Myeloma



Volume 22 Number 4 Fall 2022
Today
TECVAYLI™ (teclistamab-cqyv) Receives Accelerated Approval
•
•
Oncology
®
A publication of the International Myeloma Foundation
This edition of Myeloma Today is supported by Amgen
Bristol Myers Squibb • Janssen Oncology • Karyopharm Therapeutics • Legend Biotech • Sanofi
Takeda
for Patients in Late Relapse: Dr. Adam Cohen discusses treatment with ABECMA
or CARVYKTI® for patients with myeloma after 4 or more prior lines of therapy. PAGE 7
The first-in-class bispecific BCMA-directed CD3 T-cell engager TECVAYLI TM Myeloma cell T cell Tecvayli activates the T cell to release cytotoxic granules that kill the myeloma cell
for Patients in Early Relapse: Dr. Joseph Mikhael discusses the surge in options for patients with myeloma after 1–3 prior lines of therapy. PAGE 6












The latest information on COVID-19 variants and vaccination for myeloma patients covid19.myeloma.org Diversity and inclusion are integral aspects of the myeloma community diversity.myeloma.org Contact the IMF InfoLine with your myeloma-related questions and concerns infoline.myeloma.org Learn about FDA-approved therapies for myeloma medications.myeloma.org Robin Tuohy rtuohy@myeloma.org will help you find a multiple myeloma support group support.myeloma.org Myeloma is the most common blood cancer in the Black community. Learn the risks and symptoms of myeloma, and how to get the best care. Watch Now UPDATED AND INTERACTIVE RESOURCES AT A GLANCE Connect. Be Informed. Take Charge. Take advantage of the hyperlinks in Myeloma Today by signing up for the digital edition at subscribe.myeloma.org, where you can also sign up to receive alerts about IMF events, webinars, teleconferences, and advocacy actions, as well as our e-newsletter Myeloma Minute And engage with us on social media! /myeloma @IMFMyeloma
Booklets that explain myeloma therapies and more • Tip cards on topics important to myeloma patients
Guide to Myeloma Acronyms and Abbreviations
Guide to Myeloma Terms and Definitions
Myeloma Today Summer 2022 edition publications.myeloma.org videos.myeloma.org IMF Chairman, Chief Scientific Officer, and myeloma expert Dr. Brian G.M. Durie discusses MRD and what it means for a patient to be MRD-negative. Watch Now M-POWER New York Virtual Webinar
•
•
•
•
Dear Reader,
In October, the IMF’s Asian Myeloma Network (AMN) held a comprehensive program of in-person activities in Singapore, including the two-day AMN Summit, the second AMN Master Class, and the second AMN Patient Support Seminar. Each event was received with enthusiasm and excitement. These were the first in-person AMN activities since the beginning of the COVID-19 pandemic, but a hybrid component was still incorporated in order to enable participants from China and Japan to attend virtually.
Led by Dr. Wee Joo Chng (Director, National University Health System, Singapore), Dr. Chandramouli Nagarajan (Singapore General Hospital), and Dr. Daryl Tan (Mount Elizabeth Novena Hospital, Singapore), the Singapore team hosted all the programs wonderfully. Joining yours truly was an international faculty with Dr. S. Vincent Rajkumar (Mayo Clinic, Rochester, MN), Dr. Thomas Martin (University of California San Francisco, CA), and Dr. Jean Luc Harousseau (University of Nantes, France). The IMF Team in attendance to contribute to the wellexecuted series of activities included Daniel Navid (Senior VP, Global Affairs), Diane Moran (Senior VP, Strategic Planning), Lisa Paik (Executive VP, Medical Affairs), and Annabel Reardon (Senior Director, Strategic Program Management).
At the second AMN Patient Support Seminar, discussions centered on support requirements and inter-regional cooperation. Dr. Tan led the discussions on the medical side, including how to further enhance patient support throughout Asia. The patients were actively engaged in their interaction with the experts.

I was amazed to find a chapter titled “Doctor Durie’s Recipe for Life and Cancer” in a book by Ravinder Singh. Having never put forward any type of “recipe,” it was astounding to see how a presentation can be interpreted in this fashion. A key element was my response to a question about mental attitude. I strongly supported the notion that a positive mental attitude is needed to achieve best outcomes and can have a beneficial impact on one’s well-being. It was humbling to sense the importance of my perspective to the author.
As I noted in my closing remarks in Singapore, we are at an inflection point – a moment when plans that have been in discussion for years are coming to fruition and carrying the AMN programs strongly forward. There’s a great sense of invigoration and expectation that the IMF’s Asian Myeloma Network can now dream bigger and aim for more impact as it gains prestige in the global myeloma community. The sky’s the limit in what may be learned and achieved. Onwards to 2023 and beyond!
The goal for the second AMN Master Class was to integrate global information into practical guidelines for myeloma care in Asia. Structured clinical case discussions included data summaries, test results, imaging, and more. The robust patient information made it possible for the faculty to offer precise recommendations. The AMN Master Class is an innovation in Singapore, and the young doctors were excited to hear about expert care in myeloma. Of note, the AMN was pleased to welcome doctors from the Philippines and Vietnam as first-time participants.
Dr. Brian G.M. Durie IMF Chairman Chief Scientific Officer

Please turn to page 14 to read more about the 2022 AMN events in Singapore in the report by Daniel Navid.



1.800.452.CURE toll-free in USA and Canada 1.818.487.7455 worldwide FALL 2022 3 This free issue of Myeloma Today© (Volume 22, Number 4) is dated November 15, 2022. Myeloma Today© is a quarterly (Spring, Summer, Fall, and Winter) publication of the International Myeloma Foundation, located at 4400 Coldwater Canyon Avenue, Suite 300, Studio City, CA 91604 USA A Message from the IMF Chairman & Chief Scientific Officer
Ravinder Singh, author of “The Art of My Life,” with Dr. Brian G.M. Durie
Susie Durie and Dr. Brian G.M. Durie receive a joint 2019 Honorary Doctorate for Scientific Excellence from the Vrije Universiteit Brussel
Esteemed faculty at dinner with the young doctors taking part in the second AMN Master Class
Dear Friends, Greetings!

Since January 2022, I have had the honor to serve as President & CEO of the International Myeloma Foundation. It is a privilege to support the IMF’s mission of improving the quality of life of patients with myeloma while working toward prevention and a cure for the second-most common blood cancer in the world.

The IMF is the first and largest organization focused specifically on myeloma, with a reach that extends to more than 525,000 members in 140 countries. For 31 years, the IMF has been dedicated to the interests of myeloma patients as we pursue our initiatives in Research, Education, Support, and Advocacy. These four founding principles have guided the IMF throughout the course of our existence.
The day you read this, 94–95 people in the U.S. will be diagnosed with myeloma. As a member of the patient community, I feel an especial urgency to accelerate the IMF in fulfilling its mission. We are just on the cusp of achieving our reason for being – finding a cure for myeloma.
My goal is to help write the story of the year 2030, paying it forward and carrying the baton as far and as fast as possible so those who come after me, those who come after us, can go even further. With you by our side, as our partner and our supporter, the IMF can achieve this vision.

My personal journey with myeloma began in 1995, at age 25. I was working toward my graduate degree, newly married and anticipating a full life ahead. A persistent cough seemed like nothing more than a minor annoyance. Then a call from my doctor changed my life forever. “You have multiple myeloma,” he said. “You may not make it to age 30.”

My treatment options were aggressive chemotherapy and a transplant. My family crammed into the small room where I began chemo. Even with their unwavering support, the future looked bleak… and short. Thankfully, I have now lived with myeloma for 27 years, more than half of my life.
As needed, I continue to receive treatment while I pursue my life goals, including being of service to the myeloma community. I am truly grateful to have made it this far, and I am also thankful to no longer be an anomaly. More and more patients are living with myeloma longer than seemed possible at the time of my diagnosis, and the IMF continues to help patients like me live well despite myeloma.
Biru Myeloma Patient IMF President & CEO
info@myeloma.org myeloma.org 4 FALL 2022
Letter from the IMF President & Chief Executive Officer
Yelak
Yelak Biru with Dr. Brian G.M. Durie (IMF Chairman & Chief Scientific Officer) and Dr. Joseph Mikhael (IMF Chief Medical Officer)
Yelak and his wife, Loul Haugs
Yelak has represented the U.S. as a member of the IMF’s Global Myeloma Action Network since GMAN’s inception, when he served as co-leader of the North Texas Myeloma Support Group (NTMSG)
TECVAYLI™ (teclistamab-cqyv) Receives FDA Approval




The first bispecific antibody is now available to patients in the clinical setting!
 By Dr. Joseph Mikhael IMF Chief Medical Officer
By Dr. Joseph Mikhael IMF Chief Medical Officer
On October 25, 2022, the U.S. Food and Drug Administration (FDA) granted accelerated approval to the drug teclistamab (Tecvayli™) for patients with relapsed or refractory myeloma who have had at least 4 prior lines of therapy, including a protea some inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. Teclistamab is the first-in-line bispecific B-cell maturation antigen (BCMA)-directed CD3 T-cell engager.
The term “bispecific” means that the drug has two “arms.” One arm attaches to the myeloma cell through the BCMA on the sur face of the myeloma cell. The other arm attaches to and activates a local T cell to destroy the myeloma cell. This is a remarkable method of employing a patient’s own immune system to fight against their myeloma. Teclistamab is a highly advanced form of immunotherapy.
Response rates
Most drugs that are used to treat myeloma – in a clinical setting and not in a clinical trial – have had response rates in heavily pretreated patients of approximately 25%–30%. Teclistamab, which was studied in patients with very advanced relapsed myeloma, had an overall response rate (ORR) of 61.8%. This impressive result will make it very attractive for our patients.
Teclistamab was evaluated in the MajesTEC-1 multi-center clinical trial. The efficacy population consisted of 110 patients with myeloma who had previously received at least 3 prior therapies, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody, but had not received prior BCMA-directed therapy. As noted above, the ORR was 61.8%. With a median follow-up of 7.4 months among responders, the estimated duration of response (DOR) rate was 90.6% at 6 months and 66.5% at 9 months.
Treatment with teclistamab
Teclistamab is an “off-the-shelf” drug. It can be given to a patient directly, as opposed to the process of CAR T-cell therapy that requires the harvesting of T cells from the patient in advance. The off-the-shelf availability of teclistamab may facilitate the use of this drug more widely, especially for patients who do not have access to a CAR T-cell therapy center.
However, like with every myeloma drug, there are some challenges and risks. To receive their first doses of teclistamab, most patients will initially have to be admitted to the hospital for
approximately 2 weeks. This is necessary because the drug can cause a reaction called cytokine release syndrome (CRS), where the body responds to the process of activating the T cells. For most patients, CRS is mild, but some patients will require more intensive support in the hospital setting.
Other notable side effects can include neurological toxicities and low blood counts. It is also important to note that ongoing treatment with a bispecific antibody increases the risk of infections, and it is critical to monitor and rapidly treat infections. After the first few doses of teclistamab are administered, treatment can be continued on an outpatient basis. All treatment centers that provide teclistamab must adhere to a Risk Evaluation and Mitigation Strategy (REMS) program that monitors the risks of treatments.
Important new option in the clinic
Myeloma patients and their doctors now have a new and important treatment option. The very high response rate with teclistamab could benefit many patients in a unique and sustained way. And we are not done yet! More bispecific antibody myeloma therapies are in development – some have different targets on the myeloma cell – and each new drug brings with it the potential to further help in our goal to fight and cure myeloma. This is very exciting for the myeloma community! MT
See the Spring 2022 edition Myeloma Today to read Dr. Mikhael’s article on bispecific antibodies and stay tuned for his next #WHEREISDRJOE column. Please contact the IMF InfoLine with your myeloma-related questions and concerns. Phone lines are open 9 a.m. to 4 p.m. (Pacific) Monday through Friday at 1.800.452.CURE in the US and Canada or 1.818.487.7455 worldwide. You can also email InfoLine@myeloma.org to submit your query electronically.
1.800.452.CURE toll-free in USA and Canada 1.818.487.7455 worldwide FALL 2022 5 Scientific & Clinical
CD3
BCMA
TECVAYLITM (teclistamab-cqyv)
tumor-speci c surface antigens myeloma cell
T cell
Tecvayli activates the T cell to release cytotoxic granules that kill the myeloma cell
Tecvayli binds to CD3 on the surface of the T cell
Tecvayli binds to BCMA on the myeloma cell surface
Decisions, Decisions, Decisions
Myeloma treatment options in early relapse
By Dr. Joseph Mikhael IMF Chief Medical Officer
These are very exciting times in myeloma – so many new therapies being developed, new drug combinations being used in frontline therapy, new imaging and diagnostic techniques, and so much more! But what is often forgotten is the surge in options in early relapsed disease. We typically think of “early” relapse as the treatment someone receives when they have had 1–3 prior lines of therapy. This is opposed to “late” relapse when patients have been treated with most available options.
I wanted to highlight this part of the journey for myeloma patients, in part because there are more treatment options than ever before. Indeed, we have no fewer than FIVE phase III clinical trials of triplet combinations recently published and now available. Another reason is to demonstrate an important shift in our approach to myeloma – we no longer “save the best for last” but use the most effective approach for the individual patient earlier in the disease course, including in frontline therapy, as we know it will have a greater impact on the patient’s long-term survival.
As myeloma relapses, it becomes harder to control, so giving patients longer remissions with effective combination therapies early on is a growing and important trend. This is a key topic of discussion as it may be challenging to choose when the menu of options is expanding. Of course, this is a good problem to have, as it was not long ago that we had so few options to control this awful disease. So what are these great choices? These are the five key clinical trials that have shaped the treatment of early relapse:
1. IKEMA clinical trial of Isa-Kd
This clinical trial of Sarclisa® (isatuximab), a CD38-directed monoclonal antibody, in combination with Kyprolis® (carfilzomib) and the steroid dexamethasone (Isa-Kd) is important as it demonstrates the longest progression-free survival (PFS) that we have seen in the early relapse setting, recently reported as 35.7 months. Achieving an average 3 years of PFS in relapsed myeloma is remarkable. It does require more visits to the clinic, though, as Sarclisa is given every other week after the first month (when it is given weekly). Sarclisa is still given intravenously, although after the first 2 infusions, it is given over only 75 minutes. In the IKEMA study, the Isa-Kd combination was shown to be superior when compared to Kd alone.
2. APOLLO clinical trial of DPd
The recently approved combination of Darzalex® (daratumumab) + Pomalyst® (pomalidomide) + dexamethasone (DPd) has been extensively studied. It is attractive because Darzalex Faspro® can be given as a short subcutaneous (under the skin) injection, as opposed to how Darzalex was previously given as a long intravenous infusion. This, coupled with the orally
administered Pomalyst makes DPd considerably more convenient. DPd was shown to be superior to Pd alone.
3.
BOSTON clinical trial of XVd
This unique combination incorporates the oral drug Xpovio® (selinexor), previously given in later lines of therapy but now available earlier on in the disease course. Administered weekly, as opposed to twice weekly, has made Xpovio much easier to tolerate, especially when given with two anti-nausea medications, as the drug is known to cause significant nausea. Xpovio, a nuclear transport inhibitor, has a new mechanism of action that prevents good tumor suppressors from leaving the cell nucleus. This combination also employs Velcade® (bortezomib) subcutaneously and on a weekly schedule, plus dexamethasone (XVd). The XVd combination was shown to be superior to Vd alone.
4.
CANDOR clinical trial of DKd
Although the combination of Darzalex + Kyprolis + dexamethasone (DKd) has been available for a few years, it remains an important option. Although the CANDOR study used Kyprolis twice-weekly, it is now commonly used on a weekly schedule (three weeks on, one week off) and the approval for the DKd regimen allows for either twice-weekly or once-weekly dosing. DKd is commonly used when Darzalex has not been used in the frontline setting. The DKd triplet was shown to be superior to Kd alone.
5. ICARIA clinical trial of Isa-Pd
The regimen of Sarclisa + Pomalyst + dexamethasone has been available for a few years, but it remains an important option in early relapse. The long-term follow up of the ICARIA study was presented at the IMS Annual Meeting in August 2022, showing an overall survival (OS) benefit of about 7 months when compared to Pd alone.
So, how do we decide among all of these options? Of course, this requires careful discussion with your doctor. Below are a few principles to help in making the important decision:
Do not continue the maintenance drug when relapsing. Although we “can” just add a drug to the one someone is on, we typically discontinue the drug someone is on while relapsing, as it is likely not going to help anymore.
Introduce a mechanism of action not yet seen.
One of the ways we overcome the “resistance” the disease develops to therapies is to introduce a drug that has a different method (or mechanism of action) of attacking the myeloma cells. This gives a better chance for a deeper and more durable response.
Try to use a triplet when possible.
We have consistently seen in the trials above the superiority of triplets over doublets, so we tend to prefer this more intensive approach.
info@myeloma.org myeloma.org 6 FALL 2022 Scientific & Clinical (continues on page 8)
#WHEREISDRJOE
CAR T-cell Therapy in Myeloma Myeloma
Today in conversation with Adam
Dr. Cohen, please tell us about CAR T-cell therapy and how it is used in myeloma.
Chimeric antigen receptor (CAR) T-cell therapy is a new approach to using the patient’s own immune system to attack that patient’s myeloma. CAR T-cell therapy is a very targeted, patient-specific approach to treating myeloma. One advantage is that it’s really a “one and done” treatment. After the T cells are infused, you just let them do their work. Patients who achieve remission can go without treatment for quite some time. CAR T-cell therapy may offer a break to patients with relapsed or refractory myeloma, who are otherwise rarely able to go off treatment.
Who is a candidate for CAR T-cell therapy?
Currently, the only two CAR T-cell products that are approved for myeloma by the U.S. Food and Drug Administration (FDA) are Abecma® (idecabtagene vicleucel or ide-cel) and Carvykti® (ciltacabtagene autoleucel or cilta-cel). Both of these target a molecule on myeloma cells called BCMA (B-cell maturation antigen), and are approved for patients who have had at least 4 prior lines of treatment [4 different treatment regimens], including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody. CAR T-cell therapy as currently approved is an option in late relapse for patients who don’t have a lot of treatment options left.
What is the treatment process for the patient?
The first step is we collect the patient’s T-cells through a procedure called apheresis. The patient’s blood goes through a machine that selects out the T cells while the rest of the blood is immediately returned back into the patient. The apheresis procedure takes a few hours to complete. This is similar to harvesting stem cells for transplant, except that collecting T cells does not require the patient to have any injections prior to the apheresis procedure.
What happens to the collected T cells?
They are sent to a manufacturing company where they’re transduced [modified] to express a new receptor on their surface. These T cells are engineered to recognize the patient’s myeloma cells and to become myeloma-killing cells. They are then grown in large numbers, a manufacturing process that can take 4 to 6 weeks. Often during this time, the patient will get “bridging” therapy [additional anti-myeloma therapy] to try and keep their myeloma in check.
After the manufacturing process is complete, the cells are delivered to the patient’s treatment center. Typically, we need to temporarily lower the patient’s normal white blood cell count in order to allow the engineered CAR T cells to expand after they’re infused, so patients are given 3 days of lymphodepletion
D. Cohen, MD
[chemotherapy] on an outpatient basis. Typically, the regimen used for lymphodepletion in myeloma is cyclophosphamide, a classic chemotherapy, with fludarabine added to improve outcomes.
And then the CAR T-cell product is infused?
Depending on the patient, the product being infused, and the specific treatment center, the patient may be admitted. At my center, in some cases we will begin to administer Carvykti on an outpatient basis, then admit the patient on day 5. In other cases, we admit the patient right away and administer Abecma. The patient’s engineered T cells are infused into the vein, similar to receiving a transfusion. Then we wait for those T cells to seek out and kill the myeloma cells wherever they may be in the body.
What about the response rates?
Abecma was FDA-approved on the basis of the KarMMa clinical trial, which demonstrated an overall response rate (ORR) of 73%, with 33% of patients achieving a complete remission (CR). At the highest dose level [450 million cells, the recommended dose in the FDA approval], the ORR and CR rates were closer to 80% and 39%, respectively.
With Carvykti, the ORR was 98%, higher than with Abecma. In the CARTITUDE-1 clinical trial upon which the FDA approval of Carvykti was based, roughly 80% of study patients achieved CR, demonstrating remarkable efficacy. Still, it’s hard to compare the KarMMa and CARTITUDE-1 studies, due to the differences in the enrolled patient populations, so we can’t say that one is definitively better than the other.
Please tell us about the potential toxicities.
The number one toxicity of both Abecma and Carvykti is cytokine release syndrome (CRS), which is seen in 85% to 95% of patients. This is a reflection of the CAR T cells getting activated by the myeloma cells. When CAR T cells begin to multiply, cytokines are released into the bloodstream. Cytokines are proteins that are normally released in response to infection. This can mimic a significant infection, such as a really bad flu with high fever, muscle aches, weakness, and/or loss of appetite. In some cases, patients can develop low blood pressure, difficulty breathing, or low oxygen levels.
There’s not an actual infection, but it feels like it. If this occurs, it’s usually within the first week of treatment, which is why patients are hospitalized – to manage these potential effects. Mild reactions can be managed with over-the-counter medications. More severe CRS can be treated with an effective biological therapy called tocilizumab (toci). It’s very important that patients choose treatment centers with experienced teams who know how to manage the potential toxicities.
1.800.452.CURE toll-free in USA and Canada 1.818.487.7455 worldwide FALL 2022 7 Scientific & Clinical
(continues on next page)
#WHEREISDRJOE – CONTINUED FROM PAGE 6
Assess disease, treatment, and patient factors.
These three are fundamental to therapy selection. Disease factors include how aggressive the relapse is, the risk status and the location of disease (such as plasma cell leukemia or extra-medullary disease). Treatment factors include route of administration, expected side effects, and the efficacy of the regimen. Patient-related factors include comorbidities, prior side effects, cost, and social considerations.
Always match the option with patient preference.
This is absolutely critical, especially now that we have more options. The patient should be a key partner in the decision-making process, something we call “shared decision-making.”
Until we reach a cure for myeloma, we will continue to treat relapsed disease. We have learned how important it is to treat early relapse effectively to provide patients both a longer quantity and better quality of life. I am thankful to see the list of options growing and look forward to even more to come! MT
Stay tuned for Dr. Mikhael’s next #WHEREISDRJOE column and contact the IMF InfoLine for help with your myeloma-related questions and concerns. Phone lines are open 9 a.m. to 4 p.m. (Pacific) Monday through Friday at 1.800.452.CURE in the US and Canada or 1.818.487.7455 worldwide. You can also email InfoLine@myeloma.org to submit your query electronically.
CAR T-CELL THERAPY – CONTINUED FROM PAGE 7
Does the treatment of CRS reduce the high response rates?
No, it doesn’t seem to affect the ability of CAR T cells to kill myeloma cells. In fact, the majority of patients in the clinical trials experienced CRS and yet they still achieved very high responses.
What are the other potential toxicities?
Less common than CRS, the second toxicity to watch out for is neurotoxicity. This effect on the neurologic system is seen in about 20% of patients. It is usually fairly mild and transient, ranging from headaches to mild confusion. Some patients experience word-finding difficulties. In rare cases, neurotoxicity can progress to more serious problems like seizures. There have been deaths related to this. If neurotoxicity occurs, it is usually in the first week after infusion. Neurotoxicity is managed with “toci” if it occurs in conjunction with CRS, and it also seems to respond to steroids like dexamethasone.
CRS and neurotoxicity are the upfront risks of CAR T-cell therapy. There are later side effects like low blood cell counts that can sometimes persist for weeks or, in rare cases, a couple of months after treatment. There is an increased risk of infections because the immune system is suppressed, and a patient may receive preventive antibiotics or a monthly IVIG infusion of good antibodies to help boost the immune system.
What is the durability of remissions after CAR T-cell therapy?
With Abecma, the median duration of response at the highest level was around 12 months. With Carvykti, the median duration of response is looking like it’s closer to 2 years or maybe even 27–28 months. There are patients whose disease hasn’t come back, and it’s been 3–4 years since their CAR T-cell therapy. Unfortunately, there are also some patients who do get a response, but the disease starts to come back within 3-4 months.
What factors are predictive of response?
The big challenge in the field right now is to come up with better predictors of who is likely to get a very durable remission, and who is likely to get a shorter one. We’re not quite there yet.
There are tumor-related features that can be associated with increased risk of relapse. High-risk cytogenetics continue to be a bad prognostic factor. Extramedullary disease, when the myeloma has learned to grow outside the bone marrow, has been associated with shorter duration of remission.
Part of it is the tumor biology, but the other part may be that patients who’ve had a lot of prior lines of therapy may have dysfunctional T cells or low T cell counts. Such patients may make a CAR T-cell product that doesn’t expand as well or kill the myeloma cells as effectively. This may be associated with relapse as well.
We’re stepping up the bridging therapy for patients with a higher tumor burden, trying to get the myeloma under control before beginning the CAR T-cell process.
Please share an update on CARTITUDE-2. CARTITUDE-2 is a multi-cohort phase II clinical trial that is studying the efficacy and toxicity of Carvykti in several different myeloma patient populations.
Cohort A patients had been less heavily treated, with 1 to 3 prior treatment lines and disease progression while on Revlimid® (lenalidomide). Most patients had already had a stem cell transplant. About 60% had been exposed to Darzalex® (daratumumab). The response rates to Carvykti were very impressive: 95% responded (19 out of 20 patients), 90% achieved CR, and 16 patients who tested negative for minimal residual disease (MRD).
The follow-up is still relatively short, with the median follow-up of 17 months showing that about 70% of patients (14 out of 20) were still in remission without any sign of myeloma coming back.
info@myeloma.org myeloma.org 8 FALL 2022
Scientific & Clinical
(continues on next page)
Unfortunately, one patient died due to COVID-19 and one due to sepsis. We are awaiting longer follow-up to see if the durability of response is going to be improved compared with the CARTITUDE-1 clinical trial that studied patients who were more heavily treated. This cohort provided the rationale for an ongoing phase III randomized CARTITUDE-4 clinical trial comparing Carvykti vs. a doctor’s choice of one of two standard myeloma therapies [DPd or VPd] in patients with 1 to 3 prior treatment lines.
What was learned from Cohorts B and C?
Cohort B was comprised of patients whose myeloma relapsed within 18 months after initial treatment or within 12 months of transplant. This is a group with poor prognosis, which represents an unmet need in myeloma. Data was presented earlier this year by one of my colleagues, Dr. Niels Van de Donk. The response rate to Carvykti was around 95%, with 85% achieving CR, and follow-up is ongoing.
Overview of the mechanism of action (MOA) of chimeric antigen receptor (CAR) T-cell therapy
1. The patient’s T cells are collected from the bloodstream; 2. the T cells are modified to produce receptors on their surface that recognize the patient’s myeloma cells; 3. the T cells are manufactured in large numbers; 4. the engineered T cells are reinfused into the patient to seek out and kill the patient’s myeloma cells.

5. reinfused T cells seek out and kill the patient’s myeloma cells.
Cohort C was a small group of heavily pretreated patients (median 8 prior lines of treatment) with progressive disease after prior treatment with a different BCMA-targeted therapy. At a median follow-up of 11.3 months, the ORR was around 60%, and 7 of 10 evaluable patients were able to achieve MRD-negativity, demonstrating efficacy of Carvykti after prior exposure to a BCMA-directed agent. The median durability of response was 8–11 months, and the toxicity profile was similar to what we have already seen with Carvykti.
After Carvykti, what are the options in late relapse?
Treatment of late relapse in myeloma is highly individualized, based on what therapies the patients have had before and how well they tolerated them, as well as their blood counts, etc. An emerging approach is to move from one targeted therapy to another. If a patient had CAR T-cell therapy, then relapsed after a remission, we may decide on a different drug that’s already FDAapproved for late-line treatment of myeloma – such as Xpovio® (selinexor) or Blenrep® (belantamab mafodotin) – or we may consider a clinical trial with a novel agent or a novel mechanism of action (MOA).
One drug that just received accelerated approval from the FDA is Tecvayli™ (teclistamab-cqyv), the first bispecific T-cell engager antibody for the treatment of patients with relapsed
or refractory myeloma, which is given as an injection under the skin. It activates the patient’s T cells in the bone marrow to kill the myeloma cells that are also there in the bone marrow. With Tecvayli, the T cells won’t have to be collected and engineered as with Abecma and Carvykti. As an off-the-shelf drug, Tecvayli could do this in a less complicated way. There are least 4 or 5 other bispecifics currently in development, plus several more agents that hit different targets. There’s a rich pipeline of drugs that are far along in development!
In closing, please summarize the potential of CAR T-cell therapy in early relapse. We’re testing the use of CAR T cells in early relapse, before the patients have exhausted all other treatment options, to see if this may lead to more durable responses. I think that using CAR T cells earlier in the disease course may help an even higher proportion of patients in complete response to also achieve MRD-negativity. We need larger studies and more data before we reach a definitive conclusion about the use of CAR T-cell therapy in an earlier relapse setting. Until then, we will continue to reserve it for patients in late relapse. MT
Dr. Cohen is Director of Myeloma Immunotherapy and Associate Professor of Medicine at the University of Pennsylvania, the first center to treat myeloma with CAR T-cell therapy. Dr. Cohen’s research expertise is in cancer immunotherapy and cellular therapy. The IMF thanks Janssen Oncology and Legend Biotech for their support of Myeloma Today.
1.800.452.CURE toll-free in USA and Canada 1.818.487.7455 worldwide FALL 2022 9
Scientific & Clinical
CAR T-CELL THERAPY – CONTINUED FROM PAGE 8
Reprinted with permission from Penn Medicine
Kevin Brigle, PhD, ANP VCU Massey Cancer Center
Donna D. Catamero, ANP-BC, OCN®, CCRC Mount Sinai Health System
Kathleen Colson, RN, BSN, BS Dana-Farber Cancer Institute
Deborah Doss, RN, OCN® Dana-Farber Cancer Institute
Beth Faiman, PhD, MSN, APNBC, AOCN®, BMTCN®, FAAN, FAPO Cleveland Clinic Taussig Cancer Institute
Elizabeth Finley-Oliver, MSN, ARNP, AGNP-BC
H. Lee Moffitt Cancer Center & Research Institute
Charise Gleason, MSN, NP-BC, AOCNP® Winship Cancer Institute
Michaela Hillengass, RN, ACSM-CPT Roswell Park Comprehensive Cancer Center
Tracy King, PhD, MN, RN Royal Prince Alfred Hospital, Australia
Rebecca Lu, MSN, FNP-C MD Anderson Cancer Center
Patricia A. Mangan, RN, MSN, APRN-BC Abramson Cancer Center
Ann McNeill, RN, MSN, APN John Theurer Cancer Center at HUMC
Teresa Miceli, RN, BSN, OCN® Mayo Clinic-College of Medicine
Kimberly Noonan, DNP, ANP-BC, AOCN® Dana-Farber Cancer Institute
Amy Pierre, RN, MSN, ANP-BC Memorial Sloan Kettering Cancer Center
Tiffany Richards, PhD, ANP-BC, AOCNP® MD Anderson Cancer Center
Sandra Rome, RN, MN, AOCN®, CNS Cedars-Sinai Medical Center
Mary Steinbach, DNP, APRN Huntsman Cancer Institute
Joseph D. Tariman, PhD, MBA, ANP-BC, FAAN Rutgers University
Daniel Verina, DNP, RN, ACNP-BC
Mount Sinai Medical Center
NLB annual meeting
NLB Members Gather for Annual meeting is
By Diane Moran IMF Senior Vice President, Strategic Planning
From September 16 through 18, 2022, the NLB members gathered in Boston for “Meeting XVIII” – our 18th annual meeting! Co-chaired by Beth Faiman and Donna Catamero, it was the first in-person NLB annual meeting in three years, and all participants expressed delight at being face-to-face once again to carry on the mission of the NLB – to improve the nursing care and self-care of patients with myeloma.
For 31 years, the IMF has been a leader and innovator in myeloma. The IMF is always first on every front: starting clinical conferences, educating physicians, and launching a worldwide educational program for patients and care partners. The NLB was founded by the IMF as the first professional partnership to represent oncology nurses who are experts in the care of patients with myeloma. Each year, NLB nurses meet to focus on the following agenda:
Recognizing and celebrating NLB accomplishments from the past year, Engaging in presentations and discussions that inform evolving and/or unmet needs in myeloma,
Planning and working on future NLB projects.
Donna Catamero gave a presentation on the 16-year history of the NLB. “Through our outreach we’ve impacted thousands of nurses and have directly or indirectly affected the lives of hundreds of thousands of patients. We educate nurses through our symposia, videos, and collaborative white papers and numerous other publications. For patients and care partners, we participate in seminars and workshops, develop educational videos, and hold collaborative roundtables to gain patient and care partner insights.”
The NLB nurses contributed to many activities over the course of the past year, including the Oncology Nursing Society (ONS) symposium, IMF Patient & Family Seminars, IMF Regional Community Workshops, IMF “Living Well with Myeloma” teleconferences, support group meetings, and roundtable discussions. The nurses shared personal and professional accomplishments from the past year, and were recognized for their contributions with plaques and NLB lapel pins.
The scientific and clinical updates are always highly anticipated at NLB meetings. Dr. Andrew Yee (Massachusetts General Hospital) gave a thorough scientific update on myeloma and new drugs in development, including agents such as venetoclax, bispecific antibodies, and CELMoDs. Dr. Brian G.M. Durie (IMF
held in-person for
Chairman and Chief Scientific Officer) gave an update on research in mass spectrometry and on the IMF’s Black Swan Research Initiative® (BSRI®).
Mimi Choon-Quinones (IMF Senior VP, Global Advocacy, Policy, Research, and Access) gave an overview via video of the IMF’s Beyond Medicines’ Barriers (BMB) initiative. Mimi explained the current focus of the BMB initiative on minimal residual disease (MRD) research that generates real-world data, including implementing MRD testing in Ghana and an EMEA (Europe, the Middle East, and Africa) disease registry, understanding what is important to patients and enabling the creation of a framework for reimbursement decisions, and the Multiple Myeloma Whole Disease Model to be used to support fair and equitable access to testing and treatment. Mimi invited and encouraged the NLB nurses to be involved in the BMB initiative.
Amy Pierre gave a presentation on structural racism, explaining how historical policies and laws have implications for the inequities we see today in Black patients with myeloma. “Racial inequities, and their ensuing socioeconomic and health consequences persist because of deeply rooted unfair systems that sustain the legacy of formally discriminatory practices,” Amy explained. She gave examples of structural racism, which includes political disempowerment, segregation, financial practices, environmental injustice, the criminal justice system, and data aggregation (which frequently does not encompass diverse populations).
Social determinants of health, which include economic stability, neighborhood and physical environment, education, food, community and social context, and the healthcare system, account for 30%–55% of health outcomes and help explain why disparities exist among minority communities. Amy then explained that approximately 15% of the U.S. population is Black but less than 5% of patients in clinical trials are Black. This inequity is one of the contributing factors in the persistence of racial disparities in healthcare because it is difficult to apply conclusions drawn from clinical trials to minority populations when they are insufficiently represented in those studies.
Donna Catamero then spoke about her experiences with the Mount Sinai Health System opening clinical trials in community locations across the New York City area. She noted that they have opened six additional, more-accessible trial sites, and enrolled several patients in myeloma clinical trials.
Mary Steinbach gave a presentation about Gaps in Care and Transitions of Care for Bispecifics and Cellular Immunotherapy. “The problem that we want to address is there are barriers to awareness, access,
info@myeloma.org myeloma.org 10 FALL 2022 Nurse Leadership Board
“Meeting XVIII” the first time in 3 years!
actual treatment, and then follow-up for patients, caregivers, and the healthcare team in regard to bispecific antibodies and cellular immunotherapies,” Mary explained. From her review of the literature, she highlighted the barriers that patients, their care partners, and community healthcare professionals can encounter, and proposed resources that the NLB could develop to help overcome these barriers.
The NLB nurses then broke out into groups to work on patient and provider toolkits, publications related to cell-directed therapies and novel combinations, as well as diversity in clinical trials. Each group reported on their progress at the end of the day.
Tracy King joined the meeting via video from Australia to discuss myeloma treatment in her country. We also welcomed our newest member, Rebecca Lu, presenting her with an NLB lapel pin.
The NLB thanks the IMF and the sponsors for their support, and I personally thank each NLB nurse and guest for their dedication to improving the care and self-care of patients with myeloma.

Tiffany Richards presented on CAR T-cell therapy and bispecifics in myeloma, and
Rebecca Lu presented on Waldenström macroglobulinemia (WM) and AL amyloidosis.
All the cases presented highlight patient management scenarios encountered by APs in clinical practice, such as risk stratification and treatment of the newly diagnosed patient, sequencing of therapy at relapse, ocular toxicity management, and management of hyperviscosity syndrome. The selected cases illustrate concepts of survivorship and supportive care, as well as shed light on the importance of clinical trial participation and diversity. By completing the activities in the supplement, APs can gain continuing education credits and will be able to:
Discuss symptoms and appropriate management associated with myeloma treatment regimens for patients at different stages, from newly diagnosed through heavily pretreated.
JADPRO supplement
Historically, NLB activities have always included work on a variety of educational publications, including myeloma textbooks and professional journal supplements. In this regard, the past year has been no different.
Published in July 2022, the NLB created a continuing education supplement on myeloma for the Journal of the Advanced Practitioner in Oncology (JADPRO), a professional publication for advanced practitioners (APs). The NLB’s Beth Faiman and Tiffany Richards served as guest editors for the supplement entitled Multiple Myeloma and Plasma Cell Disorders: Update on Diagnosis, Prognosis, Treatment, and Supportive Care
Authored by members of the NLB, the JADPRO supplement features Grand Rounds articles that use a case-based approach to discuss topics of importance. The following publication chapters were reviewed by the authors at the NLB annual meeting: Kevin Brigle presented on newly diagnosed myeloma,

Kim Noonan presented on relapsed myeloma,
Mary Steinbach presented on special populations in relapsed myeloma,
Develop awareness of healthcare disparities and psychosocial impacts faced by patients with myeloma.
Employ strategies for improving patient adherence and satisfaction with treatment outcomes via approaches such as setting expectations and shared decision-making.
As the data on plasma cell disorders – myeloma, AL amyloidosis, and WM – continue to grow and new diagnostic and monitoring strategies gain broader acceptance, it will be even more important for APs to be aware of the emerging data, management of unique side effects, and evolving strategies in the diagnosis and monitoring of patients with myeloma.
Visit advancedpractitioner.com/supplements to view the JADPRO supplement. MT
Visit nlb.myeloma.org to learn how the NLB is improving the nursing care and self-care of patients with myeloma via publications, symposia, multimedia, and research.
1.800.452.CURE toll-free in USA and Canada 1.818.487.7455 worldwide FALL 2022 11
Nurse Leadership Board members at “Meeting XVIII”
The 23rd Annual IMF Support Group Leaders Summit
By Robin Tuohy IMF Vice President, Support Groups






The IMF provides educational guidance to a network of support groups that empower patients and care partners with information, insight, and hope. Every year, support group leaders are invited to a Summit hosted by the IMF. The 23rd Annual IMF Support Group Leaders Summit was the first hybrid program, giving participants the option of attending virtually or in person in Phoenix, Arizona.
For four days in September, 114 support group leaders engaged in the hybrid program, representing 77 myeloma support groups. In all, 59 leaders attended in person and 55 attended virtually. We were thrilled to welcome 28 first-time attendees and to welcome back 86 veteran leaders. Our effective safety protocols ensured that all in-person participants remained protected from COVID-19.
After my welcome to all in-person and virtual attendees, Yelak Biru (IMF President & Chief Executive Officer) shared his “Story of the Future” – a fitting perspective for the Summit and of how the IMF is pressing forward into a world where myeloma will no longer be a disease without a cure.

Medical updates





Medical information is always of great interest to myeloma patients and care partners. On the first day of the Summit, Dr. Brian G.M. Durie (IMF Chairman & Chief Scientific Officer) gave a riveting update about the IMF’s research initiatives. On the last day of the Summit, Dr. Durie was joined by Dr. Yi Lin (Mayo Clinic, Rochester, MN) for a discussion of immunotherapies in myeloma. In separate sessions, Dr. Joseph Mikhael (IMF Chief Medical Officer) presented recent developments in available myeloma therapies, and the accomplishments of the IMF Nurse Leadership Board (NLB) were presented by Beth Faiman, PhD. These expert presentations demonstrated the scope of the IMF’s leadership in the field of myeloma.
Resilience
“The Myeloma Rollercoaster” was the theme of the session by Sue Dunnett, PhD (University of Edinburgh). For more than 20 years, Sue has been conducting research on resilience, both physical and mental. She is now training the IMF’s Support Group Team to give virtual and in-person presentations on resilience and how to use the worksheet tool that she’s created.
Importance of collaboration
While most Summit attendees represented U.S. support groups, the topic of international collaboration made a strong impression. As Dr. Durie stressed, what is learned from myeloma research being conducted abroad will benefit U.S. patients. The IMF’s Advocacy initiative in the U.S., which was reported on by Danielle Doheny (IMF Director, Public Policy & Advocacy), is another example of working collaboratively to advocate for the interests of patients with myeloma. The programs and services of the IMF’s Global Myeloma Action Network were discussed in a presentation by Serdar Erdogan (IMF Director, GMAN and European & Middle Eastern Patient Programs), proving to be of great interest – many Summit participants were not aware that infrastructure for clinical trials is absent in some countries, and that the IMF is trying to help with this. Also, the IMF’s Beyond Medicines’ Barriers initiative is now part of the Support Group Leader Toolkit.
Digital resources & social media




The world changed with the onset of the COVID-19 pandemic. We’ve learned to build and sustain new connections in new ways. The IMF’s Support Group Leader Toolkit has been part of that trajectory, and Jon Fitzpatrick (IMF Technology & Coordination, Support Groups) reviewed how to use it most effectively. The more we share with one another on this platform, the more beneficial it is to all.
Part of this dynamic is how social media continues to evolve as a powerful tool to amplify the voice of the myeloma community.






info@myeloma.org myeloma.org 12 FALL 2022 Support Groups
The 2022 IMF Support Group Leaders Summit brought
Summit Hybrid Program: a Big Success!





Peter Anton (IMF VP, Marketing) quantified for us how social media has increased the IMF’s capacity, and Stephanie Smith gave a stepby-step tutorial of how to use social media strategically to engage with myeloma doctors, nurses, and other patients and care partners. Digital resources and social media connect local support groups with the larger myeloma community and also help with outreach.
Diversity
“Support Group Engagement Among African Americans” was presented by Tiffany H. Williams (Support Group Leader, Orangeburg, SC). In a separate session, Dr. Mikhael addressed the IMF’s M-Power project, which aims to improve access to care and treatment outcomes of patients with myeloma in the Black community. Myeloma is the most common blood cancer in African Americans, who have a greater-than-average risk of developing myeloma but, when barriers to early diagnosis and treatment are removed, have outcomes that are as good or better than Caucasian patients.
Patient panel
Three myeloma patients with very different experiences shared their journeys with myeloma and answered questions from their peers. Nick Lenoir, who was diagnosed in 2016 at the age of 31, has had an allogeneic transplant and CAR T-cell therapy. Thomas Goode, who was diagnosed in 2005 at the age of 34, has shown tremendous resilience through a multitude of complications and went back to school during the pandemic to get another degree. Barbara Davis shared her treatment experience, the benefits of which have given her the quality of life to enjoy time with her granddaughter.

Robust agenda
The IMF team (Nancy Bruno, Kelly Cox, Sherrie Guerrero, and Kelley Sidorowicz) led discussions about the art of leadership and how to best respond to challenging scenarios that may arise in their groups. Kelley Sidorowicz (IMF Regional Director, Support




Groups) gave a stretch exercise class each day of the Summit –visit wellness.myeloma.org to try her video meditations and yoga exercises. The Summit also included presentations by our industry partners: Amgen, Janssen, Pfizer, and Takeda.


Breakout meetings



In advance of the 2022 Summit, the IMF held six online breakout sessions. Unlike prior years, when breakouts were held concurrently and leaders would attend one session of their choice, this year’s online breakouts were held sequentially pre-Summit, making it possible for leaders to attend multiple sessions. Many Summit participants chose to attend them all! Volunteer patients and care partners later presented a summary of each breakout topic at the live Summit. Subsequently, takeaways from the breakouts were incorporated into the Support Group Leader Toolkit.
Coping with loss






Leaders of the San Fernando Valley Myeloma Support Group, grief counselor Malcolm Katz and social worker Sally Weber, led the session on coping with loss. They talked about ambiguous loss – when you’re no longer able to do or perform or be who you were prior to the myeloma diagnosis – as well as physical loss. Shortly before this session began, we experienced the sudden passing of the leader of the Rhode Island Multiple Myeloma Support Group, Mirna Betania Sangiovanni (not from myeloma). The Summit agenda had planned for the session on loss, but it became crucial for us to have each other’s support in coping with the loss of Mirna.
The 2022 IMF Support Group Leaders Summit was an invaluable learning and networking experience, which was made possible by the IMF team, all the participants, and educational grants from Abbvie, Amgen, BMS, GSK, Jack’s Education Grant, Janssen Oncology, Karyopharm, Pfizer, Regeneron, and Takeda. MT
1.800.452.CURE toll-free in USA and Canada 1.818.487.7455 worldwide FALL 2022 13
together 114 support group leaders representing 77 groups
IMF’s Asian Myeloma Network Surges Ahead
By Daniel Navid IMF Senior Vice President, Global Affairs

The IMF established the Asian Myeloma Network (AMN) at a meeting in Singapore in 2011. The first of its kind in the region, the AMN has taken the lead in projects to assist the IMF in providing physician education and patient support throughout Asia. From October 13 through 16, 2022, the AMN returned to Singapore to hold three major in-person meetings, the first in-person events after two years of virtual events. Additional participants joined virtually from studios in China and Hong Kong.
AMN Summit
The AMN Summit continues to set the standard as the key myeloma forum for Asian physicians. The sixth AMN Summit was attended by 114 participants from 10 Asian countries and regions (China, Hong Kong, Japan, Korea, Malaysia, Philippines, Singapore, Taiwan, Thailand, and Vietnam). Following the model of the IMF’s International Myeloma Working Group (IMWG), the AMN Summit featured expert presentations, debates and discussions, and a series of working group sessions focused on the following myeloma research priorities in Asia:
Immunotherapy (co-chaired by Drs. Juan Du and Thomas Martin),
Treatment of high-risk myeloma (co-chaired by Drs. Wee Joo Chng and James Chim),
MRD assessment (co-chaired by Drs. Wenming Chen and Brian G.M. Durie), and
Bone/imaging in Asia (co-chaired by Drs. Kazuyuki Shimizu and Chandramouli Nagarajan).
The Summit opened with a keynote address by Dr. Sigurður Kristinsson of the iStopMM (Iceland Screens, Treats, or Prevents Multiple Myeloma) research project, the first large-scale population study in myeloma. Spearheaded by the IMF’s Black Swan Research Initiative® (BSRI®), iStopMM is of great interest in Asia, and efforts are underway to assess if a similar project might be developed in the region.
In the first session, Drs. S. Vincent Rajkumar and Daryl Tan debated frontline therapy approaches. This was followed by Drs. Juan Du and Thomas Martin comparing CAR T-cell therapy with other immunotherapies. Next, Dr. Brian G.M. Durie presented a global overview of MRD assessment and MRD educational needs in Asia, Dr. Stephen Harding delivered an update on mass spectrometry, Dr. James Chim reviewed relapse/refractory issues, Dr. Wee Joo Chng shared information on new treatments and clinical trials, Dr. Jens Hillengass talked about imaging techniques, and Dr. Jean Luc Harousseau offered an analysis on drug access challenges. Reports on the status of AMN clinical trials and the AMN Virtual Tissue Bank were also presented. A final lively discussion was focused on priority needs for treatment in Asia, as well as priorities for AMN work into the coming year. Drs. Chng and Durie noted the following AMN efforts:
1) The identification of new AMN clinical trials, incorporating MRD, and finding ways to enroll patients who are usually excluded from trials;
2) Research projects arising out of the AMN Virtual Tissue Bank; 3) The identification and treatment of high-risk myeloma, including a clinical trial;
1. Drs. Wee Joo Chng, Brian G.M. Durie, and Chandramouli Nagarajan;

2. Dr. S. Vincent Rajkumar;
3. Daniel Navid;
4. Dr. Sigurður Kristinsson;

5. Daniel Navid extends a warm welcome to the myeloma experts at the sixth AMN Summit in Singapore


6. Dr. Thomas Martin;
7. The hybrid component of the live AMN events in Singapore made it possible for many additional participants to attend from remote studios and individual sites in China and Japan;


info@myeloma.org myeloma.org 14 FALL 2022 International
Events
1 2 3 4 7 6 5
4) Development of Asian Resource Stratified Imaging Guidelines; 5) Strengthened partnership with AMN’s academic contract research organization (CRO) Singapore Clinical Research Institute (SCRI);
6) Involving young myeloma doctors via the AMN Master Class and other initiatives; and
7) Myeloma patient support throughout Asia.
AMN Master Class
The second AMN Master Class training course brought together 95 physicians who treat patients with myeloma in China, Hong Kong, Korea, Malaysia, Philippines, Singapore, Taiwan, Thailand, and Vietnam. The renowned faculty was led by Dr. Brian G.M. Durie and included Dr. Wee Joo Chng, Dr. Jean Luc Harousseau, Dr. Jeffrey Huang, Dr. Thomas Martin, Dr. Chandramouli Nagarajan, Dr. S. Vincent Rajkumar, and Dr. Daryl Tan.
The program featured a review of clinical cases and lectures on myeloma in Asia, diagnostic criteria, prognostic factors and risk stratification, immunotherapies, and treatment options for both newly diagnosed and relapsed/refractory patients. An examination was administered with certificates issued to the successful participants. Very positive feedback has encouraged the IMF to expand this program in the future.
AMN Patient Forum
The second AMN Patient Forum, chaired by Dr. Daryl Tan, brought together 81 myeloma patients and care partners in person from


Korea, Malaysia, Singapore, and Thailand, along with additional participants who joined virtually from China, Hong Kong, and Taiwan. There has been notable progress in the establishment of and work in each of these locations, as well as valuable insights about future needs for myeloma patient support. An “Asian Tool Kit” to enhance the work of the local societies is being developed. Follow-up projects are also being pursued. MT
The seventh AMN Summit, along with the next AMN Master Class and the AMN Patient Forum will be held October 18–22, 2023, in Bangkok, Thailand. Visit amn.myeloma.org for more information about AMN initiatives and activities.

8. The team hosting the sixth AMN Summit: Drs. Daryl Tan (Mount Elizabeth Novena Hospital, Singapore), Chandramouli Nagarajan (Singapore General Hospital), and Wee Joo Chng (Director, National University Health System, Singapore);

9. Dr. Jean Luc Harousseau;
10. The 2022 AMN Master Class faculty: Drs. S. Vincent Rajkumar, Thomas Martin, Jean Luc Harousseau, Wee Joo Chng, and Brian G.M. Durie;
11. Expert faculty and graduating participants of the 2022 AMN Master Class.

1.800.452.CURE
in
1.818.487.7455 worldwide FALL 2022 15
toll-free
USA and Canada
8 9 10 11
Participants of the 2022 AMN Patient Forum
Do You Know #kNOwMyeloma? Blood Cancer Awareness Month campaign reaches more than 17 MILLION in 37 countries!
 By Peter Anton IMF Vice President, Marketing
By Peter Anton IMF Vice President, Marketing
The IMF’s 2022 Blood Cancer Awareness Month (BCAM) campaign brought global recognition of myeloma as the second-most common type of blood cancer. The IMF’s actions engaged the myeloma community and inspired the general public to advocate on behalf of those most affected by the disease.

Throughout the month of September, the IMF asked one simple question: “Do You Know Myeloma?” This set the tone for the IMF’s 2022 BCAM initiatives, which centered on two key objectives:
1. KNOW Myeloma which seeks to raise awareness about the disease, and
2. NO Myeloma which aims to help eradicate myeloma by inspiring the community to advocate for the IMF’s mission of working toward prevention and a cure.
KNOW Myeloma
The KNOW Myeloma objective sought to increase knowledge about the disease by educating both the general public and those already living with myeloma. These are just some of the actions taken:
Providing essential information about myeloma, including signs and symptoms, the importance of early diagnosis, as well as important tests and more.
Providing information about the IMF’s broad range of myeloma-related resources for patients, care partners, and loved ones.
Raising awareness about the incidence of myeloma in the Black community.
Encouraging the use of the IMF’s InfoLine, which answers myeloma-related questions and concerns in a caring and compassionate manner.
Promoting engagement with the IMF’s Support Group team, which connects people with groups that play a crucial role in empowering patients and care partners with information, insight, and hope.
NO Myeloma

NO Myeloma aimed to help eradicate the disease by inspiring people to advocate for the IMF’s mission of working toward prevention and a cure. The IMF’s advocacy efforts throughout the campaign included the following:
Sharing updates on the IMF’s research initiatives, specifically on the Black Swan Research Initiative® (BSRI®) and iStopMM, the largest-ever population-based study evaluating myeloma and its earlier precursor states MGUS and SMM.
Raising awareness of approved and new treatment options, such as CAR T-cell therapy and bispecific antibody immunotherapy.
Providing information about the International Myeloma Working Group’s (IMWG) Virtual Biobank and Immune Therapy Registry to encourage tissue sampling from myeloma patients.
Advocating for early detection and diagnosis in the Black community, which is at a higher risk for myeloma.
info@myeloma.org myeloma.org 16 FALL 2022 Education & Awareness
Bringing the IMF’s M-Power project to the collective consciousness, with the objectives of improving the short- and long-term outcomes of Black myeloma patients, as well as breaking down barriers for the Black myeloma community.

Sharing the news on drug approvals by the FDA and data from ongoing myeloma clinical trials.
Advocating for increased funding for the IMF’s myeloma research initiatives.
The #kNOwMyeloma Campaign

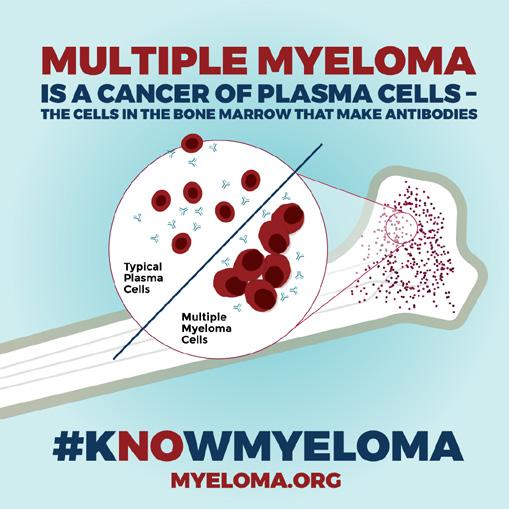

Complementing our social media efforts, the IMF’s BCAM website KnowMyeloma.org provides a rich trove of myeloma facts, news about myeloma treatment breakthroughs, and stories of hope and resilience from individuals living with myeloma. Along with the hashtags, #kNOwMyeloma, #BloodCancerAwarenessMonth, and #MyelomaWarrior, the educational infographics you see on these pages were shared across all social media channels: Twitter, Facebook, Instagram, and LinkedIn.
Apart from the shareable infographics, the campaign also actively engaged the myeloma community by testing their knowledge about the disease through a series of interactive social media quizzes.
To further fortify the objectives of the BCAM campaign, IMF Chief Medical Officer Dr. Joseph Mikhael shared his myeloma expertise through a two-part series on Facebook Live which tackled myeloma basics, facts, and stats, as well as breakthroughs in research, immune therapies, and the Black Swan Research Initiative.

The IMF’s BCAM campaign had an impact on people living with blood cancers and raised funds toward a cure for myeloma. By the end of September, the tip of the spear social campaign #kNOwMyeloma reached more than 17 MILLION people in 37 countries
We are truly grateful to our sponsors for supporting the IMF’s month-long initiatives for Blood Cancer Awareness Month: Bristol Myers Squibb, Janssen Pharmaceutical Companies of Johnson & Johnson, Karyopharm Therapeutics, Legend Biotech (Platinum Sponsors), GSK, and Pfizer (Gold Sponsors). MT
1.800.452.CURE toll-free in USA and Canada 1.818.487.7455 worldwide FALL 2022 17




info@myeloma.org myeloma.org 18 FALL 2022
Founder Brian D. Novis
Founder Susie Durie
Board of Directors
Chairman Dr. Brian G.M. Durie
Christine Battistini
Yelak Biru
Prof. Dr. Mario Boccadoro
Loraine Boyle
Susie Durie Martine Elias
George T. Hayum
Jason Katz
Benson Klein
Andrew Kuzneski, III
Dr. Robert A. Kyle
Prof. Dr. Heinz Ludwig Dr. Edith Mitchell
Charles Newman
Dr. S. Vincent Rajkumar Matthew Robinson E. Michael D. Scott
IMF Executive Team
Yelak Biru
President & Chief Executive Officer
Dr. Brian G.M. Durie
Chief Scientific Officer
Jennifer Scarne
Chief Financial Officer
Diane Moran
Senior Vice President, Strategic Planning Daniel Navid Senior Vice President, Global Affairs Lisa Paik
Executive Vice President, Medical Affairs
Dr. Joseph Mikhael
Chief Medical Officer
Lynn K. Green, Ed.D. Senior Vice President, Philanthropy
Mimi Choon-Quinones, PhD, MBA
Senior VP, Global Advocacy, Access, Policy & Research
Fatima Scipione
Senior Vice President, Strategic Alliances & External Affairs
Peter Anton Vice President, Marketing
Robin Tuohy Vice President, Support Groups
IMF Staff
Betty Arevalo
Manager, Inventory Control
Nancy Bruno
Regional Director, Support Groups
Sarah Chambliss
Assistant Meeting Coordinator
Kelly Cox
Director, Support Groups and Sr Dir, Regional Community Workshops
Danielle Doheny Director, Public Policy & Advocacy
Susie Durie Director, Global Patient Initiatives
Serdar Erdoğan
Director, GMAN and European & Middle Eastern Patient Programs
Heather Fishman Donor Relations
Jon Fitzpatrick Technology & Coordination, Support Groups
Sherrie Guerrero
Director, Human Resources
Abigail Guzman
Manager, Registration & Guest Relations
Paul Hewitt
Coordinator, InfoLine
Kevin Huynh
Web Specialist
Marya Kazakova
Editor-in-Chief, Publications
Ilana Kenville
Associate Director, Distinguished Events
Missy Klepetar Coordinator, InfoLine
Phil Lange
Accountant
Karla Lemus
Donor Relations Specialist
Jason London Manager, Marketing & Communications
Jim Needham Publication Design
Meghan O’Connor Coordinator, Meetings & Programs
Matthew Ohnsman Coordinator, Audio Visual Projects
Selma Plascencia Director, Operations
Annabel Reardon
Senior Director, Strategic Program Management
Joy Riznikove
Database Analyst
Miko Santos Web Producer
Kelley Sidorowicz
Regional Director, Support Groups
Sarah Solomon Donor Relations
Brando Sordoni
Accounting & Distribution
Rafi Stephan
Assistant to the President & Chief Executive Officer
Daria Tabota Coordinator, Marketing & Communications
Deborah Verla Coordinator, InfoLine
Jonathan Weitz Donor Relations
imfteam.myeloma.org


1.800.452.CURE toll-free in USA and Canada 1.818.487.7455 worldwide FALL 2022 19
INTERNATIONAL MYELOMA FOUNDATION




BEST OF ASH 2022 Webinar What Patients and Care Partners Need to Know Duration: 60 minutes (including Q&A)
Myeloma Foundation
Coldwater Canyon Avenue, Suite 300
City, CA 91604 USA myeloma.org 800.452.CURE Change Service Requested
©
Dedicated to improving the quality of life of myeloma patients while working toward prevention and a cure. REGISTER HERE events.myeloma.org Don’t Miss One of the Most Important Webinars of the Year! myeloma.org IF YOU MISS IT LIVE, GO HERE TO REPLAY myeloma.org/videos/best-ash-2022-webinar
Brian G.M. Durie, MD Chairman of the Board & Chief Scientific Officer, International Myeloma Foundation
International
4400
Studio
Printed in USA
2022
International Myeloma Foundation. All rights reserved.


























 By Dr. Joseph Mikhael IMF Chief Medical Officer
By Dr. Joseph Mikhael IMF Chief Medical Officer


























































 By Peter Anton IMF Vice President, Marketing
By Peter Anton IMF Vice President, Marketing
















