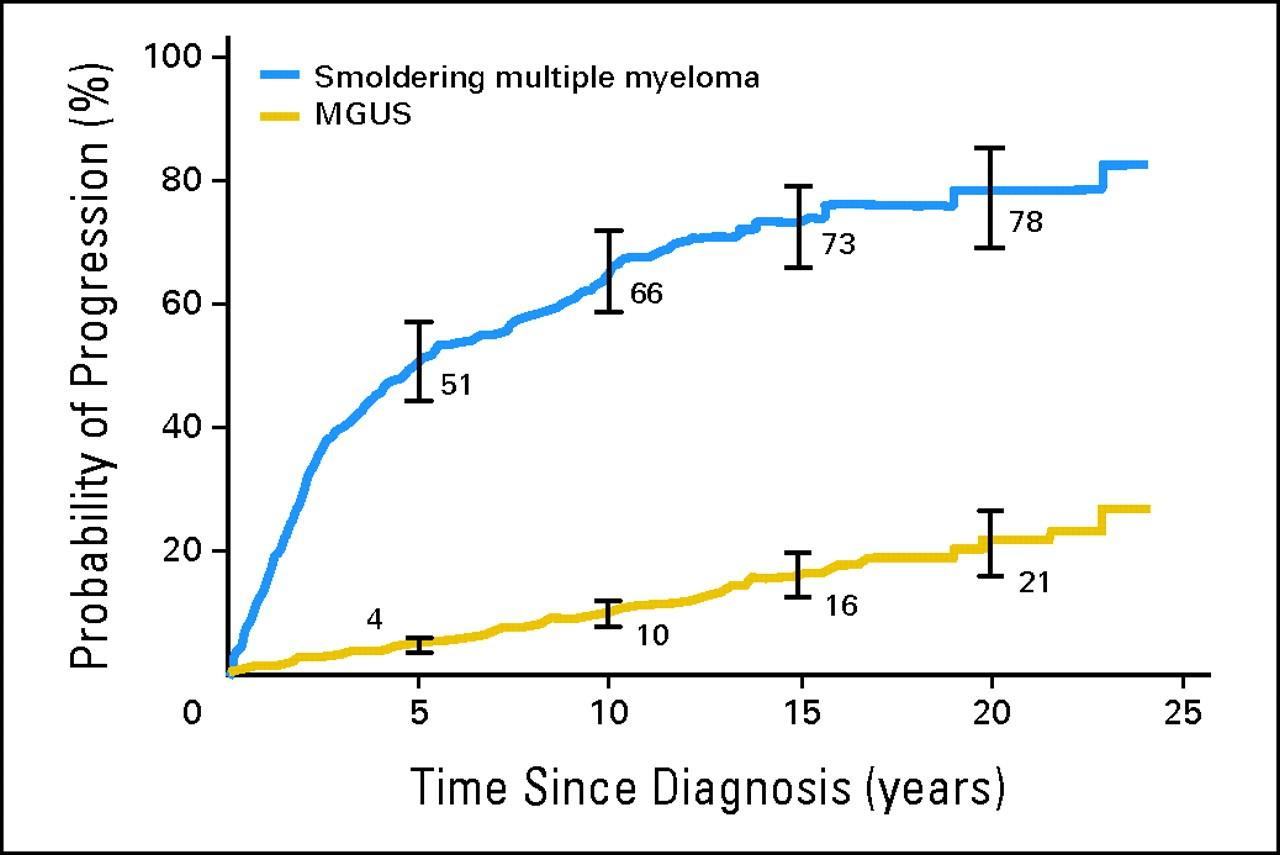
• The risk of evolution is ~1% per year for all comers
• The risk of developing myeloma or other plasma cell disorder persists over >20 years of follow-up.
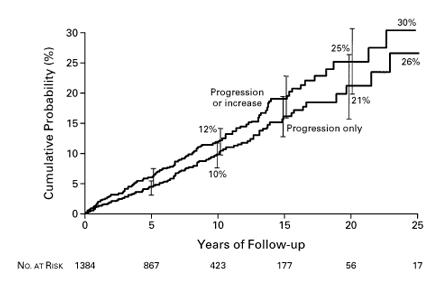
Kyle et al. N Engl J Med. 2002 Feb 21;346(8):564-9

MGUS: Risk of Progression to Myeloma
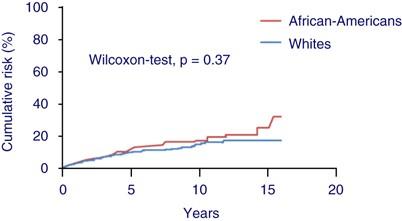
The increased risk of MM in African-Americans is due to an increased risk of MGUS, not an increased rate of progression from MGUS to MM
Landgren et al. Blood. 2006 Feb 1;107(3):904-6

Risk of Progression of MGUS to Myeloma by Race
10% per year for 5 years then 3% per year for 5 years then 1% per year thereafter

Progression of Smoldering Myeloma to Symptomatic Myeloma
Kyle et al. N Engl J
Med. 2007 Jun 21;356(25):2582-90
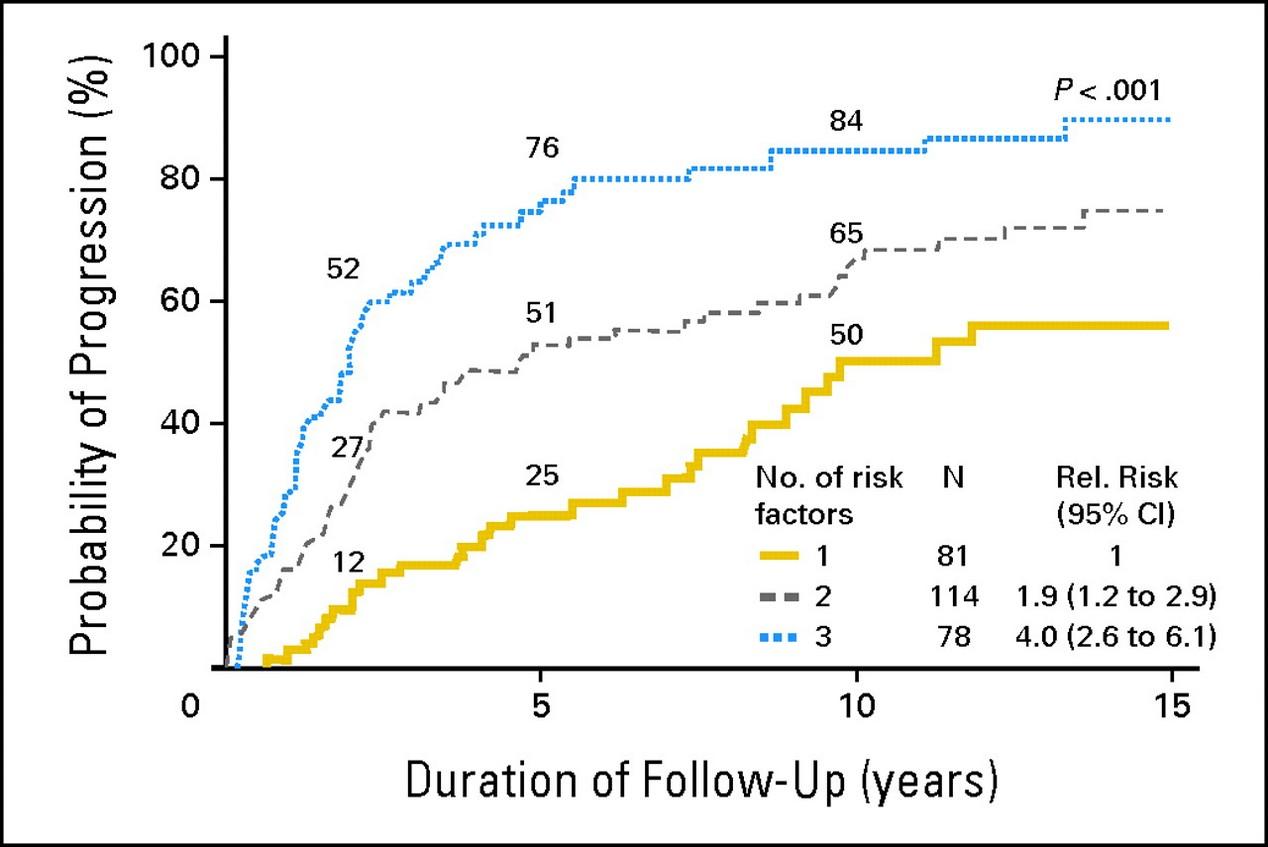
Risk Stratification of SMM: Mayo Criteria Over Time
et al. Blood. 2008;111(2):785-9
et al. Blood Cancer J 2018;8:59.

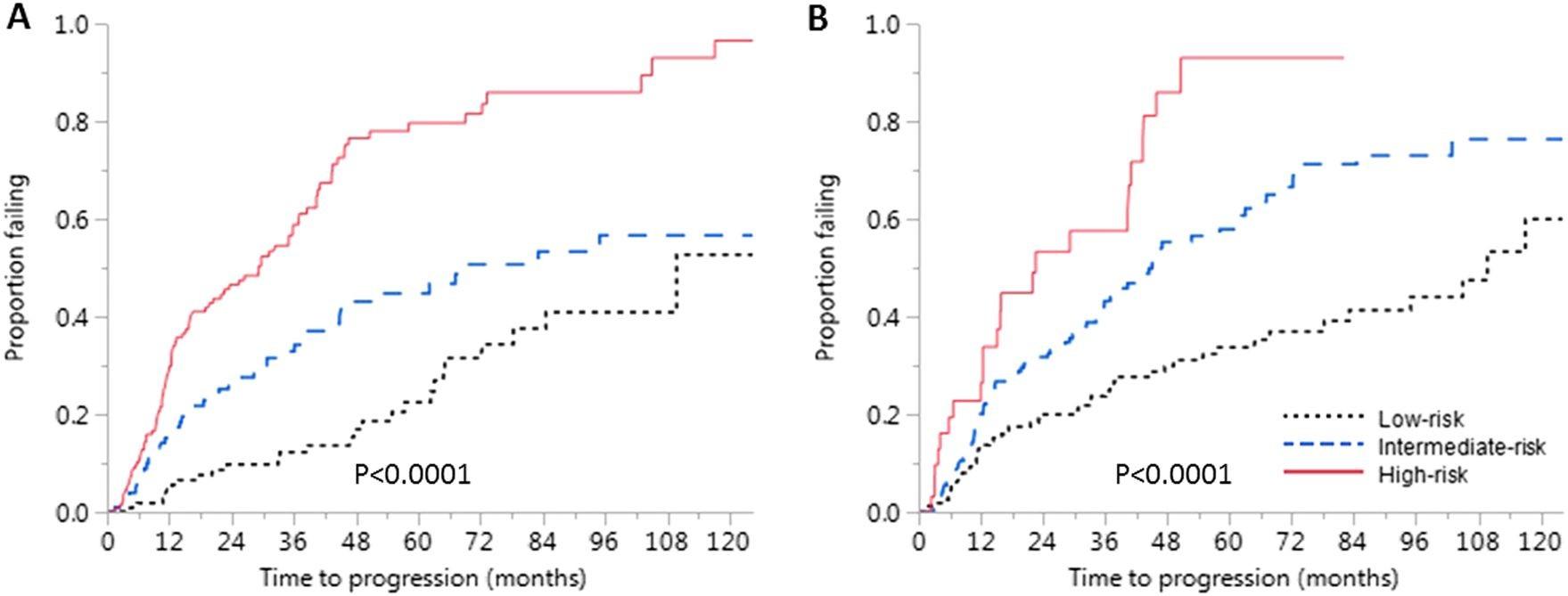
Risk 2 Yr 5 Yr 10 Yr High (≥2 RFs) 47.4% 81.5% 96.5% Intermediate (1 RF) 26.3% 46.7% 65.3% Low (0 RFs) 9.7% 22.5% 52.7% Risk 2 Yr 5 Yr 10 Yr High (3 RFs) 52% 76% 84% Intermediate (2 RFs) 27% 51% 65% Low (1 RF) 12% 25% 52% RFs: • PCs >20% • M spike >2.0 g/dL • FLCr >20 29.2 67.8 109.8 Time Median Time to Progression (mos) P a ti e n t s w i t h P r o g r e s s i o n Lakshman A,
RFs: • PCs ≥10% • M spike ≥ 3.0 g/dL • FLCr >8 Time 2008 2018 Dispenzieri
P a ti e n t s w i t h P r o g r e s s i o n
Median TTP: 1.2 vs 7.2 yrs (P < 0.01)
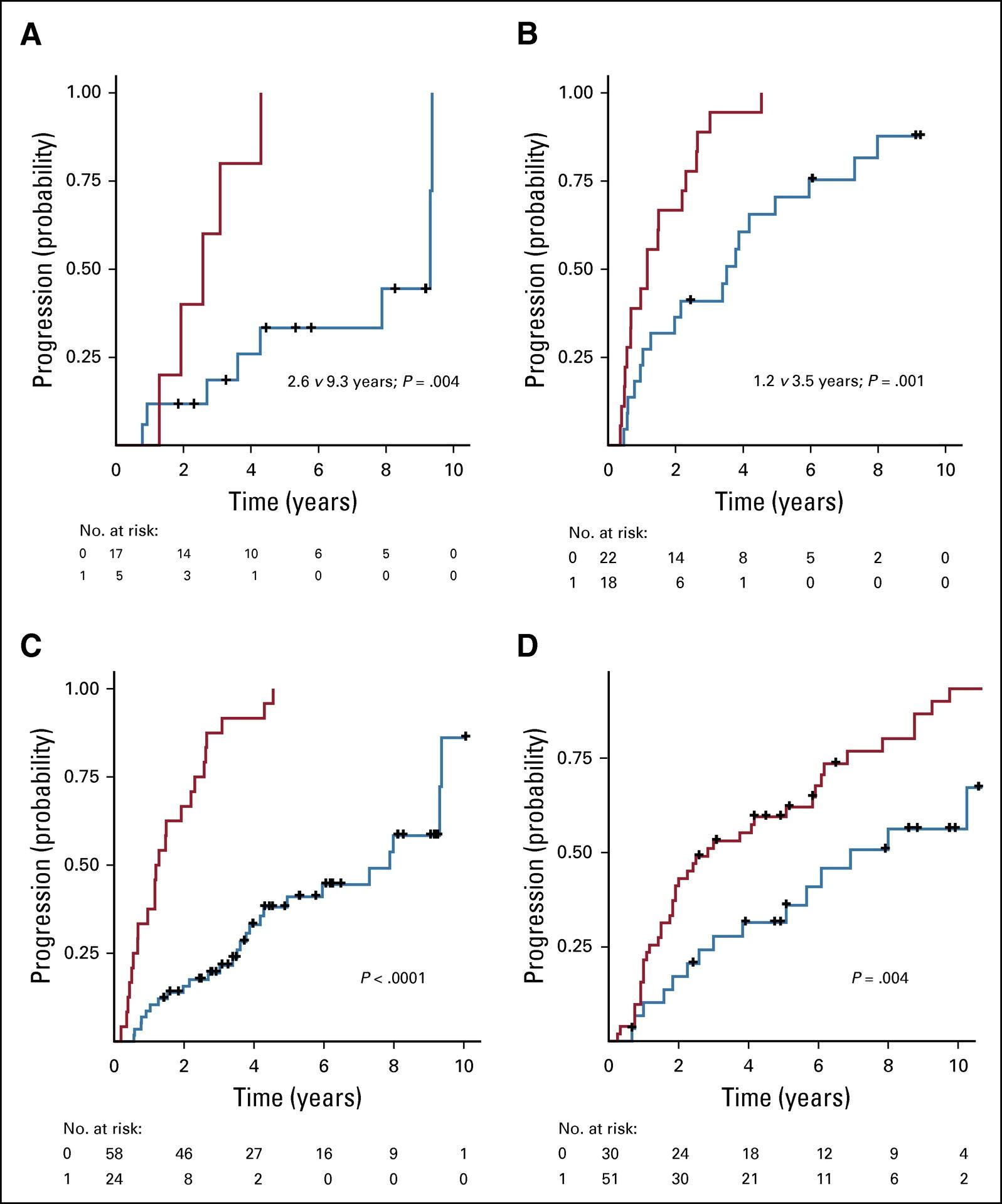
Median TTP: 2.6 vs 9.3 yrs (P = 0.004)
Genomic Risk Factors (gRFs): MAPK pathway mutations (NRAS, KRAS), MYC alterations (amplication, translocation), DNA repair pathways (TP53 mutations, del[17p], ATM SNVs)
et al. J Clin Oncol. 2020;38(21):2380-9


Median TTP: 1.2 vs 3.5 yrs (P = 0.001)
≥1 gRF 0 gRF ≥1 gRF ≥1 gRF 0 gRF 0 gRF Mayo 2018 Intermediate Risk Mayo 2018 High Risk
Bustoros
M
Evolving Smoldering Multiple Myeloma
• eMP: ≥10% ↑ in serum M protein and / or Ig within 6 mos of diagnosis (only if initial M protein was ≥3.0 g/dL) and/or ≥25% ↑ in serum M protein and/or Ig within 12 mos of diagnosis with a minimum ↑ of 0.5 g/dL in M protein or 500 mg/dL in Ig
• eHb: ≥0.5 g/dL ↓ in Hb within 12 months of diagnosis
eMP = evolving (increasing) M spike
eHb = evolving (decreasing) hemoglobin

Risk Factor Progression to Symptomatic MM within 2 Years eMP only 63.8% eHb only 64.6% eMP and eHb 81.5% Both + BMPCs ≥20% 90.5%
Ravi P, et al. Blood Cancer J
2016;6:454.
Signs and symptoms of disease because of…
• Cancerous plasma cell growth in the marrow
• Anemia
• Bone disease
• Bone pain
• Fractures
• High calcium levels
• Myeloma antibody production
• Kidney failure
• Free light chain antibodies
• Neuropathy
• Amyloidosis
•Suppression of normal plasma cell function
• Low antibody levels
• Frequent infection

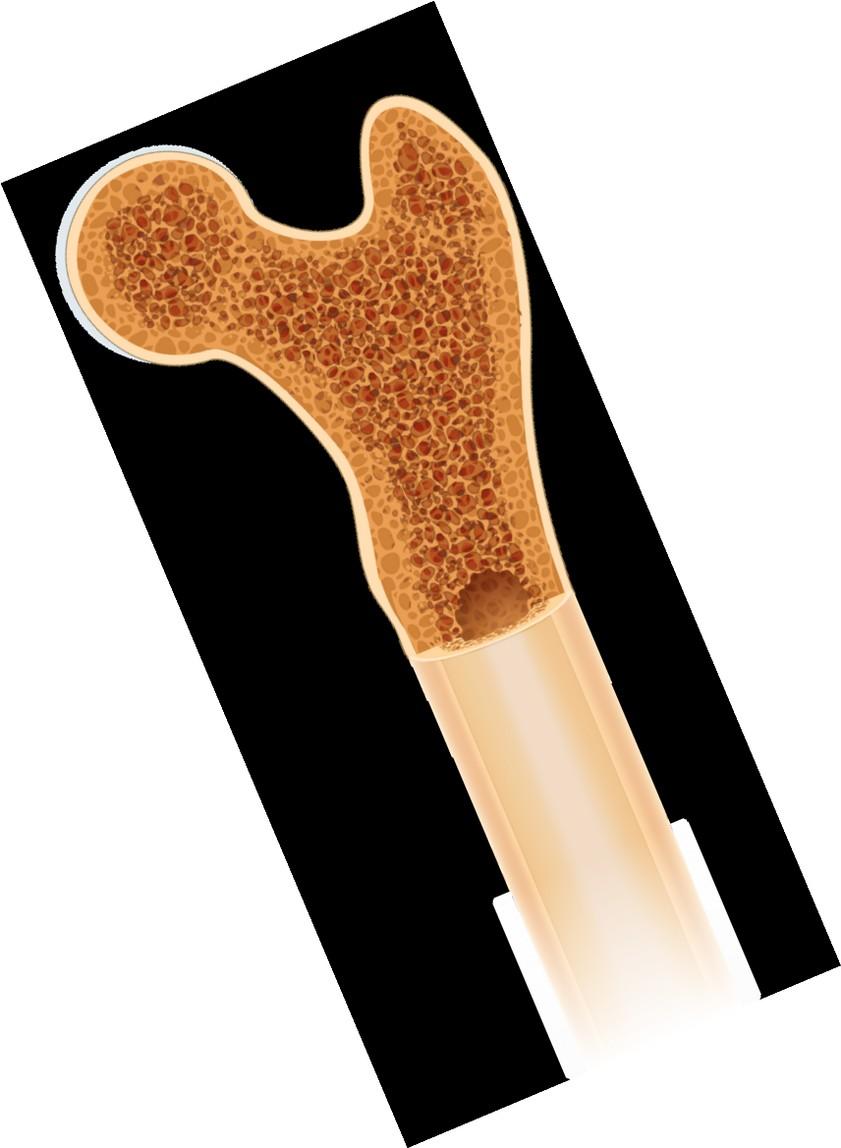
Clinical Features: Bone Disease
• Bone pain present at diagnosis in ~60% of patients.
• Lytic bone disease seen in 60% of patients at diagnosis.
• Osteopenia (bone thinning), pathologic fractures, compression fractures seen in 20% each.
• Management: kyphoplasty, surgical stabilization, radiation, zometa / xgeva
 Adapted from the American Society of Hematology (ASH) Image Bank
Adapted from the American Society of Hematology (ASH) Image Bank

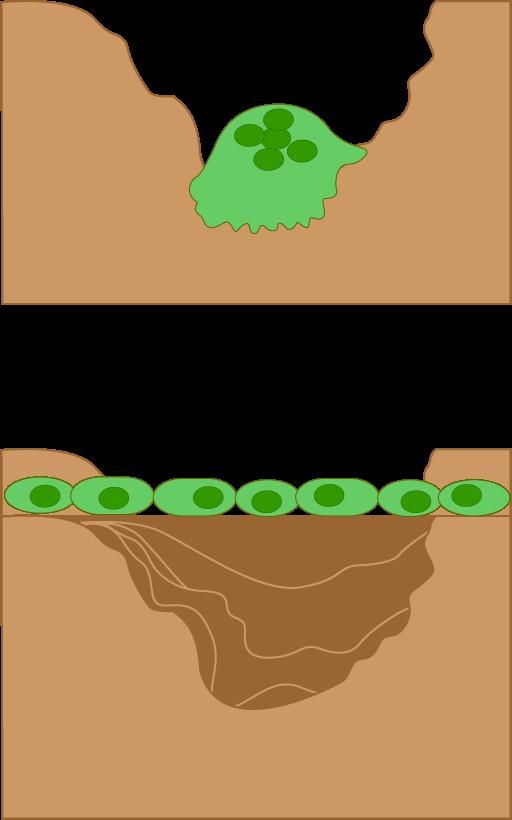


RANKL ↑ in MM
DKK-1 ↑ in MM + -

Osteoblasts: Build bone
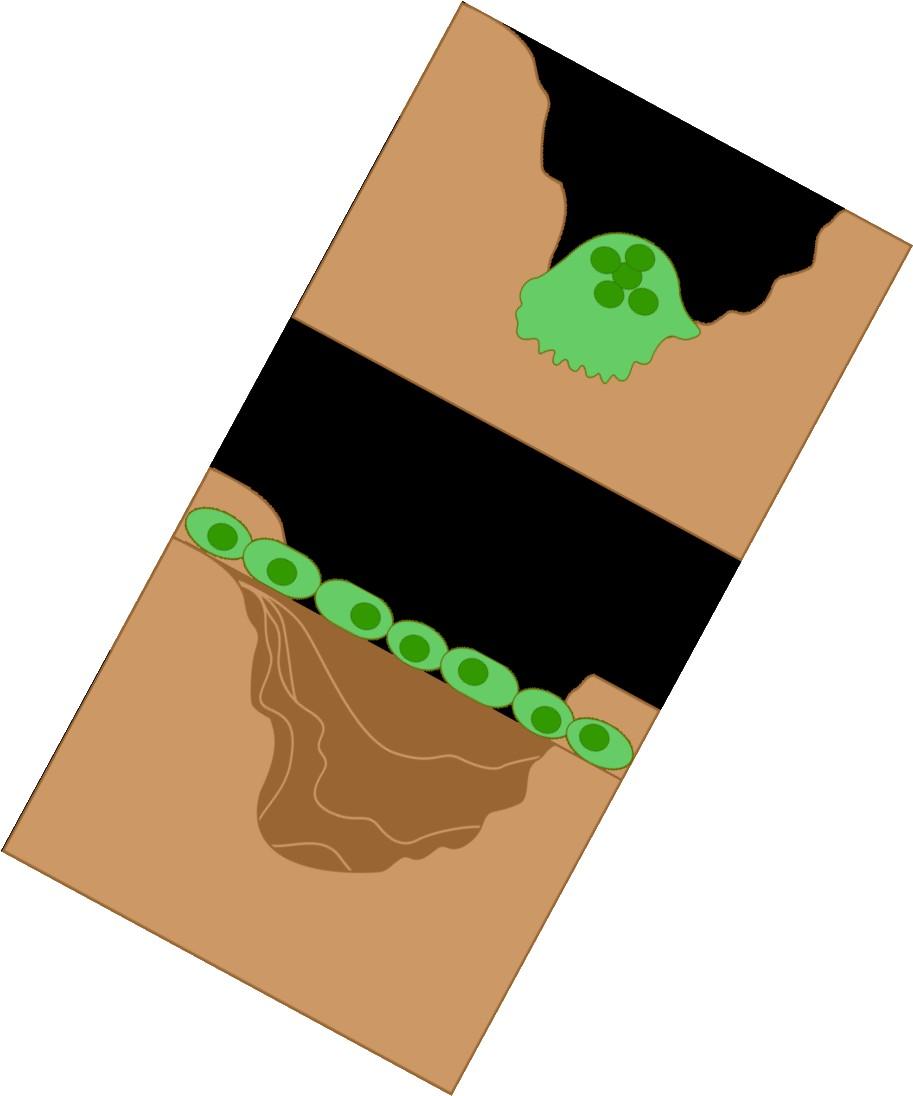
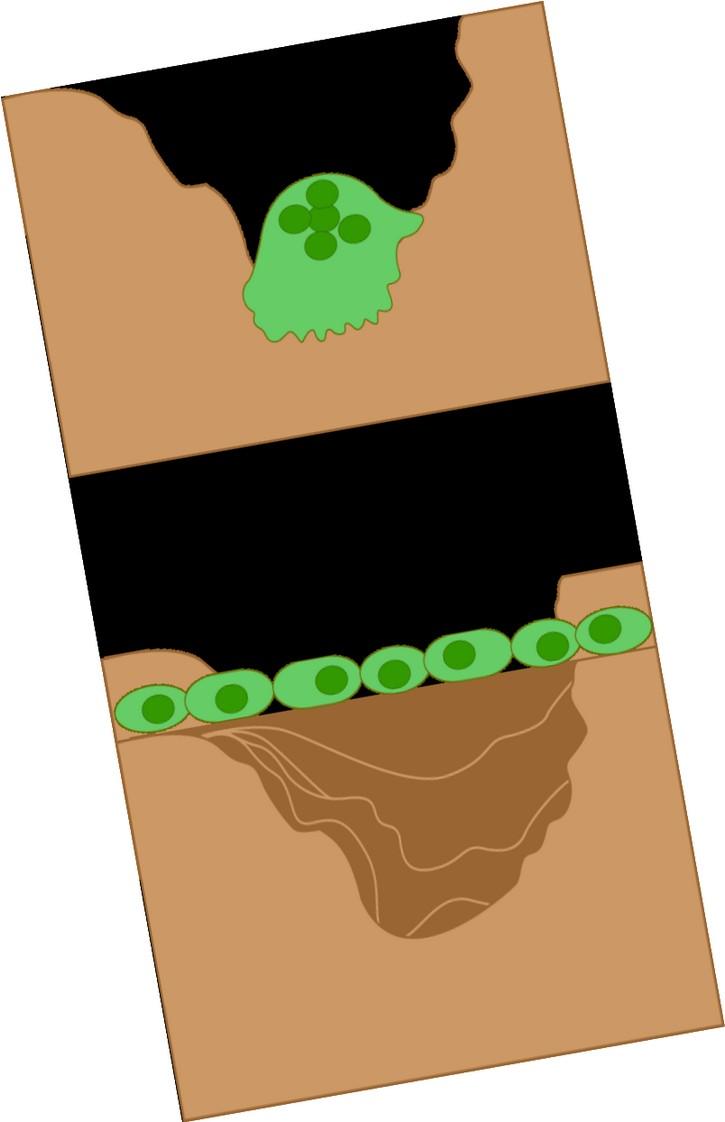
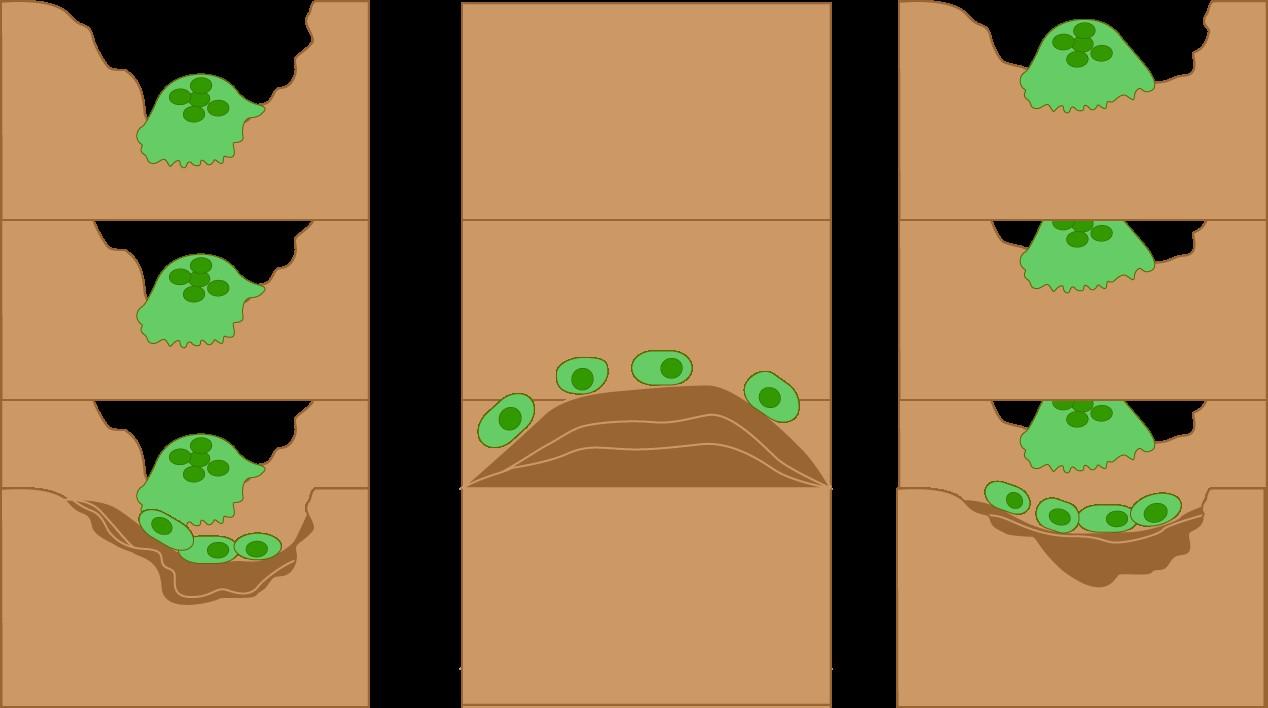
RANKL = receptor activator of NF-kappaB ligand,
DKK-1 = Dickkopf-

Bone
Myeloma
Disease down bone
Normal Myeloma
Clinical Features: Causes of Kidney Damage
• High calcium levels
• Myeloma Kidney (Cast nephropathy)
• Amyloidosis
• Kidney injury causes protein loss in urine which leads to problems with fluid retention
• Monoclonal immunoglobulin deposition disease
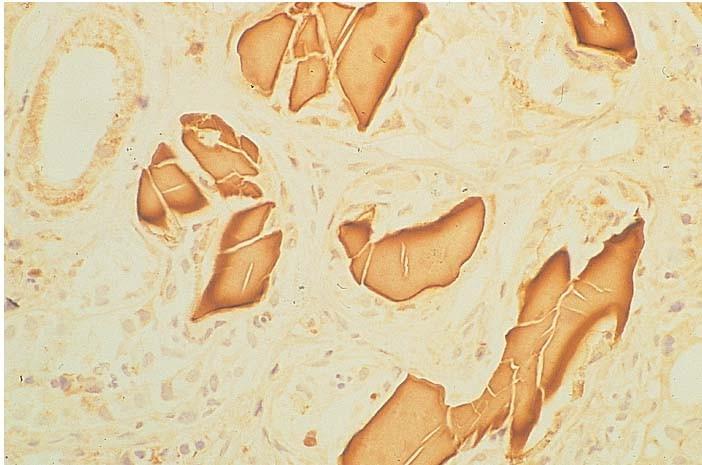
Adapted from Serum Free Light Chain Analysis. 4th Edition. AR Bradwell.

Cast Nephropathy
• The most common cause of kidney failure in myeloma pts.
• Monoclonal free light chains precipitate in the kidneys leading to damage
• Higher serum free light chain burden → higher risk of kidney injury.
• Precipitants of cast formation and worsening kidney function
• Volume depletion
• Loop diuretics (e.g. Lasix)
• Contrast dye for CT scans
• NSAIDs (e.g. ibuprofen)
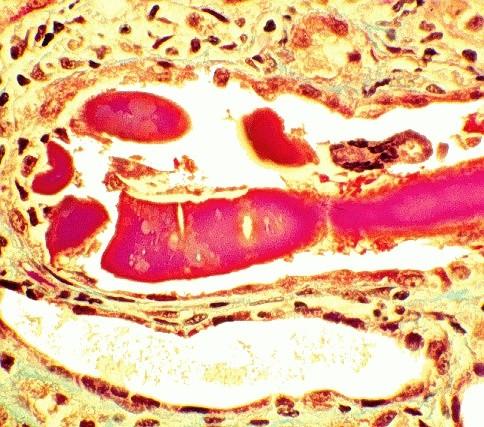
Adapted from the University of North Carolina Nephropathology Biopsy Cases.

Myeloma
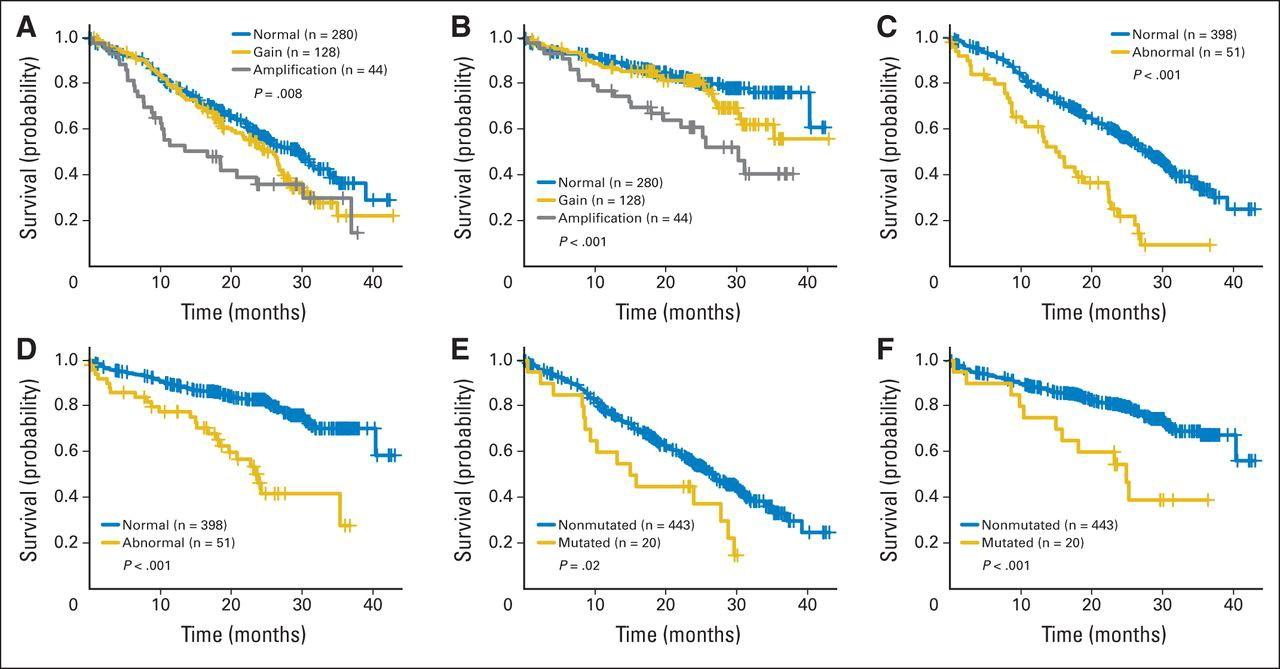
•Univariate and multivariate analysis of numerous prognostic factors
• 10,750 pts over 1981 through 2002
• β2-microglobulin and albumin emerged as a powerful, simple predictor of outcome
•Pitfalls
β2M increased in renal failure, regardless of cause
Albumin can transiently drop in the setting of acute illness (e.g. pneumonia)
PR, et al. J Clin Oncol. 2005;23:3412-3420.

International Staging System
Stage β2-microglobulin (mg/L) Albumin (g/dL) Median OS (mos) ISS I <3.5 ≥3.5 62 ISS 2 Neither stage 1 or 3 44 ISS 3 ≥5.5 Any level 29 Greipp
•R-ISS stage 1: normal LDH, no high risk cytogenetic abnormality (CA)*, ISS stage 1 disease
•R-ISS stage 2: not stage 1 or 3
•R-ISS stage 3: ISS stage 3 disease PLUS high LDH OR high risk CA
*High risk CA = del(17p) and/or t(4;14) and/or t(14;16)
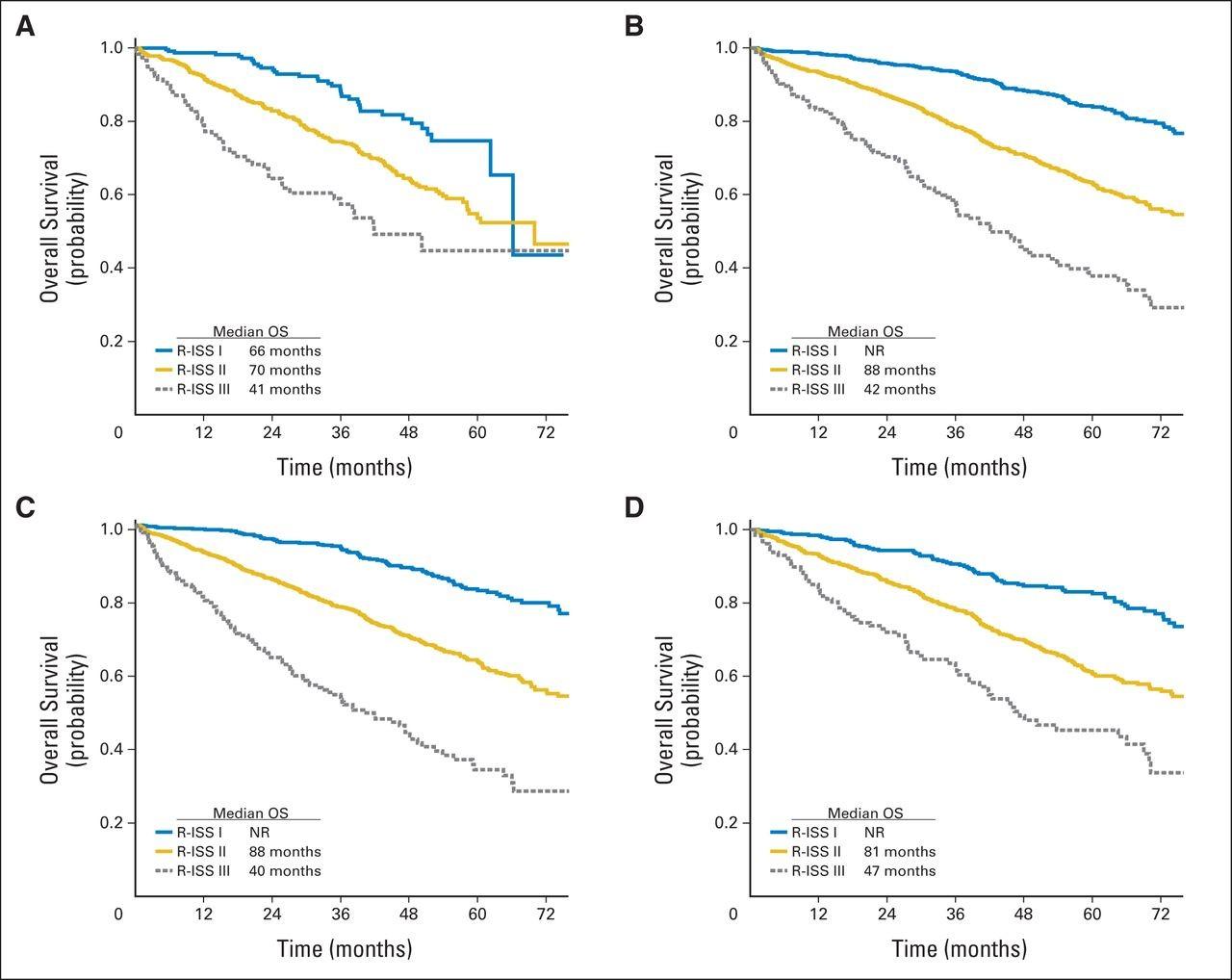

Revised International Staging System
Palumbo et al. JCO 2015;33:2863-2869
Non Transplant-Based Tx Transplant-Based Tx IMiD-Based Tx Bortezomib-Based Tx
• 1069 newly-diagnosed MM pts
• Adverse CG lesions in multivariate analysis:
• Adverse IgH translocation (4;14, 14;16, 14;20), del17p13, +1q21
• Median OS for those with 3 adverse lesions: 9.1 mos.
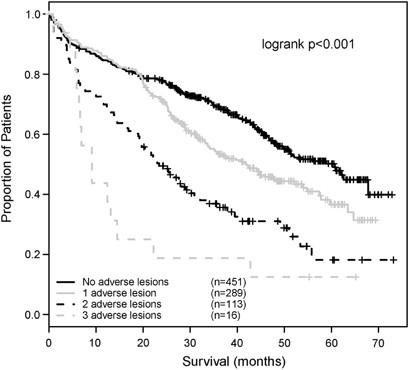
Boyd KD et al. Leukemia. 2012;26:349-55.

FISH in the MRC IX study


24
4
16
40
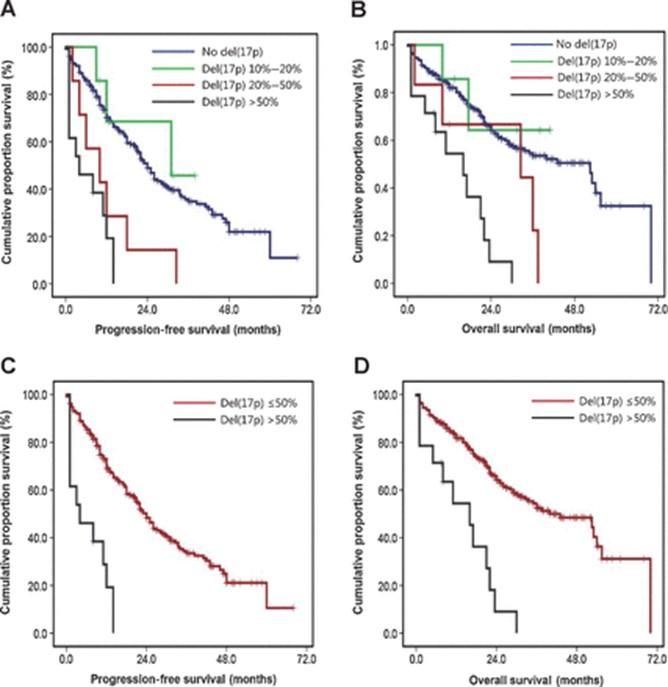
Tai T et al. Clin Cancer Res 2015;21: 2148-56 Not all del(17p) multiple myeloma is created equal
mo
mo
mo
mo (P < 0.001) (P < 0.001)
Are we overestimating the risk conferred by del(17p)?

Not all del(17p) multiple myeloma is created equal
MMRF
Study Keats
Unpublished
CoMMpass
et al:
Not
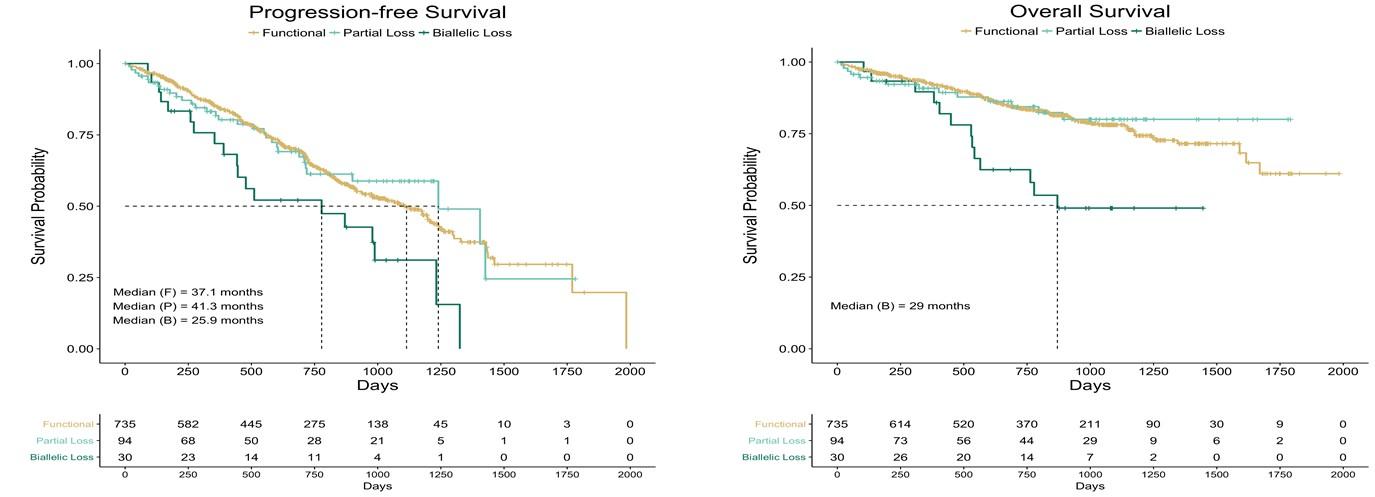
1q gain multiple myeloma is created equal P r o g r e s s i o nF r e e S u r v i v a l
v e r a l l S u r v i v a l
Gain of 1q = 3 copies; amplification of 1q = 4 copies
all
O
Walker B et al. JCO 2015;33:3911-20.
Whole Genome Sequencing in MM
• 203 patients underwent whole genome sequencing of their myeloma.
• Recurring mutations seen, some present in the majority of myeloma cells, others in subpopulations.
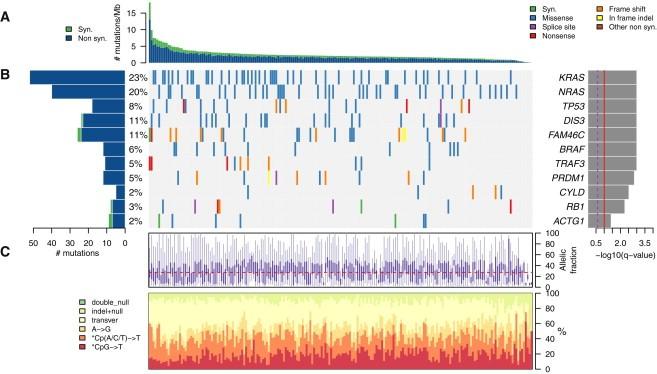

Lohr JG. et al.
Cancer Cell 2014;25:91-101.
• Array comparative genomic hybridization (aCGH) and FISH identify 3 types of MM evolution
• Genetically stable
• No new copy number alterations (CNAs, 35.7%)
• Linear evolution
• Only new CNAs (21.4%)
• Shifting dominance
• Loss and gains of CNAs, including reemergence of regions previously homozygously deleted (42.9%)
• Implications regarding optimal initial treatment strategy and choice of therapy at relapse
FISH analysis of a patient with t(4;14) MM over time

Clonal Heterogeneity in MM
Keats J J et al. Blood 2012;120:1067-76
• Paired bone marrow samples from the iliac crest and CT-guided fine needle aspirates of focal bone lesions
• 42 newly-diagnosed and 11 relapsed multiple myeloma patients
• 8 out of 13 patients with high-risk multiple myeloma by GEP70 had discordant results from the 2 sites
• 2 out of 6 patients with del(17p) multiple myeloma had discordant results from the 2 sites
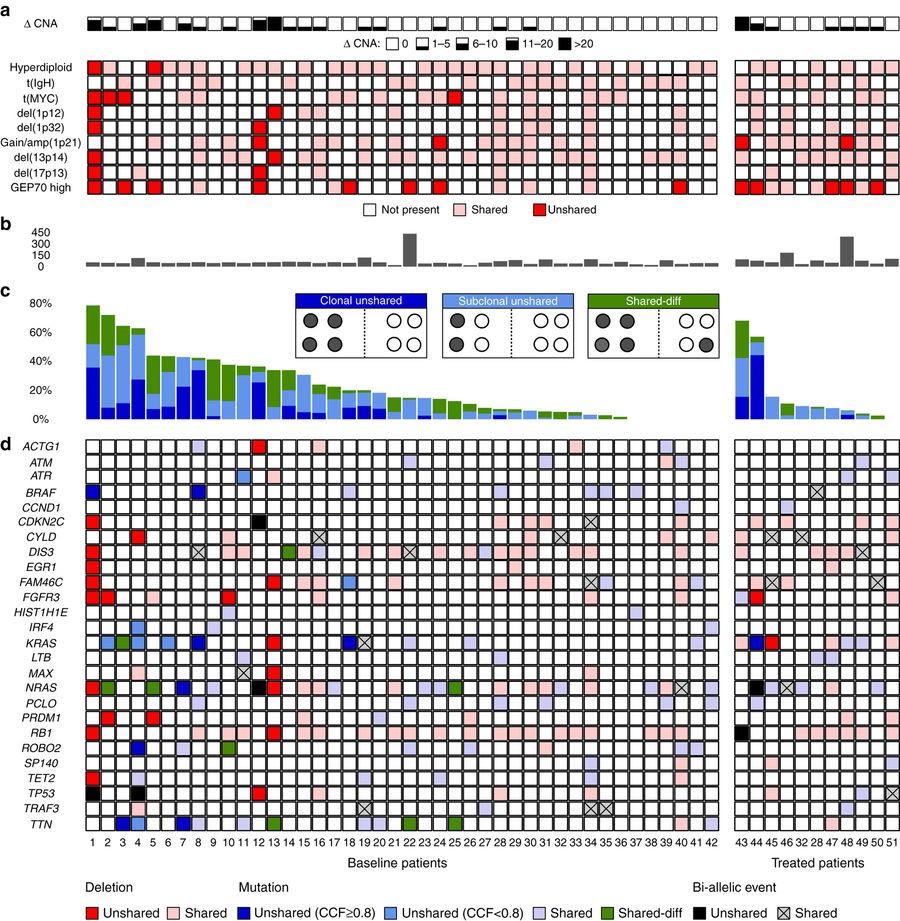
Spatial Cytogenetic Heterogeneity
Rasche L, et al. Nat Commun
2017;8:268.

Are we missing high risk disease with our current diagnostic workups?
Clonal Heterogeneity
Spatial
Rasche L, et al. Nat Commun 2017;8:268.
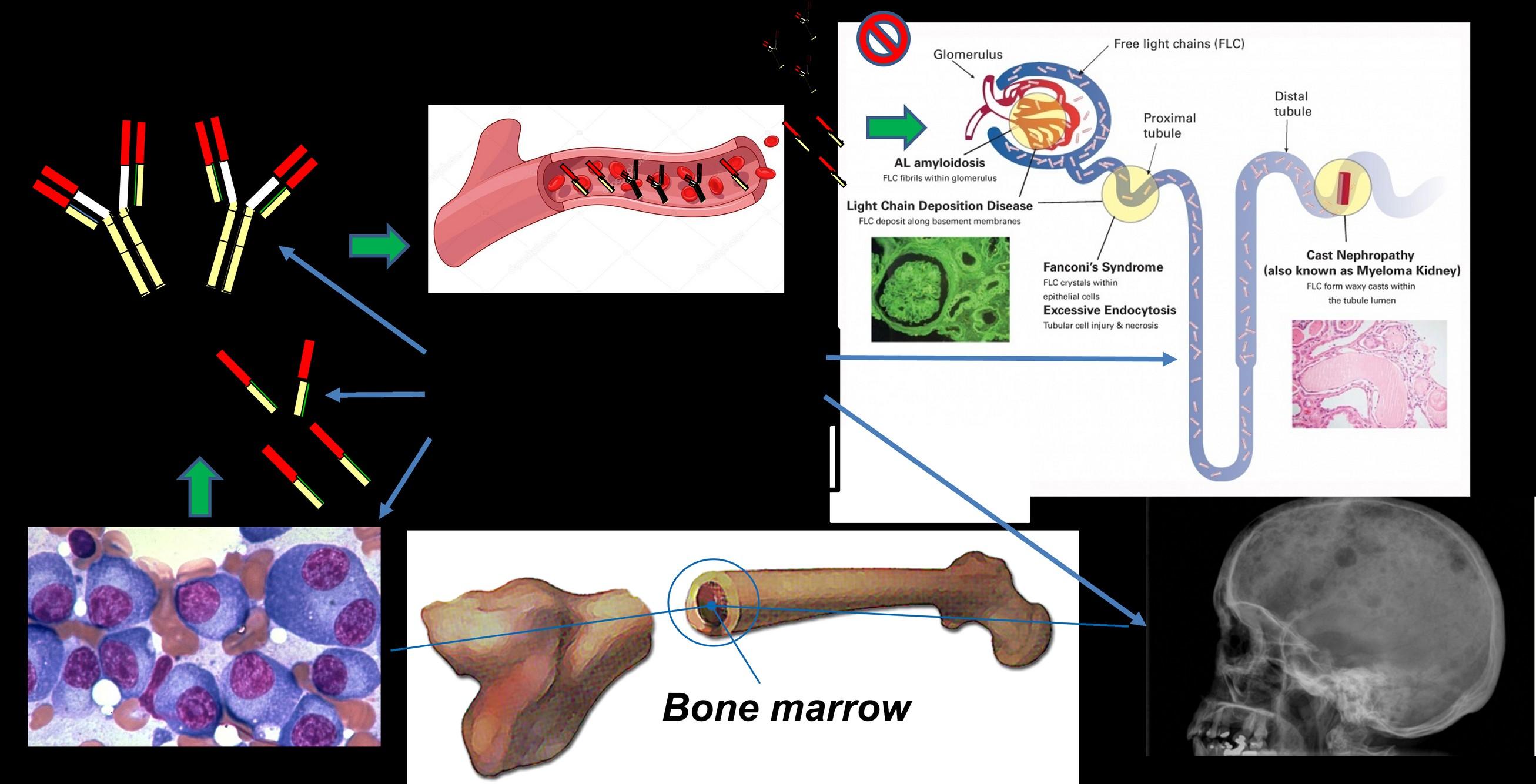

Myeloma
Measuring
What is an M-spike?
Intact antibodies
• A blood or urine marker indicating a clonal plasma cell or lymphoid population that produces immunoglobulin (Ig), also called antibody
• The M-spike (M protein):
• Heavy chain Ig
• IgG, IgA, IgM, IgD, IgE
• Light chain Ig
• Kappa or lambda Light chains

Adapted from Serum Free Light Chain Analysis. 4th Edition. AR Bradwell.
 Plasma cell
Plasma cell
Serum
Band of restricted mobility
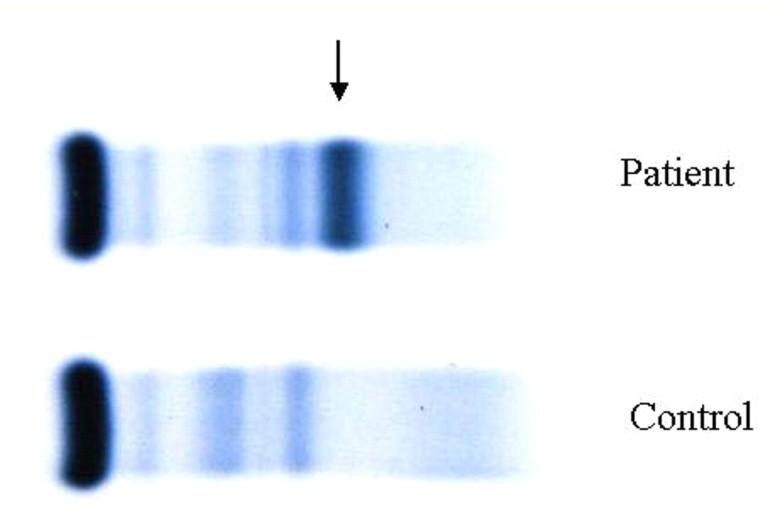
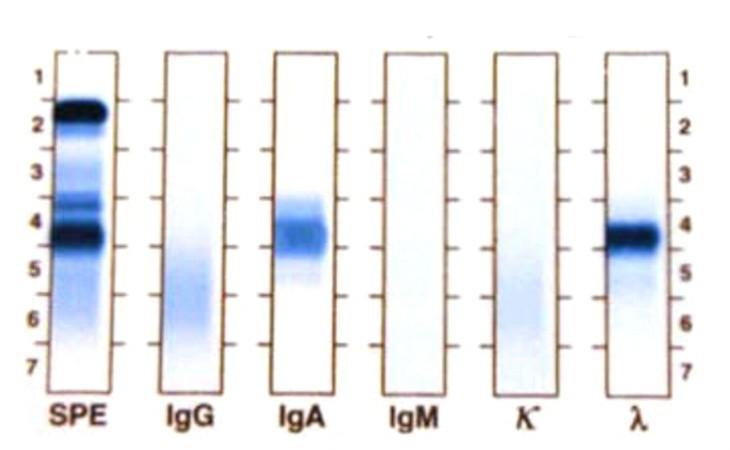

Protein Electrophoresis and Immunofixation Electrophoresis
SPEP
Monoclonal IgA lambda
Adapted from ASH Image Bank
Serum IFE
Daratumumab IFE Reflex Assay
• Daratumumab is a monoclonal IgG kappa antibody directed against CD38
• Daratumumab is picked up as an IgG kappa “M spike” on SPEP / immunofixation testing.
• To clear the daraumumab, patient samples are incubated with a mouse antibody directed against daratumumab followed by standard SPEP / immunofixation testing
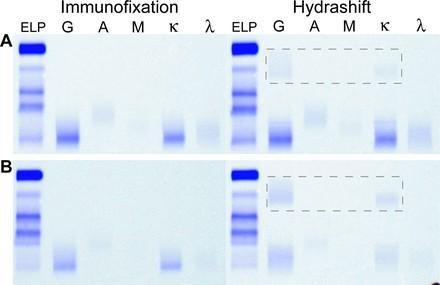

Thoren K, et al.
J Appl Lab Med 2019;3:857-63.
Serum Free Light Chain (FLC) Testing
• Up to 1000-fold more sensitive at detecting free light chains than SPEP
• Quantitative
• Can monitor response to treatment
• Lot to lot variability of reagents
• Coefficient of variability 10 – 20%
• Antigen excess
• Underestimation of light chain levels Dispenzieri et al. Leukemia. 2009 Feb;23:214-24.


• 3 patterns of myeloma disease progression after an initial response
• Paraprotein only (IgA kappa M protein only)
• Free light chain only (serum free kappa light chains only)
• Paraprotein and free light chains (IgA kappa M protein and serum free kappa light chains)
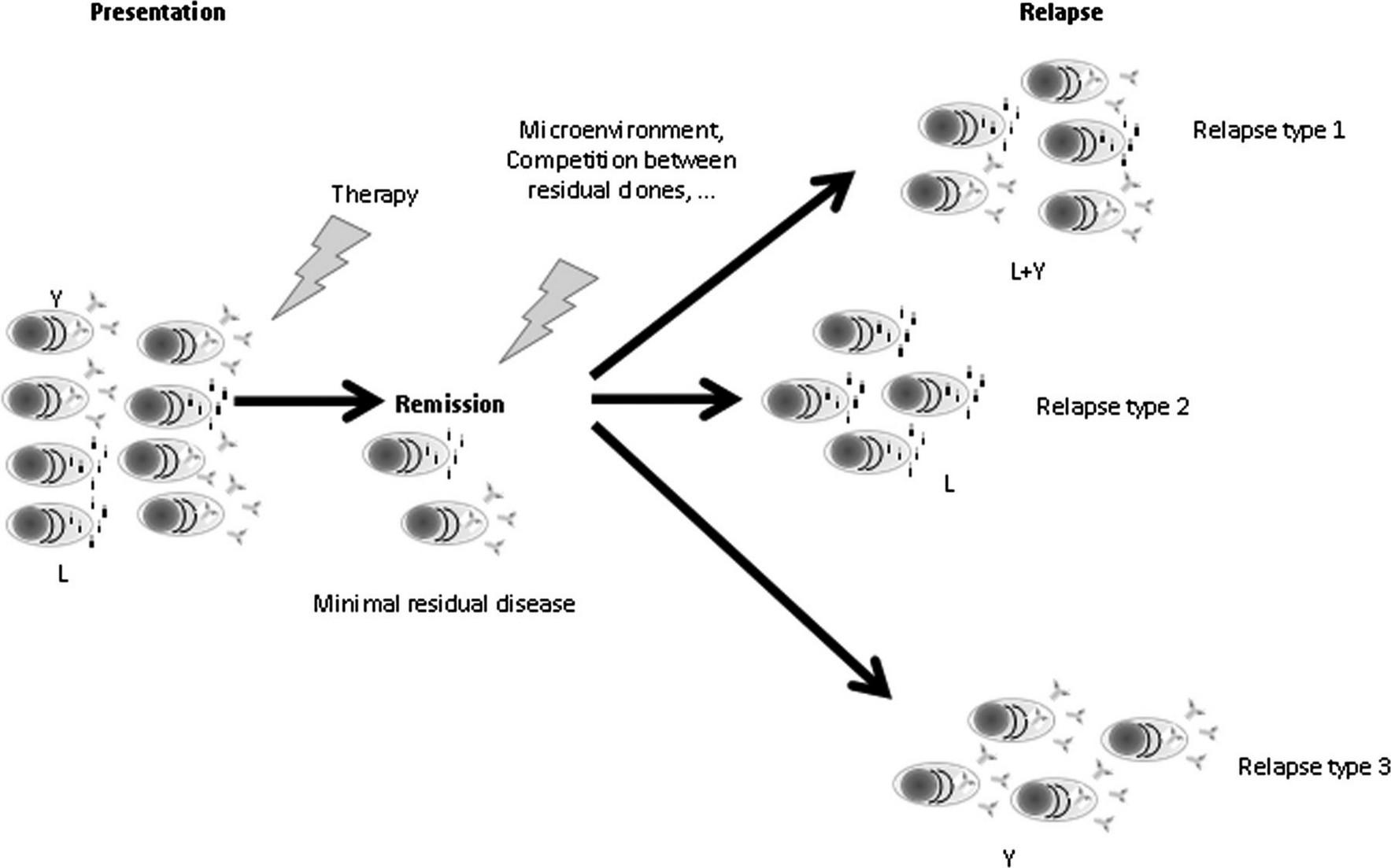

Serum FLC Escape Brioli A, et al. Blood 2014;123:3414-9. 35.2% 10.4% 49.6%
MRD and Progression-Free Survival in Multiple Myeloma: A Meta-Analysis
• 93 publications included; 85 on PFS, 48 on OS
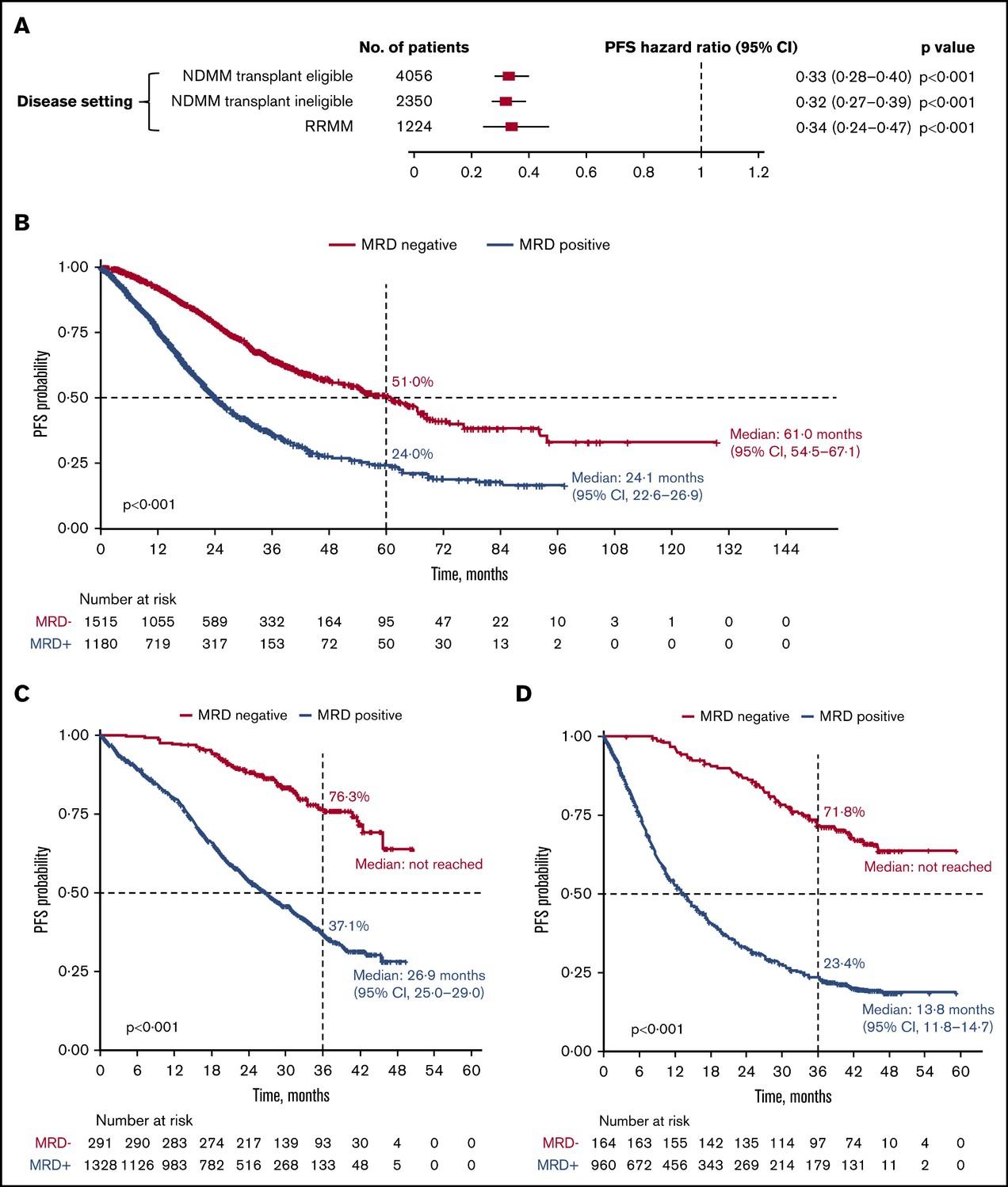
N et al. Blood Adv 2020;4:5988-99.
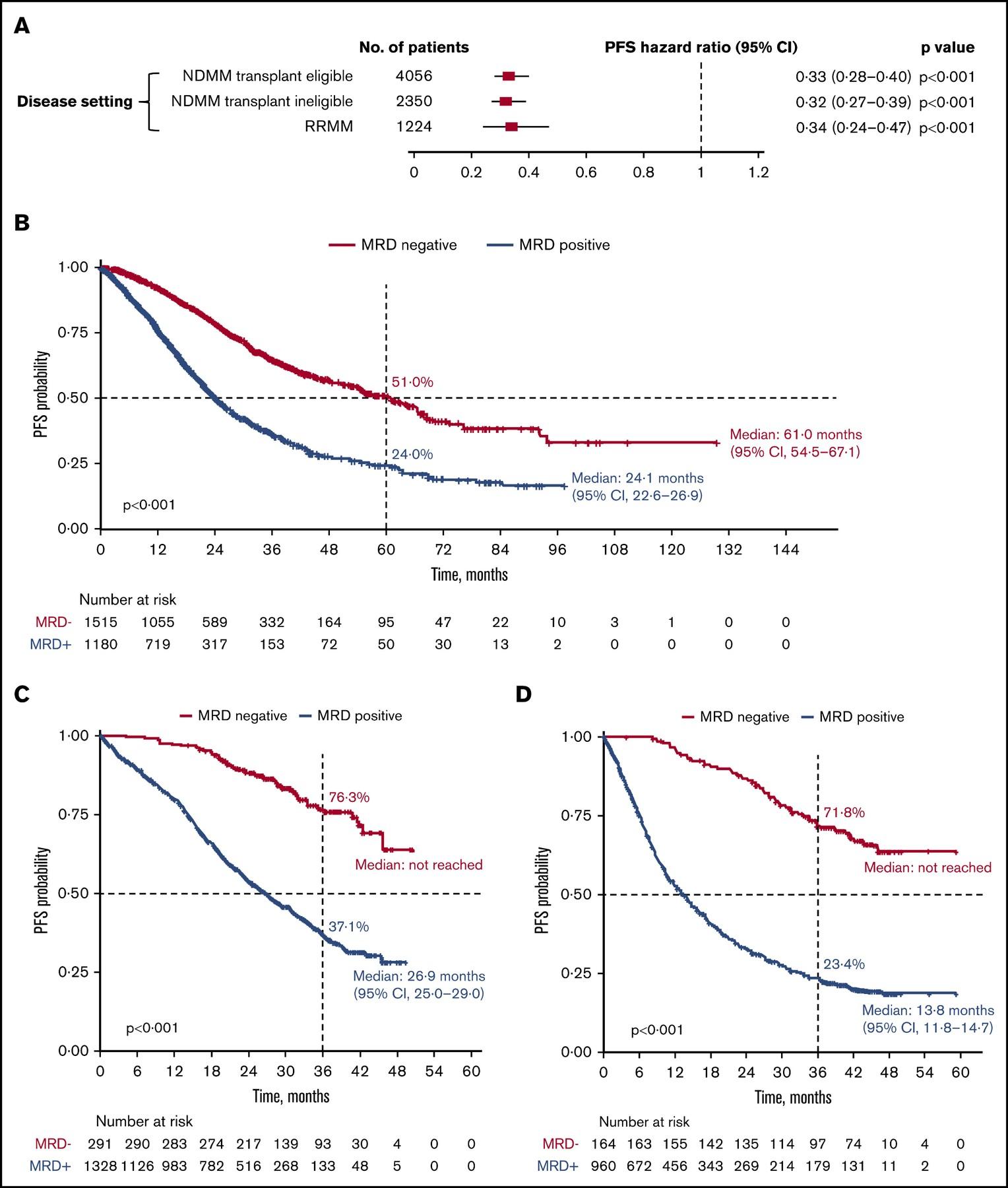
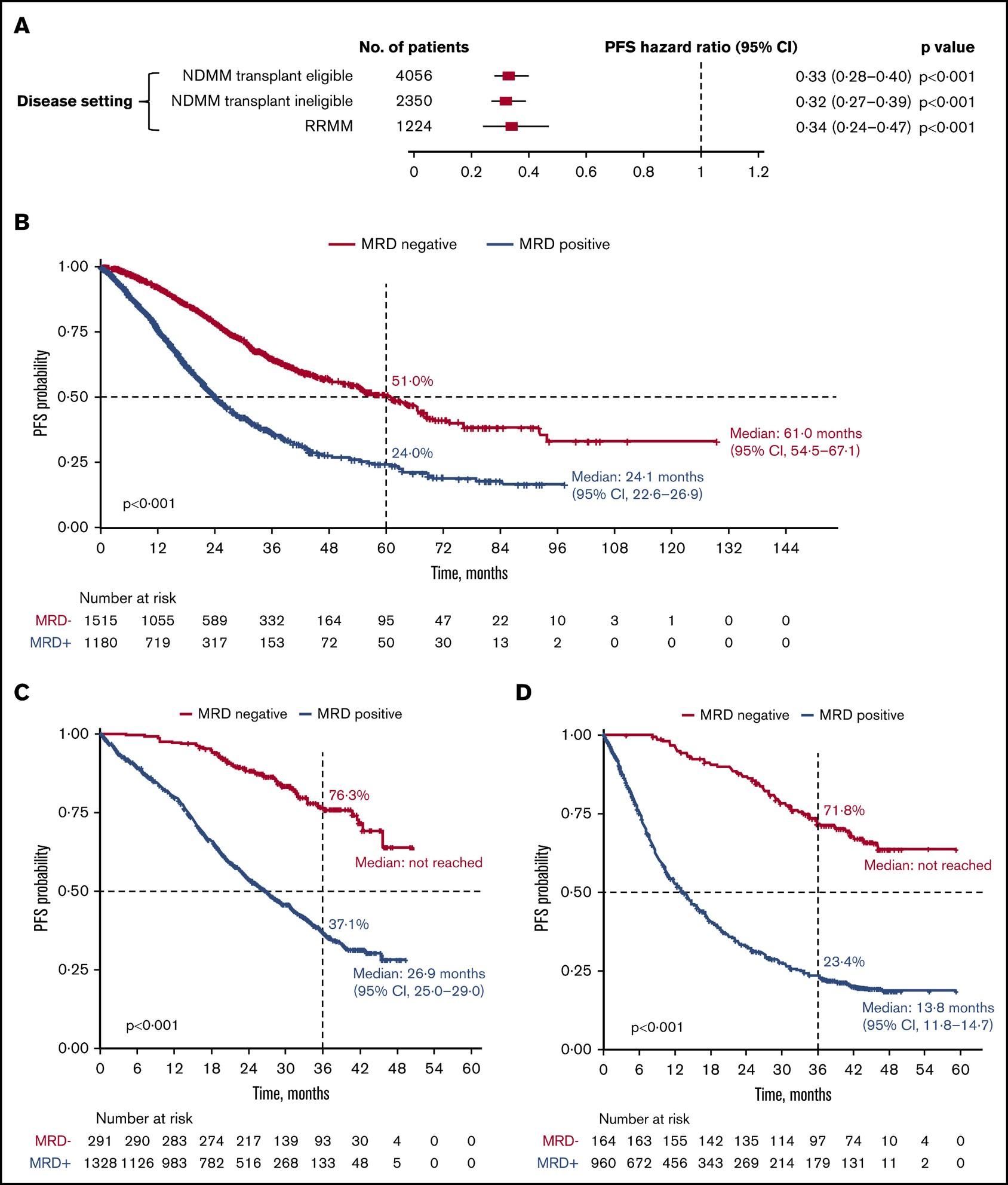
Newly-Diagnosed, Transplant Eligible Relapsed / Refractory Newly-Diagnosed, Transplant Ineligible

Munshi,
P r o g r e s s i o nF r e e S u r v i v a l P r o g r e s s i o nF r e e S u r v i v a l P r o g r e s s i o nF r e e S u r v i v a l
MRD and Overall Survival in Multiple Myeloma: A Meta-Analysis
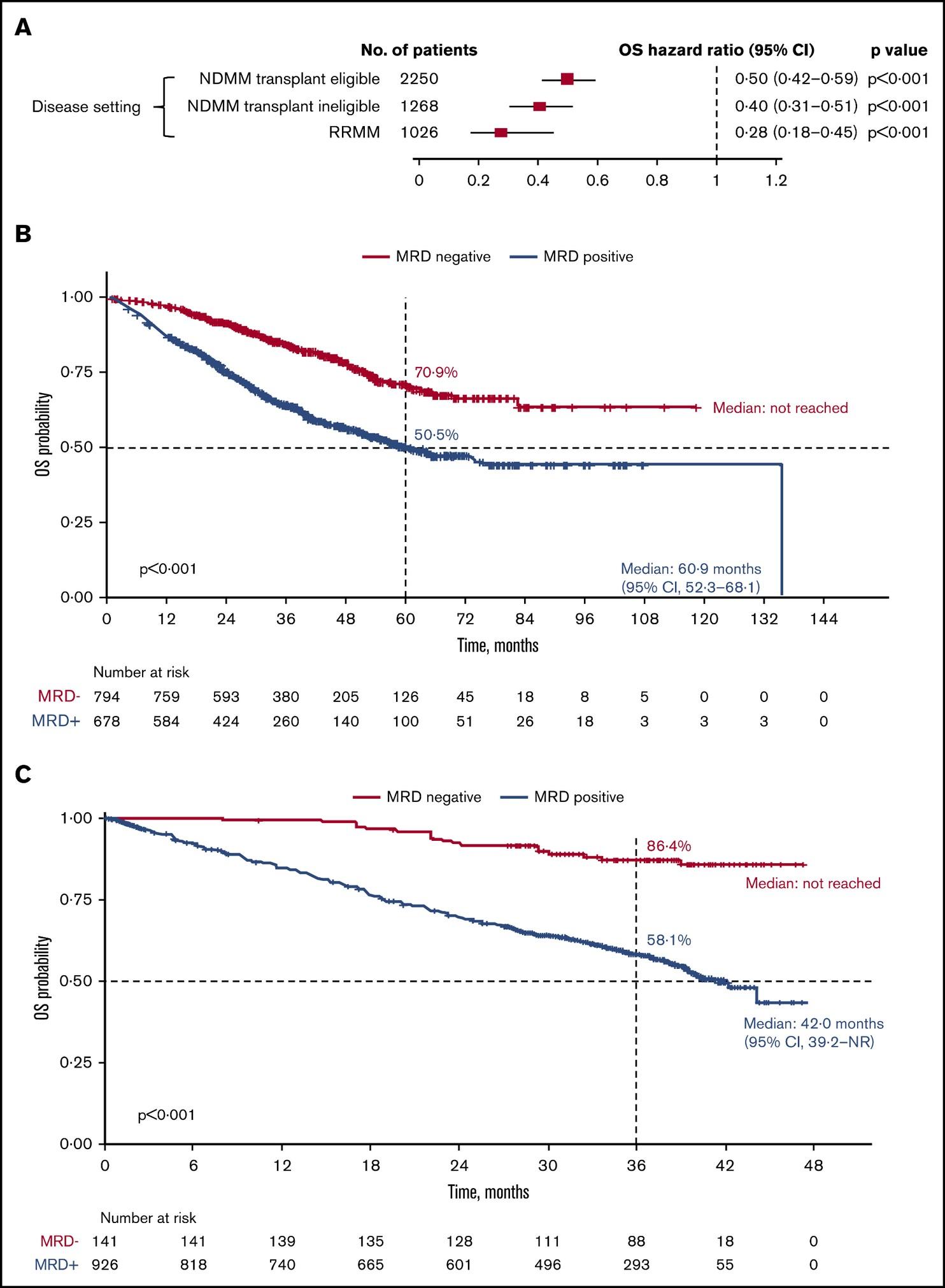
Newly-Diagnosed, Transplant Eligible

N et al. Blood Adv 2020;4:5988-99.
Relapsed / Refractory

Munshi,
O v e r a l l S u r v i v a l O v e r a l l S u r v i v a l
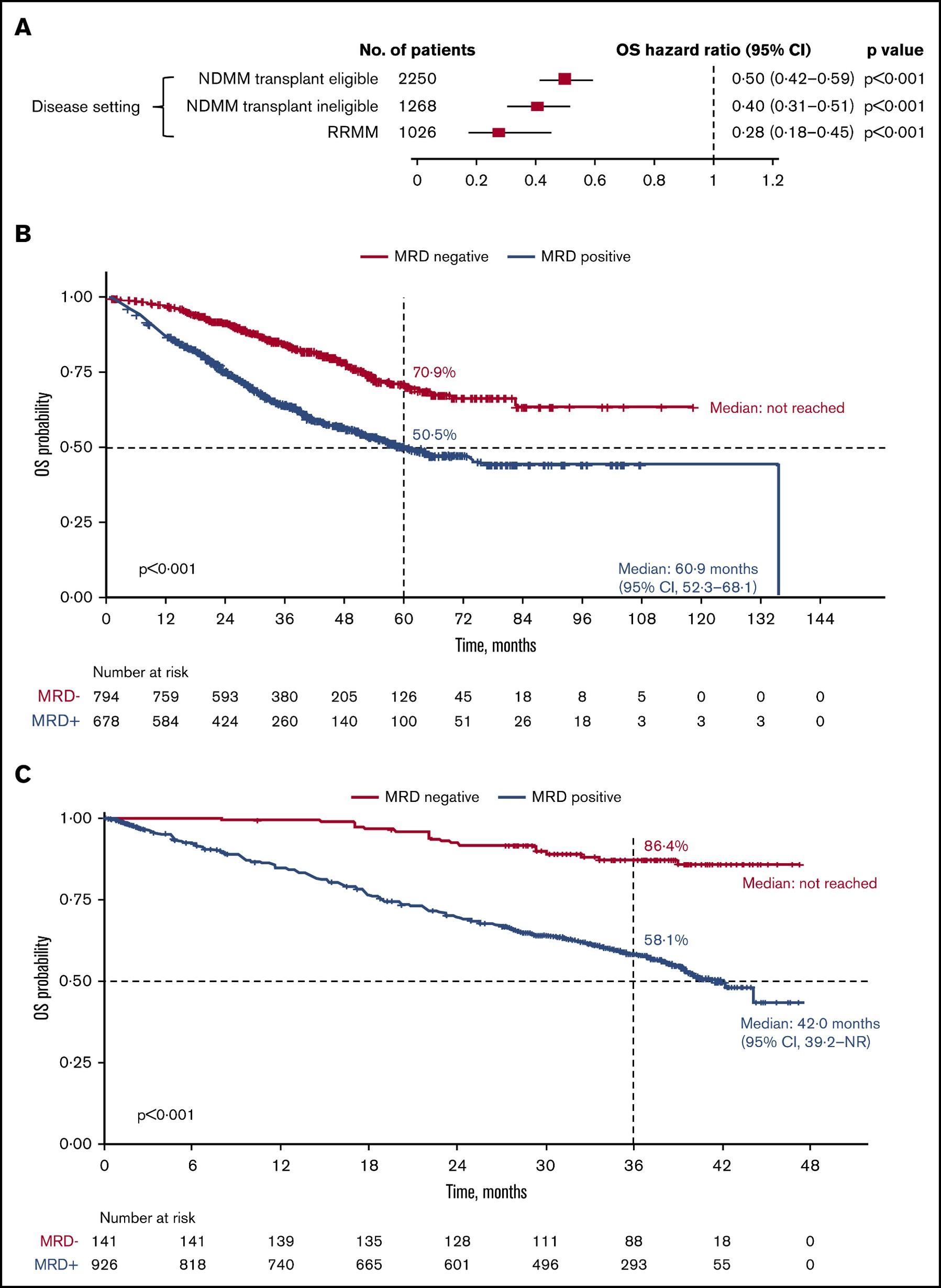
MRD: Subset Analysis of PFS and OS
Munshi, N et al. Blood Adv 2020;4:5988-99.


Survival Overall Survival
Progression-Free
Mass Spec: Performance
Sensitive… Linear…
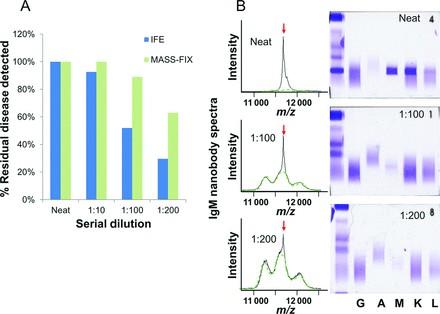
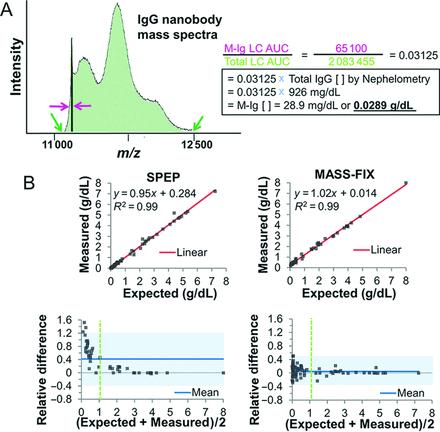
Quantifiable

Mills J, et al. Clin Chem
2016;62:1334-44.
…and
• Retrospective analysis of 61 patients who underwent induction ASCT at Mayo Clinic and achieved MRD negativity by NGF
• Median follow-up 33 months
• Patients who were also MRD- by Mass-Fix had a longer PFS and Time to next treatment
• 33-month PFS: 96% vs 72%
J, et al. Br J Haematol 2021;193:380-5.
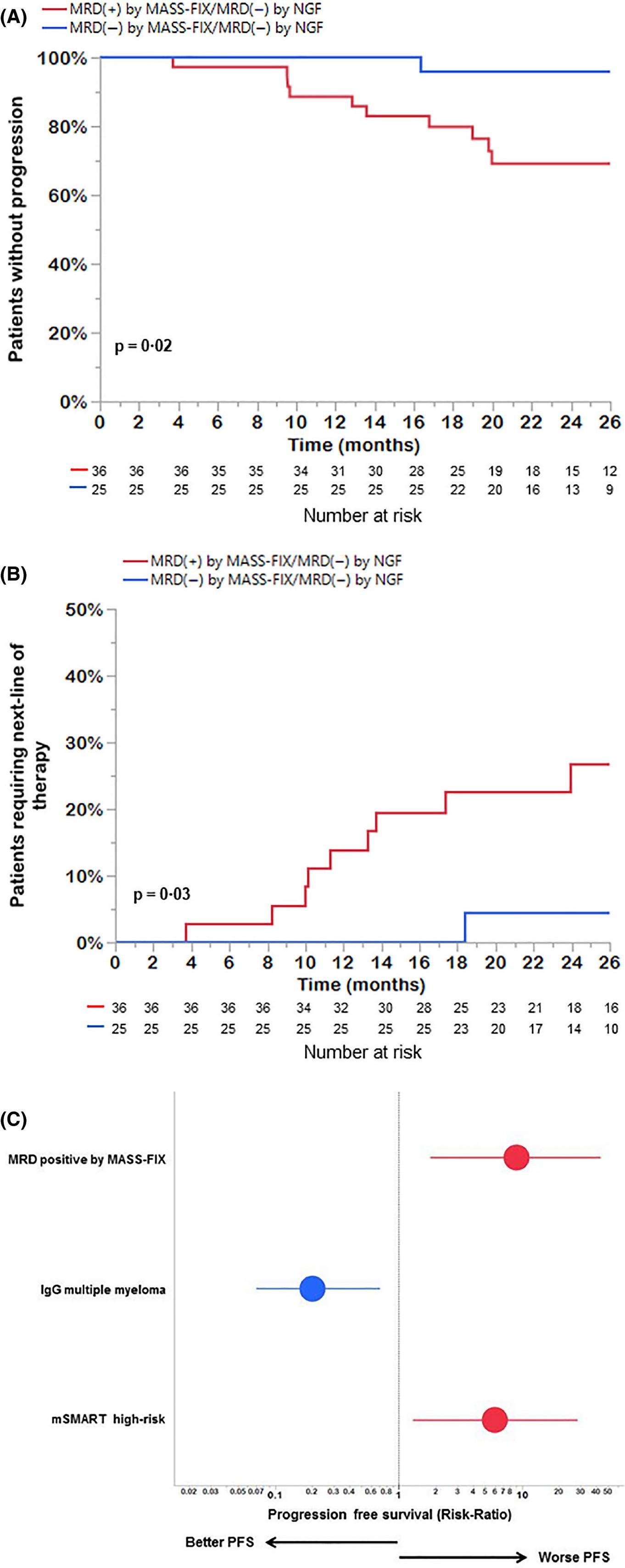

Mass Spec
Abeykoon
• Myeloma starts as a precursor form called MGUS
• Myeloma is a heterogeneous disease
• Between patients
• Within an individual patient in different areas of the body
• Within an individual patient over time
• Myeloma is complicated
• There are standard risk and high-risk versions of the disease
• A Myeloma Specialist can navigate you through the meaning of these diagnostic tests
• A thorough understanding of your disease at diagnosis is crucial to making best decisions about your care
• Peripheral blood molecular diagnostics and MRD assessment in development and will eventually replace the need for bone marrow and bone biopsies

Conclusions
Charlotte, NC
June 22, 2024
Life Is A Canvas,
You Are The Artist
Amy E. Pierre, RN, MSN, ANP-BC Memorial Sloan Kettering Cancer Center, Flatiron Health & IMF Nurse Leadership Board
Patient Education Slides 2021
YOU Can Change the Course of Myeloma in Your Community

Know the symptoms

Speak to your doctor about your risk

Ask if you should be screened




Talk to friends & family about what you’ve learned about Multiple Myeloma


@#$ % #!? %^ Be M - Powered







Note: Some patients have nonsecretory disease that does not produce detectable myeloma protein.
“Myeloma is among the worst of all cancers for delayed diagnosis.”
Drayson
M, et al. Br J Haematol. 2024;204(2):476-486.

58 Understanding Your Test Results, International Myeloma Foundation 2018.
Bone marrow
The average myeloma patient sees
their primary care doctor 3 times with symptoms and signs consistent with MM


The delay is even longer in African Americans, for many reasons:
Confounding diagnoses (like diabetes)
ACCESS to diagnostics and care
AWARENESS in primary care providers
TIMELY REFERRAL to specialists
African Americans have twice the incidence of myeloma and, on average, are diagnosed at a

How Patients With Myeloma Commonly Present
ROUTINE PHYSICAL

VISIT FOR SPECIFIC COMPLAINT

EMERGENCY ROOM
• Patient with few/no symptoms
• Abnormal blood work
• Persistent symptom or injury
• Abnormal test result (eg, x-ray)

• Severe pain—often spinal fractures
• Kidney failure


60 Brigle K, et al. J Adv Pract Oncol. 2022;13(suppl 4):7-14. Brigle K, et al. Clin J Oncol Nurs. 2017;21(5 suppl):60-76. Faiman B, et al. J Adv Pract Oncol. 2016;2016:7(suppl 1):17-29. Kurtin S, et al. J Adv Pract Oncol. 2016;7(suppl 1):59-70.
treatment!
MEDICAL EMERGENCY; need immediate
NON-EMERGENCY; More time for shared decision-making
WHAT IS AN EMPOWERED PATIENT?

“An
empowered patient is an individual who takes an active role in managing their own health and healthcare journey. They are informed, engaged, and assertive in making decisions about their health and treatment options.”


Understanding Your Test Results, International Myeloma Foundation 2018.
1. Ownership of Health: They take responsibility for their health outcomes by adhering to treatment plans, adopting healthy lifestyle behaviors, and actively managing chronic conditions.
2. Health Literacy: Empowered patients understand medical terminology, treatment procedures, and healthcare systems well. They can interpret medical information accurately and make informed choices about their health.
3. Active Participation: Empowered patients actively engage in discussions with healthcare providers, asking questions and expressing concerns.
4. Self-Advocacy: They advocate for themselves to ensure their needs, preferences, and concerns are addressed by healthcare providers. This may involve asserting their rights, seeking second opinions, or challenging recommendations that do not align with their goals or values.
5. Informed Decision-Making: They seek out information about their health condition, treatment options, and healthcare providers. They stay updated on the latest research and medical advancements relevant to their condition.

10 CHARACTERISTICS OF AN EMPOWERED PATIENT
6. Share in Decision-Making: They collaborate with healthcare providers in making decisions about their care, weighing the benefits, risks, and alternatives of treatment options based on their personal values and preferences.
7. Proactive Communication: Empowered patients take the initiative to communicate with their healthcare team, informing them about side effects, changing circumstances, or preferences
8. Resilience and Adaptability: Empowered patients demonstrate resilience in coping with health challenges and setbacks. They adapt to changes in their health status or treatment plans with a positive attitude and a willingness to explore new strategies for managing their condition.
9. Seeking Support Networks: They actively seek out support from peers, support groups, or online communities to share experiences, gather information, and find emotional support in their healthcare journey.
10. Continuous Learning and Improvement: Empowered patients embrace opportunities for learning and self-improvement in managing their health. They remain open to new information, perspectives, and treatment options that may enhance their well-being.


OF AN
10 CHARACTERISTICS
EMPOWERED PATIENT (CONTINUED)
CARE TEAM COLLAGE
Keep a contact list of your providers
Understand the different roles
Consult a specialist


















You and Your Caregiver(s) Support Network Allied Health Staff Subspecialists Myeloma Specialist General P h a r m a c i s t
64
Be M-Powered ► Ask questions ► Participate in decisions ► Communicate effectively with your entire team
Many Doctors and Nurses Believe Empowered
Patients have better Outcomes
Do patients who research their symptoms and/or treatment options typically have better or worse outcomes than patient who do not?
N=1089 physicians and
nurses
No Effect
Worse Outcomes
#REF!Nurses Physicians

65 Frellick M. Most Physicians, Nurses Say Empowered Patients Are Helpful. https://www.medscape.com/viewarticle/883783. Access date 4/30/2024.
0% 10% 20% 30% 40% 50% 60%
ge
Percenta
Barriers: Reasons Why
Shared Decision-Making Is Not Used
Practice Barriers
Limited time during patient encounter, “too busy”
Lack of commitment by all multidisciplinary team members
Nurses/Doctors might not think they make a difference.
Patient Barriers
Institutional Barrier
Scope of Practice Barrier
Administration Barrier
Patient is hesitant to “speak up”
Difference in goals between patient and caregiver
Not sure what is important to them – “trust provider’s judgment”
Lack of policy or time commitment
Laws and guidelines prohibiting autonomous practice
Inadequate support by hospital administration (types of treatments restricted)

McCarter SP, et al. Barriers and promoters for nurses’ participation in cancer treatment decision making process and patient satisfaction with treatment decision. Sigma Theta Tau International’s 26th International Nursing Research Congress. 2015. On-line: https://stti.confex.com/stti/congrs15/webprogram/Paper71335.html
What to Communicate
•Family History
− Risk factors also include Black race, male, age, obesity
•Symptoms
− Don’t ignore or self-medicate for pain
− Address changes with your healthcare providers
•Side Effects
•Questions About Test Results and Treatments


•YOUR Priorities, Preferences and Goals for Treatment

The GOOD News: Many, Many Treatment Options for MM
Immunotherapies
Dexamethasone
Frontline Relapse
Velcade® (bortezomib)
Darzalex® (daratumumab)
Maintenance
Velcade® (bortezomib)
Kyprolis® (carfilzomib)
Ninlaro® (ixazomib)
Darzalex® (daratumumab)
Empliciti® (elotuzumab)
Sarclissa® (Isatuximab)
Thalomid® (thalidomide)
Revlimid® (lenalidomide)
Revlimid® (lenalidomide)
Thalomid® (thalidomide)
Revlimid® (lenalidomide)
Pomalyst® (pomalidomide)
CelMods
• Iberdomide
• Mezigdomide
Neuropathy
Carfilzomib: Cardiac
SoluMedrol®
Dexamethasone
Prednisone
Prednisolone
SoluMedrol®
Elrexfio™ (elranatamab)
Tecvayli® (teclistamab)
Talvey™ (talquetamab)
Melphalan + ASCT
Abecma® (Idecabtagene Vicleucel)
Carvykti® (ciltacabtagene autoleucel)
Blenrep®* (Belantamab mafodotin)
Infusion reaction
Blenrep: Keratopathy
Other CAR-T
Xpovio® (Selinexor)
Doxil (liposomal doxorubicin)
Infection risk
CAR-T: CRS and neurotoxicity
Venclexta® (venetoclax)
Myelosuppression, GI
Selinexor: Low sodium

-Mibs -MAbs -Mides Steroids Alkylators Bispecific Antibody Cellular Therapies Others
Prednisone Prednisolone
Melphalan Cyclophosphamide
+ ASCT
Melphalan
Melphalan Cyclophosphamide Bendamustine
DVT/PE
Infusion reaction
See steroid slide Myelosuppression
Pending FDA Approval
effects 68 ASCT = autologous stem cell transplant; CAR-T cell therapy; CRS = cytokine release syndrome; DVT = deep vein thrombosis; PE = pulmonary embolism *Withdrawn from FDA but still available in certain situations IMF Nurse Leadership Board ONS Symposia 2022; NCCN Guidelines. Multiple Myeloma. V3.2024. Accessed March 25, 2024.
Noted Side
DISCUSS SYMPTOMS AND SIDE EFFECTS
Myeloma cells in excess can cause symptoms
Treatments for myeloma kill myeloma cells
but
can cause symptoms
• Calcium elevation
• Renal dysfunction
• Anemia
• Bone pain
• Fatigue
• Infection
• Other symptoms
• Myelosuppression
• Peripheral neuropathy
• Diarrhea
• Fatigue
• Deep vein thrombosis
• Infection (eg, shingles)
• Other symptoms
Myeloma and Treatments
Both Contribute to How You Feel

PATIENT-REPORTED SYMPTOMS
A meta-analysis identified the most common patient-reported symptoms and impact on QOL, and were present at all stages of the disease.
Symptoms resulted from both myeloma disease and treatment, including transplant, and were in these categories:
Physical

• Fatigue
• Constipation
• Pain
• Neuropathy
• Impaired Physical Functioning
• Sexual Dysfunction

Psychologica
l
• Depression
• Anxiety
• Sleep Disturbance
• Decreased Cognitive Function
• Decreased Role & Social Function
Financial

• Financial burden (80%)
• Financial toxicity (43%)

Ramsenthaler, et al. 2016. https://doi.org/10.1111/ejh.12790. 70
Immunotherapy Side Effects
Cytokine Release Syndrome (CRS)
Immune effector cell–
associated neurotoxicity syndrome (ICANS)
Neuro Toxicity

Infection

71 CRS = cytokine release syndrome; ICANS = immune effector cell–associated neurotoxicity syndrome.
Physical
Immunotherapies: UNIQUE SIDE EFFECTS

CRS
IS A COMMON BUT USUALLY MILD SIDE EFFECT WITH CAR T

CRS = cytokine release syndrome. Oluwole OO, Davila ML. J Leukoc Biol. 2016;100:1265-1272. June CH, et al. Science. 2018;359:1361-1365. Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Brudno JN, Kochenderfer JN. Blood Rev. 2019:34:45-55. Shimabukuro-Vornhagen, et al. J Immunother Cancer. 2018;6:56. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.

CRS Fever
Headache Nausea / vomiting Shortness of Breath Diarrhea Weakness Confusion
Fatigue
72 Physical
Immunotherapies: UNIQUE SIDE EFFECTS




Headache Confusion Altered wakefulness Hallucinations Ataxia Apraxia Facial nerve palsy Tremors Seizures Encephalopathy Neurotoxicity CRS = cytokine release syndrome; ICANS = immune effector cell–associated neurotoxicity syndrome; ICE = Immune Effector Cell Encephalopathy screening tool; MRI = magnetic resonance imaging. Brudno JN, Kochenderfer
Blood.
JN.
2016;127(26):3321-3330. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.
NEUROTOXICITY IS A RARE
SERIOUS SIDE EFFECT OF CAR T 73 Physical
BUT

INFECTION CAN BE SERIOUS FOR PEOPLE WITH MYELOMA
[P]reventing infections is paramount.
Infection remains the leading cause of death in patients with multiple myeloma. Several factors account for this infection risk, including the overall state of immunosuppression from multiple myeloma, treatment, age, and comorbidities (e.g., renal failure and frailty).
IMWG Consensus guidelines and recommendations for infection prevention in multiple myeloma; Lancet Haematol.2022;9(2):143–161.

Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
As recommended by your healthcare team: Infection Prevention Tips
Good personal hygiene (skin, oral)
Environmental control (avoid crowds and sick people; use a high-quality mask when close contact is unavoidable)
Immunizations: Flu, COVID, RSV & and pneumococcal vaccinations; avoid live vaccines
Preventative and/or supportive medications (next slide)
IMWG = International Myeloma Working Group; HCP = healthcare provider. Raje NS, et al. Lancet Haematol.2022;9(2):143–161. IMF Nurse Leadership Board ONS Symposia 2023.

Physical
MEDICATIONS CAN REDUCE INFECTION RISK
Type of Infection Risk
Viral: Herpes Simplex (HSV/VZV); CMV
Bacterial: blood, pneumonia, and urinary tract infection
PJP (P. jirovecii pneumonia)
Fungal infections
COVID-19 and Influenza

Medication Recommendation(s) for Healthcare Team
Consideration
Acyclovir prophylaxis
Consider prophylaxis with levofloxacin
Consider prophylaxis with trimethoprim-sulfamethoxazole
Consider prophylaxis with fluconazole
Antiviral therapy if exposed or positive for covid per institution recommendations
IgG < 400 mg/dL (general infection risk) IVIg recommended
ANC < 1000 cells/μL (general infection risk)
Consider GCSF 2 or 3 times/wk (or as frequently as needed) to maintain ANC > 1000 cells/μL and maintain treatment dose intensity
Some people receiving BCMA-targeting therapies have experienced infections that are less common like CMV, PJP and fungal infections
ANC = absolute neutrophil count; BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; CMV, cytomegalovirus; GCSF = granulocyte colony-stimulating factor; HSV = herpes simplex virus; IVIg = intravenous immunoglobulin; PJP = Pneumocystis jirovecii pneumonia; VZV = varicella zoster virus.
Raje NS, et al. Lancet Haematol.2022;9(2):143–161.

Physical
MANAGEMENT OF ORAL SIDE EFFECTS
Dry Mouth
OTC dry mouth rinse, gel, spray are recommended. Advise patients to avoid hot beverages. Initiate antifungal therapy for oral thrush
Dexamethasone oral solutions “swish and spit” have been tried but with no proven benefit yet. Sour citrus or candies before meals are also recommended. Taste Changes
Dental Care
Attention to oral hygiene.
Regular dental cleaning and evaluation. Close monitoring for ONJ, oral cancer and dental caries
ONJ = osteonecrosis of the jaw; OTC = over the counter.

Dysphagia
Weight Management
Dietary modifications with small bites, eating upright, and sips with food can help manage symptoms.
Some medications lead to weight gain, others to weight loss.
Dry mouth leads to taste changes which can lead to anorexia.
Meet with a Nutritionist
Consider diet changes, supplements
Monitor weight
Education and emotional support are key strategies to manage oral toxicities.
Catamero D, Purcell K, Ray C, et al. Presented at the 20th International Myeloma Society (IMS) Annual Meeting Nurse Symposium; September 27–30, 2023; Athens, Greece.

Physical
GI SYMPTOMS: PREVENTION & MANAGEMENT
Diarrhea may be caused by medications and supplements
Laxatives, antacids with magnesium
Antibiotics, antidepressants, others
Milk thistle, aloe, cayenne, saw palmetto, ginseng
Sugar substitutes in sugar free gum
Avoid caffeinated, carbonated, or heavily sugared beverages
Take anti-diarrheal medication
Imodium®, Lomotil®, or Colestid if recommended
Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
Welchol® if recommended

Constipation may be caused by
• Opioid pain relievers, antidepressants, heart or blood pressure medications, others
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency
Increase fiber
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®

Fluid intake can help with both diarrhea and constipation, and good for kidneys.
Discuss GI issues with health care providers to identify causes and make adjustments to medications and supplements.

Smith LC, et al. CJON.2008;12(3)suppl:37-52. Faiman B. CJON. 2016;20(4):E100-E105.
Physical
77

Management of Skin and Nail Side Effects
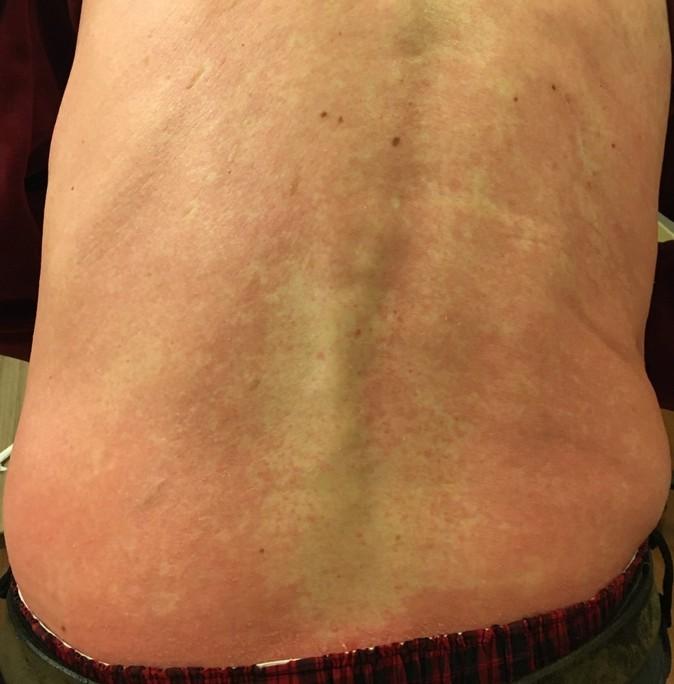
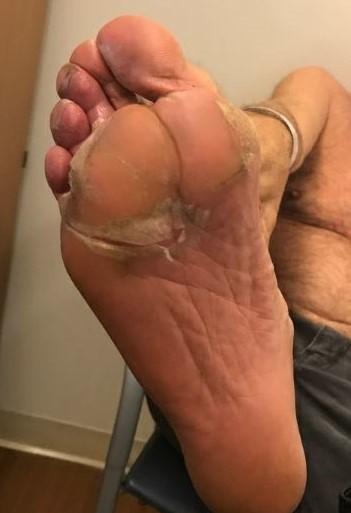
Skin Rash:
•Prevent dry skin; apply lotion
•Report changes to your care team
•Medication interruption or alternative, as needed
•Steroids:
• Topical for grades 1-2,
• Systemic and topical for Grade 3
•Antihistamines, as needed
Nail Changes:
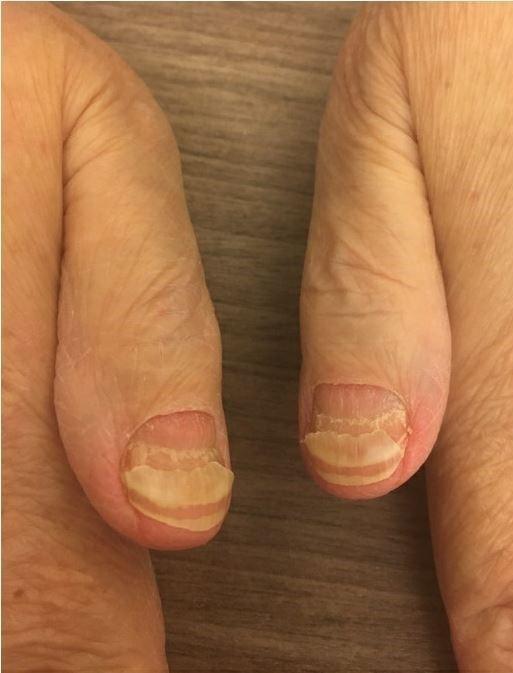
•Keep your nails short and clean. Watch for “catching and tearing”
•Apply a heavy moisturizer like Vaseline or salve. Wear cotton hand coverings to bed
•A nail hardener may help with thinning
•Tell the team if you have signs of a fungal infection, like thickened or discolored nails
 Photos: Mount Sinai Hospital, NY, NY
Nurse Leadership Board
Photos: Mount Sinai Hospital, NY, NY
Nurse Leadership Board
targeting GPRC5D
Seen more commonly with treatments
Physical
The Bright Dark Side to Steroids

Steroid Synergy
Steroids are a backbone and work in combination to enhance myeloma therapy
Managing Steroid Side Effects
Consistent schedule (AM vs. PM)
Take with food
Stomach discomfort: Over-the-counter or prescription medications
Medications to prevent shingles, thrush, or other infections
Do not stop or adjust steroid doses without discussing it with your health care provider


Steroid Side Effects
• Irritability, mood swings, depression
• Difficulty sleeping (insomnia), fatigue
• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Increase in blood pressure, water retention

• Blurred vision, cataracts
• Flushing/sweating
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increase in blood sugar levels, diabetes

Rajkumar SV, et al. Lancet Oncol. 2010;11(1):29-37. King T, Faiman B. Clin J Oncol Nurs. 2017;21(2):240-249.
& 79 Physical Psychological
PAIN PREVENTION AND MANAGEMENT
Pain can significantly compromise quality of life
Sources of pain include bone disease, neuropathy and medical procedures
Management
Prevent pain when possible
Bone strengtheners to decrease fracture risk; anti viral to prevent shingles; sedation before procedures
Interventions depends on source of pain
May include medications, activity, surgical intervention, radiation therapy, etc
Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)

Tell your health care provider about any new bone pain or chronic pain that is not adequately controlled


Physical
Faiman B, et al. CJON. 2017;21(5)suppl:19-36.
80
PERIPHERAL NEUROPATHY MANAGEMENT

Peripheral neuropathy happens when there is damage to nerves in the extremities (hands, feet, limbs).
Damage can be the result of myeloma, treatment or unrelated conditions (i.e. diabetes).
Symptoms:
•Numbness
•Tingling
•Prickling sensations
•Sensitivity to touch
•Burning and/or cold sensation
•Muscle weakness
Prevention / management:
•Bortezomib once-weekly and/or subcutaneous administration
•Massage area with cocoa butter regularly
•Neuroprotective Supplements:
• B-complex vitamins (B1, B6, B12)
• Green tea
•Safe environment: rugs, furnishings, shoes
Report symptoms of peripheral neuropathy early to your healthcare provider; nerve damage from neuropathy can be permanent if unaddressed
If neuropathy worsens, your provider may:
•Adjust your treatment plan
•Prescribe oral or topical pain medication
•Suggest physical therapy

Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Tariman, et al. CJON.2008;12(3)suppl:29-36. Zhao T, et al. Molecules. 2022;27(12):3909.
Physical
•Risk Factors
UNDERSTANDING CHANGES TO KIDNEY FUNCTION
• Active multiple myeloma (light chains, high calcium)
• Other medical issues (ex: Diabetes, dehydration, infection)
• Medications (MM treatment, antibiotics, contrast dye)
• Poor Nutrition
•Prevention
• Stay hydrated – drink water
• Avoid certain medications when possible (eg, NSAIDs), dose adjust as needed
•Treatment
• Treatment for myeloma
• Hydration
• Dialysis



Many myeloma patients will experience kidney issues at some point; protecting your kidney function early and over time is important

et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76.
82
Brigle
Physical
K,
ADDITIONAL SUPPORTIVE CARE OPTIONS

Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:6076. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.

DVT/PE Prevention Bone Health Fatigue Anxiety
Medications Blood thinners Ex: Aspirin, DOACs Bone Strengthening Agents Calcium Vitamin D Stimulant medications Red Cell Transfusion (anemia) Anti-depressants Anti-anxiety Nonmedication Therapies Compression stockings Radiation Surgery Immobilization Physical therapy Relaxation Meditation Therapy Relaxation Meditation Lifestyle Options Activity Stop smoking Weight loss Activity Activity Improved sleep Activity Improved sleep
Supportive
Physical
Have these conversations…
• Whenever your treatment stops working
• Whenever you start a new treatment
• Whenever there is a change in your life priorities
• Whenever you have a question or concern Ask Important Questions at Your Appointments • What Can I Expect Now? • What Can I Expect In the Future?



Available for download at myeloma.org
WHEN TO DISCUSS PRIORITIES
HOW AND
TIPS FOR COMMUNICATING WITH YOUR TEAM
• Reflect on what’s important to you before each visit
How well will treatment work? Quality of life? Risks and side effects?
• Write out your questions
• Take notes
• Bring along a listener

• Ask for time to consider your options
• Continue to learn about Myeloma so you are more comfortable with the conversation

HEALTHY BEHAVIORS FOR PATIENTS & CARE PARTNERS
• Stress reduction, management
• Rest, relaxation, sleep hygiene
• Maintain a healthy weight, eat nutritiously
• Activity / exercise / prevent falls, injury
• Stop smoking
• Mental health / social engagement
• Sexual health / intimacy
• Complementary or alternative therapy


• Have a PCP for general check ups, preventative care, health screenings, vaccinations
• Have specialists for dental care, eye exams/screening, skin cancer screening




86
DEVELOP A CARE NETWORK
•Multiple studies demonstrate that strong social ties are associated with
• Increased longevity including people with cancer
• Improved adherence to medical treatment leading to improved health outcomes
• Lower risk of cardiovascular diseases
• Increased sense of purpose & life satisfaction
• Improved mood and happiness
• Reduced stress and anxiety
• Enhanced resilience
Martino J, et al. Am J of Lifestyle Med. 2015;11(6):466-475. Yang YC, et al. Proc Natl Acad Sci U S A. 2016;113(3):578-583. Pinquart M and Duberstein PR. Crit Rev Oncol Hematol. 2010;75(2):122–137.
•Strategies for enhancing social connection
• Deepen existing relationships with family, friends, and loved ones
• Build new relationships by participating in a support group, joining clubs or organizations, or volunteering


Tip: Start with small steps outside your comfort zone. Call a loved one you haven’t spoken to in a while. Invite a person you’d like to know better for lunch, coffee, or a walk.
Hetherington C. Healthnews. https://healthnews.com/longevity/healthspan/soci al-connection-and-longevity/#:~:text=Research %20consistently%20demonstrates%20that %20people,of%20fulfillment%20in%20your%20life. Accessed Feb 1 2024.



























IMF OFFERS MANY RESOURCES You are not alone! eNewsletter: Myeloma Minute Website: http://myeloma.org IMF Videos Download or order at myeloma.org IMF InfoLine 1-800-452-CURE 9am to 4pm PST


Questions? 89

COFFEE BREAK
Thank you to our sponsors!








Frontline Therapy
 Cindy Varga, MD
Cindy Varga, MD
Atrium Health, Levine Cancer Institute
Charlotte, NC
Frontline Therapy in Multiple Myeloma
Cindy Varga, MD
Clinical Associate Professor
Plasma Cell Division
Levine Cancer Institute Atrium Health
June 22nd, 2024
Disclosures
•Janssen (advisory board), Arcellx (advisory board)
OUTLINE
•How do we treat newly diagnosed MM now?
•How do we choose the right therapy?
•Is there still a role for stem cell transplant in this era?
•How do we treat transplant eligible patients?
•How do we treat transplant ineligible patients?
•How do we manage or prevent adverse events?
•What is to come?
•Conclusions
TREATMENT PARADIGM
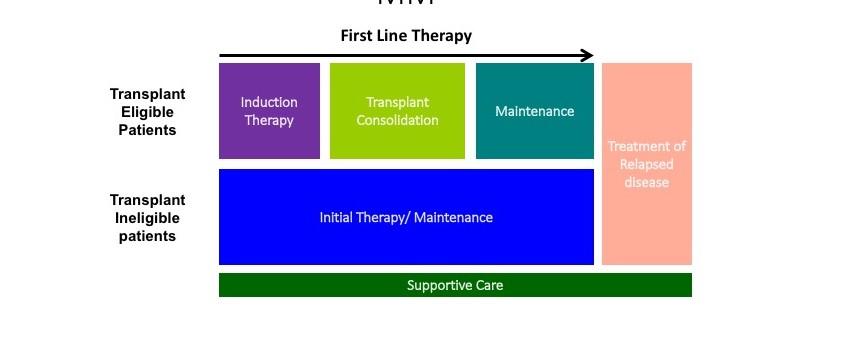
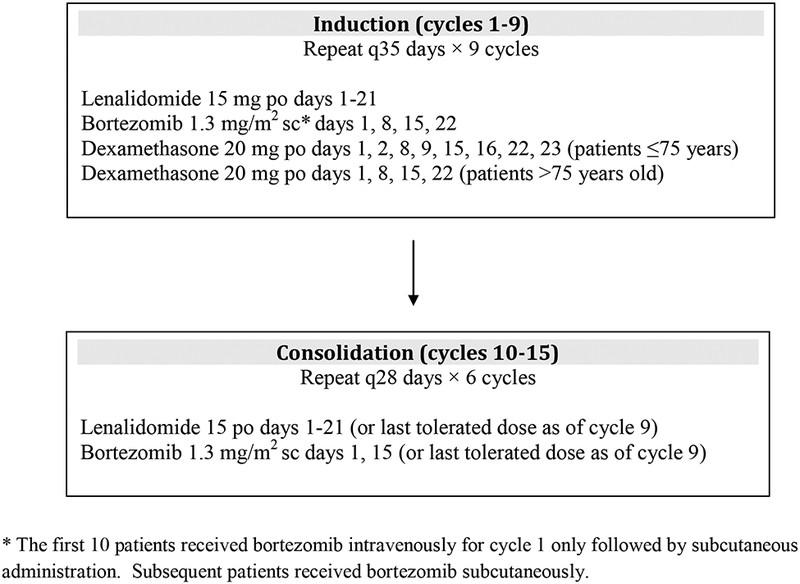
TREATMENT PHASES
Induction
Goal
First phase of Treatment
Destroy as many cancer cells as possible in order to achieve (induce) a remission
Duration fixed # cycles of chemo +/ASCT
Consolidation
Goal
Duration
Maintenance
Goal
Duration
Second phase of treatment
Further deepen response
Finite number of cycles (1-2) using the same induction regimen
Third phase of treatment
Prolong remission and prevent disease relapse
Lower dose, usually 1 agent
Until progression or intolerance
THE THREE PILLARS


Immunomodula tor
• Thalidomide • Lenalidomide (Revlimid) • Pomalidomide

Proteasome
Inhibitor
• Bortezomib (Velcade) • Carfilzomib (Kyprolis) • Ixazomib (Ninlaro) Anti-CD38 • Daratumumab • Isatuximab
Autologous Stem Cell Transplant
What is high risk disease?
Cytogenetics
• t(4;14)
• t(14;16)
• t(14;20)
• del(17p)
• gain/amp(1q)
• High risk GEP
Laboratory Features
ISS stage III, R-ISS III
Elevated LDH
Functional High Risk
Primary Refractory Disease
Rapid Progression (disease kinetics)
Clinical Features Plasma cell leukemia
Extra medullary disease
Selecting Appropriate Treatment
Patient Factors
Age/frailty
Performance status
Lifestyle
Distance from infusion site
Patient preference
Caregiver support
Comorbidities
Cardiac issues
Disease Factors
Kidney Dysfunction Low blood counts Disease burden: ISS
Rate of progression
Marrow burden
CRAB symptoms
Extramedullary disease
Cytogenetics
Toxicity
Treatment Factors
Myelosuppression
Infections
Neuropathy
Secondary cancers
Ocular toxicity
Cost
Administration route
‒
‒
‒
‒
‒
‒
‒
‒
‒
‒
Management/Prevention of Adverse Events
•
Immunomodulatory
•
• ASA or
Anti-CD38 •
(URTI, PNA)
Proteasome Inhibitor
Peripheral Neuropathy
Agents
Thrombosis
blood
Infections
Infection Management
Stay current on vaccinations
‒ COVID 19
‒ Pneumococcal
‒ Influenza
Antibacterial and antifungals if persistent low WBC
IVIG if recurrent infections
HOW DO YOU TREAT TRANSPLANT INELIGIBLE PATIENTS?
IS TRIPLET BETTER THAN DOUBLET?
SWOG S0777: Triplet versus Doublet
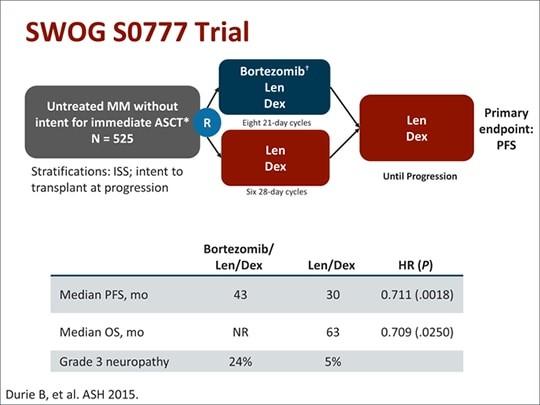
SWOG: Response Rates
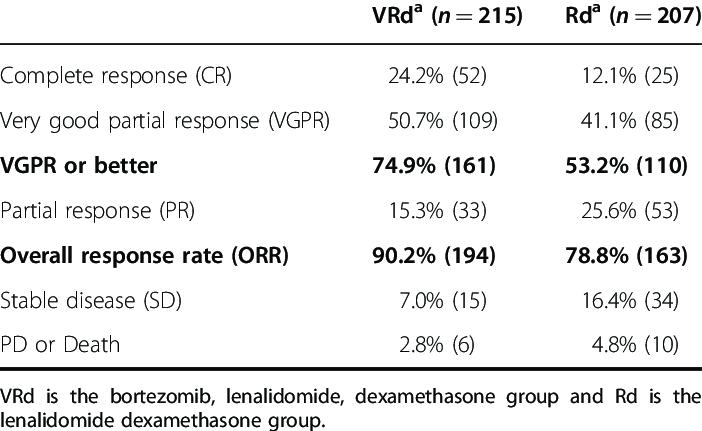
SWOG S0777: Survival
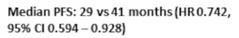
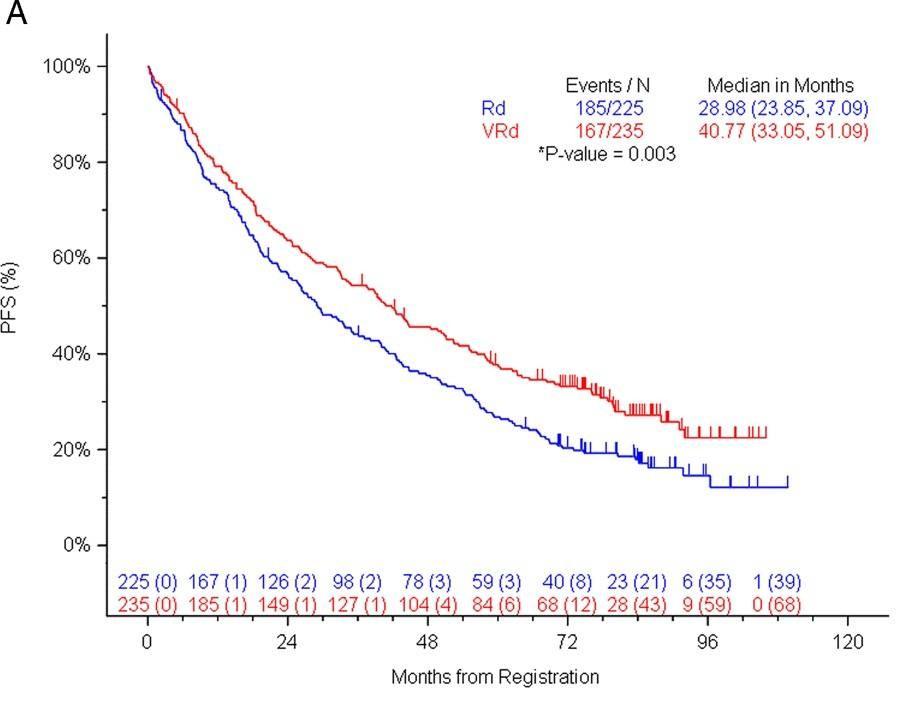

> 3 Peripheral Neuropathy 33%
Grade
CONCLUSIONS: TRIPLE BEATS DOUBLET
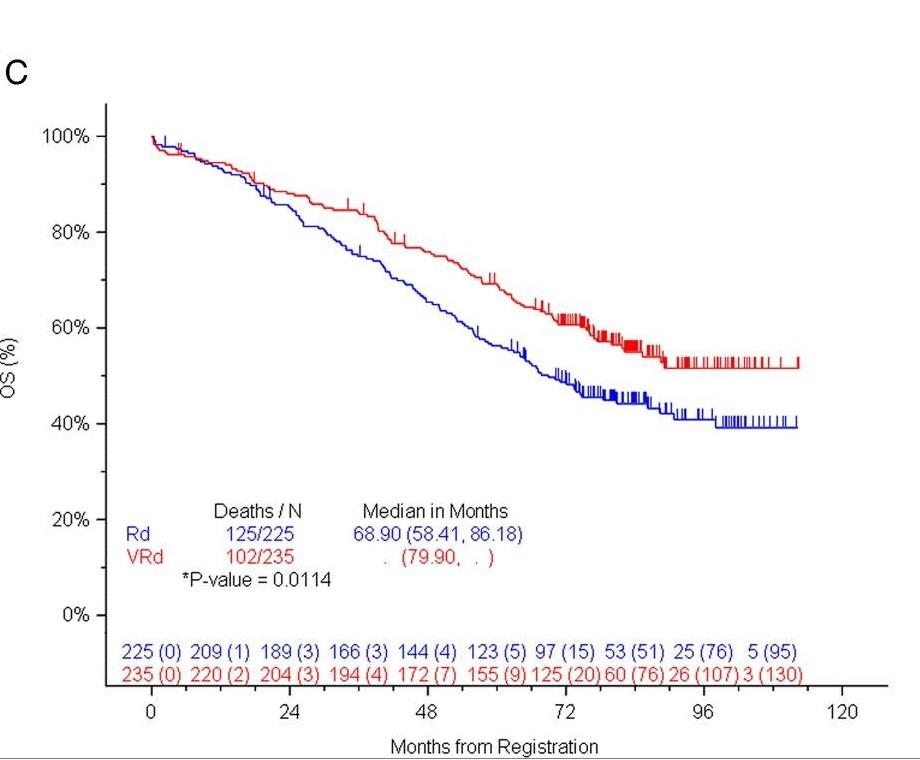
Addition of bortezomib to Rd (VRd) induction: ‒ Significantly longer PFS, overall survival Higher rate of neuropathy in RVd arm
VRd represents a new standard of care
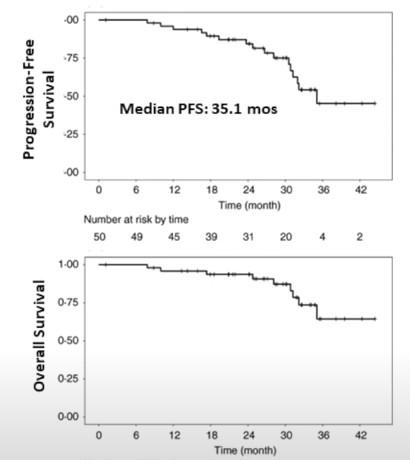
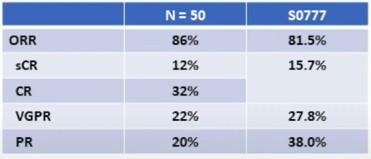
RVDlite: Can we dose reduce?
Grade > 3 peripheral neuropathy only 2%
O’Donnell et al. BJH 2018
ENDURANCE: KRd vs. VRd
Stratified by intent for SCT at PD (yes vs no)
Newly diagnosed, previously untreated MM; ECOG PS 0-2; no high-risk features*; no plasma cell leukemia; no grade ≥ 2 PN; no heart failure or MI < 6 mos (N = 1087)
*t(14;20), t(14;16), del(17p), LDH > 2 x ULN.
Induction
= 542)
Stratified by induction regimen (VRd vs KRd)
= 545)
Coprimary endpoints: PFS after induction, OS with maintenance
Secondary endpoints: ORR, MRD, TTP, OS, safety
QoL assessed during and after induction
Maintenance
Lenalidomide
15 mg PO days 1-21
24 four-wk cycles
Lenalidomide
15 mg PO days 1-21 until PD or excess toxicity
Observation until PD

Kumar. ASCO 2020. Abstr LBA3. Slide credit: clinicaloptions.com
VRd (n
KRd
(n
ENDURANCE: Survival From Induction Randomization

Slide credit: clinicaloptions.com Kumar. ASCO 2020. Abstr LBA3.
VRd KRd Median PFS, Mos (95% CI) 34.4 (30.1-NE) 34.6 (28.8-37.8) HR (95% CI) 1.04 (0.83-1.31; P = .742) PFS P F S ( % ) Mos Patients at Risk, n KRd 545 401 252 187 127 83 59 38 25 13 3 VRd 542 377 243 183 114 73 43 31 26 14 0 10 0 80 60 40 20 0 0 6 1 2 1 8 24 30 36 VRd KRd 42 48 54 60
Reproduced with permission.
Completely Different Side Effects
Nonhematologic Treatment-Related AEs ≥ 2%
Peripheral neuropathy
Thromboembolic event
Diarrhea
Acute
Generalized
*Not required reporting.

Slide credit: clinicaloptions.com
Kumar. ASCO 2020.
Abstr LBA3. Reproduced with permission.
Rash
infection
Dyspnea Hyperglycemia Fatigue
Lung
failure
Hypertension Heart
kidney injury
Edema, limbs
muscle weakness
Grade ≥ 3 0 1 2 3 4 5 6 7 8 % VRd (n = 527) KRd (n = 526)
Insomnia Hypotension
ENDURANCE: Conclusions
KRd did not improve PFS compared with VRd
VRd should remain standard of care for newly diagnosed MM unless comorbidities dictate otherwise

Kumar. ASCO 2020. Abstr LBA3.
Slide credit: clinicaloptions.com
MAIA: DARA-Rd vs. Rd
Randomized phase III trial
Stratified by ISS (I vs II vs III), region (N America vs other), age (< 75 vs ≥ 75 yrs)
Patients with ASCT-
ineligible NDMM, ECOG PS 0-2, CrCl ≥ 30 mL/min (N = 737)
Daratumumab 16 mg/kg IV
(QW cycles 1-2, Q2W cycles 3-6, Q4W cycle 7+) +
Lenalidomide 25 mg/day PO on Days 1-21 +
Dexamethasone 40 mg/wk* PO or IV
Rd
Lenalidomide 25 mg/day PO on Days 1-21 +
Dexamethasone 40 mg/wk* PO or IV
28-day cycles until progression
Primary endpoint: PFS
Secondary endpoints : ≥ CR rate, ≥ VGPR rate, MRD negativity, ORR, OS, safety

Slide credit: clinicaloptions.com
Dara-RD
Facon. ASH 2018. Abstr LBA-2.
MAIA: DRd Wins!
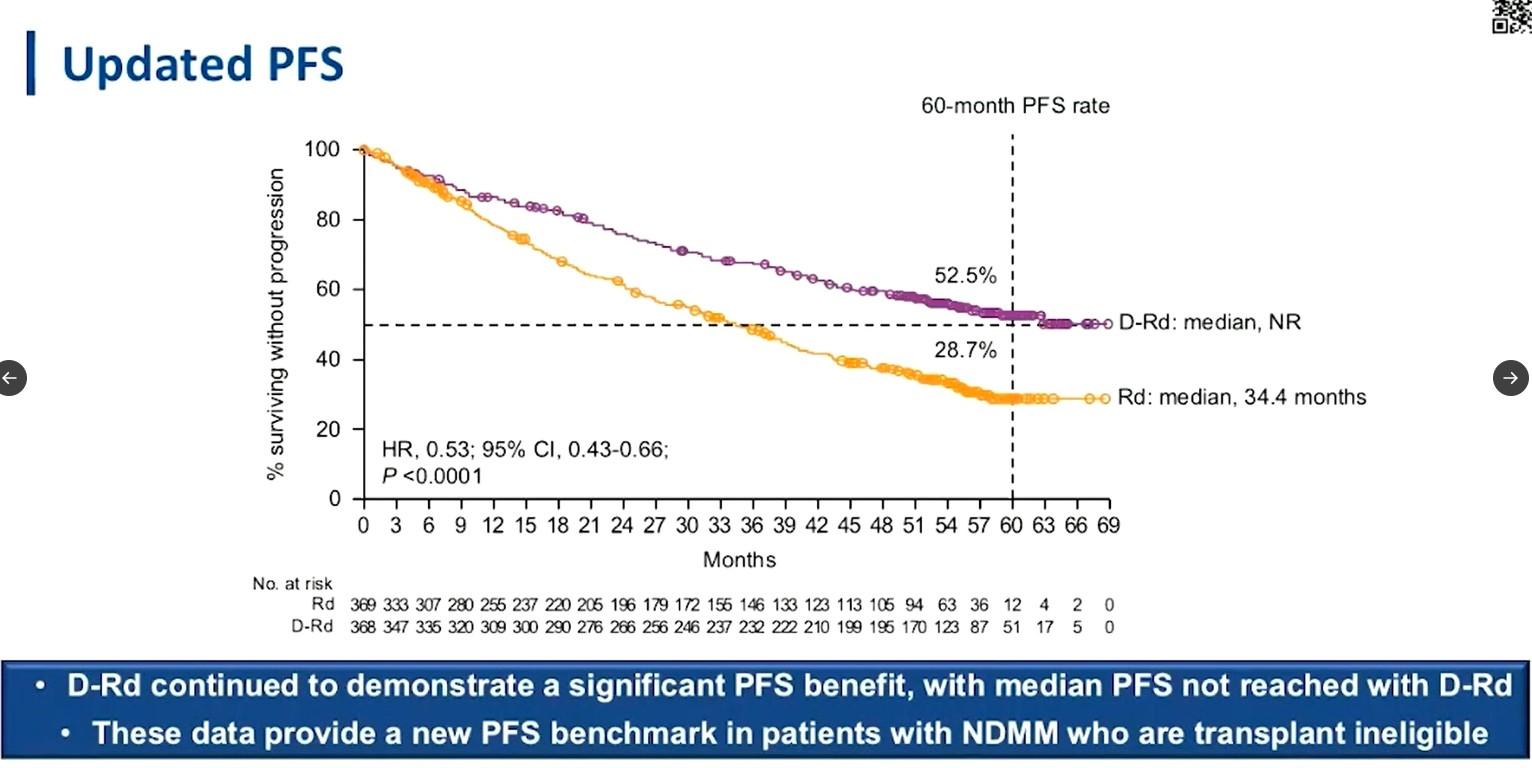
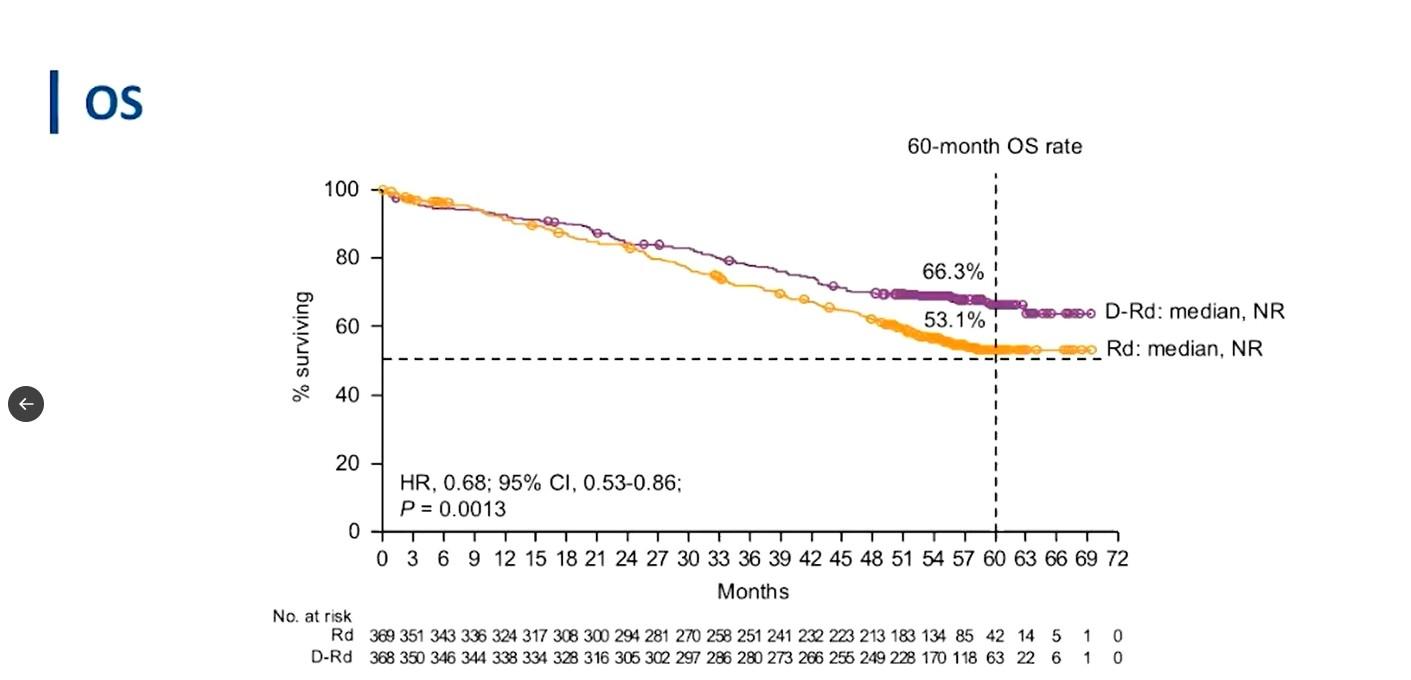

Slide credit: clinicaloptions.com

Kumar. ASH 2020. Abstr 2276. Reproduced with permission.
Slide credit: clinicaloptions.com O R R ( % ) 100 80 60 40 20 0 D-Rd Rd sCR CR VGPR PR D-Rd N = 368 Rd N = 369 D-Rd N = 368 Rd N = 369 D-Rd N = 368 (update) Rd N = 369 (update) Primary: 28.0 mos 36.4 mos Update: 47.9 mos Median Follow-up 14% 32% 17% 30% 28% 28% 13% 13% 13% 31% 17% 33% 27% 28% 13% 14% 93% 81% 93% 82% 12% 30% 17% 34% 25% 27% 15% 14% 93% 82%
MAIA: ORR (ITT Population)
MAIA: MRD Analyses (ITT Population)
MRD negativity in patients with high-risk cytogenetics: 23% with D-Rd vs 2% with Rd
ASH 2020. Abstr 2276. Reproduced with permission.

Kumar.
Slide
MRD Negativity Rate Sustained MRD Negativity M R Dn e g a ti v i t y R a t e ( % ) 25 20 15 10 5 0 D-Rd N = 368 Rd N = 369 D-Rd N = 368 Rd N = 369 D-Rd N = 368 (update ) Rd N = 369 (update ) Primary: 28.0 mos P < .0001 36.4 mos P < .0001 Update: 47.9 mos P < .0001 24 % 7 % 29 % 9 % 31 % 10 % 35 30 Median Follow-up S u s t a i n e d M R Dn e g a ti v i t y R a t e ( % ) P < .0001 P < .0001 25 20 15 10 5 0 D-Rd N = 368 Rd N = 369 D-Rd N = 368 Rd N = 369 20% 5% 16% 3% ≥ 6 mos sustained MRD negativity ≥ 12 mos sustained MRD negativity
credit: clinicaloptions.com
CONCLUSIONS: Triplet wins!
•The addition of Dara:
• reduced the risk of progression or death by 46%
• tripled the rate of MRD negativity
•DRd now the standard of care for transplant ineligible patients
DRd vs VRd?
Retrospective analysis of data from Acentrus EMR database comparing time to next treatment or death with Dara-Rd or VRd in 643 patients* between January 2018 and May 2023

Slide credit: clinicaleducationalliance.com:
Hansen.
Time to Next Treatment/Death
32% of patients treated with DRd vs 51% of patients treated with VRd received a subsequent line of therapy or died Median TTNT/death: 37.8 mo vs 18.7 mo HR: 0.58 (95% CI: 0.35-0.81); P <.001 *After
treatment weighting 120 Time From Index Date (Mo) P a ti e n t s R e m a i n i n g o n T r e a t m e n t ( % ) 100 80 60 40 20 0 0 6 1 2 1 8 2 4 3 0 3 6 302 (100) 341 (100) 171 (57) 161 (47) 83 (28) 74 (22) 18 (6) 27 (8) Patients at Risk, n (%) DR d VR d DR d VR d
ASH 2023. Abstr 543.
inverse probability of
IS QUADRUPLET BETTER THAN TRIPLET?
BENEFIT: Isa-RVd vs. Isa-Rd
Multicenter, open-label, randomized, phase III trial
Stratified by age (<75 vs ≥75 yr), cytogenic risk by FISH, treatment center
Patients aged 65-79 yr with NDMM who are nonfrail and transplant-ineligible; no prior systematic treatment; measurable disease; ECOG PS ≤2 (N = 270)
Primary endpoint: MRD (10-5) at 18 mo
Key secondary endpoints: ORR (CR, ≥ VGPR), MRD− CR (10

Slide credit: clinicaloptions.com
-5), PFS, OS, safety
Leleu. Nature Medicine. 2024. [Epub]. Leleu. ASCO 2024. Abstr 7501. Isatuximab
1, Days
15 cycle 2-12
Rd*
135) Cycle
28-day cycles Isatuximab 10 mg/kg IV Day 1 + R* + Bortezomib 1.3 mg/m2 SC Day 1, 15 Isatuximab 10 mg/kg IV Day 1 + R* Isatuximab
cycle
Days
15 cycle
Rd* + Bortezomib
SC Days
Isatuximab + R* *R:
Cycles
28-day cycles Cycle 19 onwards 28-day cycles Weekly velcade
IV 10 mg/kg QW cycle
1,
+
(n =
13-18
10 mg/kg IV QW
1,
1,
2-12 +
1.3 mg/m2
1, 8 ,15 (n = 135)
lenalidomide 25 mg PO Days 1-21, d: dexamethasone 20 mg IV QW.
1-12
BENEFIT: Rate of MRD− in ITT

Slide credit: clinicaloptions.com
sensitivity
Leleu. Nature Medicine. 2024. [Epub]. Leleu. ASCO 2024. Abstr 7501. OR:
(95% CI:
6.62) P <.0001 OR: 2.97 (95% CI: 1.605.50) P = .0005 OR: 3.16 (95% CI: 1.895.28) P <.0001 OR: 2.74 (95% CI: 1.544.87) P = .0006 12 Mo 18 Mo 10-5 10-5 10-6 10-6 21 51 13 32 26 53 17 36 P a ti e n t s ( % ) Isa-VRd Isa-Rd 60 50 40 30 20 10 0
Isa-VRd significantly improved 12- and 18-mo MRD- rate and at 10-5 or 10-6
vs Isa-Rd
3.88
2.27-
IMROZ:
Study Design
International, randomized, open-label phase III trial
Stratified by age (<70 vs ≥70 yr), R-ISS stage (I or II vs III vs not classified), and China vs nonChina
Patients 18 to ≤80 yr of age with symptomatic NDMM not considered for transplant due to older age or comorbidities (N = 446)
(4 x 6-wk cycles)
(4-wk cycles) 3:2
Isatuximab* + VRd† (n = 265) VRd† (n = 181)
Isatuximab‡ + Rd§ (n = 265) Rd§ (n = 181)
*Isa IV (C1 only) 10 mg/kg Q1W; Isa IV (C2-4) 10 mg/kg Q2W. †V: SC 1.3 mg/m2 on D1,4,8,11,22,25,29,32; R: PO 25 mg on D1-14 and 22-35; d: IV/PO 20 mg on D1,2,4,5,8,9,11,12,15,22,23,25,26,29,30,32,33.
‡Isa IV (C5-17) 10 mg/kg Q2W; Isa IV (C18+) 10 mg/kg monthly. §R: PO 25 mg on D1-21; d: IV/PO 20 mg on Q1W.
Primary endpoints: PFS
Secondary endpoints: CR rate, MRD− CR (NGS 10-5) rate, ≥ VGPR rate, OS
Facon. ASCO 2024. Abstr 7500. Facon. NEJM. 2024;[Epub].
Until PD, unacceptable toxicity, or patient withdrawal
Crossover from Rd to Isa-Rd allowed upon progression

Slide credit:
clinicaloptions.com
Continuous
Induction
Treatment
IMROZ:
PFS in ITT Population, Interim Analysis
follow-up: 59.7 mo (IQR: 56.0-63.2)

Slide credit: clinicaloptions.com
Facon. ASCO 2024. Abstr 7500. Facon. NEJM. 2024;[Epub]. Parameter, n (%) Isa + VRd (n = 265) VRd (n = 181) Median PFS, mo NR 54.34 HR (98.5% CI) 0.60 (0.41-0.88) P value <.001 Patients at Risk, n Isa-VRd VRd Mo P F S ( % ) 60-mo PFS rate: 63.2% 100 80 60 40 20 0 0 6 12 18 24 30 36 42 48 54 60 66 72 265 181 243 155 234 141 217 121 201 104 190 96 177 89 164 81 153 70 104 51 43 20 2 2 0 0
Isa-VRd VRd 60-mo PFS rate: 45.2%
Median
ALGORITHM FOR TRANSPLANT INELIGIBLE
Transplant Ineligible
Standard Risk
DRVd or Isa-RVD?
RVd x 8-9 cycles
Until Progression Len maintenance until progression
Transplant Ineligible High Risk
DRVd or Isa-RVD?
RVd x 12 cycles
Len + bortezomib maintenance until progression
DRd
HOW DO YOU TREAT TRANSPLANT ELIGIBLE PATIENTS?
DETERMINATION: RVd + ASCT vs. RVd
Multicenter, randomized, open-label phase III trial conducted in 56 sites within the United States
Stratification by ISS disease stage, cytogenetic risk
Patients aged 18-65 yr with symptomatic NDMM following 1 cycle of VRd; ECOG PS 0-2 (N = 722)
VRd in 21-day cycles: R 25 mg/day PO Days 1-14; V 1.3 mg/m 2 IV/SC Days 1, 4, 8, 11; d 20/10 mg PO Days 1, 2, 4, 5, 8, 9, 11, 12. R maintenance 10 mg/day during Mo 1-3, 15 mg/day from Mo 4 onward.
Primary endpoint: PFS
Key secondary endpoints: DoR, TTP, OS, QoL, safety
Richardson. ASCO 2022. Abstr LBA4. Richardson. NEJM. 2022;[Epub].

Slide credit: clinicaloptions.com
S t e m c e l l c o l l e c ti o n Induction Consolidation Maintenanc e Until PD VRd cycles 2-3 (n = 357) VRd cycles 2-3 (n = 365) VRd cycles 4-8 VRd cycles 4-5 R (n = 291) R (n = 289) Melphalan
ASCT
200 mg/m2 + ASCT (n = 310)
DETERMINATION: PFS (Primary Endpoint)

Slide credit: clinicaloptions.com
HR: 1.53 (95% CI: 1.23-1.91; P <.001) VRd + ASCT 67.5 (58.6-NR) VRd alone 46.2 (38.1-53.7) Median PFS, Mo (95% CI) 1.0 0.8 0.6 0.4 0.2 0.0 P r o b a b i l i t y Transplantation VRd Alone 84 0 12 24 36 48 60 72 Months Since Randomization 365 357 276 250 226 187 191 160 160 126 118 96 77 60 42 40 No. at Risk Transplantation VRd Alone
Median follow-up: 76 mo Richardson. ASCO 2022. Abstr LBA4. Richardson. NEJM. 2022;[Epub].
DETERMINATION: PFS by Cytogenetic Risk
Median follow-up: 76 mo

Slide credit: clinicaloptions.com
Richardson. ASCO 2022. Abstr LBA4. Richardson. NEJM. 2022;[Epub]. High-Risk Cytogenetics Standard-Risk Cytogenetics VRd + ASCT 82.3 VRd alone 53.2 Median PFS, Mo HR: 1.38 (95% CI: 1.07-1.79) 1.0 0.8 0.6 0.4 0.2 0 P r o b a b i l i t y o f P F S 0 12 24 36 48 60 72 84 Time From Randomization (Mo) 274 268 212 197 175 156 94 83 58 50 VRd+ASCT VRd-alone 151 134 126 109 29 34 Patients at Risk HR: 1.99 (95% CI: 1.21-3.26) VRd + ASCT 55.5 VRd alone 17.1 Median PFS, Mo 1.0 0.8 0.6 0.4 0.2 0 P r o b a b i l i t y o f P F S 0 12 24 36 48 60 72 84 Time From Randomization (Mo) 66 66 45 36 37 19 16 8 12 6 VRd+ASCT VRd-alone 29 16 24 11 8 3 Patients at Risk
DETERMINATION: PFS by MRD at Start of Maintenance
Preliminary Analysis of MRD at Start of Maintenance

Slide credit: clinicaloptions.com
Richardson. ASCO 2022. Abstr LBA4. Richardson. NEJM. 2022;[Epub].
Event VRd Alone (n = 108) VRd + ASCT (n = 90) MRD negative (10-5) by NGS, % 39.8 54.4 Odds ratio (95% CI) 0.55 (0.30-1.01) MRD-Status 5-year PFS, % HR (Unadjusted 95% CI) VRd + ASCT 53.5 0.91 (0.46-1.79) VRd alone 59.2 MRD-status Median PFS, Mo HR (Unadjusted 95% CI) VRd + ASCT 50.6 1.67 (0.98 – 2.85) VRd alone 33.4 1.0 0.8 0.6 0.4 0.2 0 P r o b a b i l i t y o f p r o g r e s s i o nf r e e s u r v i v a l Time since MRD evaluation at start of maintenance (months) 108 0 12 24 36 48 60 72 84 96 Patients at risk VRd + ASCT, MRDVRd alone, MRDVRd + ASCT, MRD+ VRd alone, MRD+ 49 43 41 65 47 37 32 39 37 33 26 32 32 28 20 25 25 22 15 15 19 16 11 14 13 11 6 10 3 5 2 3 3 1 2 0 0 0 0 0 + + + + + + + + + + ++ + ++ ++ + + ++ ++ + + + + + + ++ + + + +++ + + + ++++ + ++++++ + + + + + ++ + + ++ ++ + + + + + + + + ++ ++++ + ++ + +
DETERMINATION: OS (Key Secondary Endpoint)

Slide credit: clinicaloptions.com
Richardson. ASCO 2022. Abstr LBA4. Richardson.
2022;[Epub]. Events, n (%) 5-Yr OS, % HR (Adjusted CI) P Value VRd alone 90 (25.2) 72.9 1.10 (0.73-1.65) >.99* VRd + ASCT 88 (24.1) 80.7 *CI
P value
control
overall family-wise error rate for secondary outcomes. 1.0 0.8 0.6 0.4 0.2 0.0 P r o b a b i l i t y Transplantation VRd Alone 84 0 12 24 36 48 60 72 Months Since Randomization 365 357 353 332 324 313 300 285 275 258 228 214 165 143 95 88 No. at Risk Transplantation VRd alone
NEJM.
and
adjusted using Bonferroni correction to
for
DETERMINATION: Conclusions
ASCT prolonged median PFS but similar OS
ASCT let to higher rates of MRD-negative responses at start of maintenance
Support personalized treatment based on lack of an OS difference 1. Richardson. ASCO 2022. Abstr LBA4. 2. Richardson. NEJM. 2022;[Epub].

Slide credit: clinicaloptions.com
GRIFFIN: DRVd vs. RVd
Transplant-eligible adults with ND MM; ECOG PS ≤2; CrCl ≥30 mL/min* (N = 207)
Multicenter, open-label, randomized phase II trial Laubach. ASH 2021. Abstr 79.
Induction: Cycles 14
D-VRd in 21-day cycles
D: 16 mg/kg IV D1, 8, 15
V: 1.3 mg/m2 SC D1, 4, 8, 11
R: 25 mg PO D1-14
d: 20 mg PO D1, 2, 8, 9, 15, 16 (n = 104)
VRd in 21-day cycles
V: 1.3 mg/m2 SC D1, 4, 8, 11
R: 25 mg PO D1-14
d: 20 mg PO D1, 2, 8, 9, 15, 16 (n = 103)
Consolidation: Cycles 5-6† Maintenance: Cycles 732‡
D-VRd in 21-day cycles
D: 16 mg/kg IV D1
VRd: as in induction
D-R in 28-day cycles
D: 16 mg/kg IV D1 Q4W or Q8W
R: 10 mg PO D1-21 of C7-9 and 15 mg PO D1-21 of C10+§
VRd in 21-day cycles
VRd: as in induction R in 28-day cycles
R: 10 mg PO D1-21 of C7-9 and 15 mg PO D1-21 of C10+§
*Lenalidomide dose was adjusted in patients with CrCl ≤50 mL/min. †Consolidation began 60-100 days after transplant. ‡Patients completing maintenance phase were permitted to continue single-agent lenalidomide. §15 mg administered only if tolerable.
Primary endpoint: sCR by end of consolidation with 1-sided α = 0.1
Key secondary endpoints: rates of MRD negativity, ORR, ≥VGPR, CR, PFS, OS

Slide credit: clinicaloptions.com
AS CT
GRIFFIN: Responses Deepen Over Time

Slide credit: clinicaleducationalliance.com:
Voorhees. Lancet Haematol. 2023;10:e825. 135 10 0 8 0 6 0 4 0 2 0 0 P a ti e n t s ( % ) End of induction End of postautologous HSCT consolidation Final analysis D-VRd Group sCR CR VGPR PR SD, PD, or NE 26 % 53 % 7 % 12 % 8 % 39 % 9 % 42 % 2 % 13 % 16 % 67 % 1 % 1 % 3 % CR or better, 19% CR or better, 52% CR or better, 83% 10 0 8 0 6 0 4 0 2 0 0 End of induction End of postautologous HSCT consolidation Final analysis VRd Group 35 % 43 % 6 % 7 % 19 % 31 % 10 % 32 % 8 % 17 % 12 % 48 % 8 % 8 % 14 % CR or better, 13% CR or better, 42% CR or better, 60%
GRIFFIN: At Follow Up of 4y, mPFS was NOT REACHED

Slide credit: clinicaleducationalliance.com: 92.2 %
Overall Survival Voorhees. Lancet Haematol. 2023;10:e825. Progression-Free Survival 136 100 80 60 40 20 0 P F S ( % )
3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 HR 0.45 (95% CI: 0.210.95) P = .032 3-yr PFS 4-yr PFS 89.0 % 87.2 % 80.7 % 70.0 % 100 80 60 40 20 0 P F S ( % )
0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 HR 0.90 (95% CI: 0.312.56) P = .84 3-yr PFS 4-yr PFS 92.7 % 92.7 % 92.2 % DVRd VRd DVRd VRd
Mo Since Randomization 0
Mo Since Randomization
GRIFFIN: MRD-Negativity Rates Over Time
Negative MRD rates were higher for D-RVd compared with RVd and continued to deepen and improve over time

Slide credit: clinicaleducationalliance.com:
D-RVd End of Induction End of Consolidation End of Study M R D N e g a ti v e ( % ) 8 0 7 0 6 0 5 0 4 0 3 0 2 0 1 0 0 105 106 64 36 11 1 22 50 10-5 threshold 10-6 threshold 10-5 threshold 10-6 threshold Voorhees. Lancet Haematol. 2023;10:e825. End of Induction RVd End of Consolidation End of Study 8 20 30 3 16 105 106 137
PERSEUS
: DRVd vs. RVd
International, randomized, open-label phase III trial
Stratified by ISS stage and cytogenetic risk
Adults aged 18-70 yr with transplanteligible NDMM; ECOG PS ≤2 (N = 709)
Induction: Cycles 1-4 (28-day cycles)
(n = 355)
Consolidation: Cycles 56 (28-day cycles)
Maintenance: Cycles 7+ (28-day cycles)
(n = 354)
Dosing: D 1800 mg SC QW (induction cycles 1-2)/Q2W (induction cycles 3-4 and consolidation)/Q4W (maintenance); V 1.3 mg/m 2 SC on Days 1, 4, 8, 11; R 25 mg PO on D1-21 (induction and consolidation)/10 mg PO on Days 1-28 (maintenance); d 40 mg PO/IV on Days 1-4, 9-12. *D stopped after 2 yr in those with ≥CR and sustained MRD negativity (10-5) for 12 mo. †Restart D if confirmed loss of CR without PD or MRD recurrence.
Primary endpoint: PFS
Key secondary endpoints: ≥CR rate, overall MRD-negativity rate (proportion of patients achieving MRD negativity and ≥CR), OS
Current analysis evaluates deepening of response and MRD negativity during maintenance

Slide credit: clinicaloptions.com
Rodriguez-Otero. ASCO 2024. Abstr 7502. NCT03710603.
D-VRd
D-VRd D-R x ≥2 yr* AS CT
VRd
VRd R until PD
D-R until PD R† MRDMRD +
PERSEUS Primary Analysis: PFS Subgroup Analysis

Slide credit: clinicaleducationalliance.com:
Sonneveld. ASH 2023.
2024;390:301. Subgroup D-VRd n/N VRd n/N D-VRd mo VRd mo
Male Female 36/211 14/144 61/205 42/149 NE NE NE NE 0.51 (0.34-0.77) 0.29 (0.16-0.53) Age <65 yr ≥65 yr 30/261 20/94 84/267 19/87 NE NE NE NE 0.30 (0.20-0.46) 0.97 (0.52-1.81) Race White Other 47/330 3/25 95/323 8/31 NE NE NE NE 0.42 (0.30-0.60) 0.40 (0.11-1.50) ISS disease stage I II III 18/186 19/114 13/55 35/178 43/125 25/50 NE NE NE NE NE 41.9 0.46 (0.26-0.81) 0.37 (0.22-0.64) 0.42 (0.22-0.83) Type of multiple myeloma IgG Non-IgG 28/204 13/78 58/185 31/96 NE NE NE NE 0.36 (0.23-0.57) 0.46 (0.24-0.88) Cytogenetic risk
Standard High Indeterminate 25/264 24/76 1/15 62/266 38/78 3/10 NE NE NE NE 44.1 NE 0.35 (0.22-0.56) 0.59 (0.36-0.99) 0.16 (0.02-1.56) ECOG performance-status score 0 ≥1 28/211 22/134 60/230 43/124 NE NE NE NE 0.42 (0.27-0.66) 0.41 (0.25-0.69) D-VRd Better VRd Better 0. 1 1. 0 10. 0
Abstr LBA-1. Sonneveld. NEJM.
Sex
Median PFS Hazard Ratio for PD or Death (95% CI) 139
PD or Death
PERSEUS
MRD Analysis: Cumulative MRD-Negativity Rates in ITT Population
to
Rate of deeper MRD negativity (10-6) approximately doubled with D-VRd → D-R vs VRd → R
Deeper MRD negativity (10-6) increased by ~30% during D-R maintenance
D-VRd → D-R vs VRd → R consistently improved MRD-negativity rates (10
or 10
) across subgroups

Slide credit:
clinicaloptions.com
MRD-Negativity Rate, % D-VRd (n = 355) VRd (n = 354) End of consolidation 10-5 10-6 57.5 34.4 32.5 16.1 Up to 12 mo 10-5 10-6 65.1 43.9 38.7 20.9 Up
mo 10-5 10-6 72.1 57.7 44.9 27.4 Up to 36 mo 10-5 10-6 74.6 63.9 46.9 30.8 Rodriguez-Otero. ASCO 2024. Abstr 7502.
-5
-6
24
PERSEUS MRD Analysis: High-Risk MM
Rates were approximately doubled for MRD negativity (10-6) and sustained MRD negativity ≥12 mo with D-VRd → D-R vs VRd → R
PFS numerically improved with D-VRd → D-R vs VRd → R among patients with high-risk MM and MRD negativity (10-6)
‒ HR for PFS: 0.62 (95% CI: 0.21-1.84; P = .3853)

Slide credit: clinicaloptions.com
Rate, % D-VRd (n = 76) VRd (n = 78) MRD negativity 10-5 10-6 68.4 57.9 47.4 30.8 Sustained
negativity for
mo 10-5 10-6 48.7 30.3 25.6 14.1 Rodriguez-Otero. ASCO 2024. Abstr 7502.
MRD
≥12
IsKia EMN24: IsaKRd vs KRd
Open-label, randomized phase III trial
Stratified by centralized FISH (standard risk/missing vs high risk), ISS (I vs II and III) Induction (4 x 28-day cycles)
Transplant eligible patients aged <70 yr with newly diagnosed MM (N = 302)
IsaKRd (n = 151)
KRd (n = 151)
Isa: 10 mg/kg IV C1 D1, 8, 15, 22, followed by C2-4 D1, 15; K: 20 mg/m2 IV C1 D1 only, followed by 56 mg/m2 C1 D8, 15 and C2-4 D1, 8, 15;
R: 25 mg PO QD D1-21; d: 40 mg PO D1, 8, 15, 22
Cy 2-3 g/m2 followed by G-CSF and MEL200-ASCT MEL 200 mg/m2 followed by ASCT
(4 x 28-day cycles)
(12 x 28-day cycles)
Isa: 10 mg/kg IV C5-8 D1, 15; K: 56 mg/m2 C5-8 D1, 8, 15; R: 25 mg PO QD D1-21; d: 40 mg PO D1,8, 15, 22
Primary endpoint: MRD negativity by NGS after postASCT consolidation
Secondary endpoints: MRD negativity after induction, PFS, sustained MRD negativity
Isa: 10 mg/kg IV D1; K: 56 mg/m2 D1, 15;
R: 10 mg PO QD D1-21;
d: 20 mg PO D1, 15

Slide credit: clinicaleducationalliance.com:
Consolidation
Mobilizatio n IsaKRd KRd Gay. ASH 2023. Abstr 4. NCT04483739.
Post-ASCT
Light Consolidation
IsaKRd KRd MRD by NGS MRD by NGS MRD by NGS MRD by NGS
142
IsKia EMN24: Postconsolidation
MRD Negativity (ITT) and Response
Postconsolidation MRD Negativity
Gay. ASH 2023. Abstr 4. Outcome Isa-KRd (n = 151) KRd (n = 151) Odds Ratio P Value Postconsolidation response, % ≥ VGPR ≥ CR sCR 94 74 64 94 72 67 -- --
NGS, 10-5 NGS, 10-6 P a ti e n t s ( % ) P a ti e n t s ( % ) 100 80 60 40 Isa-KRd (n = 151) 20 0 KRd (n = 151) 77% 67% OR: 1.67; P = .049 100 80 60 40 20 0 Isa-KRd (n = 151) KRd (n = 151) 67 % 48 % OR: 2.29; P <.001
IsKia
EMN24:
MRD Negativity by Cytogenic Risk
Postconsolidation MRD Negativity
Subgroup Analysis by Cytogenetic Risk
1 HRCA was defined as the presence of one of the following high-risk cytogenetic abnormalities: del(17p13.1), r(4;14) (p16.3;q32.3), T(14;16) (q32.3;q23), gain(1q21), or amp(1q21); 2+ HRCA was defined as the presence of at least 2 high-risk cytogenetic abnormalities.
Gay. ASH 2023. Abstr 4.
P a ti e n t s ( % ) 100 80 60 40 20 0 0 HRCA 1 HRCA 2+ HRCA NGS, 10-5 NGS, 10-6 79% 72% 78% 65% 77% 53% Very high risk 0 20 40 60 80 100 0 HRCA 1 HRCA 2+ HRCA 65% 48% 69% 53% 77% 27% Isa-KRd KRd Very high risk
GMMG-HD7: IsaRVd vs. RVd
Open-label, randomized, multicenter phase III trial
Induction (3 x 6-Wk Cycles)
Isatuximab 10 mg/kg*
Bortezomib 1.3 mg/m2†
Lenalidomide 25 mg†
Adults with NDMM who are eligible for HDT and ASCT (N = 662)
Dexamethasone 20 mg† (n = 331)
Bortezomib 1.3 mg/m2
Lenalidomide 25 mg
Dexamethasone 20 mg† (n = 329)
*Cycle 1: D1, 8, 15, 22, 29; cycles 2-3: D1, 15, 29.
Maintenance (4-Wk Cycles)
Isatuximab 10 mg/kg‡ +
Lenalidomide 10 → 15 mg§
Dexamethasone 20 mg
HDT ASCT
†Bortezomib D1, 4, 8, 11, 22, 25, 29, 32; lenalidomide Days 1-14 and 22-35; dexamethasone D1, 2, 4, 5, 8, 9, 11, 12, 15, 22, 23, 25, 26, 29, 30, 32, 33.
Data cutoff: April 2021.
3 yr or PD
Lenalidomide 10 → 15 mg§
Dexamethasone 20 mg║
‡Cycle 1: D1, 8, 15, 22; Cycles 2-3: D1, 15; Cycle 4+: D1.
§Days 1-28. Increase dose to 15 mg after 3 mos
║
Dexamethasone D1, 8, 15, 22 in C1. Goldschmidt. ASH 2021. Abstr 463.
Primary endpoint: MRD negativity at end of induction (NGF, sensitivity 10-5) stratified according to R-ISS
Secondary endpoints: CR after induction, safety
MRD negativity assessed after cycle 3, HDT, 12 mos, and 24 mos as well as at end of study

Slide credit: clinicaloptions.com
║
GMMG-HD7: MRD Negativity (Primary Endpoint) and Response Rates at End of Induction
Not assessable/missing* MRD status

low:
Significant increase in ≥VGPR with IsaVRd Significant increase in ORR Slide credit: clinicaloptions.com
samples,
P <.001*
at End of Induction OR 1.83 (95% CI 1.34–2.51) Isa-VRd (n = 331) VRd (n = 329) P a ti e n t s ( % ) P = .46* P = .15* P <.001* P = .02* Response
at
of
CR ≥nCR ≥VGPR ≥PR P a ti e n t s ( % ) 50.1% 35.6% 60 50 40 30 20 10 0 100 80 60 40 20 0 Isa-RVd† RVd 90.0% 83.6% 77.3% 60.5% 41.7% 36.2% 24.2% 21.6% Goldschmidt.
Isa-VRd, 10.6%; VRd, 15.2%
*Due either to loss to follow-up, missing bone marrow
or technical failures in measurement counted as nonresponders.
Patients with MRD Negativity
Rates
End
Induction
ASH 2021. Abstr 463. Reproduced with permission.
Quadruplet vs. Triplet Question is now Settled
IsaRVd vs. RVd N/A
IsaKRd vs. KRd 77%
Trial Regimen Overall MRD neg (10-5) GRIFFIN DRVd
63% PERSEUS DRVd
75% GMMG-HD7
vs. RVd
vs. RVd
IsKia
Transplant Eligible
Standard Risk
Transplant Eligible Algorithm
DRVd or RVd x 3-4 cycles
ASCT
Len maintenance until progression
Collect & store stem cells
DRVd or RVd x 4 cycles Len maintenance until progression
Transplant Eligible High Risk
DRVd x 3-4 cycles
ASCT
Len and bortezomib maintenance until progression
CONCLUSIONS
The addition of Dara completely changed the treatment landscape of myeloma for both TE and TIE patients
‒ Deeper responses
‒ Longer remissions
‒ Very tolerable
Transplant can prolong remission but does not prolong lifespan
‒ For high risk patients, transplant upfront
‒ For standard risk patients, it is a discussion
Conclusions
Quadruplet wins over triplet in TE patients (ie. DRVd, IsaRVd, DKRd, IsaKRd)
Will quadruplet therapy be the new standard of care for TIE?
Treatment can be tweaked to improve tolerability and QOL
Important to provide aggressive supportive care
Questions?
Partnering with the IMF
By Sylvia Dsouza, Vice President, Development


WHO AM I WHAT DO I DO?

Vice President of Development for the IMF
Securing support and resources for the IMF through diverse mechanisms

Oversee a team of passionate and determined fundraising professionals who are committed to advancing the mission of the IMF
Have the incredible honor of working with dedicated volunteers from the US and across the globe.
153
Become a Partner, Be a Change Agent
Peer-to-Peer Fundraising
• Peer-to-Peer Fundraisers are created from YOUR ideas. Starting a Fundraiser is easy and fun. They also make a world of difference in the myeloma community.
• Engage your family, friends, co-workers, your network who honor your journey with myeloma and want to support you. Let them show you that you are not alone.
Join the HOPE Society (Recurring Monthly & Annual Giving Program)
• Help us cultivate the future by joining the International Myeloma Foundation's Hope Society.
• Monthly gifts starting at $10 support IMF core programs, including educational events, publications, the toll-free InfoLine, and more.
• Turn your monthly contribution into a yearly commitment.
Transformative Gifts (Major Giving and Principal Giving)



• Gifts can be designated toward a specific program, project or initiative .
Unrestricted, Direct and Endowment

154

What will your legacy be?
Planned Giving
• Join the Brian D. Novis Legacy Society and make a planned gift!
• Gain immediate tax benefits
• Potentially increase your income during your lifetime.
• Continue to fund our core programs and four pillars.
• Make a bequest (a gift from your estate)
• Include a provision in your will or living trust.
• Designated us as a beneficiary of a life insurance policy, or retirement plan (IRA, 401(k), or 403(b).

• Leave us in your will is one of the most profound ways to support the people and causes important to you.
Corporate and Foundation Gifts
• Your organization can contribute a corporate gift or foundation grant
• Provide seed funding that is necessary to accelerate the path to a cure.


155

3 Ways to Engage
Philanthropy
• Make a donation to support research, patient programs, and advocacy efforts.
• Sponsor or participate in fundraising events such as walks, runs, or galas.
• Create a fundraising campaign online to raise awareness and funds.
Community/network
• Join your local support group/become a Support Group Leader
• Volunteer your time through mentorship or support programs.
• Engage on social media to connect with others affected by myeloma.
Intellectual capacity
• Attend conferences/webinars to stay updated on the latest news.
• Offer your expertise as a speaker or panelist at events.



156
Start the Conversation




We welcome you to continue to learn more about our programs, projects, and initiatives at the IMF and find alignment with your own myeloma journey as well as ways to deepen and strengthen your engagement with us.
Reach out to the IMF Development Team to start a conversation on how you can make a difference in the lives of the people impacted by myeloma.
•Sylvia Dsouza - IMF Vice President, Development sdsouza@myeloma.org | 818.487.7455 ext. 268
157


Join us - Miles for Myeloma on Saturday, May 4

158
QUESTIONS?


LUNCH BREAK



IMF
Regional Community Workshop
Agenda after lunch 12:40 – 1:10 PM Local Patient & Care Partner Panel Patients
Ellen Herman
Ivy Walker Care Partners – Bruce Herman
Ron Walker
– 1:30 PM Maintenance Therapy, Cindy Varga, MD 1:30 – 1:40 PM Q&A 1:40 – 2:25 PM Relapsed Therapies & Clinical Trials, Pete Voorhees, MD 2:25 – 2:35 PM Q&A 2:35 – 3:00 PM Closing Remarks/Coffee & Networking
June 22nd, 2024 –
–
&
&
1:10
Thank you to our sponsors!








Local Patient & Care Partner
Ellen Herman & Ivy Walker
Bruce Herman & Ron Walker
Maintenance Therapy
 Cindy Varga, MD
Cindy Varga, MD
Atrium Health, Levine Cancer Institute
Charlotte, NC
Maintenance Therapy
Cindy Varga, MD
Clinical Associate Professor
Levine Cancer Institute Atrium Health
June 22nd, 2024
Outline
•Evidence behind maintenance therapy
•Doublet vs. monotherapy in maintenance therapy
•MRD-guided decision making
•What is in the pipeline
Maintenance
•Goal:
• Less intense therapy which would prolong disease control and possibly survival
• Long term therapy (vs. induction therapy)
•Execution
• Well-tolerated
• Convenient
• Disease sensitivity
• Not overly $$$
Transplant Ineligible
RVd x 8-9 cycles
Treatment Algorithms
Transplant Eligible
DRVd or RVd x 3-6 cycles
Len maintenance until progression
Until Progression
ASCT
Len maintenance until progression
DRd
Potential Maintenance Agents
•Immunomodulators
• Lenalidomide 10mg oral days 1-21
• Pomalidomide 2-4mg oral days 1-21
•Proteasome Inhibitor
• Bortezomib SQ every other week
• Ixazomib oral weekly days 1,8,15
• Carfilzomib IV ever other week
•AntiCD38
• Daratumumab SQ monthly
What is the rationale behind maintenance therapy?
Attal et al. NEJM 2012
IFM 2005: Len prolongs PFS
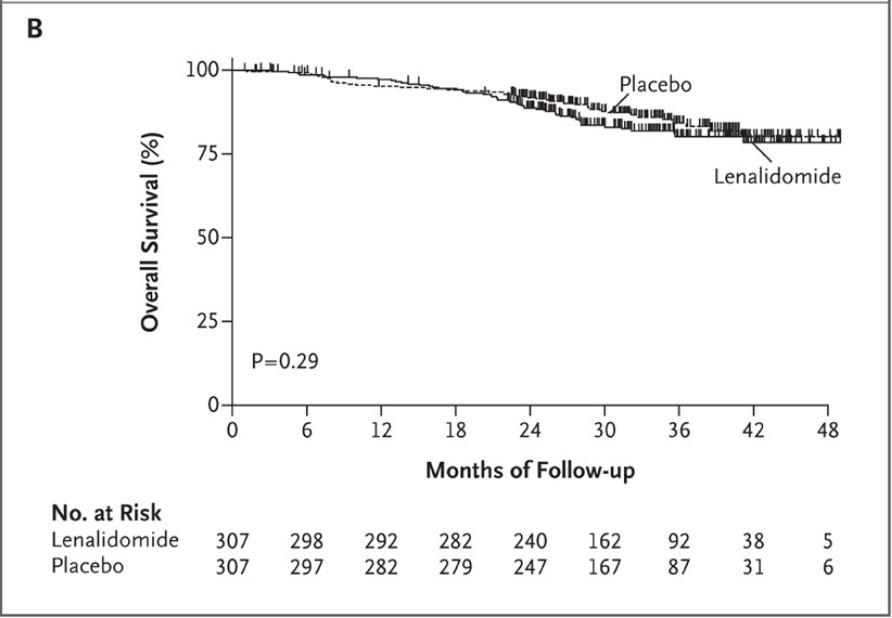
Attal et al. NEJM 2012 mPFS 41m v. 23m, p<0.001
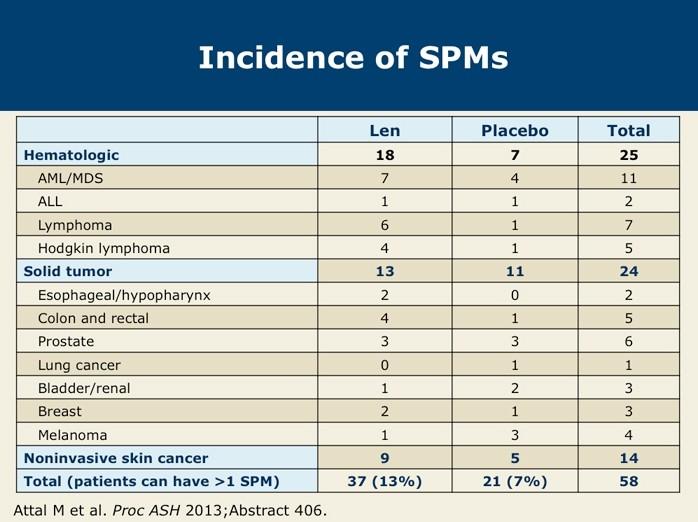
CALGB
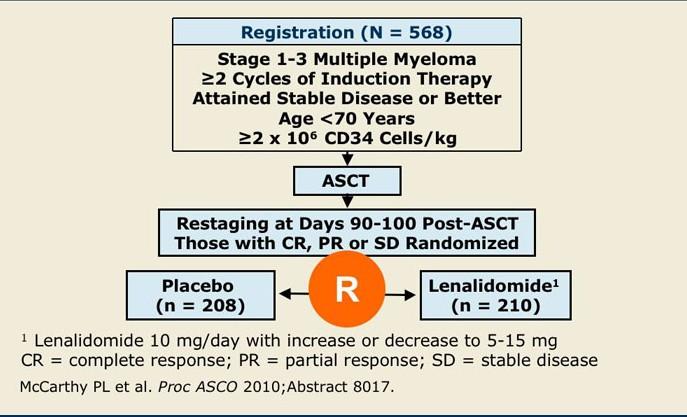
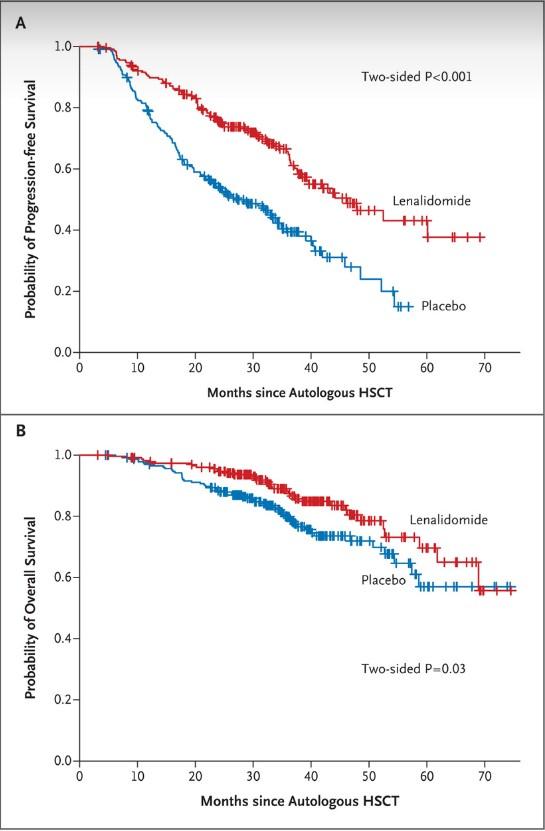
mPFS 46 mos vs. 27 mos (P<0.001)
OS at 3 years was 88% vs. 80%
al. NEJM 2012
McCarthy et
Secondary Malignancy
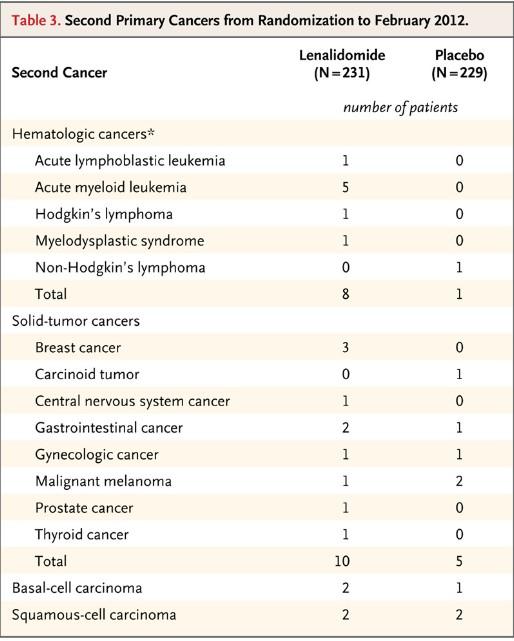
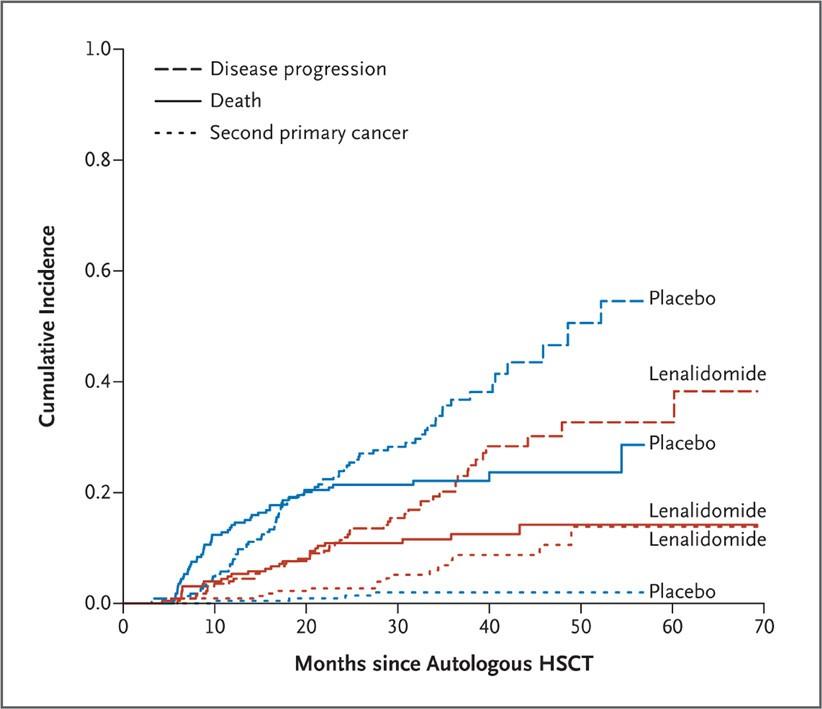
McCarthy et al. NEJM 2012
Meta-Analysis: Len doubles PFS
•3 studies
• CALGB
• IFM
• GIMEMA (Spanish)
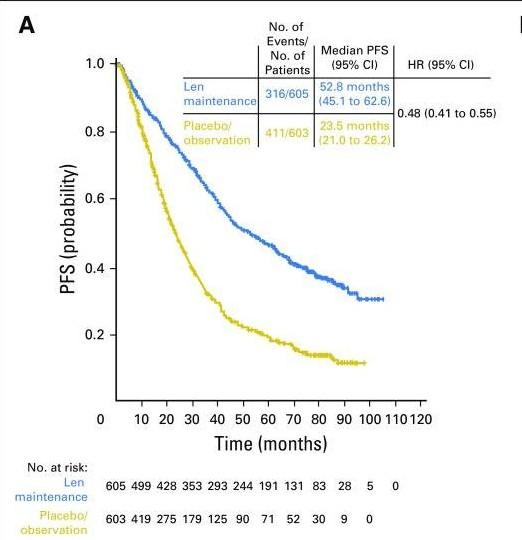
McCarthy et al. JCO 2017
Conclusions
•Len can prolong PFS after ASCT compared to placebo
• (median 46m vs. 24m, p<0.001)
•Possible overall survival benefit?
•Risk of secondary malignancy is increased on len maintenance
• 13% vs. 7%
• BUT lower risk compared to MM relapse
Does Duration Matter?
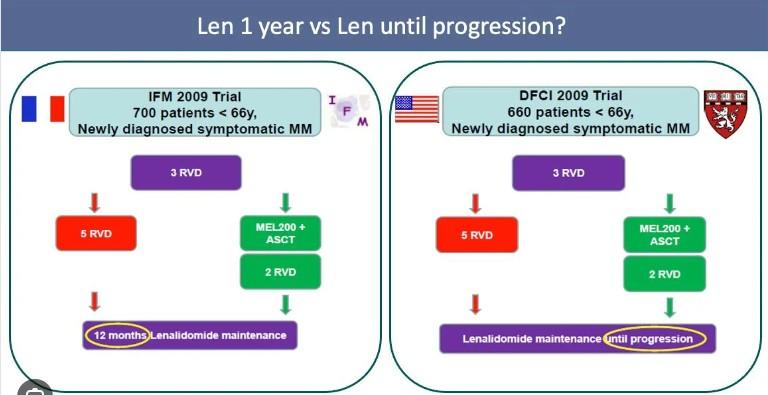
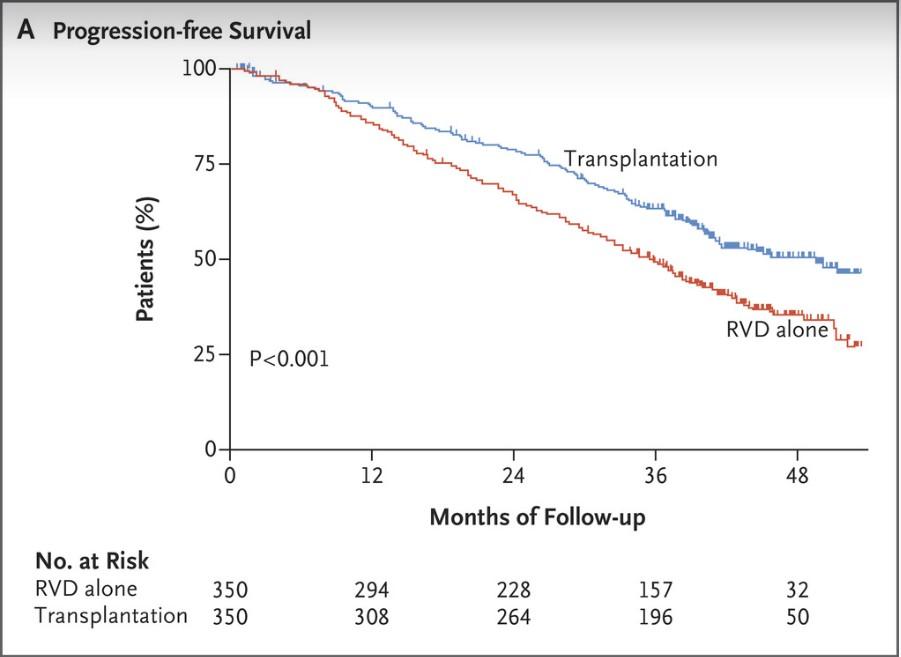
Len for 12 mos
Median PFS was longer in the DFCI trial
Len until progression
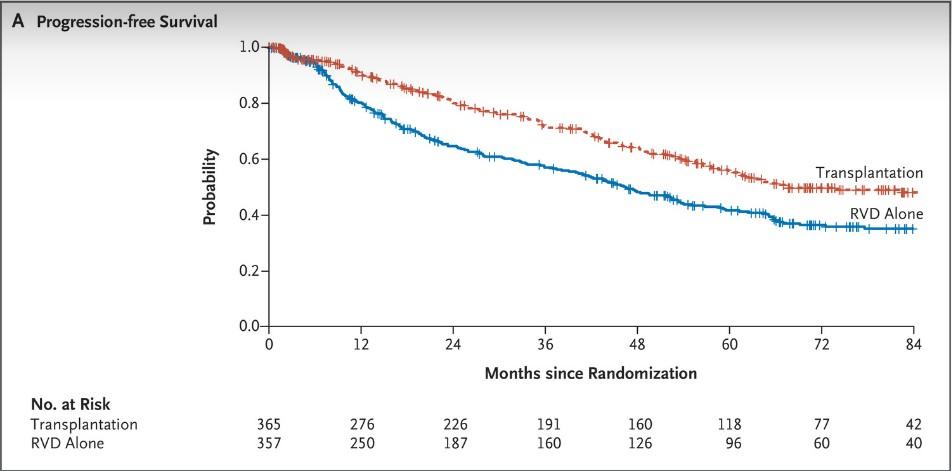
IFM DFCI mPFS 67.5 mos vs. 46.2 mos
mPFS
50 mos vs. 36 mos
What is the optimal duration?
Myeloma XI Trial: What is the Optimum Duration?
Open-label, randomized phase III study with 3 randomizations: induction, intensification, and maintenance treatment
‒ Current analysis: maintenance with lenalidomide monotherapy vs observation following ASCT
Patients with newly diagnosed, symptomatic, transplant-eligible MM completing Myeloma XI induction protocols* and ASCT; responded to lenalidomide; without PD (N = 1248)
Maintenance Phase
Lenalidomide 10 mg/day on Days 1-21/28 (n = 730)
Observation (n = 518)
Continued until disease progression
Median follow-up: 44.7 mo (IQR: 32.4-62.7); median duration of lenalidomide therapy:
28 cycles (range: 1-96) with 45% of patients (330/730) still on therapy
Endpoints: Overall PFS, PFS2; landmark PFS by genetic risk subgroups and MRD status

Slide credit: clinicaloptions.com
Pawlyn. ASH 2022. Abstr 570.
Myeloma XI Trial: PFS From
Maintenance Randomization
Median PFS, Mo (95% CI)

Pawlyn. ASH 2022. Abstr 570. Reproduced with permission.
Slide
clinicaloptions.com
credit:
P F S ( % ) Lenalidomid
Observation
HR: 0.52 (95% CI: 0.45-0.61); P <.001
Lenalidomid e Observation Patients at Risk, n (censored) Mo 730 (4) 518 (0) 641 (13) 401 (4) 509 (51) 288 (22) 327 (174) 151 (94) 175 (295) 81 (137) 96 (359) 42 (160) 50 (394) 20 (175) 9 (432) 4 (190) 1 (438) 0 (194) 0 (439) 10 0 90 80 70 60 50 40 30 20 10 0 10 8 0 12 24 36 48 60 72 84 96
PFS
e
64 (5476) 32 (2836)
Myeloma XI Trial: PFS From Maintenance Randomization
by Cytogenetic Risk Status
Risk or Ultra-High Risk

Pawlyn. ASH 2022. Abstr 570. Reproduced with permission.
Slide credit: clinicaloptions.com
Standard
P F S ( % ) Lenalidomid e Observation 64 (5476) 36 (2746) HR: 0.40 (95% CI: 0.28-0.58); P <.0001 Median PFS, Mo (95% CI) Lenalidomid e Observation 38 (3059) 21 (1130) HR: 0.50 (95% CI: 0.35-0.70); P <.0001 Median PFS, Mo (95% CI) Lenalidomid e Observation Patients at Risk, n (censored) Mo 130 (0) 130 (0) 125 (0) 108 (1) 112 (4) 77 (5) 60 (20) 43 (27) 45 (57) 21 (37) 25 (70) 14 (41) 14 (78) 8 (43) 3 (87) 1 (49) 0 (50) 0 (50) 10 0 90 80 70 60 50 40 30 20 10 0 10 8 0 12 24 36 48 60 72 84 96 P F S ( % ) Lenalidomid e Observation Mo 151 (0) 82 (0) 122 (2) 47 (0) 60 (9) 34 (5) 60 (26) 13 (14) 33 (45) 8 (17) 14 (61) 3 (10) 6 (65) 1 (21) 1 (71) 1 (21) 0 (72) 0 (22) 10 0 90 80 70 60 50 40 30 20 10 0 10 8 0 12 24 36 48 60 72 84 96 Patients at Risk, n (censored)
Risk High
Myeloma XI Trial: PFS From
Maintenance Randomization by MRD Negativity Status

Pawlyn. ASH 2022. Abstr 570. Reproduced with permission.
clinicaloptions.com
Slide credit:
*MRD
cytometry
MRD
MRD
P F S ( % ) HR: 0.72 (95% CI: 0.55-0.95); P = .022 HR: 0.37 (95% CI: 0.27-0.50); P <.0001 Lenalidomid e Observation Patients at Risk, n Mo 299 (3) 175 (0) 273 (4) 154 (1) 213 (24) 125 (7) 127 (83) 53 (45) 63 (132) 35 (59) 35 (153) 19 (72) 19 (106) 9 (78) 0 (184) 2 (85) 0 (87) Lenalidomide Observation 59 (50-NE) 44 (35-50) Median PFS, Mo (95% CI) 10 0 90 80 70 60 50 40 30 20 10 0 10 8 0 12 24 36 48 60 72 84 96 P F S ( % ) Lenalidomid e Observation Patients at Risk, n Mo 153 (0) 122 (0) 120 (5) 79 (1) 92 (13) 39 (5) 60 (31) 23 (8) 39 (45) 9 (14) 23 (50) 3 (17) 13 (67) 1 (18) 3 (76) 0 (19) 0 (78) Lenalidomide Observation 47 (33-76) 18 (13-22) Median PFS, Mo (95% CI) 10 0 90 80 70 60 50 40 30 20 10 0 10 8 0 12 24 36 48 60 72 84 96
assessed by flow
with median sensitivity of 4 x 105 .
Negative
Positive
Conclusions
•There is ongoing PFS benefit associated with continuous Len maintenance beyond 4-5 years in the OVERALL population
•In sustained MRD-negative patients, continuation beyond 3 years appeared to be of limited value
•In MRD-positive patients, results support continuing lenalidomide until disease progression
What about Dara as
a maintenance agent?
CASSIOPEIA:
What about single agent Dara maintenance?
Open-label, global, multicenter, randomized phase III trial
Stratified by induction treatment (D-VTd vs VTd), depth of response
Patients aged 18-65 yr with transplanteligible newly diagnosed MM,
ECOG PS 0-2 (N = 1085)
Part 1
Patients with ≥PR after completion of part 1 treatment (N = 886)
Dara Q8W until PD (max of 2 yrs followed by observation until PD)
Observation until PD (maximum of 2 yrs)
Part 2
Primary endpoint (part 2): PFS (after second randomization)
Key secondary endpoints (part 2): TTP (after second randomization), rates of ≥CR, MRD negativity rates (in ≥CR at a threshold of 10-5 by NGS), OS

Slide credit: clinicaloptions.com
Moreau. ASCO 2021. Abstr 8004. Maintenanc e VTd 4 cycles Daratumumab + VTd 4 cycles ASCT ASCT VTd 2 cycles Daratumumab + VTd 2 cycles
CASSIOPEIA: PFS by Combination of Induction and Maintenance Therapy
24 months: end of treatment VTd/DARA
Comparison
VTd/DARA vs VTd/OBS
D-VTd/DARA vs D-VTd/OBS
Is Dara needed in
induction and maintenance or is one or the other adequate?

Moreau. ASCO 2021. Abstr 8004. Reproduced with permission. Slide credit: clinicaloptions.com Mo P F S ( % )
0 2 0 4 0 6 0 8 0 10 0 0 6 1 2 1 8 2 4 3 0 3 6 4 2 4 8 HR (95% Cl) P value* 0.32 (0.23-0.46) <.0001 1.02 (0.71-1.47) .9133
both
D-VTd/OBS
D-VTd DARA 229 226 217 204 198 145 76 30 0 D-VTd OBS 229 223 216 207 195 144 75 38 2 VTd/DARA 213 203 189 182 174 138 79 34 1 VTd/OBS 215 201 176 155 131 83 43 15 1
D-VTd/DARA
VTd/OBS
CASSIOPEIA: PFS by Subgroups

Moreau. ASCO 2021. Abstr 8004. Reproduced with permission. Slide credit: clinicaloptions.com Hazard Ratio (95% Cl) Hazard Ratio (95% Cl) Sex Male Female Age <50 years 50-60 years >60 years Site IFM HOVON ISS Staging I II III Cytogenetic risk High Risk Standard Risk 0.57 (0.42-0.76) 0.53 (0.35-0.81) 0.38 (0.20-0.74) 0.56 (0.39-0.79) 0.67 (0.46-0.98) 0.56 (0.43-0.72) 0.59 (0.31-1.13) 0.50 (0.32-0.78) 0.56 (0.40-0.79) 0.75 (0.44-1.29) 0.43 (0.25-0.73) 0.62 (0.48-0.82) 0.1 1 Favors DARA Favors OBS Premaintenance baseline renal function CrCl >90 mL/min CrCl ≤90 mL/min Type of MM IgG Non-IgG
0 ≥1 Induction/ASCT/consolidation
VTd D-VTd MRD Positive Negative Response VGPR or better PR 0.51 (0.38-0.68) 0.72 (0.47-1.12) 0.64 (0.48-0.87) 0.44 (0.26-0.75) 0.55 (0.40-0.76) 0.57 (0.40-0.82) 0.34 (0.24-0.47) 1.05 (0.73-1.51) 0.46 (0.31-0.67) 0.61 (0.44-0.83) 0.58 (0.45-0.75) 0.39 (0.21-0.73) 0.1 1 Favors DARA
Premaintenance baseline ECOG PS
tx group
Favors OBS
What about dual maintenance therapy?
GEM2014MAIN: Ixazomib +
Len Maintenance
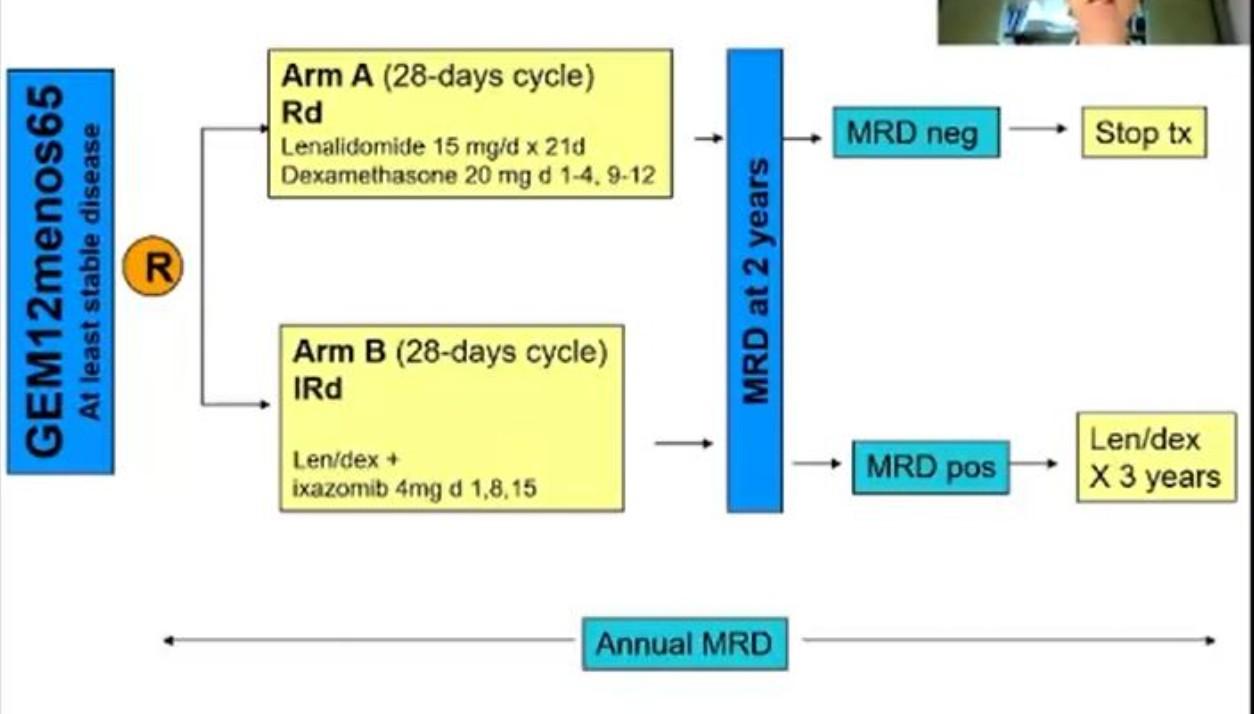
Maintenance: Rd vs. IRd
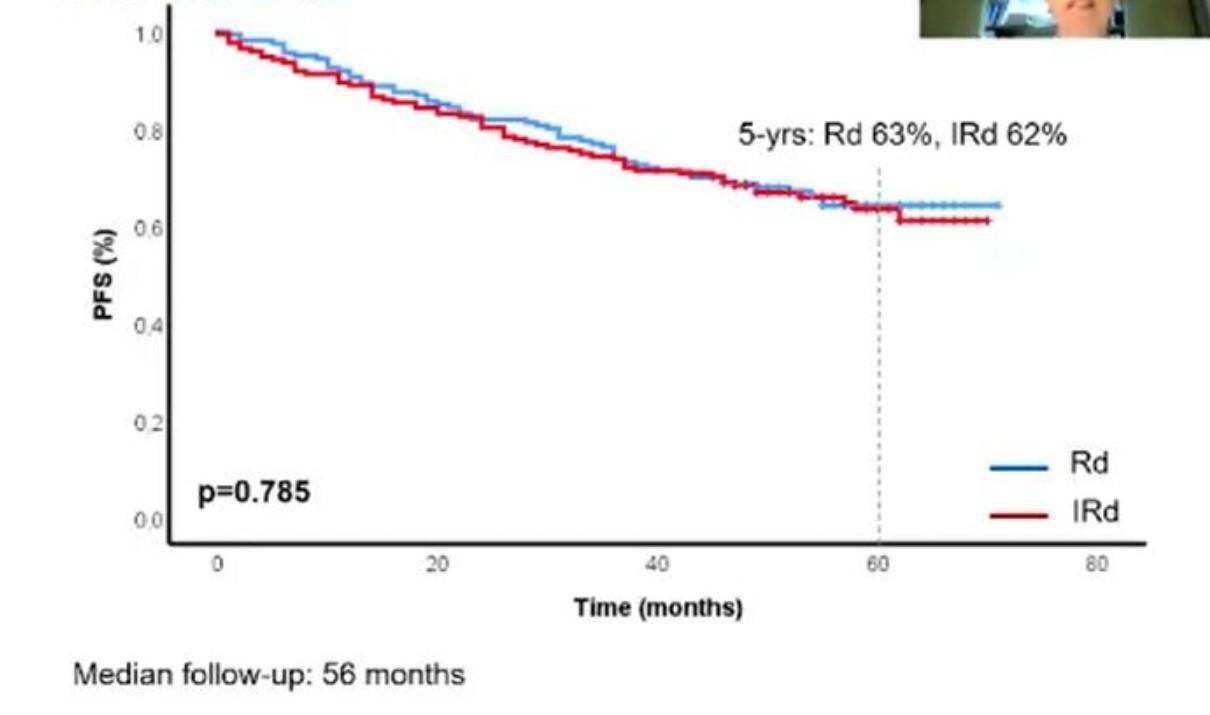
Toxicity of Adding Ixazomib
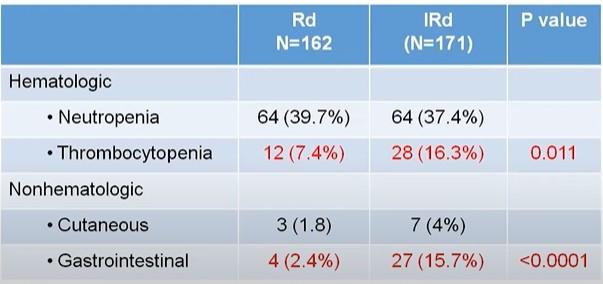
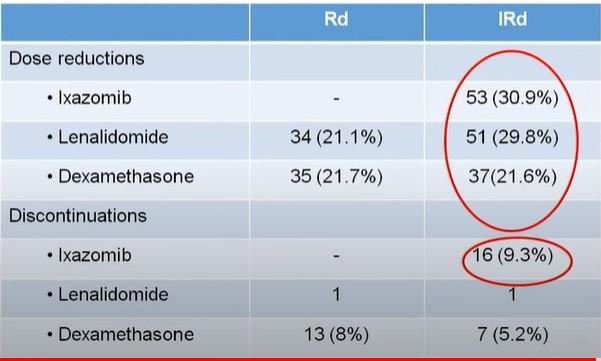
FORTE: Combination Maintenance
Multicenter, randomized, open-label phase II study
Patients with ND
MM, eligible for ASCT and < 65 yrs of age (N = 474)
4 x 28-Day Cycles
Arm A: KCd (n = 159)
Arm B: KRd (n = 158)
Arm C: KRd (n = 157) Induction
Single ASCT Arm C:
4 x 28-Day Cycles Endpoint 1:
4 x 28-Day Cycles
Arm A: KCd (n = 159)
Arm C: KRd (n = 157) Consolidation
Arm B: KRd (n = 158)

Gay. ASCO 2021. Abstr 8002. Slide credit: clinicaloptions.com
M o b i l i z a ti o n
KRd
Rate of VGPR
premaintenance VGPR, sCR, MRD negativity, safety, rate of early relapse S e c o n d R a n d o m i z a ti o n R KR
Endpoint 2:
Dosing in slide notes.
Maintenance: KR vs. R
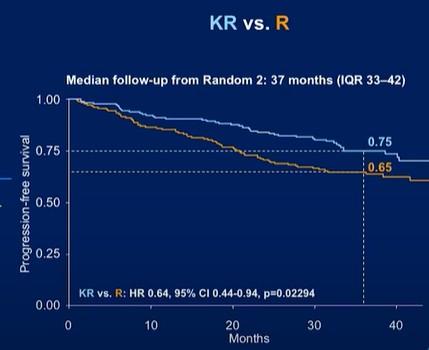
K vs. KR
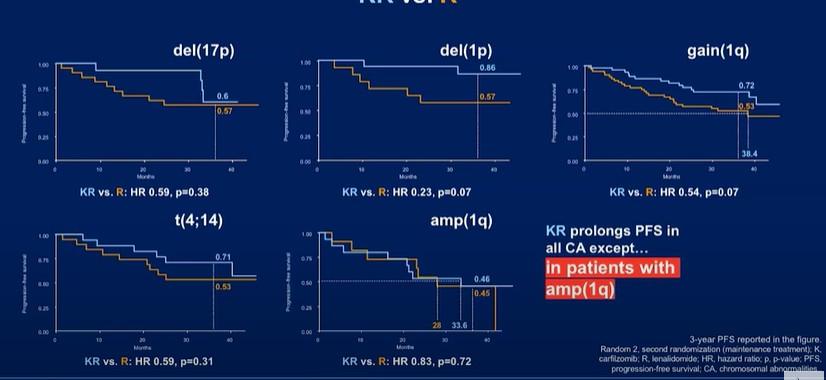
GRIFFIN Maintenance Phase Update:
Study Design
Multicenter, open-label, randomized phase II trial
Induction: Cycles 14
D-VRd in 21-day cycles
D: 16 mg/kg IV D1, 8, 15
V: 1.3 mg/m2 SC D1, 4, 8, 11
Transplant-eligible adults with ND MM, ECOG PS ≤ 2, and CrCl ≥ 30 mL/min* (N = 207)
R: 25 mg PO D1-14
d: 20 mg PO D1, 2, 8, 9, 15, 16 (n = 104)
VRd in 21-day cycles
V: 1.3 mg/m2 SC D1, 4, 8, 11
R: 25 mg PO D1-14
d: 20 mg PO D1, 2, 8, 9, 15, 16 (n = 103)
Consolidation: Cycles 5-6†
Maintenance: Cycles 7-32‡
D-VRd in 21-day cycles
D: 16 mg/kg IV D1
VRd: as in induction
D-R in 28-day cycles
D: as in consolidation Q4W or Q8W
R: 10 mg PO D1-21 of C7-9 and 15 mg PO D1-21 of C10+§
VRd in 21-day cycles
VRd: as in induction
R in 28-day cycles
R: 10 mg PO D1-21 of C7-9 and 15 mg PO D1-21 of C10+§
*Lenalidomide dose was adjusted in patients with CrCl ≤ 50 mL/min. †Consolidation began 60-100 days after transplantation. ‡Patients completing maintenance phase were permitted to continue single-agent lenalidomide. §15 mg administered only If tolerable.
Primary endpoint: sCR by end of consolidation with 1-sided α = .1
Secondary endpoints: MRD, CR, ORR, ≥ VGPR
Kaufman. ASH 2020. Abstr 549.

Slide credit: clinicaloptions.com
AS CT
Responses Deepened While on Maintenance
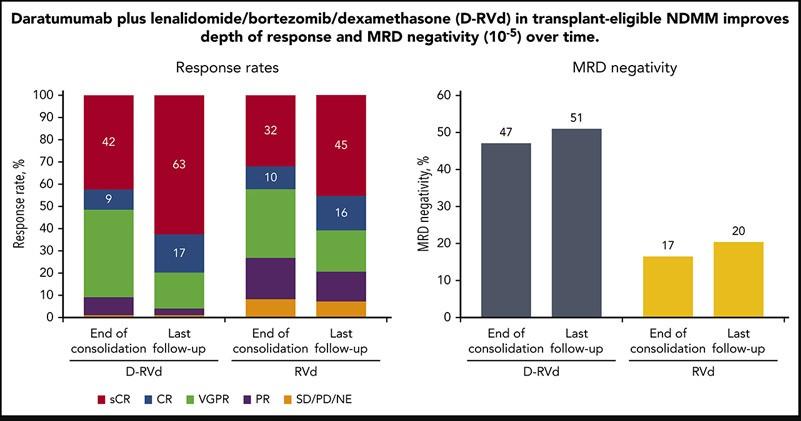
Conclusions
Cannot make any conclusions on DR vs. R maintenance
‒ no 2nd randomization
‒ Any benefit may have been related to Dara inclusion in the induction
AURIGA
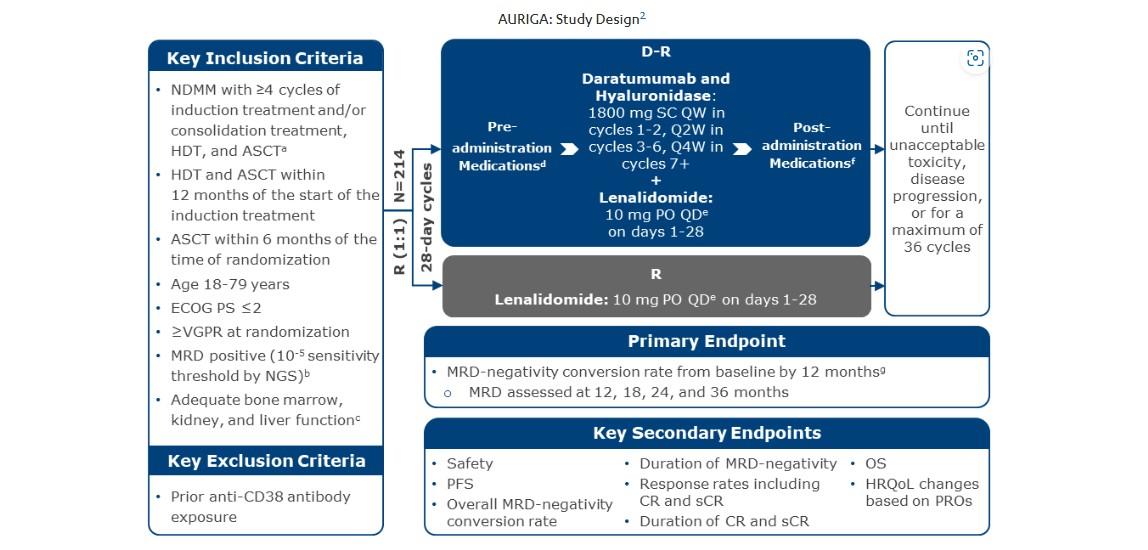
Closed
NearingEndof Enrollment
SWOG S1803
Minimal Residual Disease is the New CR!
Performed on bone marrow samples
Can detect 1 myeloma cell out of 100,000 cells
Deepest response we can achieve
Surrogate for remission duration
FDA recognizes MRD as a clinical endpoint in trials
MASTER Trial: Can you Eliminate Maintenance Therapy using MRD?
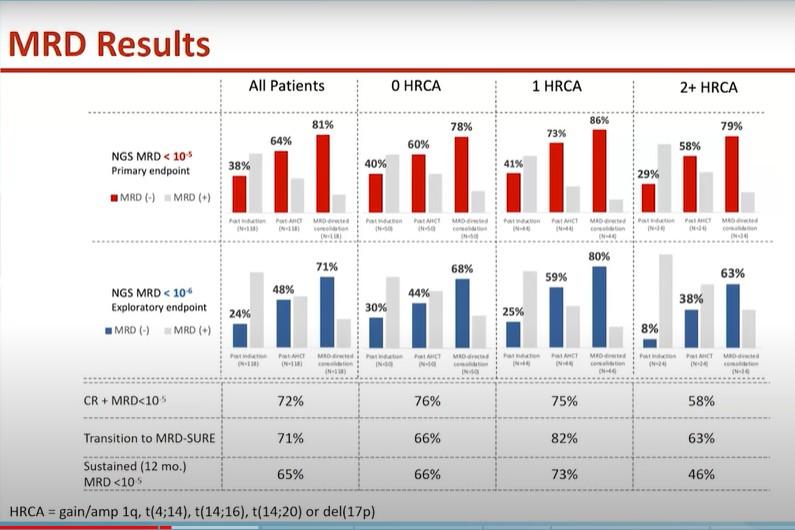
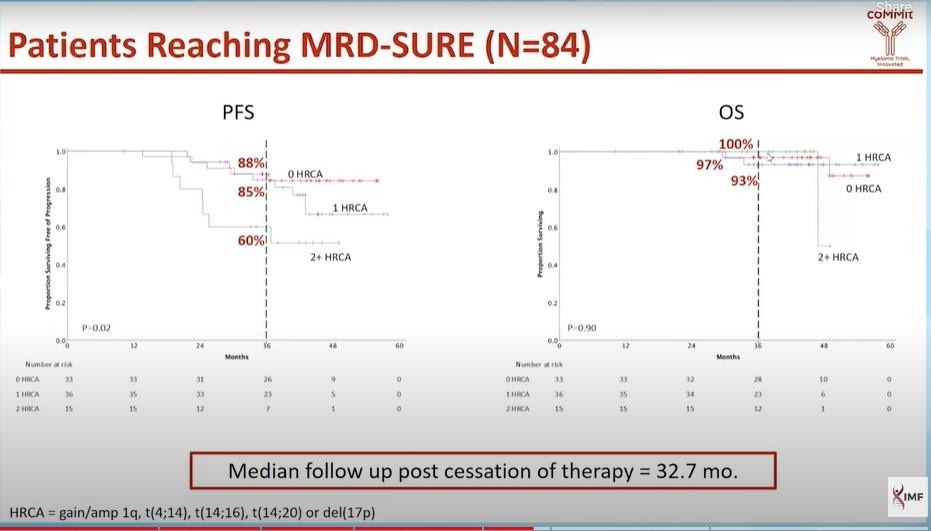
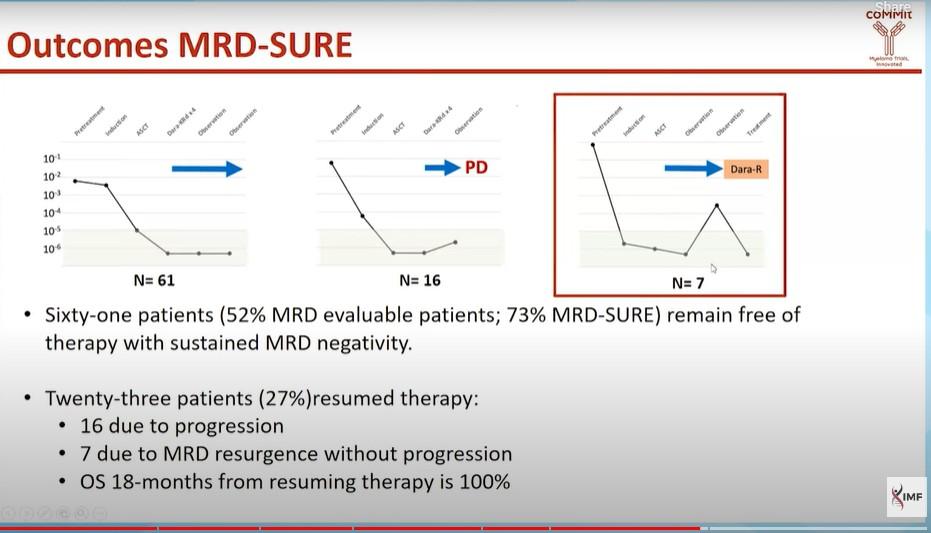
CONCLUSIONS
For NDMM with standard risk or 1 high risk cytogenetic abnormality, Dara-KRD/ASCT + MRD adapted therapy
‒ may be able to discontinue therapy and forgo maintenance with a low risk of progression
‒ Same is not true for pts with 2 high risk factors even if MRD neg = require maintenance therapy
Pipeline
EMN26: Iberdomide Maintenance
•Celmod
• Iberdomide is a novel, potent, oral cereblon E3 ligase modulator (CELMOD)
• Greater tumoricidal and immunemodulatory effects compared with Len
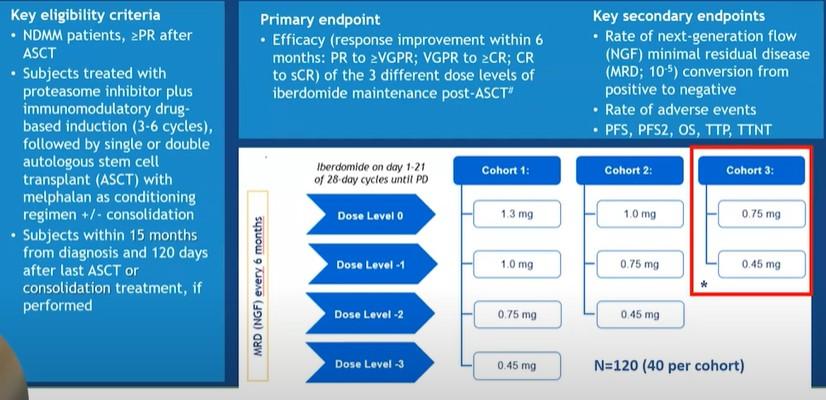
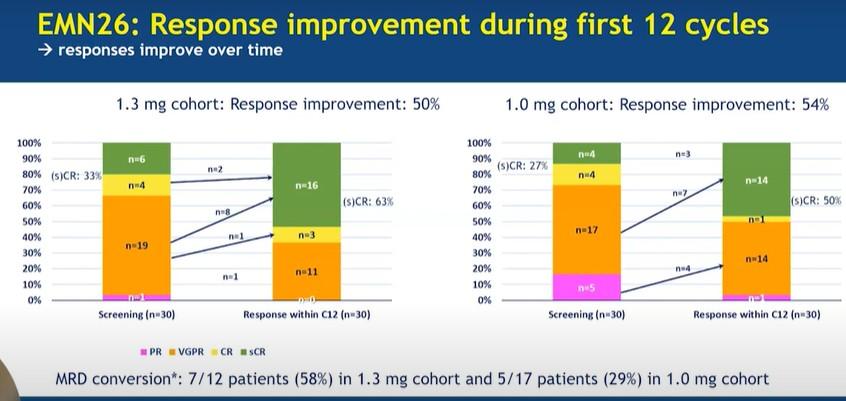
Overall “Take Homes”
•Len remains the standard of care for maintenance therapy in MM
• Convenient
• Low toxicity
•Can consider Len + PI maintenance for high-risk patients if feasible
•Dara-Len after ASCT – remains to be seen with SWOG and AURIGA trials
•Duration?
• Indefinite though we are likely overtreating some patients this way
•MRD-Adapted?
• De-escalate?
• Perhaps in standard risk patients
• Re-escalate?
Questions?

Relapsed Therapies & Clinical Trials
Peter Voorhees, MD Atrium Health, Levine Cancer Institute
Charlotte, NC
Relapsed Therapies and Clinical Trials
Peter Voorhees, M.D.
Chief, Plasma Cell Disorders Division, Atrium Health / Levine Cancer Institute
Clinical Professor of Medicine, Wake Forest University School of Medicine

National Cancer Institute Dictionary of Cancer Terms:
Progressive Disease: Cancer that is growing, spreading, or getting worse.
Relapse: The return of a disease or the signs and symptoms of a disease after a period of improvement.
Disease progression / progressive disease, disease coming out of remission, and relapse are used interchangeably. In all cases, it means that the myeloma cells are growing and dividing, and the burden of disease is increasing.
https://www.cancer.gov/publications/dictionaries/cancer-terms/

Doc, am I in Remission?
Identifying Relapse
Blood and urine studies
Regular SPEP and serum free light chain testing
Serum free light chain can replace 24-hour urine testing in many cases
Free light chain escape
When myeloma cells stop making the heavy chain and just make the light chain part of the antibody
e.g. IgA kappa myeloma changes into free kappa light chain myeloma
Picked up by serum free light chain testing.
Non-secretory and oligo-secretory myeloma.
The myeloma cells lose the ability to make any antibody or make minimal antibody
Not measurable by SPEP and serum free light chains
More frequent imaging and bone marrow biopsies required to track the myeloma

Identifying Relapse
Imaging
Bone Imaging
Skeletal survey
Low dose CT
CT, MRI, whole body PET-CT
Monitoring for disease outside of the bones (extra-medullary multiple myeloma)
PET-CT, CT, MRI
Clinical symptoms
New, progressive bone pain
Worsening fatigue

The patient is a 67-year-old Black man with IgG lambda multiple myeloma. The patient had ISS stage 2 disease and an (11;14) translocation at original diagnosis. Past medical history included diabetes and hypertension. He received 4 cycles of daratumumab, lenalidomide, bortezomib and dexamethasone induction therapy followed by high dose melphalan / autologous stem cell transplantation to which he achieved a complete response. Treatment was complicated by bortezomib-induced neuropathy with pain. Three months after transplant, he started lenalidomide maintenance therapy and has remained on it for the last 3.5 years. Aside from low grade fatigue and intermittent diarrhea and muscle cramps, he has done well with therapy and is able to live his life with minimal impact on quality of life. His bortezomib-induced neuropathy is well controlled but there is residual numbness and tingling in the feet for which he takes duloxetine.
He now presents with worsening low back pain, and his M spike has risen from undetectable to 1.1 g/dL. Plain xrays of the spine are unremarkable, but an MRI reveals a new lytic lesion with soft tissue extension at the level of L3.
What to do next?

Case
NCCN Guidelines for Relapsed Multiple Myeloma: 1 – 3 Prior

Bortezomib-Refractory Lenalidomide-Refractory Regimen Level of Evidence Level of Evidence Lenalidomide, carfilzomib, dex 1 Daratumumab, bortezomib, dex 1 Lenalidomide, daratumumab, dex 1 Pomalidomide, bortezomib, dex 1 Carfilzomib, daratumumab, dex 1 Selinexor, bortezomib, dex 1 Carfilzomib, isatuximab, dex 1 Carfilzomib, daratumumab, dex 1 Carfilzomib, pomalidomide, dex Carfilzomib, isatuximab, dex 1 Pomalidomide, daratumumab, dex 1 Carfilzomib, pomalidomide, dex Pomalidomide, isatuximab, dex 1 Pomalidomide, daratumumab, dex 1 Pomalidomide, isatuximab, dex 1 Pomalidomide, ixazomib, dex Pomalidomide, elotuzumab, dex 1 CAR T
Ciltacabtagene Autoleucel (after 1 prior therapy including an IMiD and a PI, refractory to lenalidomide) 1 Idecabtagene Vicleucel (after 2 prior therapies including an IMiD, a PI, and a CD38 antibody 1 Accessed June 2024 14 preferred regimens, 11 other recommended regimens, 13 regimens useful in certain circumstances = 38 regimens
Therapies, Preferred Regimens
Cell Therapy
PABST: The Blue Ribbon Approach to Treatment
of Relapsed Multiple Myeloma
Patient preference
Relative value of quality vs quantity of life, oral vs IV therapy
Adverse events and Comorbidities
What toxicities were experienced with prior therapy?
What co-morbidities will impact tolerability of therapy?
Biochemical vs clinical relapse/progression
Standard vs high-risk disease biology
Treatment history
Is the disease resistant to specific drug classes?
Carfilzomib refractory = bortezomib and ixazomib refractory
Pomalidomide refractory = lenalidomide refractory
Daratumumab refractory = isatuximab refractory


Back to our Patient
Patient preference: Lives close to the infusion center and wants the treatment that his doctor thinks will produce a long remission.
Adverse events and Comorbidities: Prior history of bortezomib-induced neuropathy with pain – avoid bortezomib-based therapy at relapse. Diabetes – cautiously dosed dex and get rid of it quickly; Hypertension – carfilzomib and dex ok but monitor for worsening.
Biochemical vs clinical relapse: This patient has clinical relapse with increasing pain and a new bone lesion – we need to use a therapy with a high likelihood of success and deep response.
Standard vs high risk: This patient has standard risk disease – no issues.
Treatment History: The patient has disease that has progressed on lenalidomide –avoid the use of a lenalidomide-based regimen

NCCN Guidelines for Relapsed Multiple Myeloma: 1 – 3 Prior

Bortezomib-Refractory Lenalidomide-Refractory Regimen Level of Evidence Level of Evidence Lenalidomide, carfilzomib, dex 1 Daratumumab, bortezomib, dex 1 Lenalidomide, daratumumab, dex 1 Pomalidomide, bortezomib, dex 1 Carfilzomib, daratumumab, dex 1 Selinexor, bortezomib, dex 1 Carfilzomib, isatuximab, dex 1 Carfilzomib, daratumumab, dex 1 Carfilzomib, pomalidomide, dex Carfilzomib, isatuximab, dex 1 Pomalidomide, daratumumab, dex 1 Carfilzomib, pomalidomide, dex Pomalidomide, isatuximab, dex 1 Pomalidomide, daratumumab, dex 1 Pomalidomide, isatuximab, dex 1 Pomalidomide, ixazomib, dex Pomalidomide, elotuzumab, dex 1 CAR T Cell Therapy Ciltacabtagene Autoleucel (after 1 prior therapy including an IMiD and a PI, refractory to lenalidomide) 1 Idecabtagene Vicleucel (after 2 prior therapies including an IMiD, a PI, and a CD38 antibody 1 Accessed June 2024 14 preferred regimens, 11 other recommended regimens, 13 regimens useful in certain circumstances = 38 regimens
Therapies, Preferred Regimens
CD38 Monoclonal Antibodies with Carfilzomib or
Pomalidomide in Relapsed Myeloma
Pomalidomide Carfilzomib
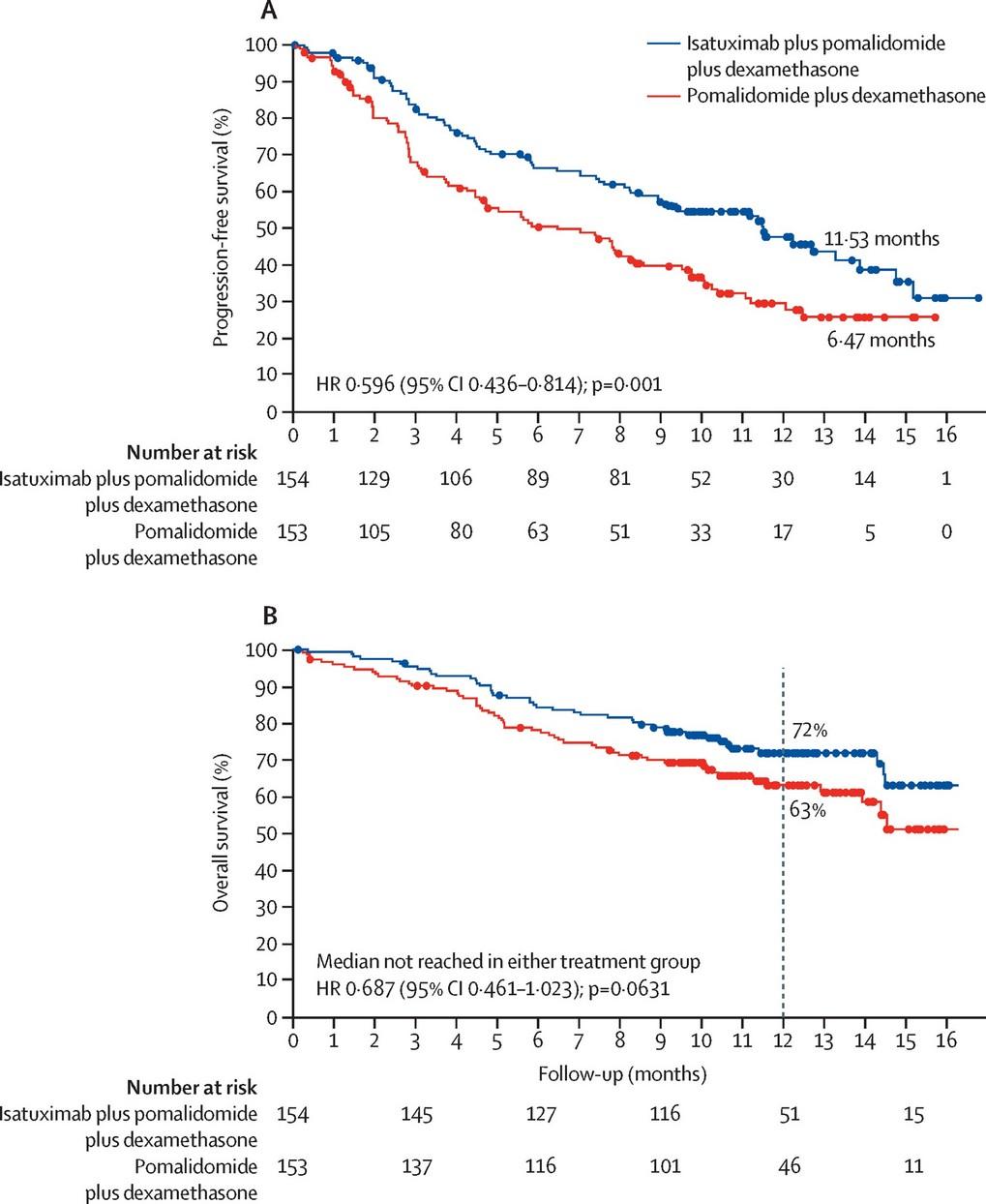
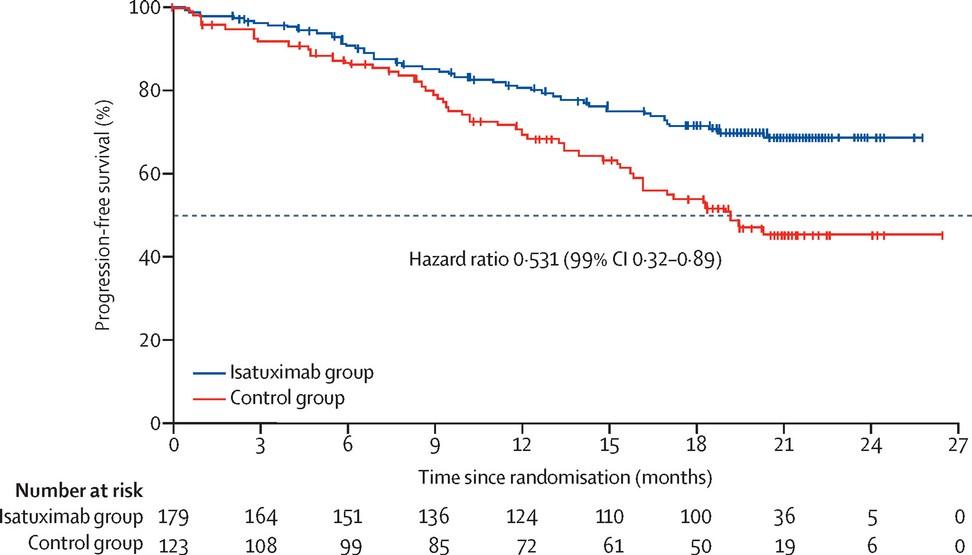
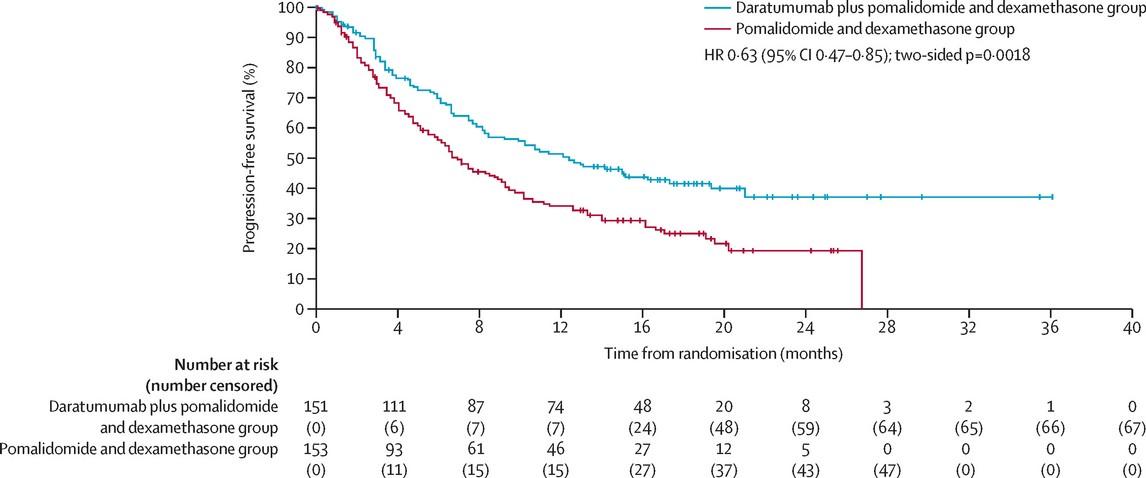
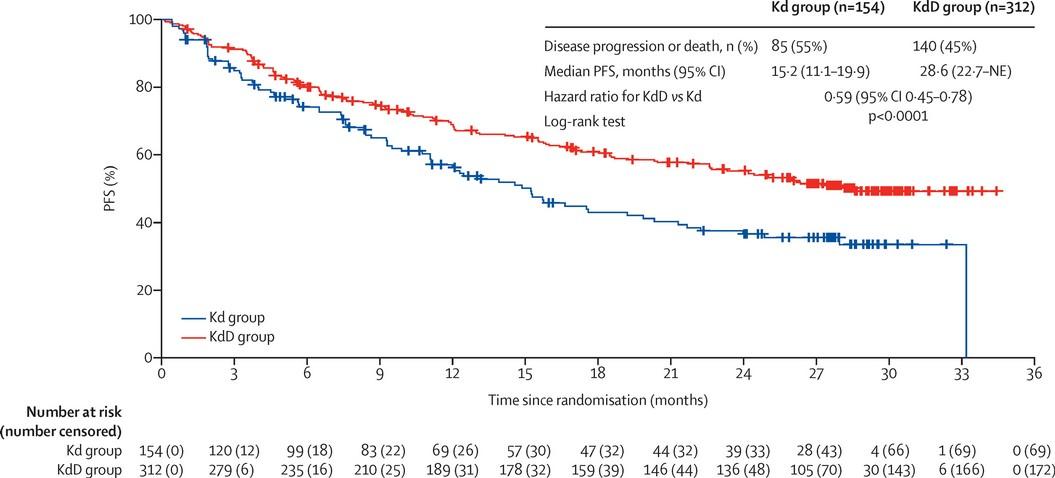
Attal, M, et al. Lancet 2019;394:2096 - 2107 Moreau, P, et al. Lancet 2021;397:2361 - 2371
Dimopoulos, M, et al. Lancet Oncol 2021;22:801-812 Usmani S, et al. Lancet Oncol 2022;23:65-76

I s a t u x i m a b D a r a t u m u m a b
The addition of CD38 antibodies to pomalidomide and carfilzomib reduce the risk of myeloma progression and death by 37% - 47%
Back to our Patient
After a discussion with their physician, the patient chooses 2nd line therapy with subcutaneous daratumumab with carfilzomib and dexamethasone. He achieves a very good partial response within 4 cycles of treatment start. One and a half years into treatment, the patient experiences new right hip pain. The M spike remains at its nadir of 0.1 g/dL. However, the serum free lambda light chain level has increased from a nadir of 24.6 mg/L to 112.2 mg/L. An MRI of the hip reveals an impending fracture of the right hip for which the patient undergoes a total hip replacement.
After recovery, what to do next?

Back to our Patient
Patient preference: The patient is tired of frequent visits to the infusion center and indefinite treatment and is interested in something that would provide him a chance to get off treatment.
Adverse events and Comorbidities: Prior history of bortezomib-induced neuropathy with pain – avoid bortezomib-based therapy at relapse. Diabetes – cautiously dosed dex and get rid of it quickly; Hypertension –dex ok but monitor for worsening.
Biochemical vs clinical relapse: This patient has clinical relapse – we need to use a therapy with a high likelihood of success and deep response.
Standard vs high risk: This patient had an unexpectedly short response to treatment. As such, he is functionally a high-risk patient now.
Treatment History: The patient has disease that has progressed on lenalidomide, carfilzomib and daratumumab – avoid the use of regimens with these agents.

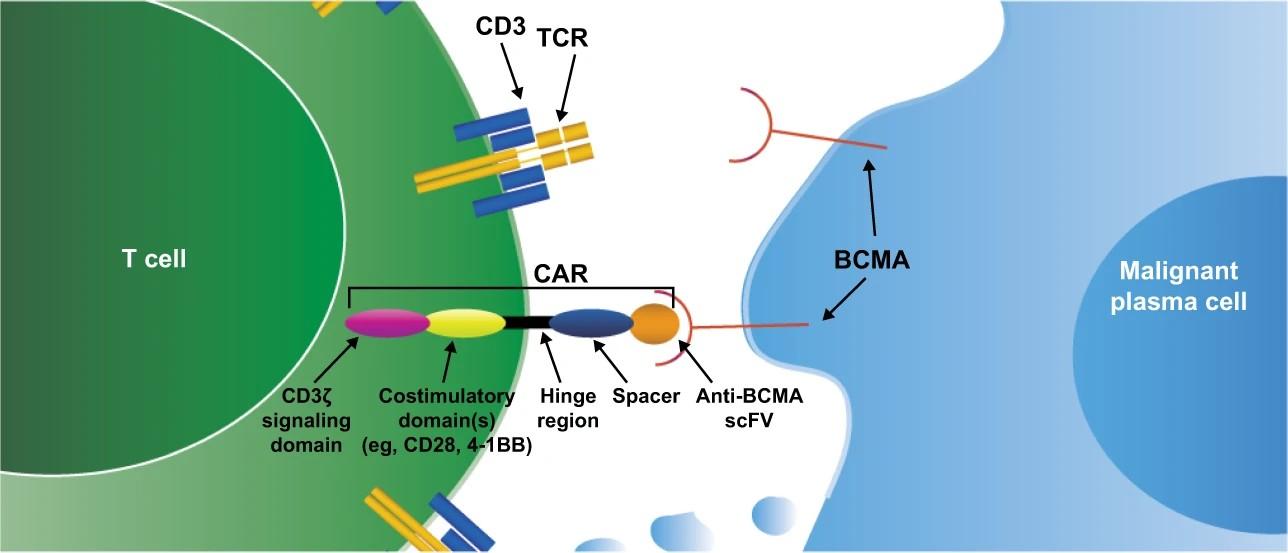
Shah N, et al. Leukemia 2020;34(4):985-1005.

CAR T Cell Therapy
BCMA-Targeted
CAR T Cell Process
Step 1: CAR T-Cell Candidate Identification/Screening
Step 2: Collection of T-Cells
Step 3: Manipulation of T-Cells into CAR T-Cells
Step 4: Lymphodepleting Chemotherapy
Step 5: Infusion of CAR T-Cells
Step 6: Post CAR T-Cell Monitoring, Complication Management, and Care
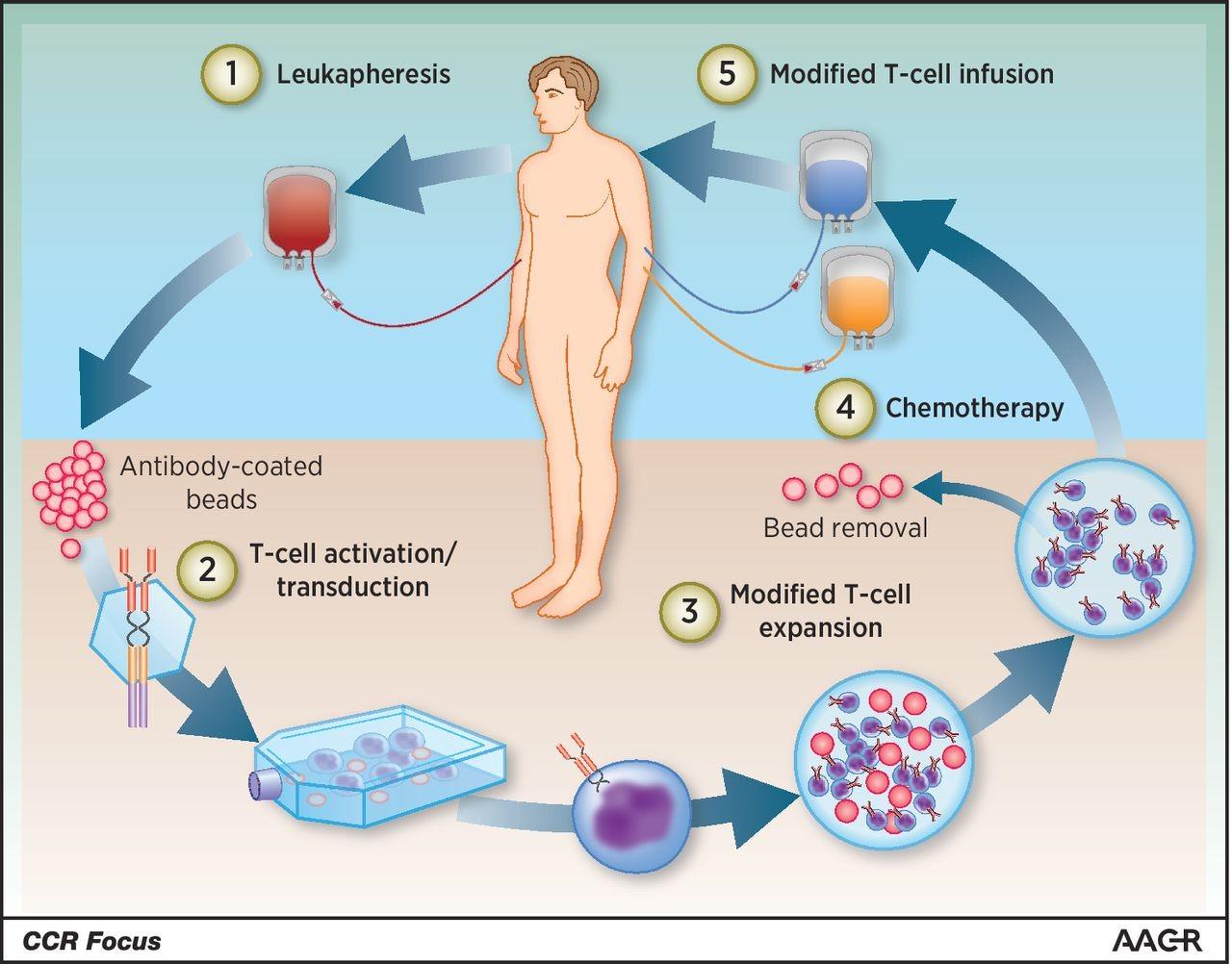

Maus MV et al. Clin Cancer Res 2016;22:1875
Complication: Cytokine Release Syndrome (CRS)
Cytokine Release syndrome (CRS) occurs when a large number of cytokines (immune substances) are released in the blood.
Onset:
CRS is most often seen within the first 14 days post CAR T-Cell infusion. It may also be seen up to 28 days post CAR T-Cell infusion.
Symptoms include:
Fever
Nausea
Headache
Low blood pressure
Fast heart rate
Difficulty breathing
Treatment:
Tocilizumab (IL-6 antagonist)
Dexamethasone

Complication: Immune Effector Cell Associated Neurotoxicity Syndrome (ICANS)
Onset:
ICANS often occurs within 5-10 days post CAR T-Cell infusion
Neuro checks are performed pre-infusion for baseline and Q12 hours (minimum) to help identify if patient has associated symptoms.
Symptoms:
Aphasia
Confusion
Delirium
Headache
Tremor
Hallucinations
Loss of consciousness
Seizures
Treatment:
Tocilizumab (IL-6 antagonist)
Steroids
Anti-Seizure medication

Other Potential Complications of BCMA-Targeted
CAR T Cell Therapies
• Low blood counts
• Infection
• Other cancers
• Myelodysplastic syndrome, acute myeloid leukemia
• Other unusual neurologic effects
• Bell’s palsy
• Neuropathy
• Parkinson-like effects

Ciltacabtagene Autoleucel:
A BCMA-Targeted CAR T Cell
Therapy
CARTITUDE-1
Phase Ib/II study of the CAR T cell product ciltacabtagene autoleucel for RRMM. High risk CGs 23.7%, Extramedullary plasmacytomas 13.4%, Median prior lines of therapy: 6 (3 – 18), Triple class refractory 87.6%, Refractory to last line 99%


0% 20% 40% 60% 80% 100% 3.1% 12.4% 82.5% ORR: 97.9% (95/97) P a ti e n t s , % sCR VGP R PR Best response = ≥VGP R: 94.9% Martin, T et al. ASH 2021. Median
CI, 25.2–NE)
PFS: 34.9 Months (95%
Median OS: Not reached
CARTITUDE-4: Phase III Study of Ciltacabtagene Autoleucel vs Investigators Choice for RRMM
Design
• 1 – 3 prior lines of therapy, lenalidomide refractory, PI exposed
• SoC regimens: Dara-Pom-Dex, Pom-Bortezomib-Dex
• 84.6% of pts assigned to Cilta-cel received it per protocol
• SoC Group: 86.7% DPd, 12.3% PVd
• Cilta-cel Group: No pts received therapy prior to apheresis, All received bridging therapy after apheresis (87.5% DPd, 12.5% PVd)
Baseline Characteristics
• Enrollment 7/2020 – 11/2021
• Median prior lines of therapy: 2 (range 1 – 3)
• 100% Len refractory, 21.3 – 23.1% dara refractory, 14.4% - 15.6% triple class refractory disease
• 59.4% - 62.9% HRCGs
Cilta-Cel: Not reached HR 0.26 (95% CI 0.18 – 0.38, P<0.001)
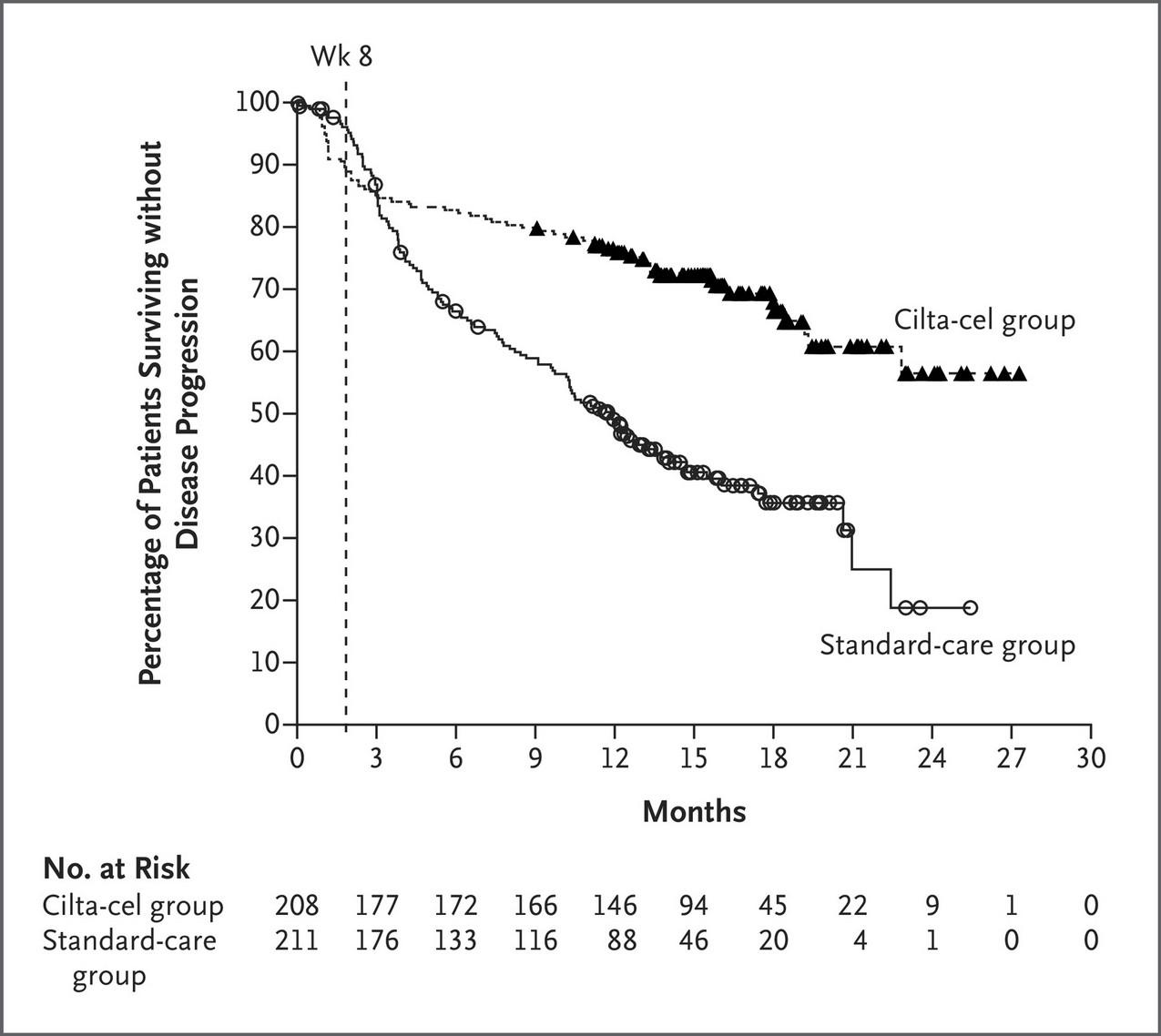
SoC: 11.8 months
*87.5% and 32.7% for those with MRD evaluable samples
Median Follow-Up: 15.9 Months

Cilta-Cel SoC ORR 84.6% 67.3% sCR 58.2% 15.2% CR 14.9% 6.6% VGPR 8.2% 23.7% PR 3.4% 21.8% MRD (10-5) 60.6%* 15.6%* San-Miguel J et al. N Engl J Med 2023;389:335-47
CARTITUDE-4: Safety
Adverse Event
*7 vs 1 patient died of COVID-19; 65.9% vs 12.5% of pts received IVIg prophylaxis, respectively
1 pt with MNT, 16 with CN palsies, 5 with peripheral neuropathy

Cilta-Cel SoC Regimens
All
All
Neutropenia 89.9% 89.9% 85.1% 82.2% Anemia 54.3% 35.6% 26.0% 14.4% Thrombocytopenia 54.3% 41.3% 31.2% 18.8% Infections* 62.0% 26.9% 71.2% 24.5% Fatigue 28.8% 1.9% 32.7% 1.0% CRS 76.1% 1.1% Neurotoxicity ICANS Other† 20.5% 4.5% 17.0% 2.8% 0.1% 2.3% SAEs 44.2% 38.9% Treatment-Related Deaths 4.8% 2.7% SPMs 4.3% 6.7%
San-Miguel
Grades Grade 3 and 4
Grades Grades 3 and 4
†
J et al. N Engl J Med 2023;389:335-47
Back to our Patient
After a discussion about pomalidomide-based treatment vs CAR T cell therapy, the patient elects to receive 3rd line therapy with ciltacabtagene autoleucel (Carvykti). He has grade 2 cytokine release syndrome while in the hospital after his CAR T cell infusion that recovers without incident. He has no ICANS but develops a Bell’s palsy 3 weeks after his CAR T cell infusion that resolves over 2 months after a course of prednisone therapy. A restaging evaluation 3 months after treatment reveals that he is in an MRD negative complete response.
3.5 years later, his M spike reappears and slowly climbs to 0.6 g/dL. He has no symptoms of disease, but a repeat PET-CT shows new FDG avid bone lesions that were not present before. The patient is placed on 4th line therapy with elotuzumab, pomalidomide and dex. He achieves a partial response but experiences disease progression 6 months later. Repeat imaging demonstrates 2 liver lesions and several enlarged lymph nodes. A biopsy of a lymph node reveals myeloma.
What to do next?

Preferred Regimens Level of Evidence
Bispecific Antibodies
Elranatamab
Teclistamab
Talquetamab
CAR T Cell Therapy
Idecabtagane Vicleucel
Ciltacabtagene Autoleucel
6 other recommended regimens, 1 useful in certain circumstances

NCCN
Guidelines for Relapsed Multiple Myeloma: ≥4 Prior Therapies
Accessed June 2024
Back to our Patient
Patient preference: The patient recognizes that his treatment options are more limited at this time and wants the therapy that is most likely to be successful.
Adverse events and Comorbidities: Prior history of bortezomib-induced neuropathy with pain – avoid bortezomib-based therapy at relapse. Diabetes – cautiously dosed dex and get rid of it quickly; Hypertension – dex ok but monitor for worsening.
Biochemical vs clinical relapse: This patient has biochemical relapse.
Standard vs high risk: Once high risk, always high risk.
Treatment History: The patient has disease that has progressed on lenalidomide, pomalidomide, carfilzomib, elotuzumab and daratumumab – avoid the use of regimens with these agents. He has already received CAR T cell therapy.

et al. Leukemia 2020;34(4):985-1005.

Therapy Shah
BCMA-Targeted Bispecific Monoclonal Antibody
N,
AMG420
Teclistamab
MajesTEC-1: Phase I/II Study of Teclistamab in RRMM
• Phase II enrollment 3/2020 – 8/2021
• 17% with EMM, 25.7% with HRCGs
• Median Prior Lines of Therapy: 5 (2 – 14)
• 77.6% triple class refractory, 89.7% refractory to last line of therapy
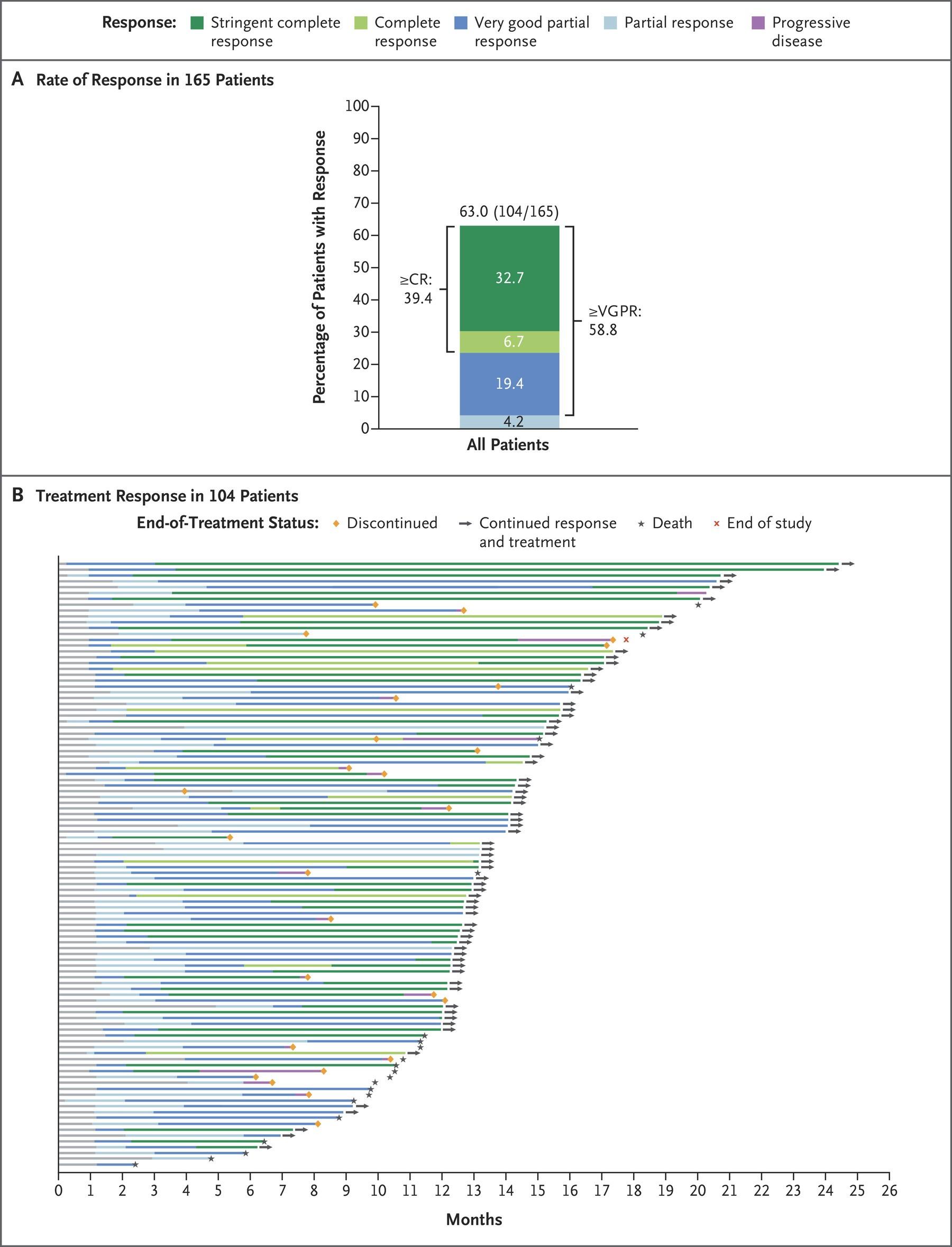
• Neutropenia 70.9% (64.2% ≥grade 3), hypogammaglobulinemia 74.5%
• Infections 76.4% (44.8% ≥grade 3)
• 12 COVID-19 deaths
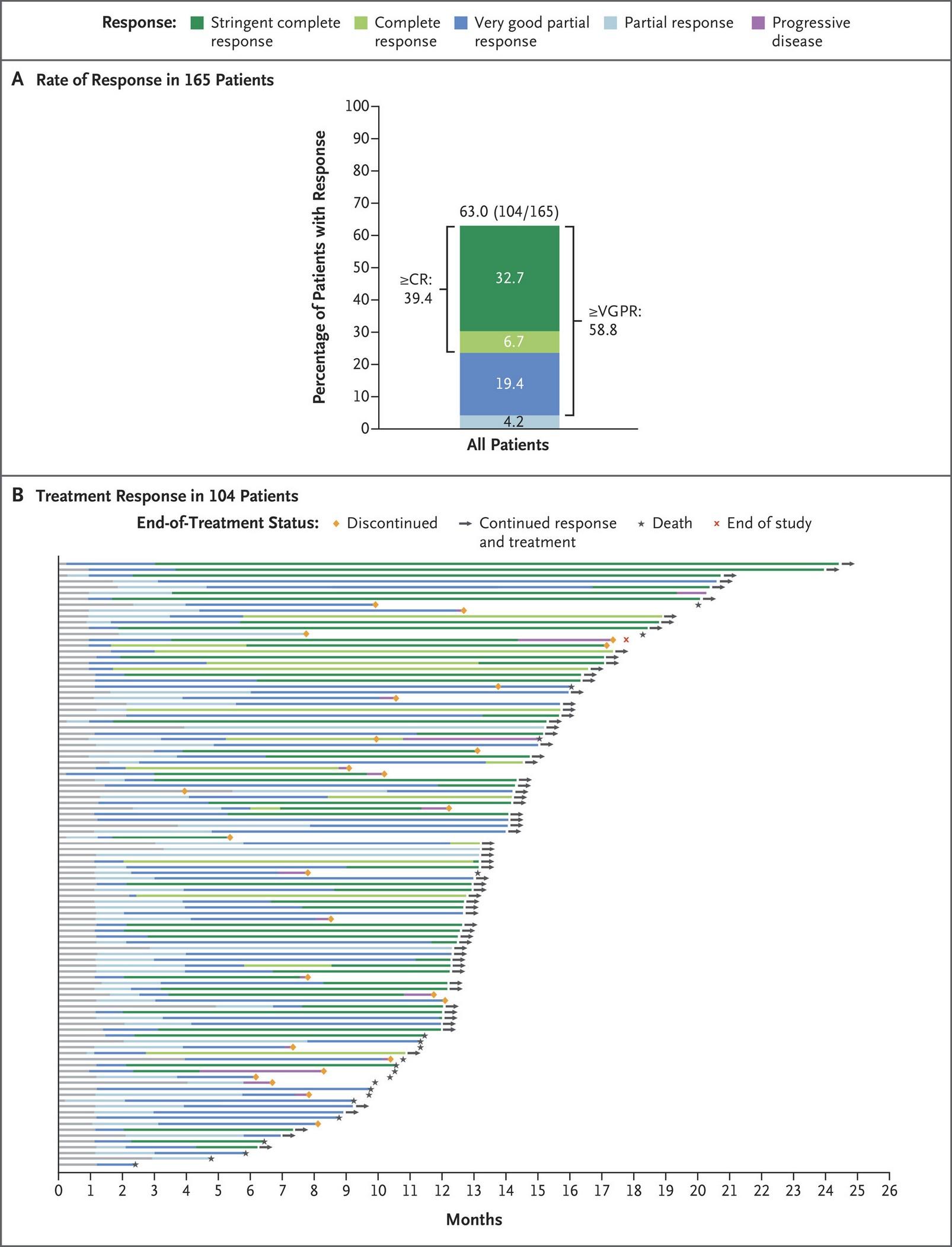
• Median DoR: 18.4 mos (95% CI 14.9 – NE mos)
• Median PFS: 11.3 mos (95%
8.8 – 17.1 mos)

CI
N=165 Moreau P, et al. N Engl J Med
2022;387:495-505.
Relapsed / Refractory Multiple Myeloma: MonumenTAL-1
Key objectives
• Describe the efficacy and safety at the RP2Ds
Key eligibility criteria
• Adults with measurable MM
• Phase 1: Progression on or intolerance to all established therapies, ECOG PS 0–1
• Phase 2: ≥3 prior lines of therapy that included a PI, an IMiD, and an anti-CD38 antibody, ECOG PS 0–2
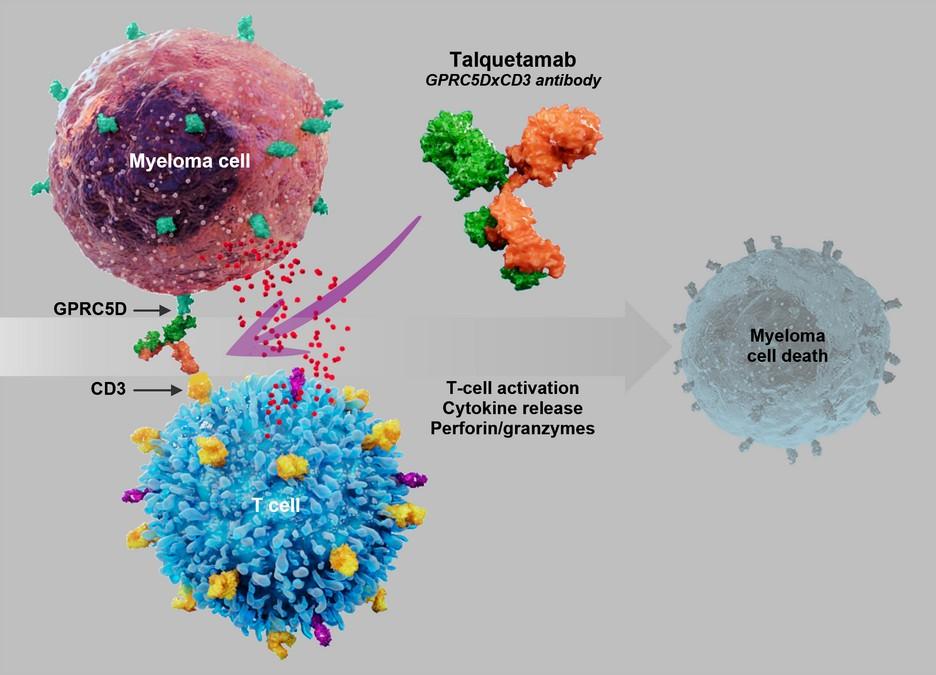
RP2D 0.4 mg/kg QW SC
Prior anti-BCMA ADC treatment allowed T-cell redirection therapy naive
(Phase 1 [n=21] + Phase 2 [n=122]: N=143)
RP2D 0.8 mg/kg Q2W SC
Prior anti-BCMA ADC treatment allowed T-cell redirection therapy naive
(Phase 1 [n=36] + Phase 2 [n=109]: N=145)
Prior
T-cell redirection (QW and Q2W)
Previously exposed to T-cell redirection therapies
Dosed with either 0.4 mg/kg weekly SC or 0.8 mg/kg Q2W SC
(Phase 1 [n=17] + Phase 2 [n=34]: N=51)

Talquetamab, a GPRC5D-Directed Bispecific Monoclonal Antibody, for
Chari A et al. ASH 2022, Abstract 157.
Talquetamab for Relapsed / Refractory Multiple
Myeloma: MonumenTAL-1 Key Phase II Efficacy Results
0.8 mg/kg Q2W: 11.9 months Key eligibility criteria (0.4 mg/kg QW / 0.8 mg/kg Q2W): HR CGs 31.1% / 28.9%, Extramedullary disease 23.1% / 26.9%, ISS stage 3 disease 19.6% / 24.3%, 5 median prior lines of therapy, Triple class refractory 74.1% / 69.0%
• Median Time to Best Response
0.4 mg/kg QW: 2.2 months
0.8 mg/kg Q2W: 2.7 months • Median Duration of Response
0.4 mg/kg QW: 9.3 months
0.8 mg/kg Q2W: 13.0 months • Median Progression-Free Survival
0.4 mg/kg QW: 7.5 months

0 20 40 60 80 100 14.7% 15.9% 25.9% 24.8% 9.8% 12.4% 23.8% 20.0% PR VGPR CR sCR 74.1% (106/143) 73.1% (106/145) ≥VGPR: 59.4% 0.4 mg/kg SC QW 0.8 mg/kg SC Q2W P a ti e n t s ( % ) ≥VGPR: 57.2% 100% 80% 60% 40% 20% 0%
•
•
•
Chari A et al. ASH 2022, Abstract 157.
•
•
•
Talquetamab for Relapsed / Refractory Multiple
Myeloma: MonumenTAL-1 Key Phase II Safety Results
*Opportunistic infections seen in 3.5% and 2.8%, respectively.

0.4 mg/kg QW 0.8 mg/kg Q2W All Grades ≥Grade 3 All Grades ≥Grade 3 Neutropenia 34.3% 30.8% 28.3% 22.1% Thrombocytopenia 27.3% 20.3% 26.9% 16.6% Infections* 57.3% 16.8% 50.3% 11.7% CRS 79.0% 2.1% 72.4% 0.7% Neurotoxicity 10.7% 1.6% 10.1% 1.8% Skin-Related AEs 55.9% 0.0% 67.6% 0.7% Nail-Related AEs 51.7% 0.0% 43.4% 0.0% Dysgeusia 48.3% NA 46.2% NA Dry Mouth 25.2% 0.0% 36.6% 0.0% Dysphagia 23.8% 0.0% 22.8% 2.1% Loss of appetite 17.5% 1.4% 20.0% 1.4% Weight loss 39.9% 2.1% 32.4% 1.4%
Chari A et al. ASH 2022, Abstract 157.
Talquetamab after Exposure to T
• Median prior lines of therapy: 6 (3 – 15)
• 70.6% (N = 36) prior CAR T cell therapy, 35.3% (N = 18) prior bispecific antibody therapy (3 patients received both)
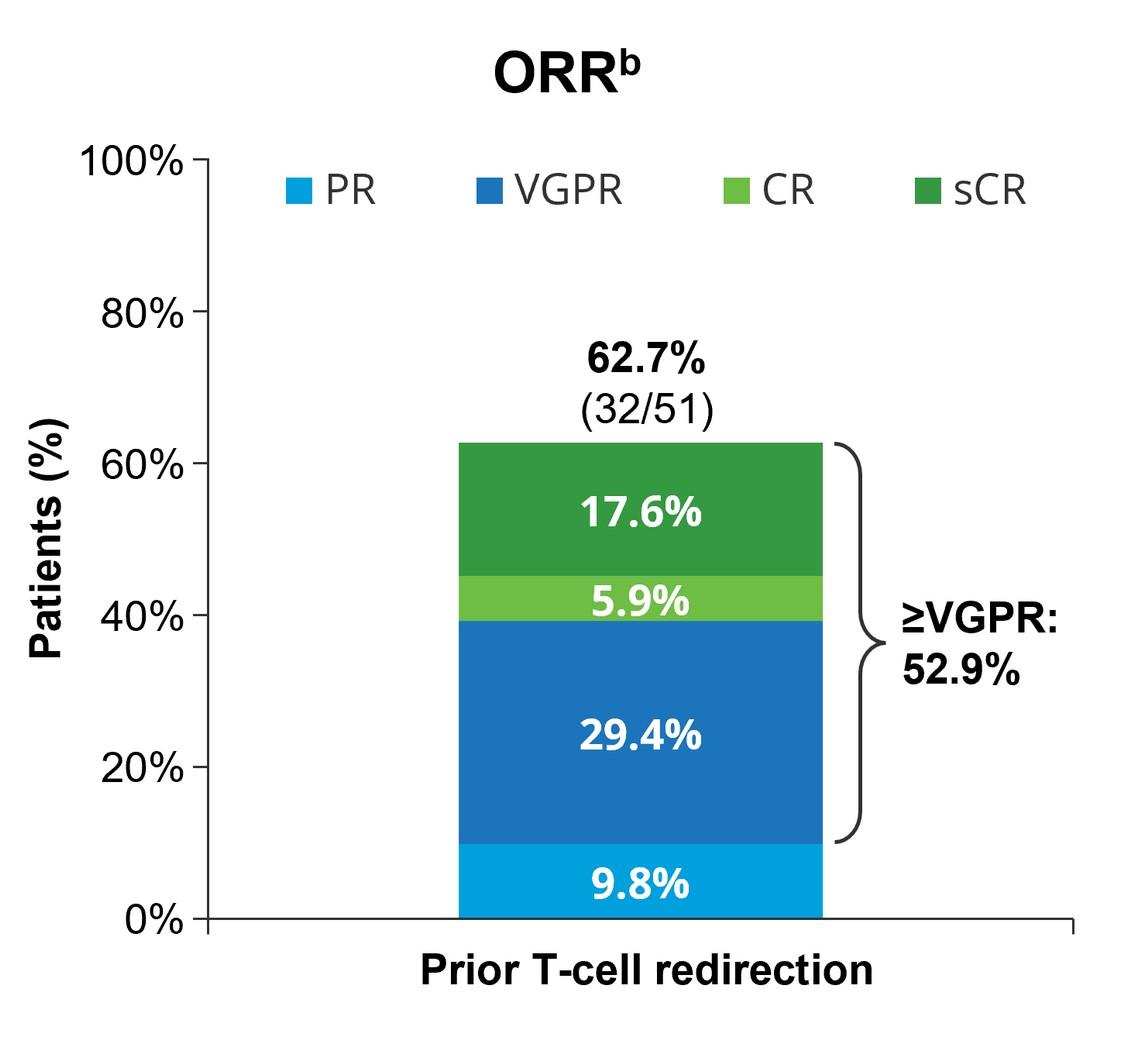
• ORR with prior CAR T: 70.6%
• ORR with prior bispecific antibodies: 44.4%
• Median DoR: 12.7 months (range 3.7 months
- not reached)
• Median follow-up: 11.8 months (1.0 –25.4 months)
• 56.3% of patients censored

cell Redirecting Therapy
A et al. ASH 2022, Abstract 157.
Chari
Back to our Patient
After a discussion about bispecific antibody therapy, the patient elects to receive treatment with on the RedirecTT-1 study, a phase II study evaluating the combination of two bispecific monoclonal antibodies, teclistamab and talquetamab for the treatment of extra-medullary myeloma. 4 cycles into treatment, he has achieved a complete response. 8 cycles into treatment, the patient is feeling better, and the taste changes are improved.

Phase I Study of Talquetamab + Teclistamab in RRMM
(RedirecTT-1)
Tec 3.0 mg/kg + Tal 0.8 mg/kg Q8Wks
Baseline Characteristics
• Median age: 65 (41 – 80)
• EMM: 32.4%
• HRCGs: 33.3%
• Median prior lines of therapy: 4 (2 – 10)
• 76.5% triple refractory
88.2% refractory to last line of therapy
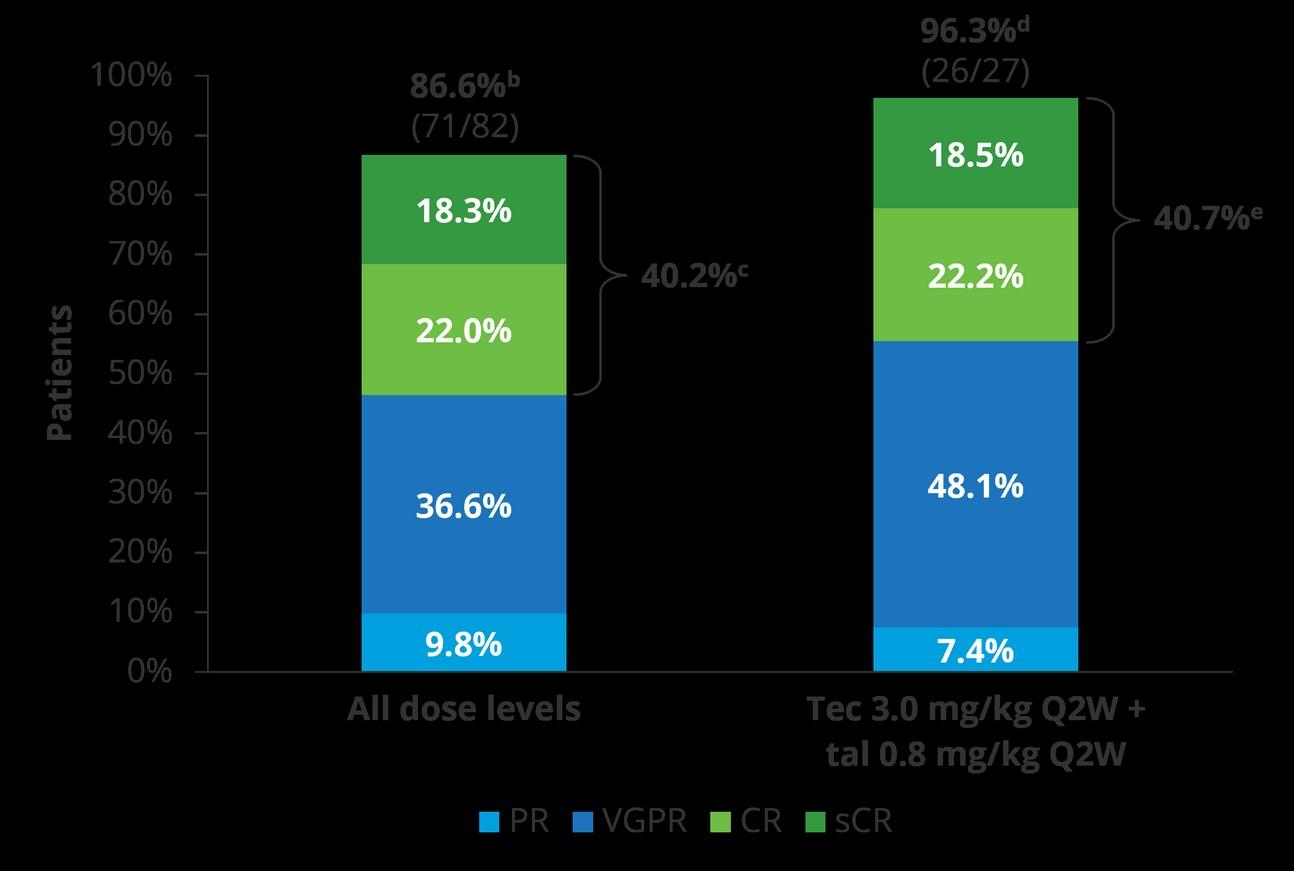
to
• Infections (all cohorts): 83.9% (52.7% grade 3/4)
• Death due to drug-related TEAE: 6.5%
• 81.7% with ≥1 postbaseline IgG value <400 mg/dL or hypogammaglobulinemia TEAE (all grade 1 or 2)

Cohen Y, et al. ASCO 2023.
All dose levels (N=93) Tec 3.0 mg/kg Q2W + tal 0.8 mg/kg Q2W (n=34) Median follow-up, months (range) 13.4 (0.3–25.6) 8.1 (0.7–15.0) Median DOR, months
CI) NE (NE–NE) NE (NE–NE) Median time
first
months (range) 1.97 (0–7.7) 1.48 (0–4.0) Median time to best response, months (range) 3.98 (1.1–15.7) 3.22 (1.4–10.7) Median PFS, months (95% CI) 20.9 (13.0–NE) NE (9.9–NE) 9-month PFS rate (95% CI) 70.1 (58.0–79.4) 77.1 (50.8–90.5)
(95%
response,
• There are numerous options available for patients whose myeloma has relapsed.
• Outcomes for patients with relapsed myeloma are better than ever and continue to improve.
• Immunotherapy, particularly T cell-redirecting therapy with CAR T cell therapy and bispecific antibodies, plays a key role in the treatment of patients with relapsed myeloma.
• Clinical trial participation is how better therapy gets to myeloma patients. If a study is a good fit for your circumstances, get involved!!!

Conclusions
Thank you to our sponsors!








Upcoming IMF Events

Patient and Family Seminars
Minneapolis – July 18th & 19th – JW Marriott
Minneapolis Mall of America

Los Angeles – August 16th & 17th, 2024 – Hilton Los
Angeles/Universal City; please check the website for updates!

Thank you for attending today’s program!
June 22nd, 2024, International Myeloma Foundation’s
Regional Community Workshop –
Charlotte














































 Yelak Biru President & CEO, 28
Yelak Biru President & CEO, 28











 Myeloma 101
Myeloma 101