2024 Los Angeles Patient and Family Seminar
August 16-17, 2024



THANK YOU TO OUR SPONSORS!










August 16-17, 2024



THANK YOU TO OUR SPONSORS!









More than 1 year since diagnosis
Stem cell transplant recipient
Less than 1 year diagnosed


Hotel Guests: Please put parking on your room, and the IMF will cover this directly with the hotel.
Meeting Attendees: Please See Meghan at the registration desk for a voucher.









Myeloma Stompers
Meets virtually the 2nd Friday of each month at 10AM San Francisco Bay Area Myeloma Support Group
San Gabriel Valley Myeloma Support Group
Meets in-person the 1st Monday of each month at 6:30PM
Upland, CA Myeloma Support Group
Meets hybrid the 1st Friday of each month at 10AM

Meets virtually the 3rd Saturday of each month at 10AM

Westlake Myeloma Support Group
Meets virtually the 2nd Saturday of each month at 11AM
San Fernando Valley Myeloma Support Group
Meets in-person the 3rd Wednesday of each month at 7PM
Sacramento Area Myeloma Support Group
Meets virtually the 1st Saturday of each month at 10AM

San Diego Multiple Myeloma Support Group
Meets hybrid the 2nd Monday of each month at 6:30PM
Inland Empire, CA
Myeloma Support Group
Meets hybrid the 3rd Saturday of each month at 10:30AM
Orange County
Myeloma Support Group

Meets in-person every other month & virtually the alternate months on the 1st Thursday of each month


Los Angeles Multiple Myeloma Support Group
Meets virtually on the 3rd Saturday of each month at 10:30AM
Santa Cruz Multiple Myeloma Support Group
Meets virtually the 1st Monday of each month at 4:30PM
Rancho Mirage, CA
Myeloma Support Group
Meets virtually on the 1st Thursday of each month at 3PM

Special interest groups are designed as a supplemental support for specific populations of patients, in addition to their local Support Groups
Las Voces de Mieloma
Designed for Spanish speaking patients only

New!
Care Partners Only
Designed to address the needs of care partners only

Smolder Bolder
Created for people living with Smoldering Multiple Myeloma
Living Solo & Strong with Myeloma
Designed for patients without a care partner

High Risk Multiple Myeloma
Designed to address the needs of the high-risk MM population
MM Families
For patients/care partners with young children


Please be sure to complete your program evaluation today.
Questions 1 – 5 can be completed before the program begins.
Questions 7 & 8 can be answered after each presentation.
If you are attending Friday program only, we ask that you turn the survey in at the end of the day.
If you are coming back for the Saturday sessions, please hold onto your survey, bring it back tomorrow and turn it in at the end of the program.
We greatly appreciate your time and feedback!
Joseph Mikhael, MD, MEd, FRCPC, FACP

Chief
Medical Officer, International Myeloma Foundation
Professor, Translational Genomics Research Institute City of Hope Cancer Center

Teresa Miceli, RN, BSN, OCN
International Myeloma Foundation InfoLine Advisor & Nurse Leadership Board; Mayo Clinic-Rochester
Teresa Miceli, RN BSN OCN
International Myeloma Foundation - InfoLine Advisor, NLB Member, Support Group Leader (MMSS, Smolder Bolder)
Mayo Clinic – Myeloma Nurse Navigator


Individual Beliefs & Preference s
Transplant
Eligible Patients
Individual Care Partner
Family
Initial Therapy

Transplant (ASCT) Maintenance
Transplant
Ineligible Patients
Everyone
Social Network & Obligations
Treatment of Relapsed disease
Consolidation / Maintenance Continued therapy
Myeloma Symptom s & Treatment
Options
Supportive Care
Employm ent & Finances
beyond the clinical-trial setting: understanding the balance between efficacy, safety and tolerability,

“The aim of shared decisionmaking is to ensure that: - Patients understand their options and the pros and cons of those options.
- Patient's goals and treatment preferences are used to guide

https://www.ahrq.gov/cahps/quality-improvement/improvement-guide/6-strategies-for-improvin g/communication/strategy6i-shared-decisionmaking.html#6i1



Identify that a decision is needed: The HCP informs the patient that a decision is to be made and that the patient's opinion is important (Choice talk).
Understand the options:
The HCP explains the evidence-based options and their pros and cons. The patient expresses their preferences, and the HCP supports the patient in decision-making (Option talk).
Come to a decision:
The HCP and patient discuss the patient's wish to take part in the decision making and incorporate the patient's values and preferences into the decision (Decision talk).
Follow-up: Review and evaluate the decision, adjust as needed


Patients, regardless of age, want to be a part of treatment decision-making
Requires staying informed
Reduces uncertainty and alleviates concerns
Decisions reflect personal and family values
Promotes patient and care partner engagement and sense of empowerment
Positive impact on QOL and continuation on therapy

“The 'efficacy' of treatment means different things to different patients, and treatment decision-making in the context of personalized medicine must be guided by an individual's composite definition of what constitutes the best treatment choice.” Terpos, et al. Terpos, et al.
https://www.ahrq.gov/cahps/quality-improvement/improvement-guid e/6-strategies-for-improving/communication/strategy6i-shared-decisi onmaking.html#6i1



Disease-derived
Time: Stage, risk stratification, Urgent intervention needed vs time to consider options
Treatment: Availability/access, effectiveness, toxicity, current research
Patient-derived
Choon-Quinones, Mimi, Hose D, Kaló Z, Zelei T, Harousseau JL, Durie B, Keown P, Barnett M, Jakab I. Patient and Caregiver Experience Decision Factors in Treatment Decision Making: Results of a Systematic Literature Review of Multiple Myeloma Decision Aids. Value Health. 2023 Jan;26(1):39-49. doi: 10.1016/j.jval.2022.04.003. Epub 2022 May 22. PMID: 35613958.
Provider-derived
Time limitations
Support for patient involvement
Provider bias and preference
Understanding complex treatment options
Physical and emotional wellness
Comfort in speaking up “Doctor knows best”
Financial, Cultural and Religious factors
Care partner & social network, transportation
https://www.ahrq.gov/sites/de fault/files/wysiwyg/cahps/qua lity-improvement/improveme nt-guide/6-strategies-for-impr oving/communication/cahps-s trategy-section-6-i.pdf



























Consider your priorities
Consider your goals/values/preferences
Include your care partner/network in the discussion
Be a part of the conversation, create a dialog
Ask questions & Express your goals/values/preferences
Ask for time to consider options, if needed
Arrive at a treatment decision together
Arrange follow up to review and adjust the plan, if needed







Know the members of your care teamUnderstand their different roles
Myeloma specialist and General Heme/Onc
Primary care: for health screening, general check ups, vaccinations
Sub-specialists: specialty needs
Keep a contact list of your providers

Primary Care Provider (PCP)


Subspecialists






Medications: Bring a current list of prescribed and over-thecounter
Questions: Prioritize questions & concerns including financial issues
Paperwork needing medical signature (ex FMLA, prior authorizations)
Updates: Medical or life changes since your last visit
Symptoms: How have they changed (improved, worsened, stable)? Keep a symptom diary. Bring it along
Communicate effectively so your health care team can help
“Next Steps”: Future appointments, medication changes, plan of care. Ask for the information in writing or on your patient portal
Include a care partner, especially for pivotal appointments


Is telemedicine an option?
What is the process and what technology is needed?
Are labs needed in advance? Do you need an order?
Preparation is similar for “in-person” appointment PLUS:
Location: quiet, well-lit location with strong Wi-Fi is best
Yourself: Do you need to show a body part - wear accessible clothing
Vital signs (blood pressure, temp, heart rate, weight) selfserve blood pressure cuff is available at many pharmacies and for purchase
Include a care partner, especially for pivotal appointments Check with your healthcare team –

Care partners assist in many ways
Attending medical appointments, being present to learn and discuss possible treatment options and alert the medical team of side effects to treatment
Some treatment options available only if care partner support exists
Care partners can be one person or a rotation of many people
Building a partnership is based in good communication
Finding the balance:

- helping the patient with needed activities while maintaining a sense of independence
- allowing the care partner to have time for good self-care
Myeloma causes the highest burden of symptoms, most commonly effecting people of older age with other medical issues. Care partner support is valuable in SDM Terpos, et al. 2021; Soong, et al., 2023
Care Partner Tip Card https://www.myeloma.org/resource-library/tipcard-care-partners
Credit: https://www.mmtoldtrue.com/community/care-partner-corner






Over the next two days:
Evaluate where you are at in the process (What decisions need to be made?)
Absorb the information being presented (What are the options?)
Consider how the information impacts you and your family (What are your preferences?)
Create questions that will lead to better understanding (What more do I need to know before making a decision?)
Become an active member of your health care team


Bylund CL, Eggly S, LeBlanc TW, Kurtin S, Gandee M, Medhekar R, Fu A, Khurana M, Delaney K, Divita A, McNamara M, Baile WF. Survey of patients and physicians on shared decision-making in treatment selection in relapsed/refractory multiple myeloma. Transl Behav Med. 2023 Apr 15;13(4):255-267. doi: 10.1093/tbm/ibac099. PMID: 36688466.
Chari A, Romanus D, DasMahapatra P, Hoole M, Lowe M, Curran C, Campbell S, Bell JA. Patient-Reported Factors in Treatment Satisfaction in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Oncologist. 2019 Nov;24(11):1479-1487. doi: 10.1634/theoncologist.2018-0724. Epub 2019 Aug 1. PMID: 31371520; PMCID: PMC6853123.
Choon-Quinones, Mimi, Hose D, Kaló Z, Zelei T, Harousseau JL, Durie B, Keown P, Barnett M, Jakab I. Patient and Caregiver Experience Decision Factors in Treatment Decision Making: Results of a Systematic Literature Review of Multiple Myeloma Decision Aids. Value Health. 2023 Jan;26(1):3949. doi: 10.1016/j.jval.2022.04.003. Epub 2022 May 22. PMID: 35613958.
Rifkin RM, Bell JA, DasMahapatra P, Hoole M, Lowe M, Curran C, Campbell S, Hou P, Romanus D. Treatment Satisfaction and Burden of Illness in Patients with Newly Diagnosed Multiple Myeloma. Pharmacoecon Open. 2020 Sep;4(3):473-483. doi: 10.1007/s41669-019-00184-9. PMID: 31605300; PMCID: PMC7426337.
3718 Cytokine Release Syndrome: The Patient, Caregiver and Healthcare Professional Experience. Janelle Soong, Giuseppe De Carlo, Naziah Lasi-Tejani, Sumanjit K. Sethi, Natacha Bolaños, Martine Elias, Yelak Biru, Solène Clavreul, G. Scott Chandler, Klaus Finzler, Yann Nouet, Antonio Giuseppe Del Santo. Blood (2023) 142 (Supplement 1): 3718
Terpos E, Mikhael J, Hajek R, Chari A, Zweegman S, Lee HC, Mateos MV, Larocca A, Ramasamy K, Kaiser M, Cook G, Weisel KC, Costello CL, Elliott J, Palumbo A, Usmani SZ. Management of patients with multiple myeloma beyond the clinical-trial setting: understanding the balance between efficacy, safety and tolerability, and quality of life. Blood Cancer J. 2021 Feb 18;11(2):40. doi: 10.1038/s41408-021-00432-4. PMID: 33602913; PMCID: PMC7891472.
https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
https://www.ahrq.gov/cahps/quality-improvement/improvement-guide/6-strategies-for-improving/communication/strategy6i-shared-decisionmaking.ht ml#6i1



Wendy Thomas, RN, MSN, CHPN
Palliative Care Nurse Specialist, Kansas University Medical Center
KC Area Support Group Leader

Wendy Thomas, RN, MSN, CHPN



Wendy Thomas, RN MSN CHPN
• Outpatient Palliative Care
Nurse Navigator
• Kansas City Area Myeloma
Support Group Leader
About me:
• Nurse 27 years
• 14 years in blood and marrow transplant
• 8 years in palliative care
• 10 years as a myeloma support group leader
• Worked for the University of Kansas Health System for 17 years
• Based at the Bloch Cancer Care Pavilion, Westwood Kansas


• Discussing and preparing for future medical care decisions
• Important at any stage of life
• Crucial for anyone with a serious illness
• Goes into effect ONLY when you are unable to speak for yourself




• Who would you want to speak for you if you were unable to speak for yourself?
• Do your family/loved ones know what your wishes would be in a healthcare emergency?
• Do you know what your wishes would be?
• Are you confident they could carry out your wishes?


DPOA & Healthcare Directive


CA requires notary or two adult witnesses to signature



Code Status
• What is a Code Status?
– Cardiopulmonary Resuscitation or CPR
• Why do they keep asking?
– Code status expires at discharge
– Out of hospital DNR
– Living will
• How aggressive do you want care to be?
– ICU
– Mechanical Ventilation
– Medically administered nutrition


• No pulse, not breathing
• One of the few treatments that patients must choose to NOT have performed
• A physician order is to NOT perform CPR
• Older people and people with cancer may have & quality of life
• You can CHOOSE to allow a NATURAL death if you prefer




Pulse present &/or still breathing
• Full treatment – most aggressive ICU and intubation with mechanical ventilation
• Midlevel treatment – less aggressive Antibiotics, fluids, medication to support blood pressure, transfusions
• Best supportive care – least aggressive Treat with dignity and respect, comfort-focused medical treatment
• DNR doesn’t equal non-aggressive care


• Patients should complete with your healthcare provider
• Requires healthcare provider signature
• Original form stays with patient
• Copy should be provided to all of your healthcare providers/health systems
• Some states have transportable DNR laws




• Physician Orders for LifeSustaining Treatment
• Provides more control over end-of-life care to seriously-ill patients



• Make certain your family/loved ones know the location
• Give a copy to your healthcare providers/health systems
• Easy to find in case of emergency
• These documents DO NOT belong in your safe deposit box
• Fridge and beside table good locaitons


Planning ahead
• Eases the burden for family/loved ones
• Protects your assets
• Allows you to manage your personal effects

New Complications
• The electronic era brings new challenges
• Cellphones, computers, online accounts, social media and photos

• Bank Accounts
• Bill Pay

• Property Cell Phone
• Access
• Contacts

• Photos Passwords
• Account Log-in
• Social Media
• EVERYTHING!


Talking with your loved ones about your healthcare wishes brings comfort





National Healthcare Decision Day: April 16th
• Go Wish Cards
• ACP Bubble Map
• Coalition for Compassionate Care of CA
• Social Worker








Robin Tuohy Vice President, Patient Support

Return at 2:45
THANK YOU TO OUR SPONSORS!











Joseph Mikhael, MD, MEd, FRCPC, FACP
Chief Medical Officer, International Myeloma Foundation
Teresa Miceli, RN, BSN, OCN
International Myeloma Foundation Nurse Leadership Board Member
Joseph Mikhael, MD, MEd, FRCPC, FACP
Professor, Applied Cancer Research and Drug Discovery, Translational Genomics Research Institute (TGen), City of Hope Cancer Center
Chief Medical Officer, International Myeloma Foundation
Consultant Hematologist and Director, Myeloma Research, Phase 1 Program, HonorHealth Research Institute
Adjunct Professor, College of Health Solutions, Arizona State University


Teresa S. Miceli RN BSN OCN
Mayo Clinic, Rochester, MN
• Mayo Associate
• Assistant Professor of Nursing
• Myeloma Research RN Navigator
International Myeloma Foundation
• InfoLine Advisor
• Nurse Leadership Board
• Support Group Leader
NCI Myeloma Steering Committee








Rate of New Cases per 100,000 Persons by Race/Ethnicity & Sex How common is Myeloma?



Percent of New Cases by Age
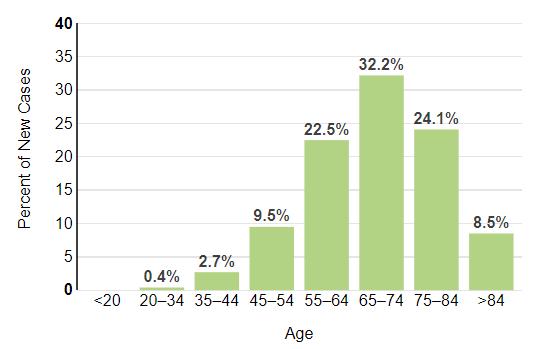
https://seer.cancer.gov/statfacts/html/mulmy.html; dated



Biochemical or Symptomatic Progression/Relapse
Environmental Factors:
• Exposure to some chemicals
• Radiation exposure
Examples:
Agent Orange
Burn pits
Pesticides, Herbicides
Firefighter/First Responder exposures
Individual Factors:
• Age
• Family History of related disorders
• Personal History of MGUS or SMM
• Obesity
In most cases, the honest truth
WE DON’T KNOW















Heavy Chain = M-Spike

cells





Condition MGUS1-4 (Monoclonal Gammopathy of Undetermined Significance)
1-5,8 (Smoldering Multiple Myeloma)
• AL-Amyloid
• POEMS
• Light or Heavy Chain Deposition Disease
• MGRS = Renal
• MGNS = Neuro
Presence of Myeloma Defining Events
Likelihood of progression
1. Kyle RA, et al. N Engl J Med. 2007;356:2582-90.
2. IMWG. Br J Haematol. 2003;121:749-57.
3. Jagannath S, et al. Clin Lymphoma Myeloma Leuk. 2010;10(1):28-43.
* In clinical trial
5. Mateos M-V, et al. Blood. 2009;114:Abstract 614.
6. Durie BG, Salmon SE. Cancer. 1975;36:842-854.
7. Durie BG, et al. Leukemia. 2006;20(9):1467-1473.



4. Kyle RA, et al. Curr Hematol Malig Rep. 2010;5(2):62-69.
8. Rajkumar SV, et al. Lancet Oncology 2014; 15:e538-e548.




Test Name
CBC + differential
Complete metabolic panel
Beta-2 Microglobulin (B2M)
Lactate Dehydrogenase (LDH)
Serum Immunofixation and Protein electrophoresis (SPEP+IFE)
Immunoglobulins (G, A, M, D, E)
Free light chain assay with kappa/lambda ratio
Urine immunofixation & protein electrophoresis (UPEP+IFE)
What it means

Hemoglobin, WBC, Platelets
Creatinine, Calcium, Albumin, Liver function
Part of staging and risk stratification
Measures the level of normal and clonal protein Identifies the type of clonal protein

Measures the level of normal and clonal protein Identifies the type of clonal protein





Imaging:
– Skeletal survey: Series of X-rays; less sensitive than other techniques
– Whole body low dose (CTWB-LD CT )
– Positron Emission Tomography (PET/CT)
– Magnetic Resonance Imaging (MRI)

Healthy bone versus myeloma bone disease









Bone marrow genetics
• Cytogenetics
• Fluorescence in situ hybridization (FISH)
• Next generation sequencing (NGS)







International Staging System (ISS) (only β2M and albumin)
Result
β2M < 3.5 mg/L; serum albumin ≥ 3.5 g/dL 2 β2M < 3.5 mg/L; serum albumin < 3.5 g/dL; or β2M 3.5 to 5.5 mg/L, irrespective of serum albumin
Risk FISH Results*









Transplan t Eligible Patients
Transplan t Ineligible Patients Consolidation / Maintenance Continued therapy Everyone Supportive Care


Clinical Experience Research Results Your Preference TREATMENT DECISION





(thalidomide)
(lenalidomide)



Peptide Drug Conjugate*
BCMA Targeted Antibody Drug
Conjugate (ADC)*
Bispecific Antibodies
(Melphalan Flufenamide)
Blenrep (belantamab mafodotinblmf) Bela, Belamaf, or B
Abecma (idecabtagene vicleucel)
Carvykti (ciltacabtagene vicleucel)
Tecvayli (teclistimab)
Talvey (Talquetamab)
Elrexfio (Elranatamab)
Cevostamab, Iberdomide, Mezigdomide, Venetoclax
Linvoseltamab, LCAR-B38M, ABBV-383 ……………………………
* These agents are currently off the market but available through special programs



























Negative by next generation flow (NGF) (minimum sensitivity 1 in 10-5 nucleated cells or higher)*
mCR AND normal Free Light Chain ratio, Bone Marrow negative by flow, 2 measures
CR AND negative PCR
Complete Response: Negative immunofixation (IFE); no more than 5% plasma cells in BM; 2 measures
Very Good Partial Response: 90% reduction in myeloma protein
Partial Response: at least 50% reduction in myeloma protein
Minimal Response
Progressive Disease: At least 25% increase in identified myeloma protein from lowest level Stable Disease: Not meeting above criteria
MRD = Minimal Residual Disease
sCR = Stringent Complete Response; BM = Bone Marrow


Kumar, S., Paiva, B., Anderson, K. C., Durie, B., Landgren, O., Moreau, P., ... & Dimopoulos, M. (2016). International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. The lancet oncology, 17(8), e328-e346.
Biochemical or Symptomatic Progression/Relapse
• Not every relapse requires immediate therapy
• Each case is different
Symptomatic or extramedullary disease
Asymptomatic biochemical relapse on 2 consecutive assessments
Consider Treatment
Patient-/Disease-Specific Monitor Carefully
Asymptomatic high-risk disease or rapid doubling time or extensive marrow involvement Consider Observation Monitor Carefully
Initiate Treatment




Bi-Specific Antibodies
Talquetamab






Antibody Drug
Elotuzumab
Bi-Specific Antibodies



Bi-Specific Antibodies
Antibody Drug
Daratumumab and Darzalex Faspro Isatuximab

Immune Therapies
Ide-cel CAR-T
Cilta-cel CAR-T
Teclistamab
Other CAR-Ts
Other Bi-Specific Antibodies



VD
Rev/Dex
CyBorD
VTD
VRD
KRD
D-VMP
DRD
Tandem ASCT (?)
Nothing
Thalidomide?
Bortezomib
Ixazomib
Lenalidomide
Combinations
D-VRD
Isa-VRD
D-KRD
Isa-VRD “More” induction?
Daratumumab?
Carfilzomib?
Lenalidomide + PI
ASCT, autologous stem cell transplant; CAR, chimeric antigen receptor; Cy, cyclophosphamide; d- daratumumab; D/dex, dexamethasone; isa, isatuximab; K, carfilzomib; M, melphalan; PDL1, programmed death ligand-1; PI, proteasome inhibitor; Rev, lenalidomide; V, bortezomib.
Speaker’s own opinions.
Bortezomib
Lenalidomide
Carfilzomib
Pomalidomide
Selinexor
Panobinostat
Daratumumab
Ixazomib
Elotuzumab
Isatuximab
Belantamab mafodotin*
Melphalan flufenamide*
Idecabtagene autoleucel
Ciltacabtagene autoleucel
Teclistamab, Talquetamab
Elranatamab
CAR T Cell Therapy
Bispecific/Tri-specific Antibodies
Cell Modifying Agents
Venetoclax*
PD/PDL-1 Inhibition?
Small Molecules


* These agents are currently off the market but available through special
Biochemical or Symptomatic Progression/Relapse


Control is the immediate priority with active disease Cure remains the overall goal
Defining “Cure” has many considerations:
Minimal Residual Disease Negative (MRD-)
Time Off Therapy
Functional Cure
Requiring Treatment Stable or Unmeasurable Disease, Receiving Treatment
Unmeasurable Disease, Receiving No Treatment Active Disease




















Danielle Doheny Director of Public Policy and Advocacy, International Myeloma Foundation
Informing and influencing policymaking on the critical healthcare issues that directly impact myeloma patients.

Principles Priorities Grassroots






We believe in the value of working to improve healthcare policies to ensure there are no barriers to care as patients navigate their myeloma journey.

The IMF is the voice of the myeloma community in Washington, DC - informing and influencing critical policy decisions.


Our advocacy priorities are determined by the following three principles:
1. Ensure Equitable Access to Care
2. Eliminate Financial Barriers
3. Secure Research Funding

1. ENSURE ACCESS TO CARE
INSURANC
E REFORM: DRUG ACCESS
INSURANC
2. ELIMINATE FINANCIAL BARRIERS
3. SECURE RESEARCH FUNDING Step Therapy Protocols Safe Step Act
H.R. 2630 / S. 652
E REFORM: DRUG ACCESS PBM Reform PBM Reform Act
H.R. 5378 / S. 1339
INSURANC E REFORM: COINSURANCE
INSURANC E REFORM: COPAYS Copay Accumulators HELP Copays Act H.R. 830 / S. 1375 Oral Parity Cancer Drug Parity Act H.R. 6301 / S. 2039
FEDERAL FUNDING
ANNUAL APPROPS
Annual Appropriations
NIH: National Cancer Institute, National Institute on Minority Health, ARPA-H
CDC: Comprehensive Cancer Control Initiative
DoD: Congressionally Directed Medical Research Program (CDMRP) for Myeloma.
MEDICARE REFORM:
PHYSICIAN ACCESS
Tele-Health/Medicine
Telehealth Modern. Act
H.R. 7623 / S. 2016
MEDICARE REFORM: ANNUAL COST LIMITS
Inflation Reduction Act implementation Cap & Smoothing (MPPP), Drug Pricing
CLINICAL TRIAL DIVERSITY
Focus on underserved, POC, rural settings and socioeconomically disadvantaged groups




Grassroots Advocacy is the critical component to influencing policy decisions
The IMF brings advocates to Capitol Hill to share their experience with lawmakers.
Together, we champion legislative priorities that directly impact the lives of millions of patients and elevate the voices of of the myeloma community.
The IMF Grassroots Advocacy Program is multi-faceted and growing
• Advocacy Training & Leadership Development
• Policy and Legislative Education
• Grassroots Campaign Planning
• Health Policy Forums & Roundtables
• Advocacy Resource Development
• Storytelling and Personal Narratives





No copays for vaccines under Part D
Insulin copays limited to $35/month


Expanded Eligibility for the Federal Extra Help Program (Low-Income Subsidy Program) to help pay premiums, deductibles, coinsurance, etc.
$3,250 annual cap (approx.) on out-of-pocket spending for prescription drugs under Part D (eliminating 5% coinsurance in catastrophic phase)

$2,000 Annual Cap in out-of-pocket spending for prescriptions under Part D
Option for a monthly payment program to “smooth out” total out-of-pocket spending throughout the year, with an overall monthly maximum
• Patients will need to enroll into the program (opt-in)
• The earlier in the year you join the program, the more you can benefit
• Your monthly bill may fluctuate somewhat
• No one will pay more than $2000 for the year
$ Thousands Per Year




Congressional “Lobby Day” Focus on Cancer Research Funding
Lobby Congress during annual appropriations process and ask for increased research funding via:
1. NIH (National Cancer Institute, National Institute on Minority Health & Health Disparities, ARPA-H)
2. CDC: Comprehensive Cancer Control Initiative
3. DOD: Congressionally Directed Medical Research Program (CDMRP) (myeloma-related research)

Congressional Briefing Focused on Oral Parity
Educate Congressional staff about the issue of Oral Parity and request their support to co-sign/advance the Cancer Drug Parity Act.
• One of the most widely attended Congressional Health Briefings of 2024 (attendance was 3x the average)
• Met with staff from key Congressional Committees
• Possible “IMF Lobby Day” in Sept. to move legislation









Joseph Mikhael MD, MEd, FRCPC
Chief Medical Officer, International Myeloma Foundation
Professor, Translational Genomics Research Institute, City of Hope Cancer Center
Yelak Biru
President and CEO , International Myeloma Foundation




• Provide The Rationale For Clinical Trials
• Outline The Phases Of Clinical Trials
• Discuss The Risks And Benefits Of Clinical Trials
• Listen To Patients Who Have Been On A Clinical Trial



Remember some of the important principles of clinical trials:
• The drive of research has brought us to where we are
• No one is expected to be a “guinea pig” with no potential benefit to them
• Research is under very tight supervision and standards


• Open, clear communication between the physician and the patient is fundamental






MYTH: If I participate in a clinical trial, I might get a placebo, not active treatment
MYTH: If I participate in a clinical trial, I can’t change my mind
• Phase 1 and 2, everyone gets active treatment
• Phase 3 standard of care vs new regimen: often standard regimen with/without additional agent in MM trials
• Patients can withdraw their consent for clinical trial participation at any time

MYTH: Clinical trials are dangerous because they have new medicines and practices
• Some risk is involved with every treatment, but medicines are used in clinical trials with people only after they have gone through testing to indicate that the drug is likely to be safe and effective for human use



MYTH: Clinical trials are expensive and not covered by insurance
• Research costs are typically covered by the sponsoring company
• Standard patient care costs are typically covered by insurance
• Check with clinical trial team/insurers; costs such as transportation, hotel, etc may not be reimbursed and are paid by patient


• Every patient is unique and must be viewed that way
• Benefits of trials are numerous and include:
• Early access to “new” therapy
• Delay use of standard therapy
• Contribution to myeloma world – present and future
• Financial access to certain agents
• Must be balanced with potential risks
• “Toxicity” of side effects
• Possibility of lack of efficacy


Identify a target for therapy in the laboratory
Confirm the anticancer activity in laboratory and animal studies
Clinical trials (human studies) to determine safety, dosing and effectiveness
The whole process costs millions of dollars and years of effort!

• Most agents are tested in lab models
• Various “myeloma cell lines”, also known as “in vitro”
• Next step is animal model
• We are more like mice than you think!!
• Earliest study in Phase I is called “First in Human”
• Often uses extremely low dose of drug to ensure safety




• All patients receive the experimental therapy
• Phase 1 trials find the optimal dose of a new drug or drug combination
• Patients get higher doses as the study continues
• Determine side effects of new drugs or combinations


• Explore how the drug is metabolized by the body
• Important for all stages of myeloma



• Determine if a new drug or combination is effective against the cancer
• May be added to a Phase 1 study once the ideal dose is found
• Patients usually receive the experimental therapy


• In some cases, the study may include two “arms” comparing either two different doses or a different treatment (another combination of drugs)



• Highest form of clinical evidence.
Typically, a large number of patients are required… usually required for full FDA approval
• Patients receive either an experimental therapy (one or more drugs) or the current standard treatment
o The patient is randomly assigned to a treatment—a process called “randomization”


o Neither the physician or the patient can determine which treatment is given
• May be placebo controlled, if no standard treatments are available
• Very closely monitored for effectiveness and side effects



ANIMAL STUDIES: Examine safety and potential for efficacy


FIRST INTRODUCTION OF AN INVESTIGATIONAL DRUG INTO HUMANS
• Determine metabolism and PK/PD actions, MTD, and DLT
• Identify AEs
• Gain early evidence of efficacy, studied in many conditions; typically, 20 to 80 patients; everyone gets agent
EVALUATION OF EFFECTIVENESS IN A CERTAIN TUMOR TYPE
• Determine short-term AEs and risks; closely monitored
• Includes up to 100 patients, typically
GATHER ADDITIONAL EFFECTIVENESS AND SAFETY INFORMATION
COMPARED TO STANDARD OF CARE
• Placebo may be involved if no standard of care exists; hundreds to several thousand patients
• Often multiple institutions; single or double blind; sometimes open label
AGENTS IN NEW POPULATIONS OR NEW DOSE FORMS



Possible benefits:
• Patients will receive, at a minimum, the best standard treatment


• If the new treatment or intervention is proven to work, patients may be among the first to benefit
• Patients have a chance to help others and improve cancer care



• New treatments or interventions under study are not always better than, or even as good as, standard care
• Even if a new treatment has benefits, it may not work for every patient


• Health insurance and managed care providers do not always cover clinical trials



Patients may:
• Be unaware of clinical trials
• Lack access to trials
• Fear, distrust, or be suspicious of research
• Have practical or personal obstacles
• Face insurance or cost problems
• Be unwilling to go against their physicians’ wishes


• Not have physicians who offer them trials
• Have a disconnect with their healthcare team



There has been a lack of diverse representation in clinical trials in myeloma.
• In the U.S., approximately 20% of all myeloma patients are of African descent, but only 5%–8% of patients in myeloma clinical trials are of African descent.
This is significant for the following reasons:
• All patients of all races and ethnicities should be able to benefit from clinical trials.
• Diverse patient representation in clinical trials is required to ensure that the outcomes are applicable to all patients.


Reasons for underrepresentation in clinical trials are complex and include:
• systemic racism, accessibility of clinical trials, sensitivity to diversity by medical professionals
• misconduct in medicine in the past, the lack of trust in the system, and more.

[P]eople from racial and ethnic minorities and other diverse groups are underrepresented in clinical research. This is a concern because people of different ages, races, and ethnicities may react differently to certain medical products.
– FDA

Leadership and commitment


Community engagement practices
Investigator hiring, training, and mentoring practices



Patient engagement practices
FDA = US Food and Drug Administration.
Regnante JM, et al. J Oncol Pract. 2019;15(4):e289-e299. FDA website. Clinical Trial Diversity. Accessed March 27, 2024. https://www.fda.gov/consumers/minority-health-and-health-equity/clinical-trial-diversity.
US Cancer Centers of Excellence: Strategies for Increased Inclusion of Racial and Ethnic Minorities in Clinical Trials
How does the study work? How often will I need to see my doctor or visit the cancer center?
Will I need to undergo additional tests?
What is currently known about the new drug or combination?


What benefits can I expect?
What side effects should I expect? Who should I notify if I have side effects?


Can I take my vitamins or other medications?
Can I get the treatment with my local doctor?
Will my insurance pay for my participation in the clinical trial?





• Discuss with your physician if you are eligible for a clinical trial
• Work with your physician to determine the best trial for you
• Meet with the clinical research nurse or trials coordinator to discuss the trial
• Carefully review the provided “Informed Consent”
• Describes the study and any potential safety concerns related to the experimental medication













• Day 1 Recap
• Welcome Reception and Networking: Club Room
• Day 2 Announcements
• Saturday breakfast at 7:00 – 8:00 am: Ballroom A
• Program begins at 8:00 am
• Hotel check-out is 11:00 am

THANK YOU TO OUR SPONSORS!








