

Welcome Announcements
 Brian G.M. Durie, MD Chairman of the Board & Chief Scientific
Officer of International Myeloma Foundation
Brian G.M. Durie, MD Chairman of the Board & Chief Scientific
Officer of International Myeloma Foundation
Thank You for Joining!
2023 West Coast Patient and Family Webinar May 17, 2023

A replay of this program will be made available shortly after the conclusion. Slides will also be made available.

Agenda
– all times are listed in Pacific Time Zone
4:00 – 4:05 PM – Welcome and Announcements w/ Dr. Brian G.M. Durie
4:05 – 4:30 PM – Myeloma 101 Dr. Brian G.M. Durie, International Myeloma Foundation
4:30 – 4:45 PM – Q&A
4:45 – 5:10 PM – Taking the Reins of Your Myeloma Care

Ann McNeill, The John Theurer Cancer Center, Hackensack
University Medical Center, IMF Nursing Leadership Board
5:10 – 5:25 PM – Q&A
5:25 – 5:35 PM - BREAK
Agenda
– all times are listed in Pacific Time Zone
5:35 – 6:05 PM – Frontline Therapy
Dr. Rafat Abonour, Indiana University School of Medicine
6:05 – 6:20 PM – Q&A
6:20 – 6:45 PM – Relapse & Immune Therapies
Dr. Saad Usmani, Memorial Sloan Kettering Cancer Center
6:45 – 7:00 PM – Q&A 7:00 PM – Closing Remarks

Thank you to our Sponsors!













IMF Patient and Family Seminar
Brian G.M. Durie, MD
International Myeloma Foundation


Studio City, CA

Myeloma 101
Myeloma 101: What Patients Need to Know


 Brian G.M. Durie, MD
Brian G.M. Durie, MD
➢ Long Remissions Common

➢ CURE on the Horizon

Importance of Early Diagnosis

➢ Screening can be a new tool
➢ Start discussions with HR SMM

Potential New Myeloma or Smoldering Myeloma
Potential New Myeloma or Smoldering Myeloma
Any Myeloma Defining Events?
CRAB, >60% PC, FLC >100, MRI >1 focal
No Myeloma Defining Events (SMM)


High Risk SMM (Median TTP ~2 years)
Intermediate or Low Risk SMM
Treat as Myeloma
Early Therapy with Len or Rd x 2 years
Clinical Trials
Observation
Evolution of Treatment Options 15 Years Ago

First Therapy Relapse
VAD (Chemo) +

Velcade and Revlimid







Treatment Options in 2023






Median PFS: 47.3 vs 35 mo




Median PFS: 67.5 vs 46.2 mo
Benefit: maintenance until PD!
GRIFFIN Study Design: VRd +/- Dara



GRIFFIN Responses






Alkylators
Steroids
Anthracyclines









Active Drugs in MM
Anti-SLAMF7 monoclonal antibody (moAB)
Elotuzumab
Selinexor (XPO1 inhibitor)
Venetoclax (BCLinhibitor, unique MOA)

Anti-BCMA CAR-T
Cilta-cel
Ide-cel
JCARH125
Anti-CD38 moABs
Daratumumab
IMiDs
Thalidomide
Lenalidomide
Pomalidomide
Isatuximab
Felzartamab (MOR202)
TAK 079
SAR 442085
TAK 573 (Modakafusp Alfa)
Anti-BCMA Bispecifics
Teclistamab
Linvoseltamab (REGN5458)
Alnuctamab
Elranatamab
TNB 383B
Novel Bispecifics
Talquetamab (GPRC5D/CD3)
Proteasome Inhibitors
Bortezomib
Carfilzomib
Ixazomib
Anti-BCMA antibody drug conjugate

Belantamab*
CELMoDs
Iberdomide
Mezigdomide
Cevostamab
(FcRH5/CD3)
*Belantamab was recently withdrawn from the US market
What Are The Options?
• Immune approaches


• BCMA ADC – Belantamab*
• BCMA CAR T – (Ide-cel, Cilta-Cel)
• BCMA Bispecific - Teclistamab
• Targeted agents
• XPO1 inhibitor - Selinexor
BCMA ADC- Belantamab
• Venetoclax – Bcl2 inhibitor
*Belantamab was recently withdrawn from the US market
Key Points for Ongoing Care
Keep Track of Follow-up Tests
- CBC; SPEP/UPEP; Freelite; SCANS; BM if needed
- FiSH for t[11;14] or high-risk [17P-; 1q+…]
Alert Doctor to Changes (myeloma/side effects)


Be Aware of Potential Future Options
Be Proactive in Seeking Expert Advice
Set Expectations with Your Primary Doctor


Q&A

Taking The Reins of Your Multiple Myeloma Care
 Ann McNeill RN, MSN, APN
John Theurer Cancer Center at HMH
IMF Nurse Leadership Board Member
Ann McNeill RN, MSN, APN
John Theurer Cancer Center at HMH
IMF Nurse Leadership Board Member
Today’s Topics
STABLE OF TREATMENT
Myeloma and treatment options, side effects, symptom management, & supportive care
FINDING YOUR GAIT
Know your care team, telehealth & meeting prep, & shared decision making
STABLE OF TREATMENT



FINDING YOUR GAIT

GOING THE DISTANCE
GOING THE DISTANCE
Healthful living, infection prevention, renal and bone health
Stable of Treatment
Treatment options, side effects, symptom management, and supportive care

Treatment Goals
Myeloma Therapies


Rapid and effective disease control
Durable disease control
Improved overall survival
Minimize side effects
Allow good quality of life
Supportive Treatment
Prevent disease- and treatment-related side effects
Optimize symptom management
Allow good quality of life
Discuss goals and priorities with your healthcare team.
Stable of Treatment Options
-Mibs -MAbs -Mides Steroids Alkylators
FRONTLINE Velcade® (bortezomib)
Darzalex® (daratumumab)
Thalomid® (thalidomide)
Revlimid® (lenalidomide)
Dexamethasone
Prednisone
Prednisolone
SoluMedrol
Melphalan
Cyclophosphamide
MAINTENANCE
Velcade® (bortezomib)
Ninlaro® (ixazomib)
RELAPSE Kyprolis® (carfilzomib)
Ninlaro® (ixazomib)
Darzalex® (daratumumab)
- ECOG clinical trial
Darzalex® (daratumumab)
Empliciti® (elotuzumab)
Sarclissa® (Isatuximab)
Revlimid® (lenalidomide)
Thalomid® (thalidomide)
Revlimid® (lenalidomide)
Pomalyst® (pomalidomide)
• CelMods
Dexamethasone
Prednisone
Prednisolone
SoluMedrol
Melphalan
Cyclophosphamide
Bendamustine

PENDING FDA APPROVAL
‒ Iberdomide
‒ CC-92480
NOTED SIDE
EFFECTS
Carfilzomib: Cardiac
STABLE OF TREATMENT FINDING YOUR GAIT GOING THE DISTANCE
ImmunoTherapy Others Cellular Therapies
ASCT
Tecvayli™
Teclistamab
Xpovio® (Selinexor)
Doxil (liposomal doxorubicin)
• Bispecific Antibodies
‒ Talquetamab
‒ Cevostamab
• Antibody Drug Conjugates*
‒ Belantamab
mafodotin
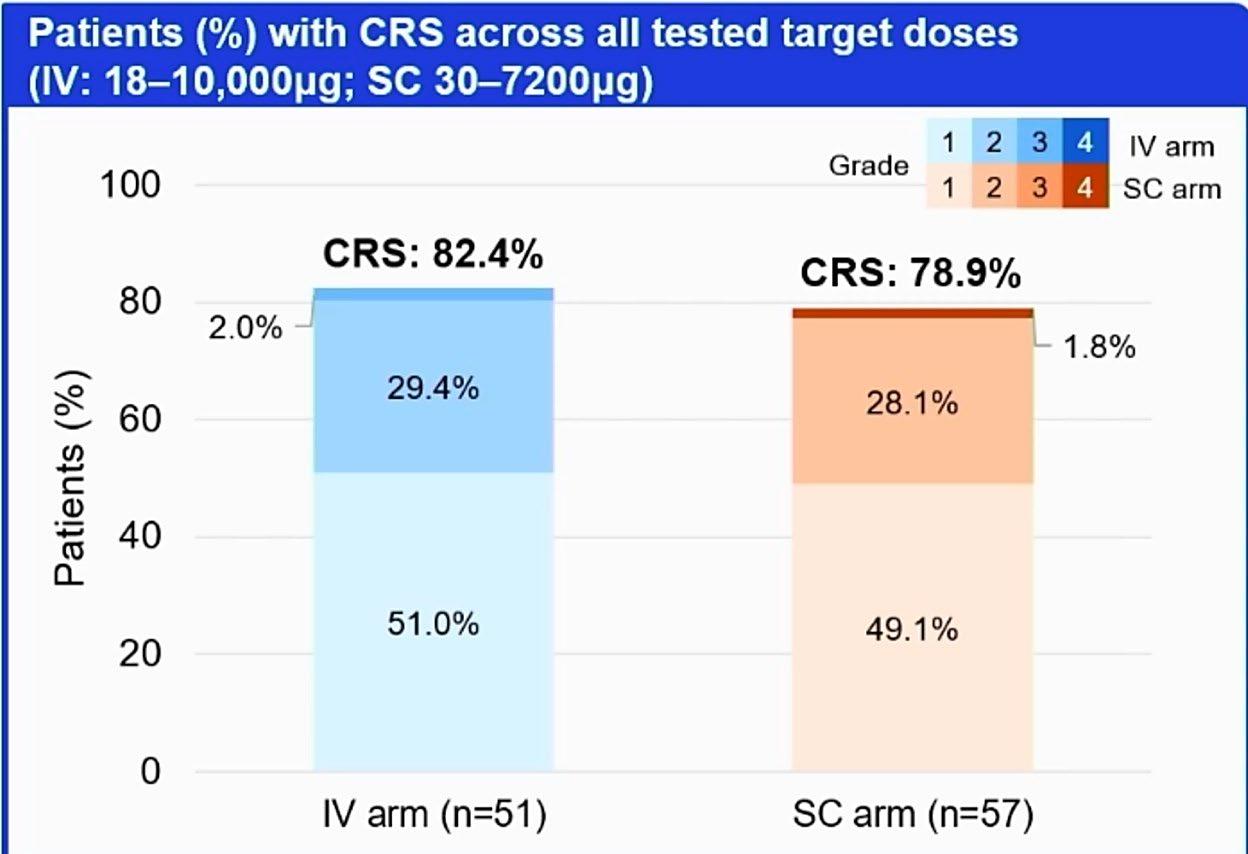
CRS and neurotoxicity; infection risk;
Blenrep: Keratopathy
Venclexta® (venetoclax)
ASCT
CAR-T cell therapy
Ide-Cel
Cilta-Cel
Myelosuppression, GI
Selinexor: Low sodium
Other CAR-T
Infection risk
CAR-T: CRS and neurotoxicity
CAR T: A New Treatment Approach

STABLE OF TREATMENT
FINDING YOUR GAIT GOING THE DISTANCE

CAR T: Tips
• Ask for a referral to CAR T-cell therapy center before relapse
– Insurance preauthorization required (weeks, maybe longer)
– Must have sufficient blood count and organ function to be eligible

– Must be able to wait or have bridging therapy

– Manufacturing CAR T-cell therapy is limited: center-specific “wait list” processes

• In patient for ≈ 1 week when CAR T administered
• Patients need a caregiver and must stay within proximity of CAR T-cell therapy center for ≈ 1 month


• No driving for 8 weeks
• One and done…BUT will need ongoing monitoring; some patients need transfusion support
• CRS, neurotoxicity, or infection are possible side effects

Horse of Another Color:
• Different bispecific antibodies have differences in efficacy, side effects

– About 7 in 10 patients responded
– CRS is common
– Some had skin/nail disorders
• Tecvayli™ (teclistamab) is the first but more expected
• Off-the-shelf treatment; no waiting for engineering cells



• Route of administration and dosing schedule will vary depending on product
• CRS, neurotoxicity, or infection are possible side effects
Bispecific Antibodies also Target BCMA MM cell death
ANTIBODIES
Cytotoxic cytokines
BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; MM = multiple myeloma; scFV = single chain fragment variable. Shah N, et al. Leukemia. 2020;34(4):985-1005. Yu B, et al. J Hematol Oncol. 2020;13:125.
Bispecific antibody



CAR
CRS

CAR T and Bi-specific Antibodies: Unique Side Effects
NEUROTOXICITY
Neurotoxicity is a rare but serious side effect
Tremors
Facial nerve palsy
Apraxia
CRS = cytokine release syndrome; ICANS = immune effector cell–associated neurotoxicity syndrome; ICE = Immune Effector Cell Encephalopathy screening tool; MRI = magnetic resonance imaging.

Infection Awareness & Prevention Infection Prevention Tips
Good personal hygiene (skin, oral)
Environmental control (wash hands, avoid crowds and sick people, etc)
Growth factor (Neupogen [filgrastim])
Immunizations (NO live vaccines)
STABLE OF TREATMENT FINDING YOUR GAIT GOING THE DISTANCE

Medications (antibacterial, antiviral)
As recommended by your health care team
COVID: The Best Way to Prevent Illness Is to Avoid Being
Exposed
to the Virus
Spread mainly through respiratory droplets that are produced by cough, sneezing and talking. More droplets with louder talking, yelling, singing
Get COVID Vaccine + Booster: Excellent protection against severe disease, but vaccine effectiveness may be lower in people with compromised immune systems
Wear a High-quality Mask: Respiratory droplets can spread disease; a high-quality mask can prevent exposure to airborne viral particles
Avoid Crowds & Sick People
Physical Distance & Outdoors: Close contact and indoor locations increases risk of spread
Wash Your Hands: Less common to get from a hard surface
CDC website. How to Protect Yourself & Others. Accessed June 17, 2021.
• Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
• Compromised immune function comes from multiple myeloma and from treatment.
• Infection is serious for myeloma patients!
Infection Guidelines
Type of prophylaxis
Recommendation(s)
HSV/VZV Standard prophylaxis recommended for all patients with MM
PJP
STABLE OF TREATMENT FINDING YOUR GAIT GOING THE DISTANCE

Opportunistic infections have been noted with bispecific agents and CAR T therapy; consider PJP prophylaxis
Bacterial & Fungal Standard prophylaxis recommended for ANC < 500 cells/μL
COVID-19 Consider monoclonal antibody therapy for patients receiving BCMA-bispecifics or CAR T therapy; based on institution-specific protocols
Hypogammaglobulinemia IVIg for IgG < 400 mg/dL or recurrent infections
GCSF 2 or 3 times/wk (or as frequently as needed) to maintain ANC > 1000 cells/μL and maintain dose intensity
BCMA = B-cell maturation antigen; GCSF = granulocyte colony-stimulating factor; HSV = herpes simplex virus; IVIg = intravenous immunoglobulin; PJP = Pneumocystis jirovecii pneumonia;
VZV = varicella zoster virus
National Comprehensive Cancer Network. Prevention and Treatment of Cancer -Related Infections. Version 3.2022. Published October 28, 2022. Accessed May 1, 2023.
https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf
Supportive Care to Address Side Effects
Don’t change horses midstream

• It is important to stay on myeloma treatment to control myeloma cells and get the most from each treatment.
• Responses deepen over time.
• Talk to your team if side effects are bothersome. Your team may be able to help, but only if they know.
Non-medication
Symptoms of Multiple Myeloma
A meta-analysis identified the most common patient-reported symptoms and their impact on QOL. Symptoms were present at all stages of disease. Symptoms resulted from both disease and treatment, including transplant, and were in these categories:
Physical
• Fatigue
• Constipation
• Pain
• Neuropathy
• Impaired Physical Functioning
• Sexual Dysfunction

Psychological
• Depression
• Anxiety
• Sleep Disturbance
• Decreased Cognitive Function
• Decreased Role & Social Function
Financial
• Financial burden (80%)
• Financial toxicity (43%)
Ramsenthaler, et al. 2016. https://doi.org/10.1111/ejh.12790.
Steroids: The Good, The Bad, The Ugly
Steroid Synergy
• Steroids are a backbone and work in combination to enhance myeloma therapy

Managing Steroid Side Effects
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over-the-counter or prescription medications
• Medications to prevent shingles, thrush, or other infections
STABLE OF TREATMENT FINDING YOUR GAIT GOING THE DISTANCE
Steroid Side Effects
• Irritability, mood swings, depression
• Difficulty sleeping (insomnia), fatigue
• Blurred vision, cataracts
• Flushing/sweating
• Increased risk of infections, heart disease
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Muscle weakness, cramping
• Weight gain, hair thinning/loss, skin rashes
• Increased blood pressure, water retention
• Increased blood sugar levels, diabetes
Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR, Eastern Cooperative Oncology Group (2010)
Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 11(1):29–37. King T, Faiman B. Steroid-Associated Side Effects: A Symptom Management Update on Multiple Myeloma Treatment . Clin J Oncol Nurs. 2017 Apr 1;21(2):240-249. doi: 10.1188/17.CJON.240-249. PMID: 28315528.
GI Symptoms:
Prevention & Management
Diarrhea may be caused by medications and supplements
Avoid caffeinated, carbonated, or heavily sugared beverages
Take anti-diarrheal medication if recommended
Constipation may be caused by medications and supplements
Increase fiber
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil®, Citrucel®,
Benefiber®
Fluid intake can help with both diarrhea and constipation and helps kidney function. Discuss GI issues with health care providers to identify causes and make adjustments to medications and supplements.

Pain Prevention and Management
Pain can significantly compromise quality of life
Sources of pain include bone disease, neuropathy and medical procedures
• Management
– Prevent pain when possible
• Bone strengtheners to decrease fracture risk; anti viral to prevent shingles; sedation before procedures
–
Interventions depends on source of pain
– May include medications, activity, surgical intervention, radiation therapy, etc
– Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)

–
Scrambler therapy for neuropathy
Tell your health care provider about any new bone pain or chronic pain that is not adequately controlled.
Peripheral Neuropathy Management
Peripheral neuropathy:
Damage to nerves in extremities (hands, feet, or limbs)
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness
Prevention / management:
• Bortezomib once-weekly or subcutaneous administration
• Massage area with cocoa butter regularly
• Supplements:
– B-complex vitamins (B1, B6, B12)
– Folic acid, and/or amino acids but do not take on day of Velcade® (bortezomib) infusion
• Safe environment: rugs, furnishings, shoes

Report symptoms of peripheral neuropathy early to your health care provider; nerve damage from neuropathy can be permanent if unaddressed.
If neuropathy worsens, your provider may:
• Change your treatment
• Prescribe oral or topical pain medication
• Suggest physical therapy
Why the Long Face?

98.8%
STABLE OF TREATMENT FINDING YOUR GAIT GOING THE DISTANCE

Fatigue is the most commonly reported symptom. Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression
>35% of patients
~25% of patients
Often, people do not share these symptoms with their provider. Talk to your provider about symptoms that are not well controlled or if you have thoughts of self harm. Help is available.
Rest and Relaxation Contribute to Good Health
• Adequate rest and sleep are essential to a healthful lifestyle
–
Shortened and disturbed sleep increase risk of
– Heart related death
–
–
–
–
Increase anxiety
Weakened immune system
Worsened pain
Falls and personal injury
• Things that can interfere with sleep
–
–
–
Medications: steroids, stimulants, herbal supplements
Psychologic: fear, anxiety, stress
Physiologic: sleep apnea, heart issues, pain

Sleep hygiene is necessary for quality nighttime sleep and daytime alertness
– Engage in exercise but not too near bedtime
– Increase daytime natural light exposure
– Avoid daytime napping
– Establish a bedtime routine - warm bath, cup of warm milk or tea
• Associate your bed ONLY with sleep
• Sleep aid may be needed
– Avoid before bedtime:
• Caffeine, nicotine , alcohol and sugar
• Large meals and especially spicy, greasy foods
• Computer screen time
Rod NH et al 2014. PloS one. 9(4):e91965; Coleman et al. 2011. Cancer Nurs. 34(3):219-227.National Sleep Foundation. At: http://sleepfoundation.org/ask-the-expert/sleep-hygiene
Mustian et al. Journal of clinical Oncology. Sep 10 2013;31(26):3233-3241; Stan DL, et al. Clin J Oncol Nurs. Apr 2012;16(2):131-141; Zeng Y et al., Complementary therapies in medicine. Feb 2014;22(1):173-186.
Financial Burden
• Financial burden comes from
• Medical costs
– Premiums
– Co-payments
– Travel expenses
– Medical supplies
• Prescription costs
• Loss of income
– Time off work or loss of employment
– Caregiver time off work
• Funding and assistance may be available
– Federal programs
–
–
Pharmaceutical support
Non-profit organizations
– Websites:
• Medicare.gov
• SSA.gov
• LLS.org
• Rxassist.org
• NeedyMeds.com
• HealthWellFoundation.org
• Company-specific website


YOU









Be empowered
Ask questions, learn more
Participate in decisions
Communicate with your team
Understand the roles of each team member and who to contact for your needs







Participate in support network





















Going the Distance
Healthful living, infection prevention, renal and bone health

Healthful Living Strategies: Prevention
Maintain renal health

• Myeloma management
• Hydration
• Avoid renally-toxic medications
– Dose adjust to renal function
• Diabetes management
Preventative health care
Health screenings, vaccinations
Prevent falls, injury, infection
Stop smoking
Dental care
Protect your bones
Nutrition, Calcium + D supplement
Weight-bearing activity and/or walking
Bone strengthening agents
Manage stress
• Rest, relaxation, sleep hygiene
• Mental health / social engagement
• Complementary therapy
Maintain a healthy weight
Nutrition
Activity / exercise
An ounce of prevention is worth a pound of cure.
Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56.
Benjamin FranklinBrigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19- 36. Faiman B, et al. CJON. 2011;15suppl:66- 76. Miceli TS, et al. CJON. 2011;15(4)suppl:9- 23.
Healthful Living Strategies: Keep Active
Movement therapies can reduce stress, promote sleep
Yoga, Pilates, Tai Chi
• Shown to improve sleep and sleep quality
• Improved quality of life & mood
Do:
• Keep a log or journal of your activity
FINDING YOUR GAIT GOING THE DISTANCE
• Notify your healthcare provider about sudden onset of pain, progressive weakness, headaches, blurred vision, numbness, and tingling
• Dehydration can lead to low blood pressure, falls
• Consider weightlifting limits
Do Not:
• Overdo it
• Force exercise
• Try things without discussing with provider
Myeloma bone disease may affect your ability to do certain movement activities. Review your activity interests with your health care provider!
Boullosa DA, et al., Jul 2013;45(7):1223-1228. Faiman B et al., Clinical Journal of Oncology Nursing. 2008;12(0):53-62;
. 2011;15:9-23; Coleman EA et al.,Oncol Nurs Forum. May 2008;35(3):E53-61.

Nurse Leadership Board
In 2006, the IMF founded the Nurse Leadership Board® as a professional partnership to represent oncology nurses who are experts in the care of multiple myeloma patients at leading medical centers. The NLB is improving the nursing care and self-care of patients with multiple myeloma via publications, symposia, multimedia, and research.



Agenda after break
all times are listed in Pacific Time Zone
5:35 – 6:05 PM – Frontline Therapy
Dr. Rafat Abonour, Indiana University School of Medicine
6:05 – 6:20 PM – Q&A
6:20 – 6:45 PM – Relapse & Immune Therapies
Dr. Saad Usmani, Memorial Sloan Kettering Cancer Center
6:45 – 7:00 PM – Q&A
7:00 PM – Closing Remarks

Multiple Myeloma Rafat Abonour, M.D.
Harry and Edith Gladstein Professor of Cancer Research
Professor of Medicine, Pathology and Laboratory Medicine
Director, Multiple Myeloma, Waldenstrom's Disease and Amyloidosis Program
Indiana University School of Medicine

Treatment Goals for MM
• Symptom Control
• Ameliorate pain and other disease-related symptoms
• Prevent further organ damage
• Preserve and improve performance status and quality of life
• Disease Response and Survival
• Rapid cytoreduction to relieve symptoms
• Minimize treatment-related toxicity
• Prolong survival – Overall Survival

Depth of Response Influence Time to Progression

Minimal Residual Disease (MRD) Testing
• MRD is emerging as an important marker of lasting clinical benefit
• MRD can be tested: Using flowcytometry. Sensitive at 10-5 and can be done anytime on bone marrow samples and does not require knowing what the patient myeloma sequences were at diagnosis
− Using next generation sequences Sensitive at 10-6 but require knowing what the patient myeloma sequences were at diagnosis.
MRD Strongly Predicts outcomes




Munshi et al. Blood Advances 2020; 4:23

MRD May Abrogate Other Risk Factors = Optimal Dynamic Risk Assessment Tool

PETHEMA/GEM2012
(MRD < 2 x 10-6)



Goicochea I et al. Blood 2021;137:49
Opportunity for de-escalation
Need to employ new approaches

Downsides of Maintenance Therapy
Deferring Lenalidomide for 7 years:
No therapy
Perrot A et al. ASH meeting 2020
Most patients have not progressed in 7years despite no therapy

-”Saving” of US$ 1.5 million!

-Avoid ~3.5% absolute increase in risk of SPM
-Avoid dozens of extra lab checks/MD visits

-Avoid diarrhea, fatigue, rash
-Avoid “daily reminder I have cancer”
Implications for loss of MRD negativity



The Evolution of Myeloma Therapy
Bortezomib
Lenalidomide
Carfilzomib
Pomalidomide
Selinexor
Panobinostat
Daratumumab
Ixazomib
Elotuzumab
Isatuximab
Belantamab mafodotin
Melphalan flufenamide
Idecabtagene autoleucel
Daratumumab?
Carfilzomib?
Lenalidomide + PI
ASCT, autologous stem cell transplant; CAR, chimeric antigen receptor; Cy, cyclophosphamide; ddaratumumab; D/dex, dexamethasone; isa, isatuximab; K, carfilzomib; M, melphalan; PD-L1, programmed death ligand-1; PI, proteasome inhibitor; Rev, lenalidomide; V, bortezomib
Bispecific/Trispecific Antibodies
Cell Modifying Agents
Venetoclax?
PD/PDL-1 Inhibition?
Multiple small
Upfront therapy
• Combination therapy continues to prove superiority in providing long remission and long survival.
• The patient is at good place as options are becoming more prevalent.
• Lenalidomide, bortezomib and dexamethasone continues to be an effective regimen.

• Daratumumab in combination with lenalidomide is an excellent option for “frail patient”
• But wait there are more to come
Is three drugs better than two?



Three may better in some Patients





MAIA How about Antibodies based!
‒ Patients were enrolled in MAIA from March 2015 through January 2017
D: 16 mg/kg IV
QW Cycles 1-2, Q2W Cycles 3-6, then Q4W thereafter until PD
R: 25 mg PO Days 1-21 until PD
da: 40 mgb PO or IV
Days 1, 8, 15, 22 until PD
Rd
R: 25 mg PO Days 1-21 until PD
d: 40 mg PO
Days 1, 8, 15, 22 until PD
Cycles: 28 days
End-oftreatment visit (30 days after last dose)
Longterm follow-up
Primary endpoint
• PFS
Key secondary endpoints
• OS
• PFS2
• ORR
• CR/sCR rate
• MRD (NGS; 10–5)

MAIA is a multicentre, randomised, open-label, active-controlled, phase 3 study of D-Rd versus Rd alone in patients with NDMM who are transplant ineligible
TIE, transplant-ineligible; ECOG PS, Eastern Cooperative Oncology Group performance status; CrCl, creatinine clearance; IV, intravenous; QW, once weekly; Q2W, once every 2 weeks; Q4W, once every 4 weeks; PD, progressive disease; PO, oral; ORR, overall response rate; CR, complete response; sCR, stringent complete response; MRD, minimal residual disease; NGS, next-generation sequencing; BMI, body mass index.
aOn days when DARA is administered, dexamethasone will be administered to patients in the D-Rd arm and will serve as the treatment dose of steroid for that day, as well as the required pre-infusion medication. bFor patients >75 years of age or with BMI <18.5 kg/m2, dexamethasone was administered at a dose of 20 mg QW.
Treatment Exposure and Patient Disposition

Median duration of follow-up, 56.2 months
42% of patients in the D-Rd arm and 18% of patients in the Rd arm remained on treatment; more patients discontinued Rd for AEs
Overall Response Rate
• D-Rd induced deeper responses with significantly higher rates of ≥CR and ≥VGPR, compared with Rd
• With >28 months of additional follow-up, responses deepened with continued DARA therapy


Time without Relapse (PFS)

• D-Rd continued to demonstrate a significant PFS benefit, with median PFS not reached with DRd
• These data provide a new PFS benchmark in patients with NDMM who are transplant ineligible
Overall Survival
D-Rd demonstrated a significant benefit in OS, with a 32% reduction in the risk of death, in patients with NDMM who are transplant ineligible

Subsequent Therapy
‒ Median time to next treatment was not reached with D-Rd versus 42.4 months with Rd (HR, 0.47; 95% CI, 0.37-0.59; P <0.0001)
‒ 114 patients in the D-Rd arm and 186 patients in the Rd arm received subsequent therapy; of these:

• A PI-containing regimen without an IMiD was the most common first subsequent therapy
(53% vs 54% with D-Rd and Rd, respectively)
• 15% of patients in the D-Rd arm and 46% of patients in the Rd arm received daratumumab as any subsequent therapy
Conclusions
‒ After almost 5 years of follow-up, a significant OS benefit of D-Rd versus Rd given to progression was demonstrated in patients with transplant-ineligible NDMM
• The estimated 5-year OS rate was 66.3% with D-Rd and 53.1% with Rd
‒ The significant PFS benefit of D-Rd versus Rd was maintained, with a 47% reduction in risk of disease progression or death (median PFS for D-Rd, not reached)
• The estimated 5-year PFS rate was 52.5% with D-Rd and 28.7% with Rd
‒ These PFS and OS results have been achieved in a study population with 44% of patients aged 75 to 90 years
‒ No new safety concerns were identified with continuous therapy and longer follow-up
These results strongly support upfront D-Rd as a new standard of care for patients with transplant-ineligible NDMM

How about Four Drugs regimen?
Daratumumab (DARA) Plus Lenalidomide, Bortezomib, and Dexamethasone (RVd) in Patients (Pts) With Transplant-eligible
Newly Diagnosed Multiple Myeloma (NDMM):

Updated Analysis of GRIFFIN After 24 Months of Maintenance
Presented at the 63rd American Society of Hematology (ASH) Annual Meeting & Exposition; December 11-14, 2021; Atlanta, GA/Virtual
ASH2021/Daratumumab/Laubach
*Presenting author.
GRIFFIN: Study Design of the Randomized Phase
• Phase 2 study of D-RVd versus RVd in transplant-eligible NDMM, 35 sites in the United States with enrollment between December 2016 and April 2018

GRIFFIN: Responses Deepened Over Timea
• Response rates for sCR and ≥CR were greater for D-RVd versus RVd at all time points, with the deepest responses occurring after 2 years of maintenance therapy

GRIFFIN: MRD Negativitya (10–5) After 2 Years of Maintenance Therapy


GRIFFIN: Time to MRD Negativity
• Median time to MRD negativity (10–5) was 8.5 months for D-RVd and 34.6 months for RVd
– At the 10–6 threshold, time to MRD negativity was 33.9 months for D-RVd and not reached for RVd
• Median time to MRD negativity was shorter for D-RVd versus RVdb

GRIFFIN: PFS in the ITT Population
• Median follow-up: 38.6 months
• Median PFS was not reached in either group
• There is a positive trend toward improved PFS for D-RVd/DR versus RVd/R
• The separation of the PFS curves begins beyond 1 year of maintenance and suggests a benefit of prolonged DR therapy

Daratumumab, Carfilzomib, Lenalidomide and Dexamethasone (Dara-KRd), Autologous Transplantation and MRD Response-Adapted Consolidation and Treatment Cessation-Final Primary Endpoint Analysis of the MASTER Trial
COMMIT- Academic Consortium to Overcome Multiple Myeloma through Innovative Trials
 Luciano J. Costa1, Saurabh Chhabra2, Natalie S. Callander, MD3 , Eva Medvedova4, Bhagirathbhai Dholaria5, Rebecca Silbermann4, Kelly Godby1, Binod Dhakal2, Susan Bal1, Smith Giri1, Anita D’Souza2, Timothy Schmidt3, Aric Hall3, Pamela Hardwick1, Robert F. Cornell5, Parameswaran Hari2
1- University of Alabama at Birmingham; 2- Medical College of Wisconsin; 3- University of Wisconsin at Madison; 4- Oregon Health and Science University; 5- Vanderbilt University
Luciano J. Costa1, Saurabh Chhabra2, Natalie S. Callander, MD3 , Eva Medvedova4, Bhagirathbhai Dholaria5, Rebecca Silbermann4, Kelly Godby1, Binod Dhakal2, Susan Bal1, Smith Giri1, Anita D’Souza2, Timothy Schmidt3, Aric Hall3, Pamela Hardwick1, Robert F. Cornell5, Parameswaran Hari2
1- University of Alabama at Birmingham; 2- Medical College of Wisconsin; 3- University of Wisconsin at Madison; 4- Oregon Health and Science University; 5- Vanderbilt University
Treatment
Dara-KRd
• Daratumumab 16 mg/m2 days 1,8,15,22 (days 1,15 C 3-6; day 1 C >6)





• Carfilzomib (20) 56 mg/m2 Days 1,8,15
• Lenalidomide 25 mg Days 1-21
• Dexamethasone 40mg PO Days 1,8,15,22
MRD assessment by NGS
*24 and 72 weeks after completion of therapy
”MRD-SURE” -Treatment-free observation and MRD surveillance*
Best MRD response by phase of therapy

Progression-Free and Overall Survival


Conclusions
• NGS-MRD response-adapted therapy is feasible in ~96% of patients in multi center setting – 72% reaching MRD-SURE.
• Patients with standard and high-risk NDMM have similar depth of response and low risk of MRD resurgence or progression when treated with Dara-KRd/AHCT and MRDadapted treatment cessation.
• Quadruplet therapy and achievement of confirmed MRD (-) responses enables the exploration of treatment cessation and “MRD-SURE” as alternative to continuous therapy.
Effective novel consolidative strategies should be explored to clear MRD and improve outcomes in patients with ultra-high-risk MM
Addition of Isatuximab to Lenalidomide, Bortezomib and Dexamethasone as Induction Therapy for
Newly-Diagnosed, Transplant-Eligible Multiple Myeloma:
The Phase III GMMG-HD7 Trial
Hartmut Goldschmidt1,2, Elias K. Mai1, Eva Nievergall1, Roland Fenk3, Uta Bertsch1,2, Diana Tichy4, Britta Besemer5, Jan Dürig6, Roland Schroers7, Ivana von Metzler8, Mathias Hänel9, Christoph Mann10, Anne Marie Asemissen11, Bernhard Heilmeier12, Stefanie Huhn1, Katharina Kriegsmann1, Niels Weinhold1, Steffen Luntz13, Tobias A. W. Holderried14, Karolin Trautmann-Grill15, Deniz Gezer16, Maika Klaiber-Hakimi17, Martin Müller18, Cyrus Khandanpour19, Wolfgang Knauf20, Markus Munder21, Thomas Geer22, Hendrik Riesenberg23, Jörg Thomalla24, Martin Hoffmann25, Marc-Steffen Raab1, Hans J. Salwender26, Katja C. Weisel11 for the German-speaking Myeloma Multicenter Group (GMMG)
1Department of Internal Medicine V, University Hospital Heidelberg, Heidelberg, Germany; 2National Center for Tumor Diseases Heidelberg, Heidelberg, Germany;
3Department of Hematology, Oncology and Clinical Immunology, University Hospital Düsseldorf, Düsseldorf, Germany; 4Division of Biostatistics, German Cancer Research Center (DKFZ) Heidelberg, Heidelberg, Germany;


5Department of Internal Medicine II, University Hospital Tübingen, Tübingen, Germany; 6Department for Hematology and Stem Cell Transplantation, University Hospital Essen, Essen, Germany;
7Medical Clinic, University Hospital Bochum, Bochum, Germany; 8Department of Medicine, Hematology/Oncology, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany;
9Department of Internal Medicine III, Clinic Chemnitz, Chemnitz, Germany; 10Department for Hematology, Oncology and Immunology, University Hospital Gießen and Marburg, Marburg, Germany;
11Department of Oncology, Hematology and BMT, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; 12Clinic for Oncology and Hematology, Hospital Barmherzige Brueder Regensburg, Regensburg, Germany; 13Coordination Centre for Clinical Trails (KKS) Heidelberg, Heidelberg, Germany; 14Department of Oncology, Hematology, Immuno-Oncology and Rheumatology, University Hospital Bonn, Bonn, Germany; 15Department of Internal Medicine I, University Hospital Dresden, Dresden, Germany; 16Department of Hematology, Oncology, Hemostaseology, and Stem Cell Transplantation, Faculty of Medicine, RWTH Aachen University, Aachen, Germany; 17Clinic for Hematology, Oncology and Palliative Care, Marien Hospital Düsseldorf, Düsseldorf, Germany; 18Clinic for Hematology, Oncology and Immunology, Klinikum Siloah Hannover, Hannover, Germany; 19Medical Clinic A, University Hospital Münster, Münster, Germany; 20Center for Hematology and Oncology Bethanien, Frankfurt am Main, Germany; 21Department of Internal Medicine III, University Hospital Mainz, Mainz, Germany; 22Department of Internal Medicine III, Diakoneo Clinic Schwäbisch-Hall, Schwäbisch-Hall, Germany; 23Hematology/Oncology Center, Bielefeld, Germany; 24Hematology / Oncology Center, Koblenz, Germany; 25Medical Clinic A, Clinic Ludwigshafen, Ludwigshafen, Germany; 26Asklepios Tumorzentrum Hamburg, AK Altona and AK St. Georg, Hamburg, Germany

Primary endpoint: MRD negativity at the end of induction phase
Induction phase (3 x 6-week cycles)
Maintenance phase (4-week cycles)
MRD (bone marrow aspirate)
Primary endpoint:
• MRD negativity at the end of induction treatment (NGF, sensitivity 10-5) stratified according to R-ISS

Secondary endpoints:
• CR after induction
• Safety
Data cut-off:
• April 2021
ASCT, autologous stem cell transplant; CR, complete response; d, dexamethasone; HDT, high-dose therapy; Isa, isatuximab; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; NGF, next-generation flow; PD, progressive disease; R, lenalidomide; R-ISS, Revised International Staging System; Te, transplant eligible; V, bortezomib 1. ClinicalTrials.gov: NCT03617731
Patients with MRD negativity at the end of induction therapy
OR 1.83 (95% CI 1.34–2.51)
Low number of not assessable/missing† MRD status: Isa-RVd (10.6%) and RVd (15.2%)
Isa-RVd is the first regimen to demonstrate a rapid and statistically significant benefit from treatment by reaching a MRD negativity of 50.1% at the end of induction and to show superiority vs. RVd in a Phase 3 trial
*P value derived from stratified conditional logistic regression analysis †Missing NGF-MRD values were due to either patients’ loss to follow-up during induction therapy or to missing bone marrow samples or technical failures in measurement counted as non-responders, i.e. NGF-MRD positive CI, confidence interval; d, dexamethasone; Isa, isatuximab ITT, intent-to-treat; MRD, minimal residual disease; NGF, next-generation flow; OR, odds ratio; R, lenalidomide; V, bortezomib

First primary endpoint, end of induction MRD negativity by NGF (10-5), was met in ITT analysis
Response rates after induction therapy
Although the rates of CR after induction therapy did not differ between the Isa-RVd and RVd arms, there was a significant increase in ≥VGPR rates and ORR with Isa-RVd
*P values derived from Fisher’s exact test †Data adjusted per M-protein interference CR, complete response; d, dexamethasone; Isa, isatuximab; nCR near-complete response; ORR, overall response rate; PR, partial response; R, lenalidomide; V, bortezomib; VGPR, very good partial response

Addition of Isa to RVd had limited impact on safety profile
A comparable number of patients discontinued induction therapy due to AEs in the

*SOC considered as “Investigations” as defined by the CTCAE †Includes five episodes of febrile neutropenia during induction: Isa-VRd (n=3) vs. VRd (n=2) ‡Infusion-related reactions of CTCAE grade 2 or higher in the Isa-RVd arm were n=42 (12.7%) AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events d, dexamethasone; Isa, isatuximab; NA, not applicable; PT, preferred term; R, lenalidomide; SOC, system organ class; V, bortezomib
Upfront Therapy Status..Evolving
• Adding a CD38 antibody to VRD or KRD is feasible with limited incremental toxicity
• Depth of response is improved, including MRD negativity
• MRD will be an even more important measure going forward
• There is a trend to improved PFS
• This may overcome the high-risk feature of some patients
• Ultra high-risk disease remains challenging to treat with current strategies
• What about transplant?? It is still part of most upfront studies.

The future: MASTER 2 The Dream Trial
Sequential Therapy in Multiple Myeloma Guided by MRD Assessments
*MRD-SURE – Treatment-free observation and MRD surveillance




















T-cell Redirecting therapy
T-cell Redirecting therapy

Relapsed Multiple Myeloma: Novel and Cellular Therapies in 2023
 Saad Z. Usmani, MD MBA FACP Chief of Myeloma Service
Saad Z. Usmani, MD MBA FACP Chief of Myeloma Service


Disclosures
• Research funding: Amgen, BMS/Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, Takeda.
• Consulting/Advisory Board: Abbvie, Amgen, BMS, Celgene, Genentech, Gilead, GSK, Janssen, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio.

Journey to the First Relapse

























Factors in Selecting Relapsed Therapy
Patient












• Age
• Performance status
• Renal insufficiency
• Poor marrow reserve
• Neuropathy
• Comorbidities
• Cardiac disease
• Diabetes
Disease
• Risk Status
• Cytogenetics
• del [17p], t(4;14), t(14;16)
• Rapidity of relapse
• Rate of rise
• Organ damage
• Extramedullary disease
• Plasma cell leukemia
Treatment
• Previous therapy
• Depth
• Duration
• Route of administration
• Single or combination
• Cost
• Toxicity
• Myelosuppressi on
• Neuropathy
Dimopoulos MA, et al. Nat Rev Clin Oncol 2015;12(1):42-54; Baz R, et al. Support Care Cancer 2015;23(9):2789-2797; Agarwal A et al. Clin Lymphoma Myeloma Leuk. 2017;17(2):69-77.

• Thrombosis
• Risk of SPM
We have many options!
Lenalidomide combinations
• Carfilzomib, lenalidomide, dexamethasone
(KRd)
• Ixazomib, lenalidomide, dexamethasone (IRd)
• Elotuzumab, lenalidomide, dexamethasone (EloRd)
• Daratumumab, lenalidomide, dexamethasone
(DRd)
Pomalidomide combinations
• Carfilzomib, pomalidomide, dexamethasone (KPd)
• Elotuzumab, pomalidomide, dexamethasone (EloPd)
• Daratumumab, pomalidomide, dexamethasone (DPd)
• Isatuximab, pomalidomide, dexamethasone (IsaPd)
Carfilzomib combinations
• Daratumumab, carfilzomib, dexamethasone (DKd)
• Isatuximab, carfilzomib, dexamethasone (IRd)
• Carfilzomib, dexamethasone (Kd) with or without cyclophosphamide
Other notable combinations
• Selinexor, bortezomib, dexamethasone (SVd)
• Off-label use of venetoclax, dexamethasone for translocation (11;14) (VenD)

IMWG Guidelines: Treatment at Relapse
Treatment at first relapse
Not
refractory to LEN Refractory to LEN
Treatment after multiple relapses
Any first relapse options not yet used
(2 new drugs; triplet preferred)
Preferred options†: DRd or KRd
Alternatives‡: DVd, Kd, DKd, Isa–Kd, IRd, Elo–Rd, PVd, or SVd (subject to approval)
If daratumumab, isatuximab, or carfilzomib are not available: Rd, Vd, VTd, VCd, or VMP
Preferred options†: D–Kd, or Isa–Kd, or PVd
Alternatives‡: DVd or Kd
Other options: KPd, DPd, or Ipd
If daratumumab, isatuximab, carfilzomib, or pomalidomide are not available: VCd, Vd, or VMP
Investigational options and clinical trial

Autologous Stem Cell Transplant Candidate?
• SCT not performed as part of frontline therapySCT
• Durable remission after 1st SCT (≥24 months on no maintenance therapy, ≥36 months with maintenance therapy)
The Promise of T-cell redirection






 Adapted from Cho S-F et al. Front Immunol. 2018;9:1821.
BCMAbispecific antibody
BCM A Myeloma cell
BCMAbispecific T-cell engager
T cell
BCM A Myeloma cell T cell toxin
CD 3
Myeloma cell dying
Adapted from Cho S-F et al. Front Immunol. 2018;9:1821.
BCMAbispecific antibody
BCM A Myeloma cell
BCMAbispecific T-cell engager
T cell
BCM A Myeloma cell T cell toxin
CD 3
Myeloma cell dying
BCMA CARTs: Summary

KarMMa-2: Study design and baseline characteristics
Cohort 2a – early relapse after ASCT
Baseline characteristics
aResponse defined as PR or better based on IMWG criteria by investigator assessment; measured from infusion; bPatients with ≥PR (2 patients had minimal response; 4 had stable disease and 0 had progressive disease); cClopper-Pearson CI; dPatients with sCR and CR

ASCT, autologous stem cell transplant; CR, complete response; CRR, complete response rate; ORR, overall response rate; PD, progressive disease; PR, partial response; sCR, stringent complete response; VGPR, very good partial response
Usmani S et al. ASH 2022;abstract 361 (oral presentation)

GPRC5D-targeting CAR-T cell therapy: CC-95266-MM-001study

Study design
bTreatment schedule and follow-up for Part B (dose expansion) will be the same as Part A.
BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; MTD, maximum tolerated dose; RP2D, recommended phase 2 dose
Berdeja J et al. ASH 2022;abstract 364 (oral presentation)

Baseline characteristics
GPRC5D-targeting CAR-T cell therapy: Response
ORR in patients with and without experience of BCMA-targeting therapy


BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; CRR, clinical response rate; ORR, overall response rate; PR, partial response; sCR, stringent response rate; VGPR, very good response rate
Berdeja J et al. ASH 2022;abstract 364 (oral presentation)

GPRC5D-targeting CAR-T cell therapy: Safety profile TEAEs
• ICANS-type neurotoxicity was infrequent, low grade and reversible with steroid treatment: Any grade 2 (6%), none were grade 3/4
• DLTs: prolonged neutropenia and/or thrombocytopenia, 2 patients (25 x 106 and 75 x 106 CAR-T cells)

• MTD has not been reached
• No deaths related to study treatment (1 death prior to treatment)

CAR, chimeric antigen receptor; CRS, cytokine release syndrome; DLTs, dose-limiting toxicities; ICANS, immune effector cell-associated neurotoxicity syndrome; MTD, maximum tolerated dose; TEAEs, treatment-emergent adverse events
Berdeja J et al. ASH 2022;abstract 364 (oral presentation)
BsAbs – Many Different Platforms
Bispecific T-cell Engager or BiTE (Amgen)
Dual Affinity ReTargeting or DART (Janssen, Macrogenics)



Tandem diabodies or TandAb (Affimed)
T-cell dependent BsAb Xmab (Xencor, Glenmark, Amgen)
BsAb armed activated Tcells or BAT (mostly academic)

CrossMAb (Celgene, Roche)

Duobody (Genmab)
Adapted from Lejeune M et al. Front Immunol 2020 11:762.
Trifunctional Antibody or TriFAb




Teclistamab – 1st FDA Approved BsAb for MM



Many more BCMA-targeting T-cell redirecting BsAbs in development


Alnuctamab
Elranatamab
bsAb, bispecific antibody; BCMA, B-cell maturation antigen


ABBV-383 (TNB-383B)
Bahlis N et al. ASCO 2021;abstract 8006 (oral presentation); Costello C et al. EHA 2021;abstract S192 (oral presentation); Costa L et al. Blood 2019;134:abstract 143 (oral presentation); Costa L et al. EHA 2020;abstract S205 (oral presentation); Madduri D et al. ASH 2020;abstract 291 (oral presentation; Rodriguez C et al. ASH 2020;abstract 293 (oral presentation); Sebag ASH 2021;abstract 895 (oral presentation); Zonder et al. ASH 2021;abstract 160 (oral presentation); Kumar S et al. ASH 2021;abstract 900 (oral presentation)

Median prior regimens: 5

Median follow-up: 10.4 months
BICR, blinded independent central review; CR, complete response; PR, partial response; sCR, stringent complete response; VGPR, very good partial response

Bahlis N et al. ASH 2022;abstract 159 (oral presentation)

Elranatamab (BCMA-CD3 TCE): Phase 2 MagnetisMM-3 trial
Interim safety analysis (n=94) – safety profile
• All CRS events were grade 1 or 2
• Median time to onset 2 days (1 – 9)
• Tocilizumab was used in 27 pts (22.7%)
• TEAEs led to permanent elranatamab discontinuation in 19 (15.4%) patients (8 patients due to infections)

• TEAEs led to death in 21 patients; 2 treatment-related per investigatorb
a Includes preferred terms in COVID-19 (narrow) standardized MedDRA queries
b Other grade 5 TEAEs (n=19) were not treatment-related per investigator and included TEAEs at the MedDRA level of system organ class: disease progression or neoplasms benign, malignant and unspecified (n=11), infections (n=5), cardiac disorders (n=2), and respiratory disorders (n=1)
• Infections were reported in 66.7% (Grade 3/4, 35.0%) of patients

• Median time to first onset of infections was 47.5 d (range, 1.0−295.0)
• Overall, 50 (40.7%) patients received IVIG during the study.

CMV, cytomegalovirus; CRS, cytokine release syndrome; IVIG, intravenous immunoglobulin G; TEAE, treatment-emergent adverse event; TCE, T-cell engager
Bahlis N et al. ASH 2022;abstract 159 (oral presentation)
Alnuctamab: Design, baseline characteristics and safety profile
Safety profile
Phase 1 step-up dosing of alnuctamab (CC-93269)

28-day cycles
Baseline characteristics

• Median follow-up: 4.1 months
aMedian time to resolution of neutropenia (grade ≤2) was 1 week (range 0.1 x 0.1–5.1); b2 grade 1 ICANS events (duration 3 and 5 days); cMost common were COVID-19 n=8), rhinovirus (n=3).

ALT, alanine aminotransferase; CRS, cytokine release syndrome; DLT, dose-limiting toxicity; ICANS, immune effector cell-associated neurotoxicity syndrome; SC, subcutaneous
Wong S et al. ASH 2022;abstract 162 (oral presentation)

Alnuctamab: Responses
• mDOR with SC alnuctamab: not reached
• 26/29 (90%) responses are ongoing
Responses over time with SC alnuctamab

ORR with SC alnuctamab
aPatients who received 10 or 15 mg target doses.
CR, complete response; mDOR, median duration of response; MRD, minimal residual disease; PD, progressive disease; PR, partial response; Q2W, every other week; SC, subcutaneous; sCR, stringent complete response; SD, stable disease; TEAE, treatment-emergent adverse events; VGPR, very good partial response

Wong S et al. ASH 2022;abstract 162 (oral presentation)

Other targets
1. GPRC5D: TALQUETAMAB
Median prior regimens: 5
Prior belantamab: 12.6-9.0%
Median follow-up: 14.9-8.6 months
On target/off tumor toxicities :


-dysgeusia; dysphagia
-weight loss
-skin-related AEs
-nail-related AEs
mPFS
Infections:
-all grade: 57.3 / 50.3%
-grade 3/4: 16.8 / 11.7%
Chari A et al. ASH 2022;abstract 157 (oral presentation); Verkleij CPM et al. Blood Adv 2021;5(8):2196–2215


MonumenTAL-1: Talquetamab in patients with prior T-cell redirecting therapies
ORR in patients with prior T-cell redirecting

Patient’s characteristics:
• Median of 6 prior lines of therapy (range, 3–15)
• 70.6% (n=36) received prior CAR-T cell therapy and 35.3% (n=18) prior bispecific antibody therapy; 3 patients received both
Data cut-off date: September 12, 2022.
• Median follow-up: 11.8 months
• Median duration of response: 12.7 months
• 72.2% ORR (26/36; 95% CI, 54.8–85.8%) in patients with prior CAR-T cell therapy 2200%

• 44.4% ORR (8/18; 95% CI, 21.5–69.2%) in patients with prior bispecific antibody treatment
aIndependent review committee assessment of evaluable patients per 2011 IMWG response criteria; due to rounding, individual response rates may not sum to the ORR.
CAR, chimeric antigen receptor; CR, complete response; PR, partial response; ORR, overall response rate; Q2W, every other week; QW, weekly; SC, subcutaneous; sCR, stringent complete response; VGPR, very good partial response
Chari A et al. ASH 2022;abstract 157 (oral presentation)
Forimtamig (RG6234): GPRC5D x CD3 phase 1 dose escalation study


Study design
*Q3W dosing in the 18/162/7200 μg IV and the 30/150/4800 μg and 30/300/7200 μg SC cohorts; †Until progression or unacceptable toxicity; ‡doses in μg; §1 patient had previously received an ADC and CAR-T cell therapy.
ADC, antibody-drug conjugate; BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; ECOG, Eastern Cooperative Oncology Group; MTD, maximum tolerated dose; Q2W, every 2 weeks; RP2D recommended phase 2 dose; SC, subcutaneous
Carlo-Stella C et al. ASH 2022;abstract 161 (oral presentation)
Forimtamig (RG6234): Efficacy and CRS
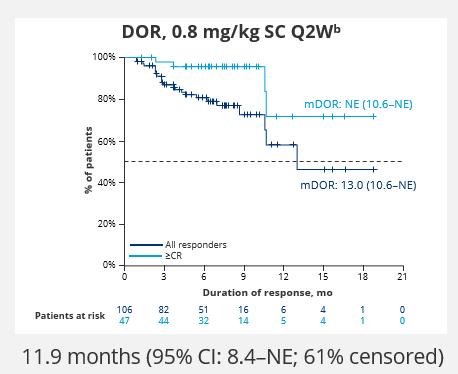
• Median duration of response was 10.8 months for the IV arm and 12.5 months for the SC arm
Forimtamig is highly active in patients with RRMM
CR, complete response; CRS, cytokine release syndrome; IV, intravenous; MRD, minimal residual disease; ORR, overall response rate; PR, partial response; SC, subcutaneous; sCR, stringent complete response; VGPR, very good partial response
• Median time to CRS onset was 5 hours in the IV arm and 24 hours in the SC arm
• Median duration of CRS was 2 days
• Safety profile was consistent with other GPRC5Dtargeting bispecific antibodies

Carlo-Stella C et al. ASH 2022;abstract 161 (oral presentation)

Cevostamab with tocilizumab pretreatment to reduce incidence of CRS
• Tocilizumab treatment pretreatment with a single 8 mg/kg dose

• Median follow-up: 12.8 months non-TCZ and 8.5 months for TCZ groups


Tocilizumab reduces CRS rates with no negative impacts on antimyeloma activity of cevostamab


ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR, complete response; CRS, cytokine release syndrome; Gr, grade; ORR, overall response rate; PR, partial response; sCR, stringent complete response; TCZ, tocilizumab; VGPR, very good partial response
Trudel S et al. ASH 2022;abstract 567 (oral presentation)
CELMoD (including IMiD agents) agents act as molecular glues

CELMoD agents alter the target protein binding properties of CRBN to promote interaction with protein substrates that would not otherwise be candidates for degradation
IBER (CC-220) is an investigational product, currently not approved by any regulatory agency

CUL4, cullin 4; DC, dendritic cell; DDB1, DNA damage-binding protein 1; IMiD agents, immunomodulatory drug agents; NK, natural killer; ROC1, regulator of cullins-1; Ub, ubiquitin
van de Donk NWCJ et al. ASH 2020;abstract 724 (oral presentation)
CELMoD Iberdomide
+ Dd or Vd in R/R MM: Phase I/II Study Design
• Open-label, dose-escalation/dose-expansion trial
Patients with R/R MM and ≥2 prior regimens (≥1 in cohort F), including len/pom and PI) who progressed within 60 days of last therapy
Dosing schedules
Cohort E (28-day cycles)
Iberdomide D1-21
Dexamethasone D1,8,15,22
Daratumumab C1-2: D1,8,15,22; C3-6: D1,15; C7+: D1
Cohort F (21-day cycles)
Iberdomide D1-14
Dexamethasone D1,8,15
Bortezomib C1-8: D1,4,8,11; C9+: D1,8
Phase II: Dose Expansion
Cohort D at RP2D
Cohort I (post BCMA) at RP2D
Cohort J1 (ND MM, ASCT ineligible)
Cohort J2 (ND MM, ASCT eligible)

Primary endpoints: identify MTD and RP2D, efficacy
Secondary endpoint: safety
RP2D of 1.6 mg/day determined for iberdomide with Dex; cohorts E, F continuing enrollment with 1.6-mg/day dose
Iberdomide + Dd or Vd in R/R MM: Safety


• reported in either cohort

Iberdomide + Dd or Vd in R/R MM: Efficacy
• High response rates in heavily exposed and highly refractory patient population
– Among 27 patients in daratumumab cohort,

26 were IMiD refractory, 15 daratumumab refractory, 13 tripleclass refractory; 4 patient refractory to daratumumab achieved PR
– Among 23 patients in bortezomib cohort, 18 were IMiD refractory, 15 PI refractory, 9 bortezomib refractory, 9 triple class refractory; durable responses achieved in patients refractory or with prior exposure to bortezomib
• Addition of daratumumab or bortezomib to iberdomide + dexamethasone shows minimal effect on pharmacodynamics

Novel CELMoD agent: Mezigdomide (CC-92480) (preclinical data)


Mezigdomide (CC-92480) is a novel CELMoD agent, with a distinct chemical structure from LEN and POM, higher affinity for CRBN, and more efficient substrate degradation
Substrate (Aiolos) degradation efficiency
CC-92480
LEN
POM
Ymin = 35
Ymin = 18
Ymin = 5
CC-92480 is an investigational product, currently not approved by any regulatory agency

Ymin is the lowest point of the dose-response degradation curve and denotes the minimum percentage protein remaining
Hansen JD et al. J Med Chem. 2020;63:6648–76

Mezigdomide: PFS and response
PFS Response rates
Activity of MEZI-DEX is promising in TCR patients as well as those with plasmacytomas and/or prior anti-BCMA therapy



aData cut-off, Sept 16 2022; b≥PR; cIncluding extramedullary soft tissue only disease as well as soft tissue bone-related plasmacytomas; dResponders without adjusting for censoring.
BCMA, B-cell maturation antigen; CR, complete response; MR, minimal response; NE, not evaluable; PD, progressive disease; PFS, progression-free survival; PR, partial response; sCR, stringent complete response; TCR, triple-class refractory; VGPR, very good partial response
Modakafusp alfa: Mode of action


Modakafusp alfa: Study design and baseline characteristics
Study design
Baseline characteristics
BCMA, B-cell maturation antigen; IMiD, immunomodulatory drug; mAb, monoclonal antibody; MTD, maximum tolerated dose; OBD, optimal biological dose; PI, proteasome inhibitor; QW/Q3/4W, weekly/every 3/4 weeks


Modakafusp alfa: Efficacy and safety in the 1.5 mg/kg

Q4W + dexamethasone expansion cohort

• Median follow-up: 5.3 months
• Median duration of response: 12.5 months
• Median PFS: 5.7 months
BCMA, B-cell maturation antigen; CR, complete response; IRR, infusion-related reactions; mAb, monoclonal antibody; MR, minimal response; PFS, progression-free survival; Q4W every 4 weeks;
PR, partial response; TEAEs, treatment-emergent adverse events; VGPR, very good partial response



MSKCC Myeloma Service


TCE, CAR T Cells









Checkpoint Inhibitors
Developmental Therapeutics
Carlyn Tan

MM Precursor diseases
Supportive Care
Bone Health
Urvi Shah

MM Precursor Disease
Nutrition & Modifiable
Risk Factors
Early Relapse
Kylee Maclachlan
MM Precursor Disease, NDMM Trials
Genomics, Immune
Profiling
Neha Korde
NDMM Clinical Trials

Digital Wearables
Supportive Care
Alex Lesokhin
RRMM Immunotherapy
TCE, Checkpoints
Inhibitors
Neoantigens
Microbiota, Immune




Profiling
Hani Hassoun

MM Supportive Care
Alliance Liaison
NDMM/RRMM Trials
Elderly and Frail
Sham Mailankody
RRMM Trials with
CAR T Cells
High-Risk Disease
Malin Hultcrantz
RRMM Trials in TCR
Antibody drug conjugates
Epidemiology
Presented by: Saad Z. Usmani, MD MBA FACP, @szusmaniMSKCC Myeloma TCT Program






Sergio Giralt
Allo/Auto HCT for MM
New Regimens
CAR T Cells
David Chung
T Cell exhaustion
Auto HCT + Vaccines
MM Immunotherapies
Gunjan Shah
HCT Toxicities
Precision Drug Dosing
CAR T Cells
Salvage Auto and Allo HCT
Saad Z. Usmani

High-Risk Disease Biology/Trials


CAR T Cells
Auto HCT for MM
Michael Scordo
HCT Toxicities
Precision Drug
Dosing
CAR T Cells
Heather Landau
Amyloidosis
HCT Toxicities
Homebound HCT
Precision Drug Dosing
Novel Regimens for Salvage
Auto






Oscar Lahoud
Auto HCT and CAR T Cells
Post HCT Therapies
Parastoo Dahi
Auto HCT and CAR T Cells
Post HCT Therapies
Thank you to our Sponsors!













Thank you for Joining!
A replay of this program will be made available on our website and shared via email with all attendees

