

Welcome!
Thank you for joining us today for the October 22, 2022 International Myeloma Foundation’s Regional Community Workshop – Eastern Midwest States.
The Workshop will begin at 10:00 AM Central Time.
2
or order at






















Website: http://myeloma.org eNewsletter: Myeloma Minute IMF Webinars Videos Download
myeloma.org
Thank you to our sponsors!







4
Audience Q&A with Panel
• Open the Q&A window,allowing you to ask questions to the host and panelists. They can either reply to you via text in the Q&A windowor answer your question live.


• If the host answers live, you may see a notification in the Q&A window.
• If the host replies via the Q&A box – you will see a reply in the Q&A window.

5
Technical Issues
•If a technical issue arises – please use the Q&A to send questions to our support team who will reach out to assist you.


•You can also email us during the webinar at meetings@myeloma.org
There is a lag in audio, can someone assist me?
6
Workshop Video Replay & Slides


As follow up to today's workshop, we will have the speaker slides and a video replay available.
These will be provided to you shortly after the workshop concludes.
We want to hear from you!

Feedback Survey
At the close of the meeting a feedback survey will pop up.
This will also be emailed to you shortly after the workshop.
Please take a moment to complete this survey.
10:00
October 22, 2022 Agenda
and Announcements
Kelly Cox, Director Support Groups & Senior Director of Regional Community Workshops

10:05

Myeloma 101 and Frontline Therapy

Craig Cole, MD, Michigan State University, Karmanos Cancer Institute
AM - Q & A with Panel
and Stretch Break
Therapy and Clinical Trials
Rafat Abonour, MD, Indiana University
How to Manage Myeloma Symptoms and Side Effects
Deborah Doss, RN,
Board
Q&A with
Retired of 40+Years Dana Farber Cancer Research Institute,
IMF REGIONAL COMMUNITY WORKSHOP
AM - Welcome
AM -
10:40
10:55 AM – Meditation
11:05 AM - Relapsed
11:45 AM -
OCN,
IMF Nurse Leadership
12:05 PM -
Panel 9
Myeloma 101 and Frontline Therapy Craig Cole, MD Michigan State University



10









The Application of Science: Multiple Myeloma 101 and Frontline Therapy International Myeloma Foundation Regional Community Workshop Saturday October 22th, 2022 Craig Emmitt Cole,M.D. Assistant Professor Director of Clinical Research Department of Internal Medicine Division of Hematology/Oncology; Hematology Section Michigan State University College of Human Medicine Karmanos Cancer Institute
Today’s Discussion
common is multiplemyeloma
▪ How
▪ Spectrum of plasma cell disorders ▪ Diagnosis of myeloma and labs ▪ Staging and riskstratification ▪ The Science behind the treatments! ▪ Up front therapy strategies:induction, transplant, and maintenance ▪ Bone support ▪ “New Stuff” 4 drug induction therapy ▪ Perspectives in the advancement of myeloma science and survival
Multiple Myeloma is a Cancer of the Bone Marrow Plasma Cells
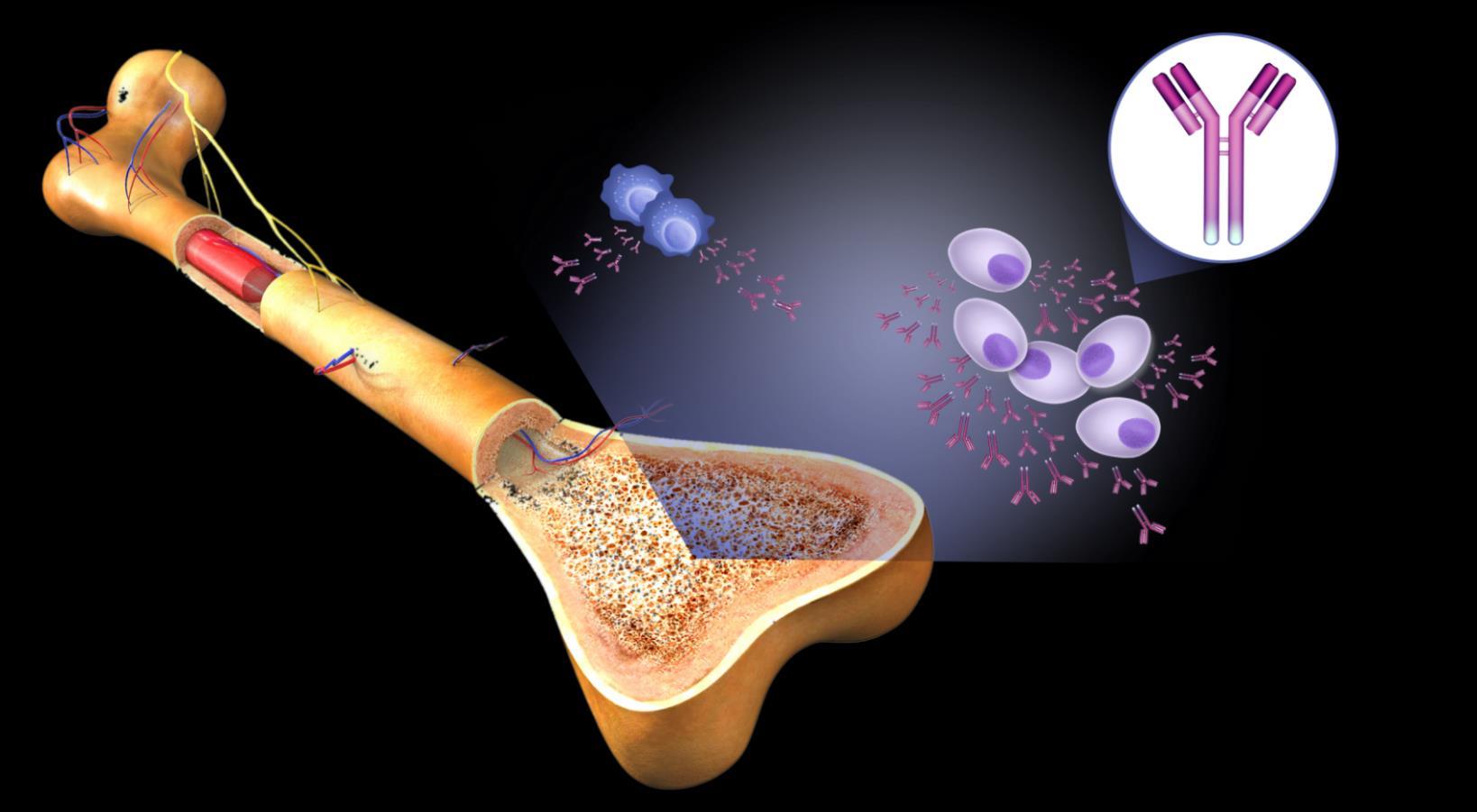

Cell
Normal plasmacells

Antibodies
Monoclonal (M) proteins







marrow
Multiple Myeloma cells
Large amounts of M proteins
Bone Bone
BONES • Surrounding bone where Myeloma cells grow is damaged/ weakened • Myeloma cells activate bone destruction blood calcium levels Calcium high Renal (kidney) failure Anemia Bone destruction Mutated Cancer
BLOOD • Myeloma is a cancer of the blood • Myeloma crowds out normal blood forming cells, causing anemia
Common Symptoms Multiple Myeloma

LowBloodCounts

Weakness Fatigue Infection
Weakness
Bone pain



Loss of
Weight loss
Campbell K. Nurs Times. 2014;110:12. About 10% to 20% of patients with newly diagnosed myeloma will not have any symptoms.
• Anemiais presentin 60% at diagnosis • May lead to anemia and infection
DecreasedKidneyFunction • Occursin overhalf of myelomapatients BoneDamage • Affects85%ofpatients • Leadsto fractures BoneTurnover • Leadsto high levels of calcium in blood (hypercalcemia)
appetite
C: Calciumelevation(>11mg/dL) R: Renal-low kidneyfunction;(serum creatinine >2mg/dL) A: Anemia–low redbloodcount(Hb <10g/dL) B: Bonedisease (≥1lytic lesionsonskeletalradiography,CT,or PET-CT)
Multiple































Myeloma Fast Facts Leukemia & Lymphoma Society. Facts and Statistics. http://www.lls.org/facts-and-statistics/facts-and-statistics-overview#Myeloma. SEER Cancer Stat Facts: Myeloma. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/mulmy.html North American Association of Central Cancer Registries (NAACCR), 2021 http://www.naaccr.org/DataandPublications/CINAPubs.aspx MultipleMyeloma 2nd most common blood cancer 34,920 new cases of myeloma in 2021 138,451 U.S. patients living with myeloma in 2021 Myeloma is most frequently diagnosed in people 65 to 74 years oldBlack Incidence: 14.1/100,000 White Incidence: 6.1/100,000















M protein over 3 g/dL (serum) or over 500 mg/24 hrs (urine) AND Plasma cells in Bone Marrow 10%–60% AND No CRAB or “SLiM” high risk features M protein under 3 g/dL AND Plasma cells in Bone Marrow <10% AND No CRAB or “SLiM” high risk features MGUS Monoclonal Gammopathy of Uncertain Significance Smoldering Myeloma MultipleMyeloma 10% risk of progression/year to active myeloma 1% risk of progression/year to multiple myeloma or related conditions Spectrum of Plasma Cell Disorders and Myeloma Malignant Plasma cells seen on any biopsy (usuallybone marrow) AND ≥1 “CRAB” feature OR have >1 SLiM ‘high risk” features: C: Calcium elevation(>11 mg/dL) R: Renal- lowkidneyfunction; (serumcreatinine >2 mg/dL) A: Anemia–low red bloodcount (Hb <10 g/dL) B: Bone disease (≥1 lytic lesions onskeletal radiography, CT, or PET-CT) S: >60% Plasma Cells on Bone Marrow biopsy Li: Serum light chain ratio >100 M: >1 lytic lesions on MRI (or PET/ CT scan) High Risk Smoldering M protein over 2 g/dL AND Plasma cells in Bone Marrow 20%–60% AND Free Lt Chain Ratio >20 “Evolving type”SMM Increase >10% protein w/in 6mo AND No CRAB or “SLiM” high risk features >46% risk of progression in 2 yr to active myeloma Observation Clinical Trials Observation Clinical Trials Close Observation Clinical Trials ?? Treatment?? Front Line Treatment Clinical Trials
Diagnosing

























Learn Your Labs!




Myeloma:
CBC • Number of red blood cells, white blood cells, and platelets CoMP • Measure levels of albumin, calcium, and creatinine. Assess function of kidney, liver, and bone status (alkaline phosphatase) and the extent of disease. Immuno Fixation • Identify the type of abnormal antibody proteins: IgG, IgA, κ,or λ Serum ProteinEP • Detect the presence and level of M protein = how much myeloma Serum FreeLight Chain • Freelite test measures free light chains (kappa or lambda) in blood = how much myeloma Urine ProteinEP • Detect Bence-Jones proteins (otherwise known as myeloma light chains) in urine (present or not present) 24 hrUrine Analysis • Determine the presence and levels of M protein and Bence Jones protein in the urine = how much myeloma LDH Lactate Dehydrogenase • Determine the level of myeloma cell production and extent of MM : USED FOR STAGE Beta2 MicroG • Determine the level of a protein that indicates the presence/extent of MM and kidney function: USED FOR STAGE

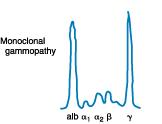
























IgG Kappa M-Protein IgG Kappa M-Protein Serum ProteinEP • Detect the presence and level of M protein = how much myeloma Monoclonal protein Treatment • Freelite test measures free light chains (kappa or lambda) in blood = how much myeloma Serum FreeLight Chain Kappa Lt. Chain Lambda Lt. Chain Kappa Lt. Chain MM Positive Kappa Monoclonal Serum Light Chains Treatment Ratio: 1
Types of Monoclonal Protein (M Protein) in Multiple Myeloma
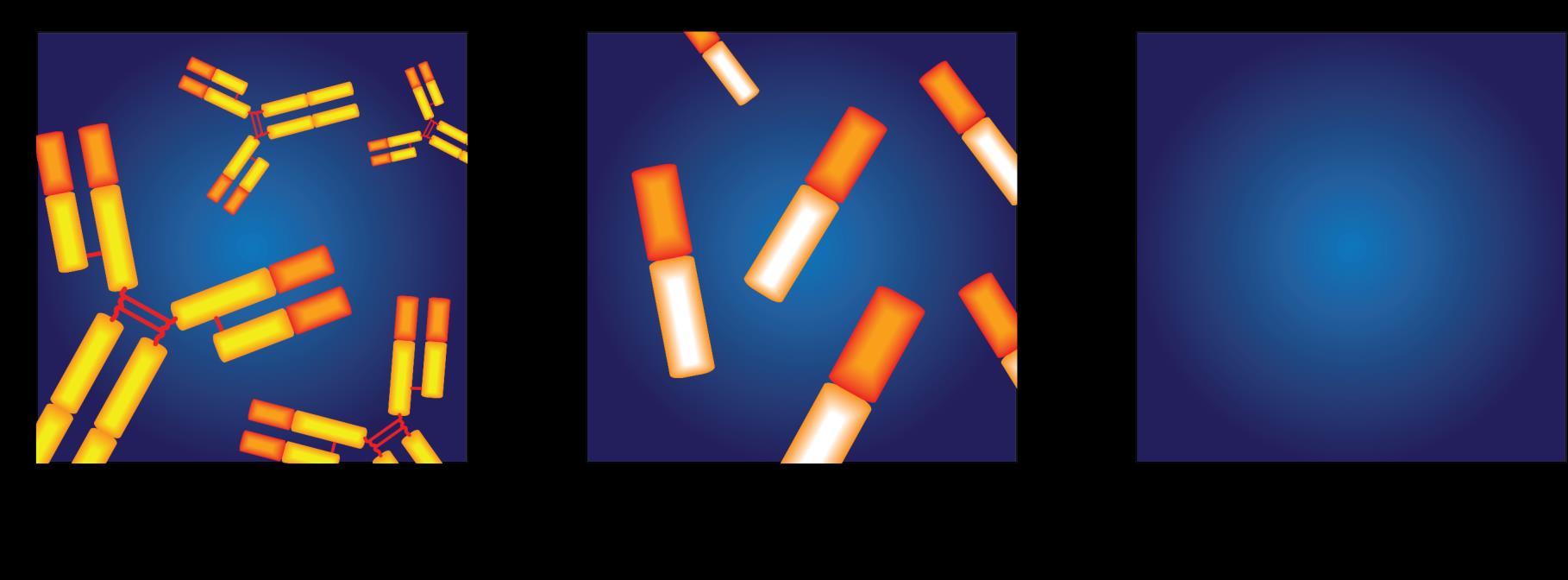
Forexample:
IgG+kappa
IgG+lambda
IgA+kappa
IgA+lambda
etc…
80%of myeloma cases
Jonesprotein
18%of all myeloma cases
Renal failure more commonin light chain multiple myeloma; creatinine >2 mg/dL in 1/3 ofcases
proteinpresent
Lessthan 3% of casesofmultiple myeloma
•
•
•
•
•
•
•
•
•
Diagnosis of

Conventional X-rays reveal punched-out lytic lesions, osteoporosis, or fractures in 75% of patients.
FDG PET/CT appears to be more sensitive (85%) than skeletal survey for the detection of small lytic bone lesions.


Multiple Myeloma •
•
Kyle RA et al. Mayo Clin Proc Jan;78(1): 2003. Nanni Cet al. European Journal of Nuclear Medicine and Molecular Imaging Vol.33:2006 Dimopoulos MA, et al. Leukemia. 2009 • Diagnosis is confirmed with bone marrow demonstrating greater than 10% involvement by malignant plasma cells with either CRAB or SLiM Malignant Plasma cells seen on biopsy AND ≥1 “CRAB” feature OR have >1 SLiM ‘high risk” features: C: Calcium elevation(>11mg/dL) R: Renal- low kidneyfunction; (serumcreatinine >2mg/dL) A: Anemia –low redblood count (Hb <10 g/dL) B: Bone disease (≥1 lytic lesionson skeletalradiography, CT, or PET-CT) S: >60%PlasmaCells onBoneMarrow biopsy Li: Serumlightchainratio >100 M:>1 lytic lesionsonMRI (or PET/CT scan)
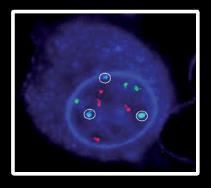
Staging Myeloma: The Importance of Genomic Testing
Conventional cytogenetic analysis (karyotyping)


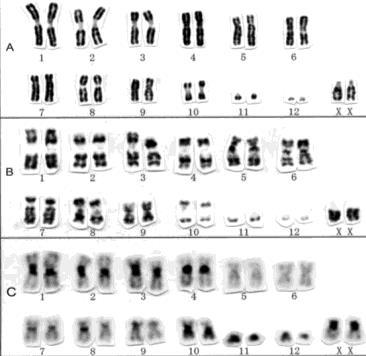
FISH (fluorescencein situ hybridization)
DNA
Advances
expressionprofiling[GEP]
whole-exomesequencing
cellnext generationsequencing
• Genetic
• Whole-genome/
• Plasma


Staging Myeloma: FISH helps to Assign Risk in Myeloma Risk Category High Risk Standard Risk Findings on Chromosome (FISH) Analysis Results in the Bone marrow FISH: • Deletion17th chromosome • Gain of chromosome1q • Translocation 4 and 14 • Translocation 14 and 16 • Translocation 14 and 20 NGS: p53 mutation (on chrom 17) FISH: • Hyperdiploid: More than 1 pair of chromosomes (Trisomies) • Translocation 11 and 14 • Translocation 6 and 14 • Others • Normal *Based on the Updated MayoStratification of Myelomaand Risk-AdaptedTherapy(mSMART)ConsensusGuidelines2013 Mikhael JR et al. Mayo Clin Proc.2013;88:360 • Double Hit Myeloma: 2 high risk genetic abnormalities • Triple Hit Myeloma: 3 or more high risk genetic abnormalities


































Stage 3 β2-microglobulin over 5.5 mg/L HIGH Lactate Dehydrogenase (LDH) High Risk Cytogenetics (FISH) Deletion17 chromosome Translocation4th and 14th Translocation14 and 16th Translocation14 and 20th AND/OR Stage 1 β2-microglobulin under 3.6 mg/L Normal Lactate Dehydrogenase (LDH) NO High Risk Cytogenetics (FISH) AND *Based on the Updated MayoStratification of Myelomaand Risk-AdaptedTherapy(mSMART)ConsensusGuidelines2013 Mikhael JR et al. Mayo Clin Proc.2013;88:360. Palumbo et al. JCO September 10, 2015 vol. 33 no. 26 2863 2869 Revised International Staging System for Multiple Myeloma From International Myeloma WorkingGroup Stage 2 Does not meet Criteria for Stage 1 or 3


Immunomodulatory Drugs ( Thalomid(Thalidomide), Revlimid(Lenalidomide Proteasome Inhibitors (Pis): Velcade(Bortezomib), Ninlaro(Ixazomib), Kyprolis(Carfilzomib) Antibodies Against Myeloma (Immunotherapy): Darzelex (Daratumumab), Sarclisa(Isatuximab), Empliciti(Elotuzumab)
IMiDs for Multiple Myeloma
How does it work?-SCIENCE!
• Direct inhibition of DNA synthesis of myeloma cells.
IMiDs for Multiple Myeloma
VEGF bFGF


How does it work?-SCIENCE!
Direct inhibition of DNA synthesis of myeloma cells.
Inhibition of blood vessel synthesis in the bone marrow.
•
•
IMiDs for Multiple Myeloma
How does it work?-SCIENCE!
• Direct inhibition of DNA synthesis of myeloma cells.
• Inhibition of blood vessel synthesis in the bone marrow.
• Inhibition of adhesion between the myeloma and bone marrow stromal cells.
IMiDs for Multiple Myeloma
How does it work?-SCIENCE!
Direct inhibition of DNA synthesis of myeloma cells.
Inhibition of blood vessel synthesis in the bone marrow.
Inhibition of adhesion between the myeloma and bone marrow stromal cells.
Inhibition of the release of the cytokines IL-6, TNF-
and IL-1β.









•
IL 6 IL 1β TNF α •
•
•
α,
IMiDs for Multiple Myeloma
•
How does it work?-SCIENCE!
Direct inhibition of DNA synthesis of myeloma cells.
• Inhibition of blood vessel synthesis in the bone marrow.
• Inhibition of adhesion between the myeloma and bone marrow stromal cells.
•
Inhibition of the release of the cytokines IL-6, TNF-α, and IL-1β.
• Activation of the body’s natural killer cells (T-cells) which attack the myeloma cells.
Rise of the IMiD Biologic Therapies
1990s, several thalidomide analogs were synthesized to increase efficacy and minimize toxicity
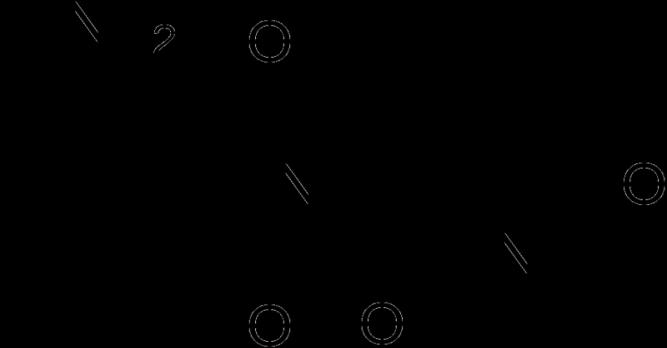
2006 FDA approves Lenalidomide (Revlimid).
Revlimid is felt to be 50 to 2000 more potent than thalidomide.
Phase 2 trial 91% new myeloma achieved responses with Lenalidomide plus dexamethasone.
2013 FDA approves Pomalidomide.
Pomalyst and dexamethasone given to multi-refractory myeloma with response rates of 35 to 65%.
Combination of pomalidomide, bortezomib, and dexamethasone in relapsed MM response rates of 72%.
(CC-220) is the newest in the class and is
in
trials
are the next class of IMiD with CC92480 drug more potent than
▪
. ▪
▪
▪
▪
▪
▪
▪ Iberdomide
now
clinical
▪ CELMODs
iberdomide Blood.2005;106:4050 4053.Blood.2013Jan14. [Epubaheadof print]. Exp HematolOncol.2012;1: 27; J ClinOncol. 2009;27(30):5008 5014. O N H N O OOThalidomide N H N O OO NH2 Lenalidomide Pomalidomide
Rise of the Proteasome
Proteasome


 R Vij et al. Br J Haem, June 2012. ; MOREAU et al. BLOOD,
R Vij et al. Br J Haem, June 2012. ; MOREAU et al. BLOOD,
AUG VOL 120(5 ); 2012
Rise of the Proteasome
Proteasome


 R Vij et al. Br J Haem,
R Vij et al. Br J Haem,
June
2012. ; MOREAU et al. BLOOD,
AUG VOL 120(5 ); 2012
Rise of the Proteasome


 R Vij et al. Br J Haem,
R Vij et al. Br J Haem,
June 2012. ;
MOREAU
et al. BLOOD, AUG VOL 120(5 ); 2012
Rise of the Proteasome
• Bortezomib(velcade)approved by the FDAin 2003 in patients with relapsed refractory myeloma.
• Several phase 2 trials in newly diagnosed myeloma with bortezomib-dexamethasoneinduction.
o Responses 66% to 90%, including 15% to 21% Complete Responses!
• 2012 FDAapproves Carfilzomib(Kyprolis); secondgenerationirreversibleProteasomeinhibitor
• In refractory myeloma with 48% response rates. Higher in combination!
• Ixazomib (Ninlaro) is new oral boronated reversible proteasomeinhibitor currently approved by the FDAin 11/2015.
Bortezomib(Velcade)
Carfilzomib(Kyprolis)



Ixazomib (Ninlaro)
R Vij et al. Br J Haem, June 2012. ; MOREAU et al. BLOOD, AUG VOL 120(5 ); 2012; Blood. 2014;124(7):1047 1055


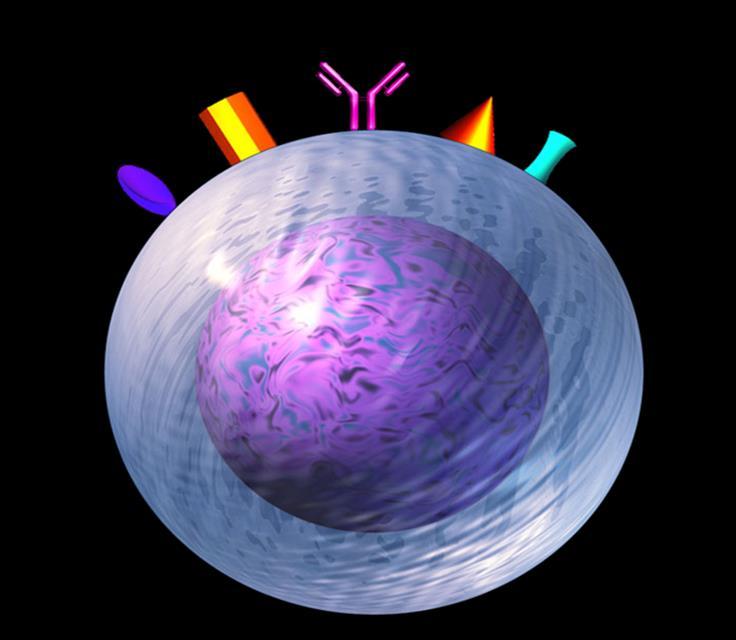




























Targets on the Myeloma Cell Surface and Therapeutic Antibodies BCMA CD38 GPRC5D SLAMF7 FcRH5 Immune Therapies Belantamab Ide cel CAR T Cilta cel CAR T Bi-Specific Antibodies Other CAR-Ts Antibody Drug Elotuzumab Bi-Specific Antibodies Antibody Drug Daratumumab and Darzalex Faspro Isatuximab TAK-079 MOR202 Bi-Specific Antibodies CAR-T Bi-Specific Antibodies CAR-T



















































Antibody and Immune System Attack Manufactured Antibody Targeting of Myeloma Antibody Receptor Natural Killer Cell Good Guys Myeloma surface targets Manufactured Anti-Myeloma Antibody Complement Proteins Complement Protein Attack Myeloma Cells Attacking the Myeloma Cell Biology Macrophages Good Guys Increase production of cytoxic macrophages Inhibition of adhesion between the myeloma and bone marrow stromal cells
Tools
for

of the Trade
Frontline Therapy Standard DrugOverview Class Drug Name Abbreviation Administration IMiD immunomodulatory drug Revlimid (lenalidomide) R or Rev Oral Thalomid (thalidomide) T or Thal Proteasome inhibitor Velcade (bortezomib) V or Vel or B Intravenous (IV) or subcutaneous injection (under the skin)Kyprolis (carfilzomib)* C or K or Car Ninlaro (ixazomib)* N or I Oral Chemotherapy Cytoxan (cyclophosphamide) C Oral or intravenous Alkeran or Evomela (melphalan) M or Mel Steroids Decadron (dexamethasone) Dex or D or d Oral or intravenous Prednisone P Monoclonal Antibodies Daratumumab (Darzalex) Dara Intravenous (IV) or subcutaneous injection (under the skin) *In Clinical Trials/ not FDAapproved








Treatment Sequence and Regimens for Active Myeloma National Comprehensive Cancer Network. The NCCN Clinical Practice Guidelines in Oncology Multiple Myeloma (Version 1.2020). http://www.nccn.org/. Accessed September 6 2020 • Revlimid • Velcade • Ninlaro • Observation • Thalidomide • Revlimid/Dara • Clinicaltrial • Velcade/Revlimid/Dex:(VRD) • Velcade/Thalomid/Dex:(VTD) • Velcade/Cytoxan/Dex:(CyBorD) • Darzalex/Revlimid/Dex:(DRD) • Darzalex/Velcade/Melphalan/Dex • Darzalex/Velcade/Thalidomide/Dex • Kyprolis/Revimid/Dex(KRD) • Darzalex/Velcade/Revlimid/Dex: Dara-RVD • Ninlaro/Revimid/Dex(IRD) • Clinicaltrials Frontline treatment Maintenance Relapsed Induction Maintenance • Stem Cell Transplant • Continue Induction • Clinicaltrial Consolidation Dara+Pomalyst+Dex Kyprolis+Pomalyst+Dex Cytoxan+Pomalyst+Dex Ninlaro+Pomalyst+Dex Elo+Pomalyst+Dex Elo+Thaliomide+Dex Dara+Kyprolis+Dex Kyprolis+Revlimid+Dex Elo+Revlimid+Dex Dara+Revlimid+Dex Dara+Velcade+Dex Elo+Velcade+Dex Panobinostat+thalidomide+ velcade+dex Cytoxan+Kyprolis+Thalidomide+dex 4 drug therapies of novel agents Ninlaro+Cytoxan+Dex Velcade+ Cytoxan+Dex Velcade+ Pomalyst+Dex Chemotherapy Selinexor+Dex Isatuximab(Sarclisa)+ Pomalyst+Dex Darzalex Faspro (under skin) (FDA approved May 1, 2020) Belantamab mafodotin (FDA approved 8/5/2020) Ide-cel CAR-T (FDA approved 3/26/2021) Cilta-cel CAR-T (FDA approved 2/28/2022) CLINICAL TRIALS! Rescue









https://www.msmart.org/mm-treatment-guidelines Dispenzieri et al. Mayo Clin Proc 2007;82:323 341; Kumar et al. Mayo Clin Proc 2009 84:1095 1110; Mikhael et al. Mayo Clin Proc 2013;88:360-376. v18 //last reviewed June 2020 Transplant Candidate Newly Diagnosed MM Not Transplant Candidate Revlimid/Velcade/Dex (RVD) or Dara-Revlimid Dex (Dara-Rd) 9 to 12 cycles RVD-light or Dara-RD Others: D-VMP,D-VTD,RD 9 to 12 cycles High Risk Standard Risk Velcade Based Maintenance of VRD or Continued Dara-Rd Maintenance Revlimid Maintenance or Continued DaraRd Maintenance Dara-RVD x 4 cycles Other: KRD High Risk Early Auto SCT ?Tandem SCT or Dara-VRD Early Auto SCT Delayed Transplant Collect & store Continue Tx for 8m Standard Risk Revlimid Maintenance +/- Dara Revlimid Maintenance +/- Dara Velcade (PI) Based Maintenance Continued Dara-Rd Maintenance Preferred
Goals
The











Model of
of Therapy:
Iceberg
Myeloma Partial response 50% reduction in M protein Very good partial response 90% reduction in M protein immunofixation positive only Complete remission No M-protein immunofixation negative Minimal Residual Dis Next Generation Molecular testing Minimal Residual Dis Flow Cytometry At diagnosis >1 Trillion Disease Burden (# of myeloma cells) Symptomatic Myeloma >1 Billion >10 Million 1 myelomacell in 100Kto 1 million normal cells
Stem Cell Transplant: Fighting Myeloma with the Left Hook!




41
N Engl J Med. 2022 Jul 14;387(2):132-147. doi: 10.1056/NEJMoa2204925 -Patients aged 18-65 yrs with symptomatic newly diagnosed MM following 1 cycle of VRd -56 sites within the United States from 2010 to 2018 Stem cell collection Induction Consolidation Maintenance Until Progression VRd cycles 2-3 (n = 357) VRd cycles 2-3 (n = 365) VRd cycles 4-8 VRd cycles 4-5 R (n = 291) R (n = 289) ASCT: Melphalan 200 mg/m2 + Stem Cell Support (n = 310) RVD +Stem Cell Transplant vs. RVD without Transplant DETERMINATION Trial ofNewly DiagnosedMM: DESIGN End Points of Study and Follow-up • Primary end point: progression-freesurvival (time to next relapse) • Secondary end points included: • Response rates, overall survival, quality of life, and adverse events • Follow-up on participantstatus: median of 6 years

















Parameter RVd- No Transplant (n = 357) RVD with Up Front Transplantation (n = 365) P Value Is it Significant? Grade 3 or 4 toxicities 78 94 YES! P<0.001 P<0.001 P<0.001 OVERALL SURVIVAL at 5 years (%) 79.2 80.7 NO Median duration of Partial Response or better, mo 38.9 54.4 YES! .003 Negative MRD 39.8 79 RVD +Stem Cell Transplant vs. RVD without Transplant DETERMINATION Trial ofNewly DiagnosedMM RESULTS • At a median follow up of 76.0 months, the risk of disease progression or death was 53% higher in the RVD alone group than in the transplantation group (P<0.001) N Engl J Med. 2022 Jul 14;387(2):132-147. doi: 10.1056/NEJMoa2204925
vs.
without
Trial ofNewly Diagnosed
Quality of Life
Global Health Status/QoL,
Physical Functioning
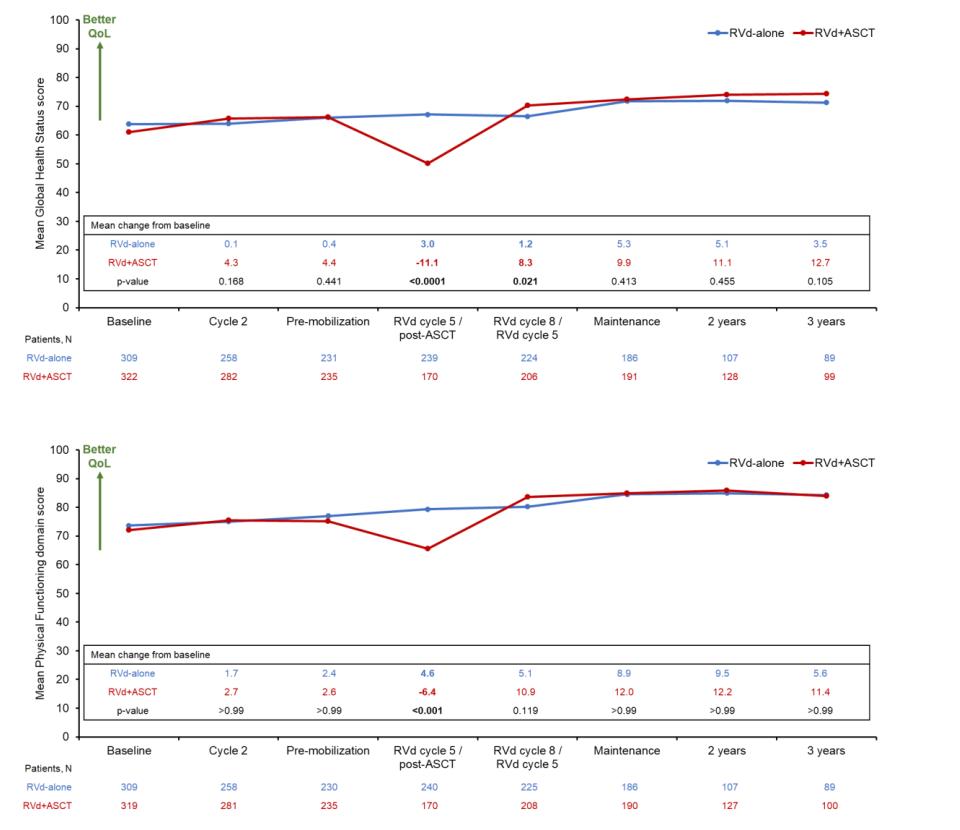
RVD +Stem Cell Transplant
RVD
Transplant DETERMINATION
MM
N Engl J Med. 2022 Jul 14;387(2):132-147. doi: 10.1056/NEJMoa2204925



















Stadtmauer EA, et al. ASH 2016. Abstract LBA 1; Journal of Clinical Oncology 38, no. 15_suppl ( ASCO May 20, 2020) 8506 8506 New Dx Myeloma After >2 cycles induction Tx ASCT eligible ≤ 70 yrs (N = 758) Melphalan 200mg/m²IV Stem Cell Transplant Revlimid Maintenance 10 mg/day for 3 cycles, then 15 mg/day* (n = 257) Consolidation Velcade 1.3 mg/m² IV Days 1, 4, 8, 11 Revlimid 15 mg Days 1-15 Dexamethasone 40 mg IV Days 1, 8, 15 Four cycles (n = 254) Revlimid Maintenance 10 mg/dayfor3 cycles, then 15 mg/day Second(Tandem) Stem CellTransplant Melphalan 200 mg/m² IV Second ASCT (n = 247) Induction regimens ▪ RVD ▪ CyBorD ▪ RD ▪ VD ▪ Others What to do After Transplant? STaMINA: Phase III Study Design RESULTS • No difference in time to relapse (PFS) or Overall Survival in standard risk patients who have two transplants, consolidation RVD therapy, or just straight to maintenance after first BMT • Straight to maintenance is the easiest! • ? If high risk patients benefit from two transplants
Analysis of the MaintenanceRevlimid (Lenalidomide) Trials
Data from 4 randomized trials of Revlimid (lenalidomide) maintenancevs. no maintenance
Involving a
of
multiple
The results of the analysis showed that Revlimid maintenancetherapy is associatedsignificant improvementin progression-freesurvivaland a modestimprovementin overallsurvival
Duration ofmaintenanceis unknown
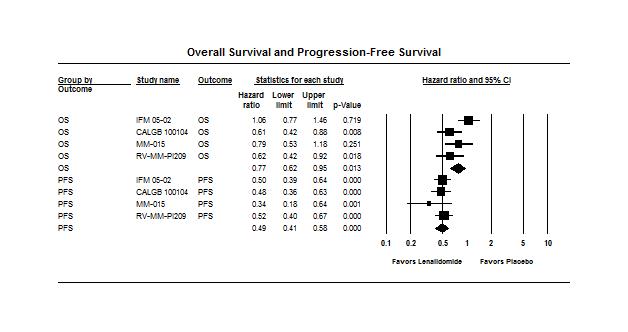
•
–
total
almost 2,000
myeloma patients •
•
Bone Support & Control of Bone Pain

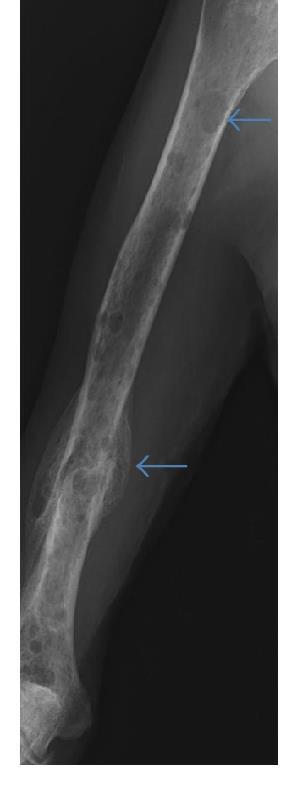
Multiple myeloma can cause weakened areas in the bone called osteolytic lesions which can compress the spinal cord or cause bone destruction. • Bone strengtheningdrugs: bisphosphonates(pamidronate& Zometa) ormonoclonal antibodies (Xgeva) are given at diagnosis and continuedfor at least 2 years • Vitamin-Dand Calcium supplementsto help bonehealing • Orthopedicsupport – Physical therapy, physical medicine consults, orthopedic/neuro surgery, radiation therapy, etc • Minimally invasive procedures: kyphoplastyor vertebroplasty • Use of medication to controlpain • Anticonvulsants and antidepressantsfor treatrelieve pain from nerve damage or numbness

GRIFFIN Randomized Phase II: Dara-RVD vs. RVD in Newly Diagnosed Multiple Myeloma Dara-VRd in 21-day cycles (n = 104) Transplant-eligible adults with Newly Diagnosed MM, with good performance status and kidney fxn (N = 207) Dara-VRd in 21-day cycles D: 16 mg/kg IV D1 VRd: as in induction Dara-R in 28-day cycles D: as in consolidation Q4W or Q8W R: 10 mg PO D1 21 of C7 9 and 15 mg PO D1-21 of C10+§ A S C T Induction: Cycles 1-4 Consolidation: Cycles 5-6† Maintenance: Cycles 7-32‡ ▪ Primary endpoint: CR by end of consolidation VRd in 21-day cycles (n = 103) VRd in 21-day cycles VRd: as in induction R in 28-day cycles R: 10 mg PO D1 21 of C7 9 and 15 mg PO D1 21 of C10+§ Randomized 1:1 Kaufman. ASH 2020. Abst.594; Voorhees PM etal. Blood. 2020 Aug 20;136(8):936-945.



















Depth of Response Stringent Complete Response (sCR) Complete Response (CR) Very Good Partial Response (VGPR) Partial Response (PR) Patients (%) 60 0 Dara-RVD (n = 99) RVD (n = 97) ≥ CR: 42.3% ≥ VGPR: 73.2% 10.3 18.6 Overall ResponseRate = 91.8%Overall ResponseRate = 99.0% End of Consolidation ≥ VGPR: Voorhees PM et al. Blood. 2020 Aug 20;136(8):936 945. Sborov et al. IMS Annual Meeting. 2022 Aug 26; Abst# OAB 057 Overall ResponseRate = 99.0% End of study 83% CR: 60% ≥ VGPR: 77% 14 Overall ResponseRate = 91.8% GRIFFIN Randomized Phase II: Dara-RVD vs. RVD in Newly Diagnosed Multiple Myeloma In the final analysis after >4 years of follow up, the addition of DARA to RVd led to a Progression Free Survival benefit favoring the Dara RVd arm with a 55% reduction in progression or death These data support use of D RVd induction/consolidation and D R maintenance as a NEW standard of care in Newly Diagnosed Myeloma
Study No. of patients Phase of study Efficacy Data Safety Data Dara-VMP vs VMP Dimopoulos MA, 2018 706 Phase III ORR = 90.9% sCR = 22.3% VGPR = 27.7% PR = 18.0% ≥VGPR = 72.9% CR+ = 45.1% Median PFS (at 27.8 months) = NR Grade 3 or 4 TEAEs = 23.7% Dara-IRD Kumar 2019 40 Phase II CR = 11% VGPR = 47% PFS = 97.5% ORR = 95% Grade ≥3 AEs = 42% Dara-RVD vs RVD followed by ASCT Voorhees 2020 D-RVD: 99 RVD: 97 Phase III ORR: DVRD=99.0% vs VRD=91.8%; 22 mo sCR: DVRD 62.6% vs RVD 45.4% MRD negativity DRVD 51.0% vs RVD 20.4% 24-mo PFS DRVD 95.8% RVD 89.8% Serious AEs were reported in 39 (39.4%) patients in the D-RVd group and 52 (51.0%) in the RVd group Dara-CVD Yimer 2018 87 NDMM (101 total) Phase II ≥VGPR = 56% CR = 9% ORR = 81% 12 month PFS = 87% OS = 99% Grade ≥3 AEs = 56% Dara-KRd Costa 2019 Landgren 2021 38 ----41 Phase II ORR = 100%; ≥VGPR = 92% after induction CR/sCR = 91% before BMT; MRD negative 65% at best response ORR = 100%; ≥VGPR = 95% after induction; PFS was 98%MRD negative 71% at 20.3 months follow-up Grade 3/4 AEs: neutropenia (n=7), infection (n=6), insomnia (n=4), hyperglycemia (n=2), rash (n=2) Isatuximab-RVD Ocio 2018 22 Phase I ORR = 93% MRD negativity = 38.5% sCR = 7.14% VGPR = 71.43% CR = 7.14% 7.5 mo PFS = 100% Grade ≥3 AEs = 46% Isatuximab-KRD for high-risk MM Abstract S204. EHA 2020 46 Phase II ORR = 100%, with PR=10%, VGPR=44% and CR=46%; 20 of 33 pts were MRD negative in ASCT eligible arm Grade 3/4 AE: neutropenia 34%, anemia10%, thrombocytopenia 14%, hypertension 12%, infection 8% Where We Are Going…4-Drug Induction for Newly Diagnosed Myeloma
It’s important to know…
What are YOUR goals of therapy
How to read your M-protein level
What is your MM risk/ stage
What are your therapy options
What is your response to tx
Know what side effects to expect so you can report them
Who is on your care team
Obtain a second opinion Ask about clinical trials


informed and empowered!
M-Protein
IgG Kappa

M-Protein
Be
Advancements in Survival of Multiple Myeloma




Blood (ASH Annual Meeting Abstracts) 2011 118: Abstract 5070; Blood Advances, 2017 Vol1(4);p282 287. • Withnew biology-basedmedication3 and 4 drug regimens the responserates are now >98% • Wehave had 31 drugs and tx indicationsFDA approvals for myeloma 2015-2022! Myeloma is not curable…yet. But is survivable now! • Withnovel therapiesare used at diagnosis, survival has improved dramatically From 3.8 years to >8 years! The 10yr relative survival rate has nearly doubledsince in the past 20 years 2019 74,814 2011 2021 138,451 54,963 2004 People in the United States living or in a Remission from Multiple Myeloma








colecrai@msu.edu


55
Audience Q&A with Panel
• Open the Q&A window,allowing you to ask questions to the host and panelists. They can either reply to you via text in the Q&A windowor answer your question live.



• If the host answers live, you may see a notification in the Q&A window.




• If the host replies via the Q&A box – you will see a reply in the Q&A window.

56
IMF

11:05 AM -
Agenda After Break

RelapsedTherapy and Clinical Trials
Rafat Abonour, MD, Indiana University
AM - How to Manage Myeloma Symptoms and Side Effects

Deborah Doss, RN, OCN, Retired of 40+Years Dana Farber Cancer
Research Institute, IMF Nurse Leadership Board
PM - Q&A with Panel
REGIONAL COMMUNITY WORKSHOP – October 22, 2022
57
11:45
12:05
Relapsed Therapy and Clinical Trials Rafat Abonour, MD Indiana University



58
How Do I Treat Relapsed Multiple Myeloma? Rafat Abonour, M.D.
Harry and Edith GladsteinProfessor of Cancer Research Professor of Medicine, Pathology and Laboratory Medicine
Director,MultipleMyeloma, Waldenstrom's Diseaseand Amyloidosis Program
Indiana University School of Medicine

When should we treat relapsed disease?

• At biochemical relapse? • When myeloma protein starts rising! • When involved free light chain starts rising! • At Clinical relapse? • CRAB criteria (Anemia, kidney failure, high calcium or new bone disease) • Extramedullary disease (myeloma growing at tumors outsides the bone)
When should we treat relapsed disease?

treatment
were based on
Some observations support better outcome when treating
relapse:
• We do not know, but we can speculate! • Most approved
in relapsed myeloma
biochemical relapse. •
biochemical
• Mayo clinic showed better overall survival when treating biochemical relapse (median PFS 125 vs 81 months)
Pros and Cons of treating Biochemical Relapse

• Con: • 25% of biochemical relapse patients will have a smoldering course. No progression for 2 years. • Pros: • Median time between biochemical relapse and clinical symptoms is about 5-6 months. • If you do not get it right at first relapse you may not get it right at subsequent relapses
Duration Decreaseswith


0 12 10 8 6 2 First Second Third Fourth Fifth Sixth TreatmentRegimen Median response duration (months) 4 Response
each relapse
Genomic Heterogeneity Affects the Course of MM
Most patients with MM have multiple distinct subclonalpopulations as a result of the expansion of genetically different myeloma cells; this causes intratumoral heterogeneity
MM is clonallyheterogeneous at diagnosis and throughouttreament
The genomic heterogeneity of MM contributes to treatment resistance and relapse
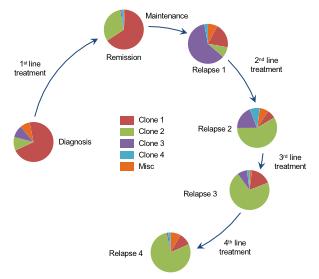
Wide variety of mutations found within a single patient may result in treatment resistance and refractory disease

Furthermore, subclones continually mutate over time, including after treatment, which may contribute to resistance and result in disease
Evolution
•
1 •
2 •
3 •
1,3,4 •
progression1,5
1. Bolli N et al. Nat Commun. 2014;5:2997. 2. Walker BA et al. Leukemia. 2014;28(2):384 390. 3. KyrtsonisMet al. Appl Clin Genet. 2010;3:41 51. 4. Keats JJ et al. Blood. 2012;120(5):1067 1076. 5. Abdi J et al. Oncotarget. 2013;4(12):2186 2207.
of ClonalPopulationsofMyeloma Cells4 6 4 Republished with permission of American Society of Hematology, from Keats JJ et al. Blood. 2012;120(5):1067 1076; viaCopyright Clearance Center, Inc.
Clonal heterogeneity in English
• Myeloma cells in each patients are a family of odds and aggressive members

• The longer this family sticks around the odder and more aggressive it becomes
• Family therapy is not going to work, you can not be politically correct here, eradicate them early.
• This is making the case for early treatment and with combination regimen
Factors influencing the choice of therapy at Relapse

Disease related
Regimen related
Patients related
•
•
•
Disease related Factors
Clinical features associated with relapse (elevated calcium, renal failure, anemia, bone lesions)
Expanding or new plasmacytomas or hyperviscosity
High-risk cytogenetics
Extramedullary disease or plasma cell leukemia
High ISS stage

•
•
•
•
•
Regimen-related Factors
Previous treatment and dose
Duration of prior response
Side effects, tolerability, and toxicity of prior treatment(s) and treatment combinations
Depth and duration of previous transplant

•
•
•
•
Patient-related Factors
status, Frailty
comorbidities

eligibility
preference
• Performance
• Patient
• Transplant
• Patient
• Cost/socioeconomics
Currently available

Prednisone Melphalan Thalidomide Bortezomib Panobinostat
Dexamethasone Cyclophosphamide Lenalidomide Carfilzomib
Doxorubicin Pomalidomide Ixazomib



















































DCEP/D PACE
Agents
Daratumumab (anti CD38) Selinexor
Isatuximab (anti CD38)
Elotuzumab (anti CS1)
Belantamab (anti BCMA + MMAF)
ABECMA
INDIANA UNIVERSITY SCHOOLOF MEDICINE Steroids Conventional Chemo ImIDs Proteasome Inhibitors HDAC inhibitor Immunologic approaches XPO inhibitor
METRO28 Carmustine Others: Venetoclax Bendamustine
Anti-Multiple Myeloma
How does your oncologist decide what to do?

It is like going to the Cheesecake factory
This is a very short menu

How does your oncologist decide what to do?
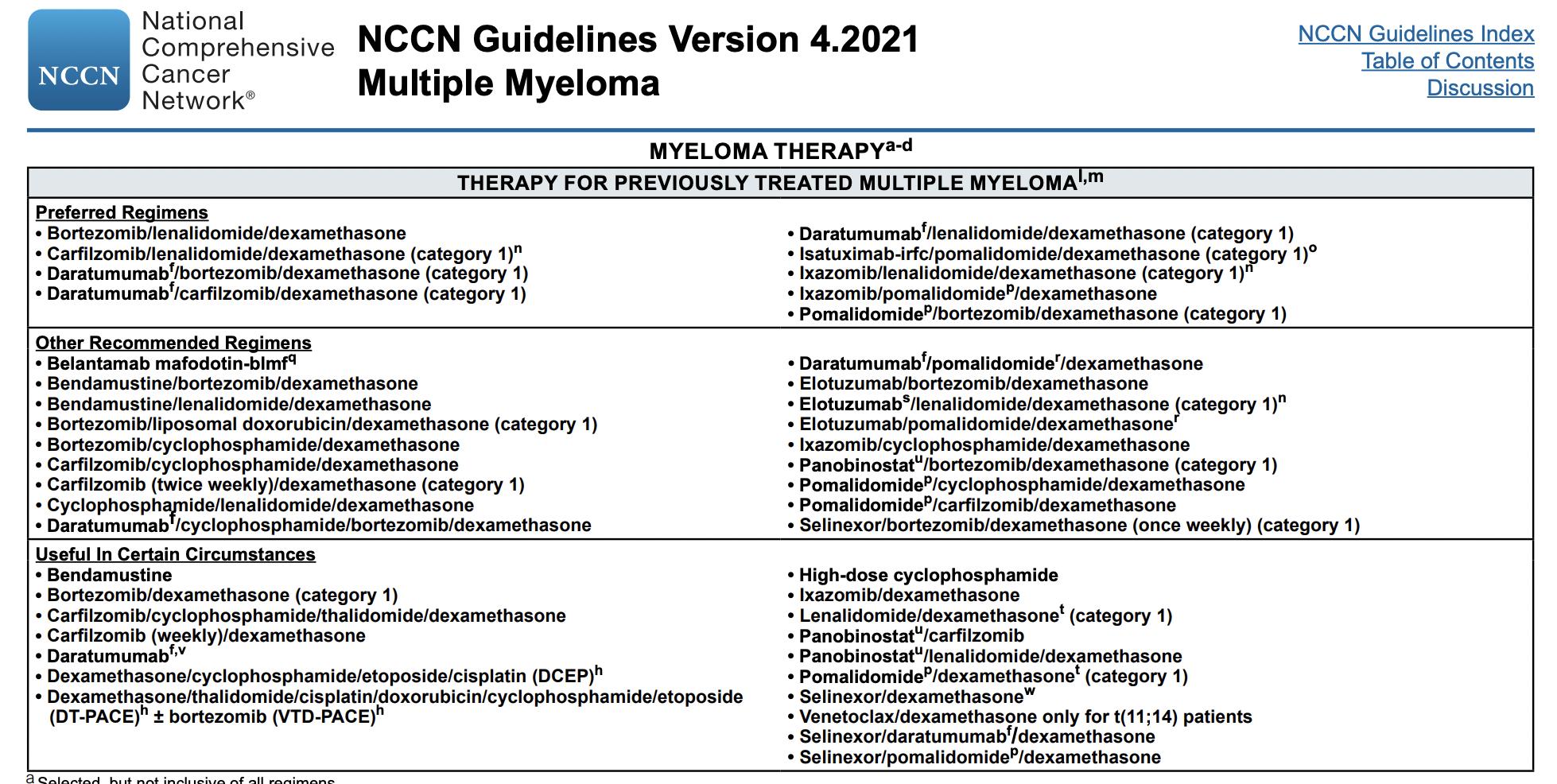

This is not a very short menu
GREAT NEWS WE HAVE OPTIONS BAD NEWSYOUR DOCTOR MAYGET CONFUSED
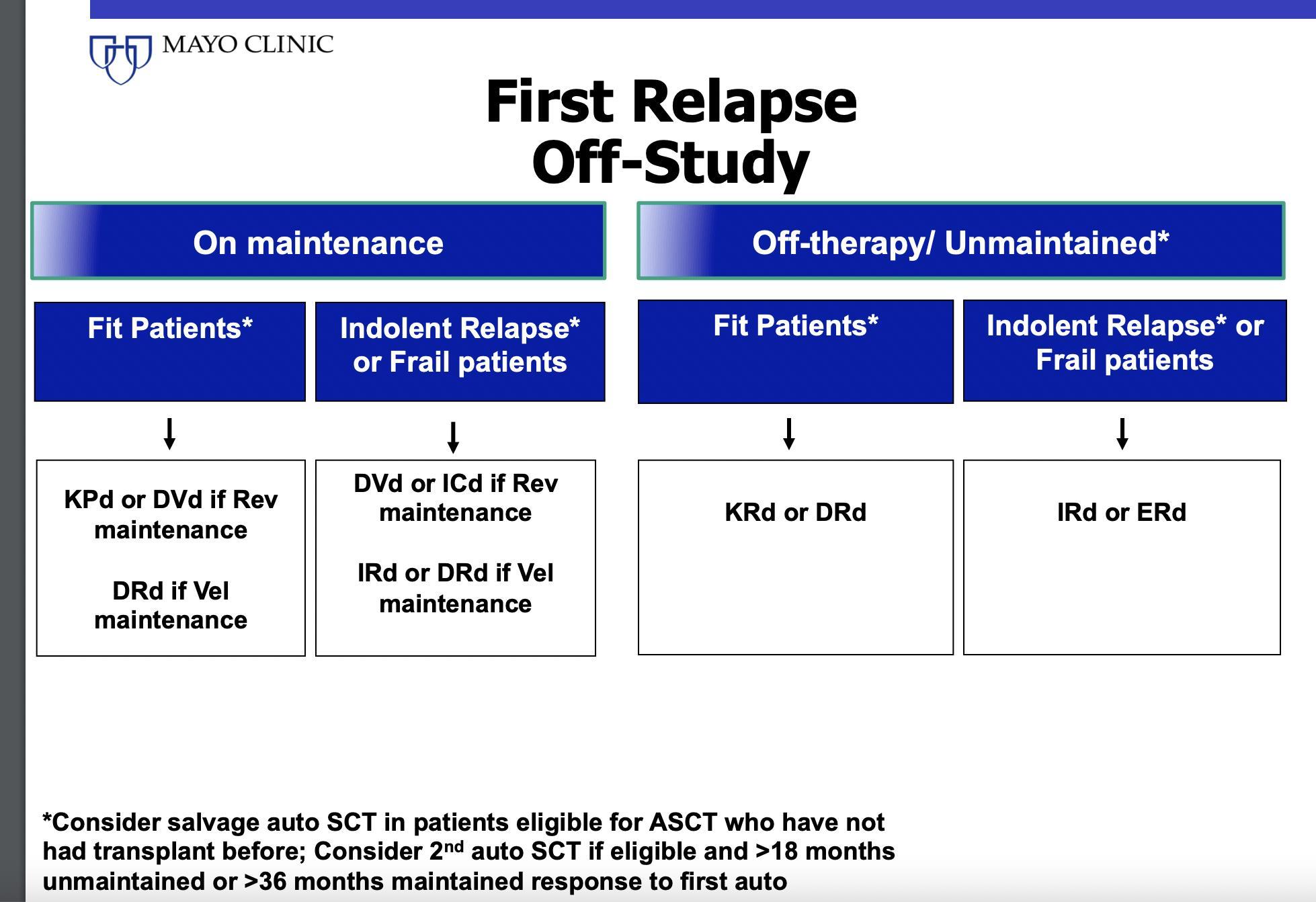
Mayo Practical Approach- First Relapse

Mayo Practical Approach- 2nd/ later Relapse
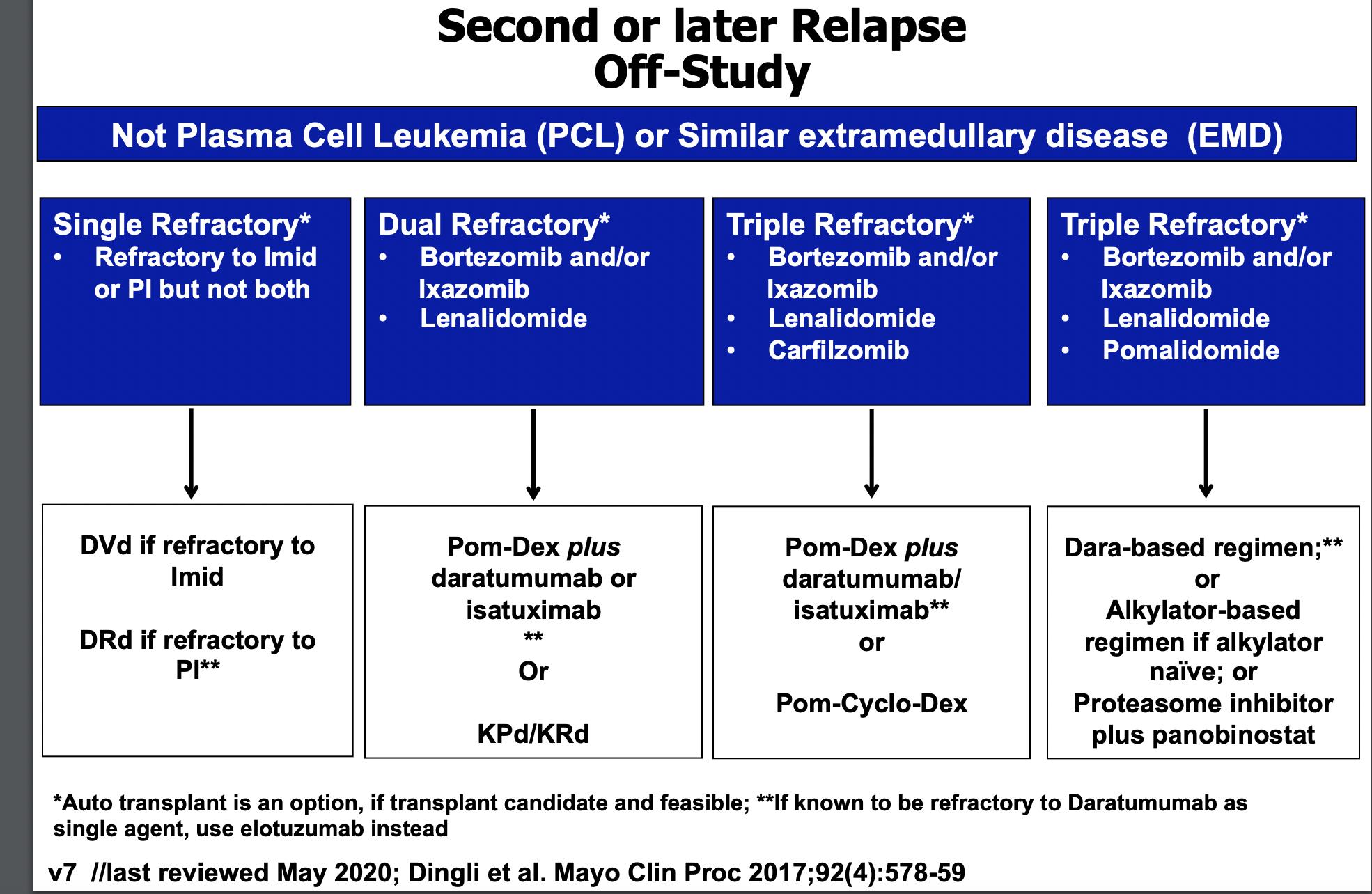


The Principles • It is not a simplealgorithmof treatment#1 then 2 then 3… • Leveragethe benefitof multiplemechanismsof action in combinationtherapy Categories: • 1-3 priorlines • Later Relapse • Refractoryto PI, IMiD and MoAb = TripleClassRefractory Principles 1. Depth of Responsematters…likely incorporate MRD soon 2. High riskvs standard risk…more aggressive Rx in high risk 3. Balanceefficacyand toxicity…initially and constantly assess 4. Overcomedrug resistance…change mechanism of action when possible
Stratification factors





Statistical analyses

Combination is Better. Cycles: 28 days DRd (n = 286) Daratumumab 16 mg/kg IV • Qw in Cycles 1 to 2, q2w in Cycles 3 to 6, then q4w until PD R 25 mg PO • Days 1 to 21 of each cycle until PD d 40 mg PO • 40 mg weekly until PD Rd (n = 283) R 25 mg PO • Days 1 to 21 of each cycle until PD d 40 mg PO • 40 mg weekly until PD Primary endpoint • PFS Secondaryendpoints • TTP • OS • ORR, VGPR, CR • MRD • Time to response • Duration of response ISS, international stagingsystem;DRd, daratumumab/lenalidomide/dexamethasone; IV, intravenous;qw,weekly;q2w,every2weeks;q4w,every4weeks;PD, progressivedisease; R, lenalidomide; PO, oral; d, dexamethasone; Rd, lenalidomide/dexamethasone; PFS, progression freesurvival;TTP, time to progression;OS, overallsurvival;ORR, overall responserate; VGPR, very goodpartial response;CR, complete response;MRD, minimal residualdisease. aOn daratumumab dosing days,dexamethasone20 mg was administeredas pre-medication on Day 1 and Day 2. Key eligibility criteria • RRMM • ≥1 prior line of therapy • Prior lenalidomide exposure, but not refractory • Creatinine clearance ≥30 mL/min Multicenter, randomized (1:1), open-label,active-controlled, phase 3 study
• No. of prior lines of therapy • ISS stage at study entry • Prior lenalidomide R A N D O M I Z E 1:1 Pre-medication for the DRdtreatment group consisted of dexamethasone 20 mg,a acetaminophen, and an antihistamine
• Primary analysis: ~177 PFS events 7 6
analysis:PFS (time without relapse
POLLUX updated
is much better with 3 drugs....) Dimopoulos MA, et al. PresentedatASH 2017(Abstract 739), oralpresentation. Median follow-up: 32.9 months (range, 0 - 40.0 months) 56% reduction in risk of progression/death for DRd versus Rd % surviving without progression 0 20 40 60 80 100 0 3 6 9 12 15 18 42 Months 30 283 286 249 266 206 249 181 238 160 229 143 214 126 203 0 0 100 183 No. at risk Rd DRd 21 24 36 89 167 36 67 111 194 DRd Rd 3927 33 5 16 80 145 1 2 Median: not reached Median: 17.5 months HR 0.44;95%CI,0.34-0.55; P <0.0001 30-monthPFSb 58% 35% Progression-free survivala HR, hazardratio; CI, confidenceinterval. aExploratory analyses basedon clinical cut off dateof October 23,2017; bKaplan Meier estimate.

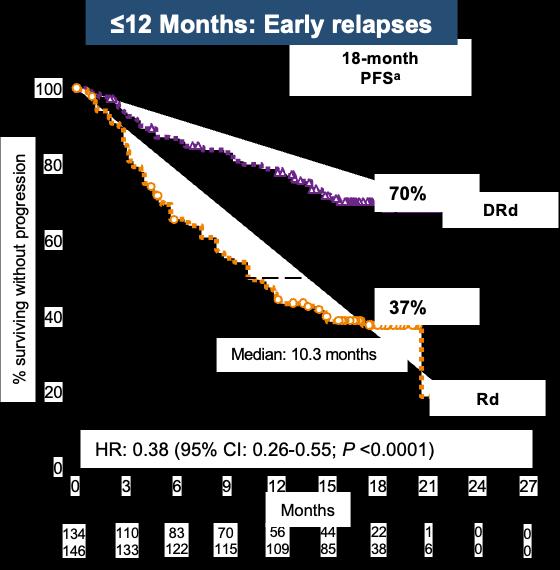
78 aKaplan Meier estimate. bResponse evaluable population. Time From Last Line of Therapy to Study Treatment of > or ≤12 Months 149 140 139 133 123 127 111 122 103 118 88 109 4 9 Rd >12 DRd >12 No. at risk 26 44 0 1 0 0 18-month PFSa DRd Rd 83% 60% % surviving without progression 0 20 40 60 80 100 0 3 6 9 12 15 18 27 Months 21 24 HR: 0.37 (95%CI: 0.23 0.61; P <0.0001) DRd is superior to Rd regardlessof time since last therapy >12 Months: Late relapses
Moreau P, et al. Presented at ASH 2016 (Abstract 1151), oral presentation
Isatuximab:



INDIANA UNIVERSITY SCHOOLOF MEDICINE
CD38 antibody • Phase III ICARIA-MM: Isatuximab + Pom/Dex vs Pom/Dex in R/R MM • Phase III IKEMAIsaKD vs KD • FDA approved isatuximab in combination with pomalidomideor carfilzomib PFS HR (95% CI) ▪ Len refractory: 0.59 (0.43-0.82) ▪ Len refractory in last line: 0.50 (0.34 0.76) ▪ Len / PI refractory: 0.58 (0.40-0.84) PFS (%) HR: 0.596 (95% CI: 0.4360.814); P = .001 20 40 60 80 100 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Mos 154 153 129 105 106 80 89 63 81 51 52 33 3 17 14 5 1 0 No. at risk Isa Pd Pd Isatuximab-Pd 11.53Pd 6.47 Median PFS, mos
Anti-MultipleMyeloma

Novel ImmunotherapeuticAgent Structures
Antibody Drug Conjugate T Cell Bispecific Antibody
T-Cell Trispecific Antibody
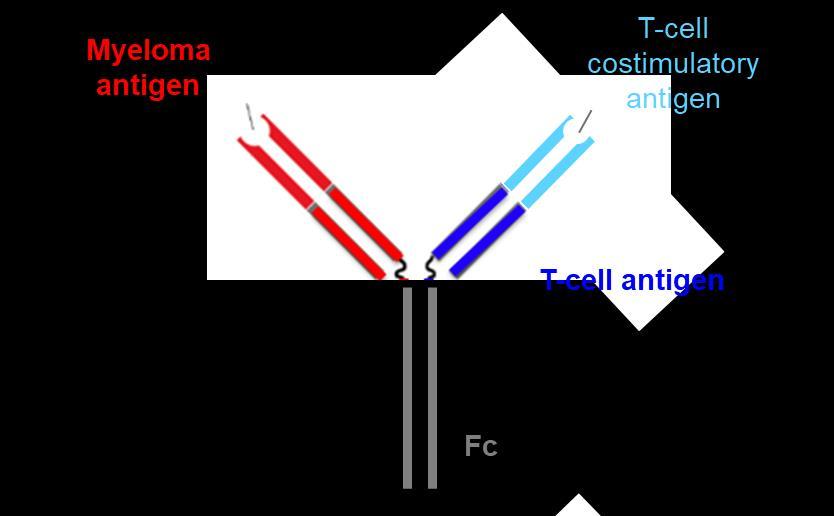
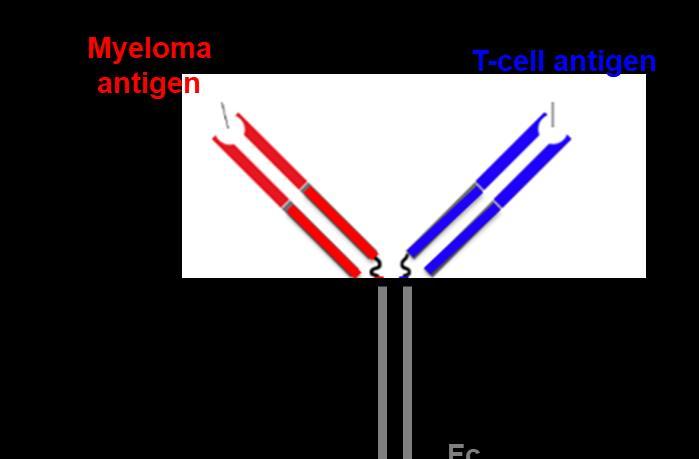
Designed ankyrin repeat proteins (DARPins)
Lancman, et al. ASH 2020
 INDIANA
INDIANA
UNIVERSITY SCHOOLOF MEDICINE
Comparison of Novel ImmunotherapeuticApproaches
Chimeric antigen receptor T cells (CAR-T)
Pros Unprecedented ORR including MRD neg in heavily pre-treated patients
One timeintervention; long chemoholiday resulting in median PFS~1 year
Bispecific antibodies
Offthe shelf
Deep responses
Limited severe CRS - ? Safety in frail elderly
Antibody-drug conjugates
Offthe shelf
Encouraging response rates
1 hour infusion every 3 weeks
Cons Manufacturing timemakes impracticalfor patients with aggressive/rapidly progressing disease
Requires complexinfrastructure – stem cell lab, RN/ ICU/ER training – thus restrictedto accredited centers CRS? role in frail elderly
Impact of bridging chemoon remissionduration
Low WBC and plts postCAR-T
Costgiven relapses even in MRD neg patients; mgmt. challenging especially if soon after flu/cy given impacton T cells
Can be given in community settings after 1st cycle
? Need for admissions with initial doses until CRS risk low
No CRS,can be given in communitysettings
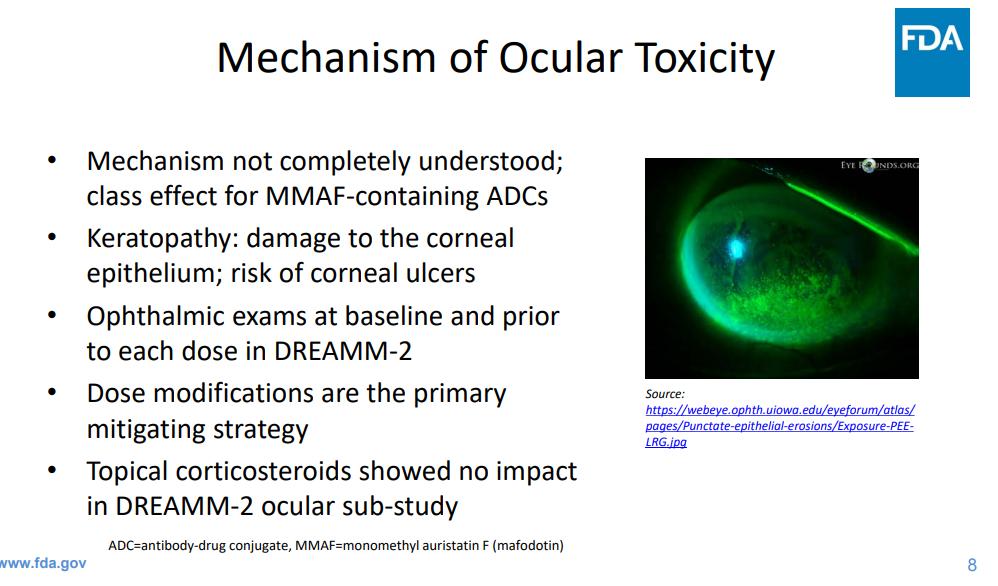
Oculartoxicity requires close collaboration with opthamology & impacton pt quality of life
Dosing/schedule to be determined
Need for continuous treatment until progression
Thrombocytopenia
Need for continuous treatment until progression
Toxicities require further study infections, neurotoxicty
Modest ORR and PFS in triple class/pentarefractory
Lancman, et al. ASH 2020
BCMA (B-cell maturationantigen)
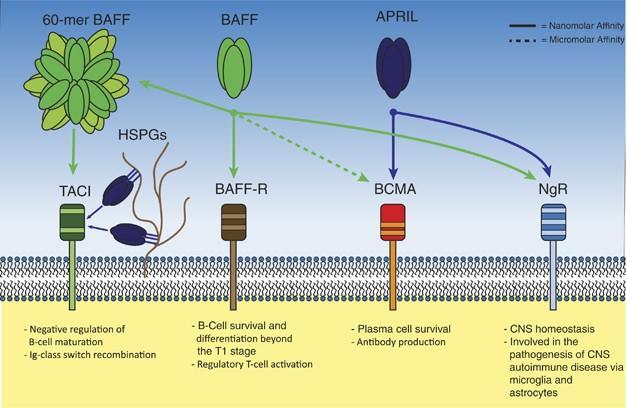
• Receptor for BAFF and APRIL
• Expressed on mature B cell subsets, PC’s, and plasmacytoid DC’s
• Maintains plasmacell homeostasis
• BCMA-/- mice have normal B cell #s, impaired PC survival
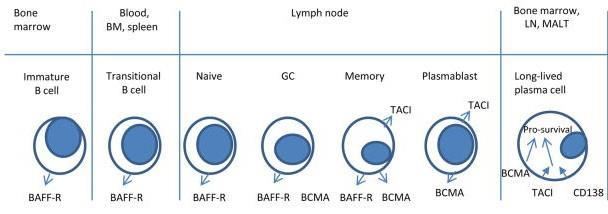 Hengeveld et al Bl Cancer J 2015 ; Maus, June, Clin Can Res 2013
Hengeveld et al Bl Cancer J 2015 ; Maus, June, Clin Can Res 2013
Belantamab
BCMA-Targeted ADC
Mafodotin:
• Belantamab mafodotin (GSK2857916): Humanized, afucosylated,IgG1 BCMA-targeted ADC that neutralizes soluble BCMA • Preclinical studies demonstrate selective, potent activity Belantamab Mafodotin –Enhanced ADCCAfucosylation –Stable in circulationLinker –MMAF (non–cell-permeable, highly potent auristatin)Cytotoxic agent Tai. Blood. 2014;123:3128. Trudel. Lancet Oncol. 2018;19:1641. Trudel. Blood Cancer J. 2019;9:37. Four mechanisms of action: 1. ADC 2. ADCC 3. Immunogenic cell death 4. BCMA receptor signaling inhibition 1 4 3 1 BCMA Effector cell x BCMA BCMA BCMA Lysosome Fc receptor ADCC ADC Cell death 2 3 4 Malignant plasma cell
DREAMM-2 trial study design
Belantamab mafodotin
• Belantamab mafodotin – ADC: Anti-BCMA mAb conjugatedto auristatin F througha non-cleavable linker Lonial S, et al. Lancet Oncol. 2020;21:207 221; https://www.fda.gov/drugs/drug approvals and databases/fda granted accelerated approval belantamab-mafodotin-blmf-multiple-myeloma FDA.gov Belantamab mafodotin 3.4 mg/kg IV (frozen) Primary Endpoint: •ORR: % of patients with ≥ PR by IMWG 2016 criteria Belantamab mafodotin 2.5 mg/kg IV (frozen) Belantamab mafodotin administered once every 3 weeks until disease progression or unacceptable toxicity Belantamab mafodotin 3.4 mg/kg IV (lyophilized) R 1:1SCREENINGR/R MM ≥ 3 prior lines of therapy N = 293 Belantamab mafodotin blmf (2.5 mg/kg): FDA accelerated approval on August 5, 2020 for R/R MM after ≥ 4 prior therapies, including an anti-CD38 monoclonal antibody, a PI, and an immunomodulatory agent ADC, antibody drug conjugate
DREAM-

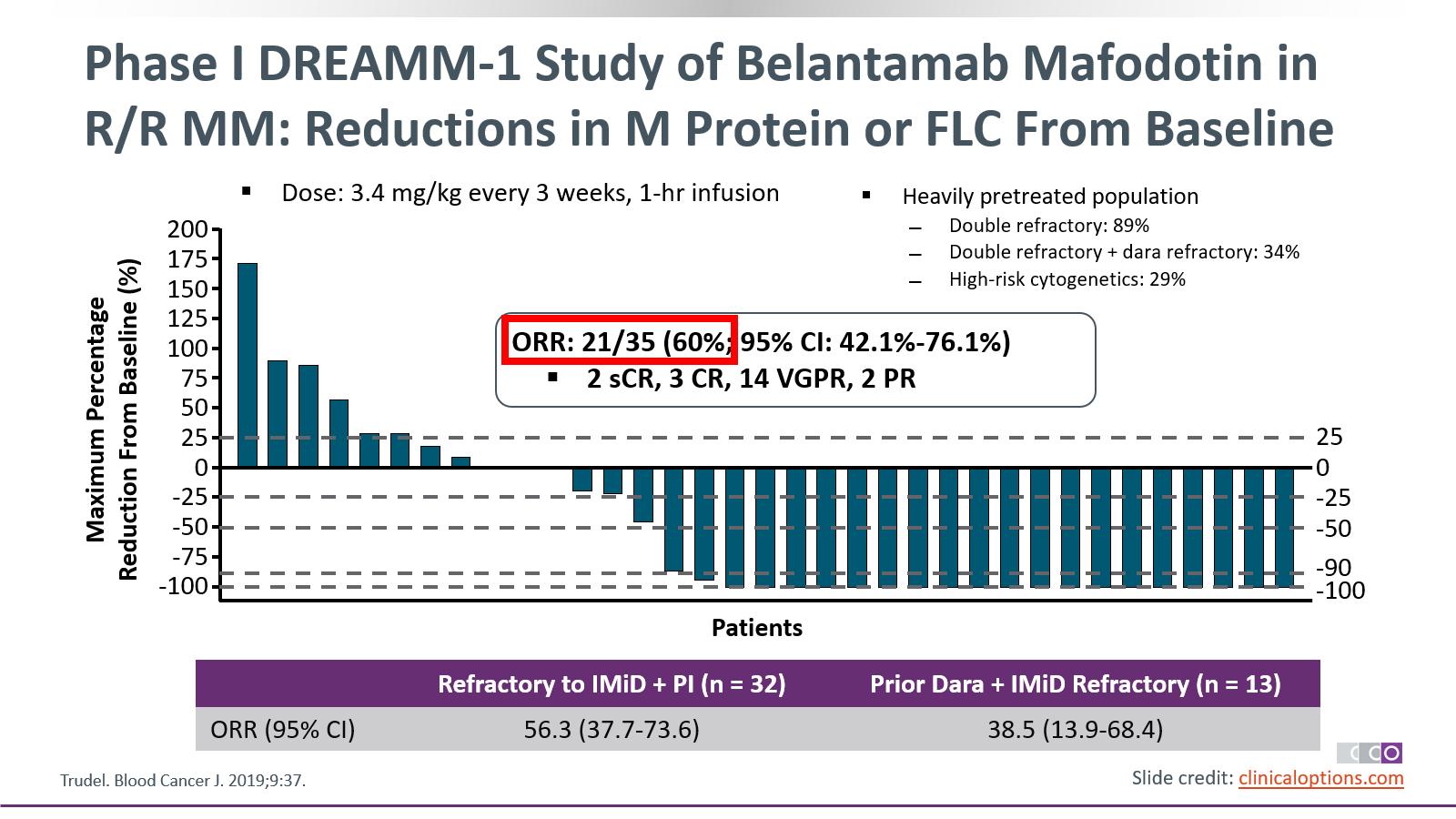
efficacy results

2
60%of respondingpatients(n=18/30) hada verygood partialresponse or better1 15% (n=15) VGPR 12% (n=12) PR (n=1) CR (n=2) sCR Overall Response Rate1 Lonial S, et al. Lancet Oncol. 2020;21:207-221
DREAMM-2
Safety overview
Number of Patients With Event (Safety Population), n (%)
Keratopathy or corneal epithelium changes 41 (43) 26 (27)
53 (54) 20 (20) 1 (1)
Thrombocytopenia 14 (15) 8 (8) 11 (12) 24 (24) 11 (11) 22 (22)
Anemia 4 (4) 19 (20) 0 12 (12) 22 (22) 3 (3)
Nausea 23 (24) 0 0 31 (31) 1 (1) 0
Pyrexia 18 (19) 2 (2) 1 (1) 21 (21) 4 (4) 0
Blurred vision 17 (18) 4 (4) 0 28 (28) 2 (2) 0
Infusion related reactions 17 (18) 3 (3) 0 15 (15) 1 (1) 0
Increased aspartate aminotransferase 17 (18) 2 (2) 0 18 (18) 6 (6) 0
Fatigue 13 (14) 2 (2) 0 21 (21) 5 (5) 0
Dry eye 12 (13) 1 (1) 0 23 (23) 0 0
Neutropenia 4 (4) 5 (5) 4 (4) 12 (12) 12 (12) 3 (3)
Belantamab Mafodotin 2.5 mg/kg (n = 95) Belantamab Mafodotin 3.4 mg/kg (n = 99) Grade 1/2 Grade 3 Grade 4 Grade 1/2 Grade 3 Grade 4
0
Lonial S, et al. Lancet Oncol. 2020;21:207-221
Belantamab Mafodotin
The Process of CAR T Cell Therapy
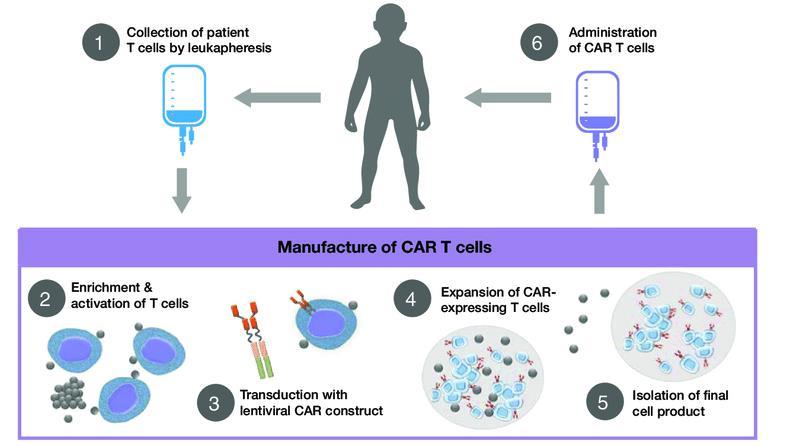
Hucks G, Rheingold SR. Blood Cancer J. 2019;doi:10.1038/s41408 018 0164 6.
Article
Idecabtagene Vicleucelin Relapsed and Refractory Multiple Myeloma
Nikhil C. Munshi, M.D., Larry D. Anderson, Jr., M.D., Ph.D., Nina Shah, M.D., Deepu Madduri, M.D., Jesús Berdeja, M.D., Sagar Lonial, M.D., Noopur Raje, M.D.,
Yi Lin, M.D., Ph.D., David Siegel, M.D., Ph.D., Albert Oriol, M.D., Philippe Moreau, M.D., Ibrahim Yakoub-Agha, M.D., Ph.D., Michel Delforge, M.D., Michele Cavo, M.D., Hermann Einsele, M.D., Hartmut Goldschmidt, M.D., Katja Weisel, M.D., Alessandro Rambaldi, M.D., Donna Reece, M.D., Fabio Petrocca, M.D., Monica Massaro, M.P.H., Jamie N. Connarn, Ph.D., Shari Kaiser, Ph.D., Payal Patel, Ph.D., Liping Huang, Ph.D., Timothy B. Campbell, M.D., Ph.D., Kristen Hege, M.D., and Jesús San-Miguel, M.D., Ph.D.

Original
N Engl J Med 2021 Volume 384(8):705-716 February 25, 2021
Efficacy
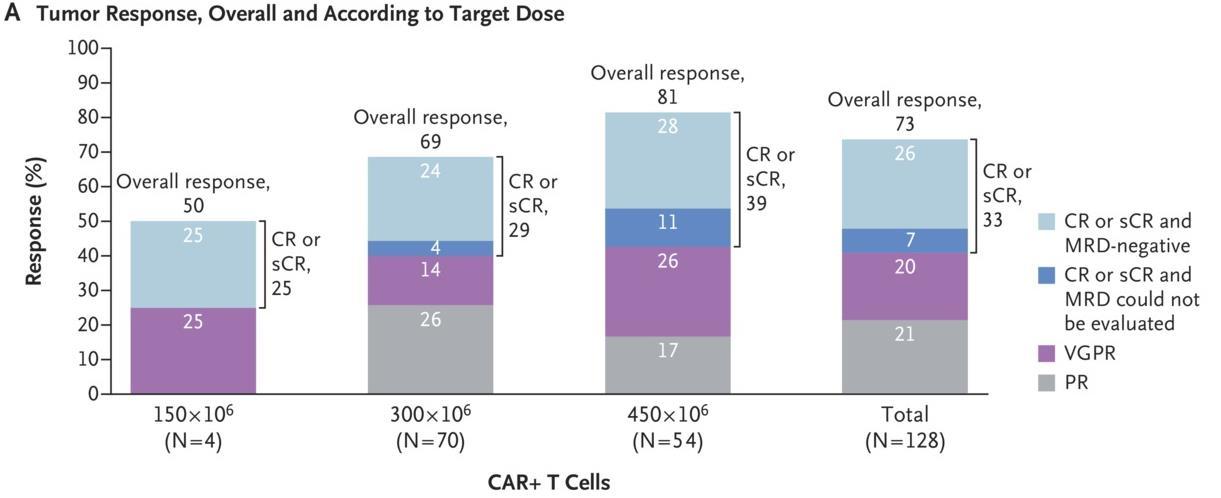
Response
–
Munshi NC, et al. N Engl J Med. 2021;384(8):705 716
Progression-free Survival, Duration of Response, and Overall Survival
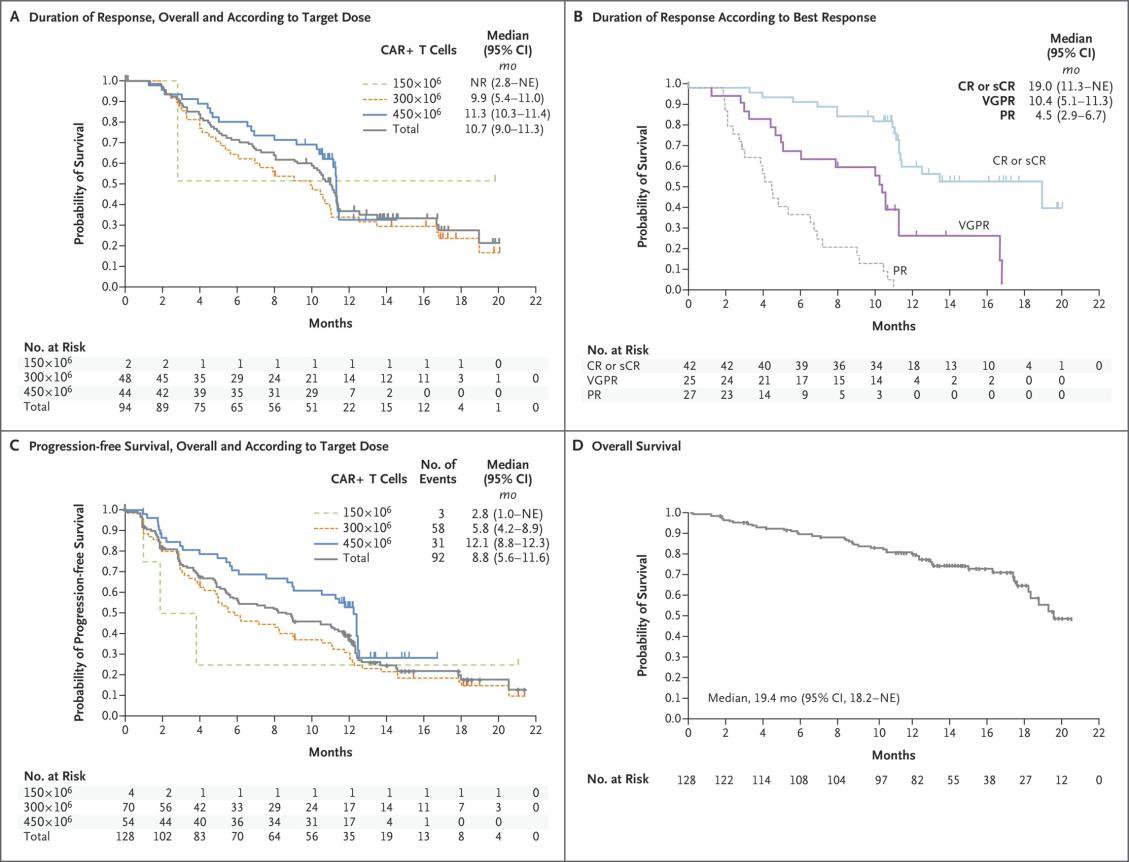
Munshi NC, et al. N Engl J Med. 2021;384(8):705 716 PFS 8.8 months OS 19.4 months
Adverse Events, Cytokine Release Syndrome, and Neurotoxic Effects in the 128 Patients Who Received Ide-Cel
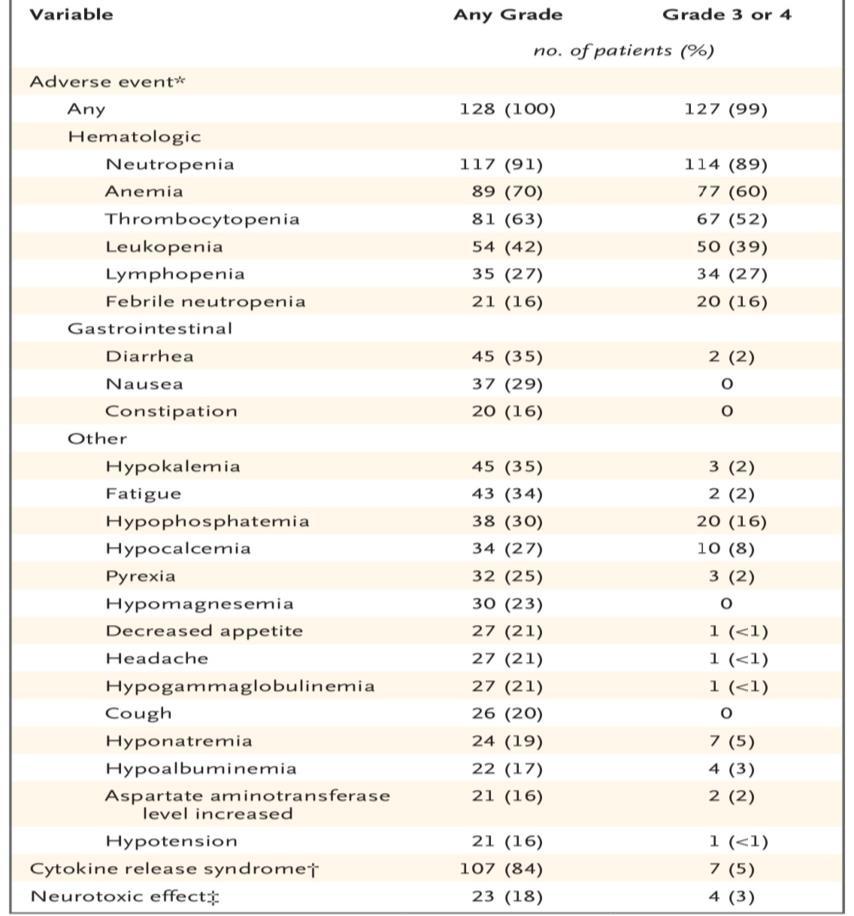
MunshiNCet al.N Engl J Med 2021;384:705-716
Munshi NC, et al. N Engl J Med. 2021;384(8):705 716

1UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA, USA;2Levine Cancer Institute, Charlotte, NC, USA; 3Sarah Cannon Research Institute, Nashville, TN, USA; 4University of Chicago, Chicago, IL, USA; 5UPMC Hillman Cancer Center, Pittsburgh, PA, USA; 6Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 7Medical College of Wisconsin, Milwaukee, WI, USA; 8Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA; 9Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA; 10City of Hope Comprehensive Cancer Center, Duarte, CA, USA; 11Memorial Sloan Kettering Cancer Center, New York, NY, USA; 12Dana Farber Cancer Institute, Harvard Medical School, Boston, MA, USA; 13Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; 14University Health Network and the Princess Margaret Cancer Centre, Toronto, ON, Canada; 15Janssen R&D, Raritan, NJ, USA; 16Janssen R&D, Spring House, PA, USA; 17Janssen R&D, Beerse, Belgium; 18Legend Biotech USA, Piscataway, NJ, USA; 19Mayo Clinic, Rochester, MN, USA; 20Mount Sinai Medical Center, New York, NY, USA
Presented at the 63rd American Society of Hematology (ASH) Annual Meeting & Exposition; December 11 14, 2021; Atlanta, GA/Virtual.
*Presenting author.
Additional information can be viewed by scanning the QR code or accessing this link: https://www.oncologysciencehub.com/ASH2021/Cilta
The QR code is intended

cel/ThomasMartin
to provide scientific information for individual reference, and the information should not be altered or reproduced in any way. Updated Results From CARTITUDE-1: Phase 1b/2 Study of Ciltacabtagene Autoleucel, a B-cell Maturation Antigen–Directed Chimeric Antigen Receptor T Cell Therapy, in Patients With Relapsed/Refractory Multiple Myeloma
Efficacy Response
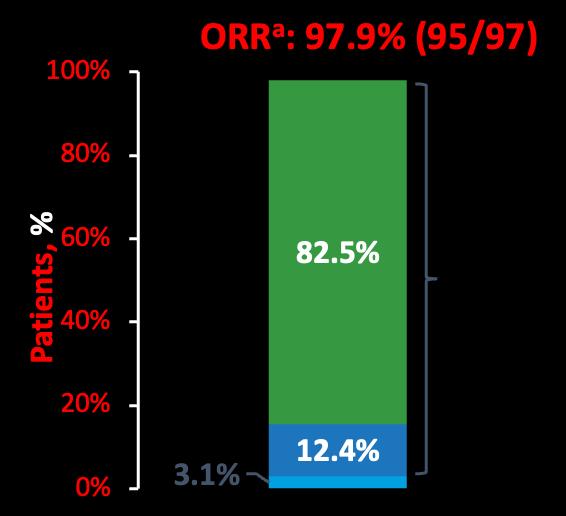
CARTITUDE-1:
• Median time to first response was 1 month (range, 0.9–10.7) • Median time to best response was 2.6 months (range, 0.9–17.8) • Median time to CR or better was 2.9 months (range, 0.9–17.8) • Median duration of response was not estimable (21.8 months NE) 98 sCR VGPR PRBest responseb = ≥VGPR: 94.9% sCR: 82.5 % Responses deepened over time from the 1year follow-up Best response at any time Median–1 year follow-up Median–2 years follow-up sCR, % 67 83
CARTITUDE-1: Conclusions
At a median follow-up of ~2 years patients treated with cilta-cel showed durable and deepening responses
• ORR remained at 98%, with sCR rates higher at a median of ~2 years than at median of ~1 year (83% vs 67%)
• 2-year PFS and OS rates were 60.5% and 74.0%, respectively
• MRD negativity (at 10 5) was achieved in 92% of evaluable patients
• PFS and OS was improved in patients with MRD-negativity sustained for ≥6 and ≥12 months
Cilta-cel has a manageable safety profile with no new safety signals observed with longer follow-up
These encouraging data suggest cilta-cel will be an important treatment option for patients with MM
• Cilta-cel is currently under further investigationin patients with MM in earlier-line settings, including patients with newly diagnosed MM (CARTITUDE-2a, CARTITUDE-4b, CARTITUDE-5c)
• Outpatientadministrationof cilta-cel is beingexplored in these studies
99
Emerging therapies in myeloma
outline
–
• CAR T cell therapy – both Auto and Allo • Bispecifics and trispecifics • Targeting BCMA but also: • GPRC5D • FCRH5 • CelMods – Iberdomide • And many combinations of the above!
Options of therapy for myeloma
Daratumumab +
Induction therapy
CAR T Cell?
ASCT eligible
Bortezomib-Lenalidomide-Dex
OR Carfilzomib-Lenalidomide-Dex
ASCT (melphalan)
Lenalidomide Maintenance
First relapse
CAR T Cell?
Bispecific antibodies? Other combos with Selinexor, Belantamab mafodotin or Melphalan flufenamide?
current
non-ASCT eligible
Bortezomib-Lenalidomide-Dex
OR Daratumumab-Lenalidomide-dexamethasone
OR Doublets (rarely)
Lenalidomide Maintenance
Daratumumab-Pomalidomide-Dex
Daratumumab-Carfilzomib-Dex
Daratumumab-Lenalidomide-Dex
Daratumumab-Bortezomib-Dex
Isatuximab-Pomalidomide-Dex
Selinexor-Bortezomib-Dex
Isatuximab-Carfilzomib-Dex
Second Relapse Carfilzomib Based Combination – Pomalidomide or Cyclophosphamide
Pomalidomide BasedCombination – Isatuximab or Elotuzumab
Third+ Relapse
Selinexor-Dex
Belantamab mafodotin
Melphalan flufenamide
CAR T Cell Therapy
ASCT, autologous stemcell transplant; CAR, chimeric antigen receptor
–
Best therapy for Relapse
• Not all relapses are the same.
• Combination therapies provide better outcomes.
• Available drugs and combinations are increasing.

• The optimal therapy is the one based on your needs.
• Weneed to get it right at first relapse.


103
How to Manage Myeloma Symptoms and Side Effects Deborah Doss, RN, OCN Retired of 40+Years Dana Farber Cancer Research Institute, IMF

Leadership Board


Nurse
104
October 22, 2022

LIFE IS A CANVAS, YOU ARE THE ARTIST
Deborah Doss, RN, OCN
Dana-FarberCancer Institute Boston, Massachusetts
Patient Education Slides2022
GALLERY OF GOALS

MYELOMA TREATMENT SUPPORTIVE THERAPIES


DISCUSS
• Rapid and effective disease control • Durable disease control • Minimize side effects • Allow for good quality of life • Improved overall survival • Prevent disease- and treatmentrelated side effects • Optimize symptom management • Allow for good quality of life
GOALS AND PRIORITIES WITH YOUR HEALTHCARE TEAM 106
PREPARE FOR VISITS & CONSIDER TELEMEDICINE
Come prepared:
• Bring a list of current medications, prescribed and over the counter
• Write down your questions and concerns. Prioritize them including financial issues

• Have there been any medical or life changes since your last visit?
• Current symptoms - how have they changed (improved, worsened, stable)? Keep a symptom diary. Bring it along
• Communicate effectively: your health care team can’t help if they don’t know
• Know the “next steps”, future appointments, medication changes, refills, etc
Check with your healthcare team –
Is telemedicine an option?
Similar planning for “in-person” appointment PLUS:
• What is the process and what technology is needed?

• Plan your labs: are they needed in advance? Do you need an order?
• Plan your location: quiet, well-lit location with strong wi-fi is best
• Plan yourself: consider if you may need to show a body part and wear accessible clothing
• Collect recent vital signs (blood pressure, temp, heart rate) self serve blood pressure cuff is available at many pharmacies and for purchase
IMF TelemedicineTipSheet.In development.
Velcade® (bortezomib)
Darzalex® (daratumumab)
Thalomid® (thalidomide)
Revlimid® (lenalidomide)
COLOR WHEEL OF TREATMENT OPTIONS

Velcade® (bortezomib)
Revlimid® (lenalidomide)
Dexamethasone
Prednisone
Prednisolone SoluMedrol
Melphalan
Kyprolis® (carfilzomib) Ninlaro® (ixazomib)
Darzalex® (daratumumab)
Empliciti® (elotuzumab)
Sarclissa® (Isatuximab)
Thalomid® (thalidomide)
Revlimid® (lenalidomide)
Pomalyst® (pomalidomide)
Dexamethasone
Prednisone Prednisolone SoluMedrol
Melphalan Cyclophosphamide Bendamustine
(Selinexor)
ASCT
(Belantamab mafodotin)
(liposomal doxorubicin)
(panobinostat)
(CAR T)
Iberdomide
CC 92480
(melphalan flufenamide)
Teclistamab, Talquetamab Cevostamab
(venetoclax)
T
Cilta-Cel
-Mibs -MAbs -Mides Steroids Alkylators ImmunoTherapy Others Cellular Therapies
Cyclophosphamide Melphalan +
Blenrep®
“Belamaf” Xpovio®
Doxil
Farydak®
Melphalan + ASCT Ide Cel
CelMods •
•
Pepaxto
“Melflufen” ADCs BSAs Ex:
Venclexta®
Other CAR
•
Neuropathy Carfilzomib: Cardiac Infusion reaction DVT/PE See steroid slide Myelosuppression Infusion reaction Blenrep: Keratopathy Myelosuppression, GI Selinexor: Low sodium Infection risk CAR T: CRS and neurotoxicity Frontline Relapse Pending FDA Approval Maintenance
Noted Side effects 108
SHADES OF “AUTO” STEM CELL TRANSPLANT (ASCT)
• There are no black and white answers to deciding to undergo a transplant
• Undergoing transplant is a commitment for both you and your care partner
• Understanding the process will help bring to focus elements needed to decide if/when to undergo transplant

Clinical Experience Data from Research
DECISION
Patient Preference
Adapted from Philippe Moreau, ASH 2015



CARE PARTNER SUPPORT
Care partner support is essential for theentire transplant and CAR T processes
• Sedated procedures; Education sessions
• Assistance with daily activities, managingmedications and alerting the medicalteam of changes
• Continued support and assistance is often needed in the early days afterreturning home. Less assistance will beneeded as time goes on.

Care partner can be one person or arotation of many people.



PATIENT-REPORTED SYMPTOMS




Physical • Fatigue • Constipation • Pain • Neuropathy • Impaired Physical Functioning • Sexual Dysfunction Psychological • Depression • Anxiety • Sleep Disturbance • Decreased Cognitive Function • Decreased Role & Social Function Financial • Financial burden (80%) • Financial toxicity (43%) A meta-analysis identified the most common patient-reported symptoms and impact on QOL, and were present at all stages of the disease. Symptoms resulted from both myeloma disease and treatment, including transplant, and were in these categories: Ramsenthaler, et al. 2016. https://doi.org/10.1111/ejh.12790. 111
MYELOMA AND
BOTH
How You Feel 112 Myeloma cells in excess numbers cause symptoms • Calcium elevation • Renal dysfunction • Anemia • Bone pain • Fatigue • Infection • Other symptoms Treatments for myeloma kill myeloma cells but can cause symptoms – Myelosuppression – Peripheral neuropathy – Diarrhea – Fatigue – Deep vein thrombosis – Infection (eg, shingles) – Other symptoms
TREATMENTS
CONTRIBUTE TO HOW YOU FEEL
MYELOMA CELLS CAN CAUSE DAMAGE TO BONES
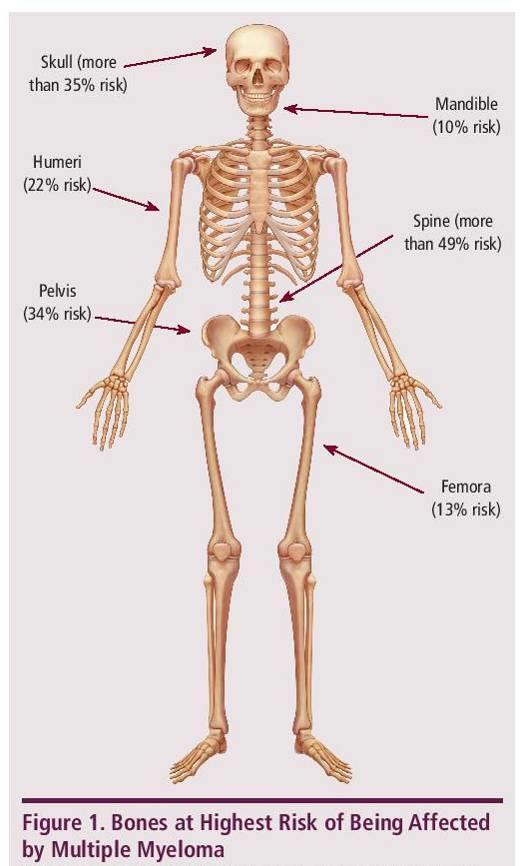
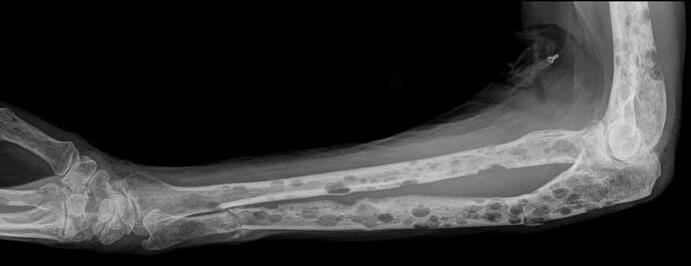
113Faiman B, etal. CJON. 2017;21(5)suppl:19-36. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23. Dimopoulous M, etal. Leukemia. 2009;23(9):1545 56. ► Approximately85% of myeloma patients develop bone disease ► Protecting bone health – Nutrition – Weight-bearing activity – Medications • Vitamin D • Calcium (if approved by doctor) • Bone strengthening agents: Zometa® (zoledronic acid), Aredia (pamidronate), or Xgeva® (denousamab) ► Report new pain to your health care provider Most myeloma patients will experience bone involvement at some point; it is important to protect your bone health This Photo by Unknown Author is licensed under CC BY SA
UNDERSTANDING CHANGES TO KIDNEY/RENAL FUNCTION
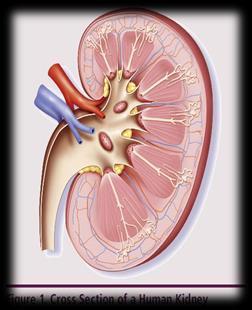

►Risk Factors – Active multiple myeloma (light chains, high calcium) – Other medical issues (ex: Diabetes, dehydration, infection) – Medications (MM treatment, antibiotics, contrast dye) ► Prevention – Drink, Drink, Drink – Avoid certain medications, when possible ► Treatment – Treatment for myeloma – Hydration – Dialysis Brigle K, et al. CJON. 2017;21(5)suppl:60 76. Faiman B, et al. CJON. 2017;21(5)suppl:19 36. Faiman B, et al. CJON. 2011;15suppl:66 76. Water glass:Cleanwateraction.org Many myeloma patients will experience kidney function problems at some point; it is important to protect your kidney function early and over time. 114
Steroid Side Effects
Steroid Synergy
Managing Steroid Side Effects



• Consistent schedule (AM vs. PM) • Take with food • Stomach discomfort: Over-the-counter or prescription medications • Medications to prevent shingles, thrush, or other infections THE BRIGHT DARK SIDE TO STEROIDS
Steroids are a backbone and work in combination to enhance myeloma therapy
• Increase in blood sugar levels, diabetes • Weight gain, hair thinning/loss, skin rashes • Irritability, mood swings, depression • Muscle weakness, cramping • Blurred vision, cataracts • Difficulty sleeping (insomnia), fatigue • Flushing/sweating • Stomach bloating, hiccups, heartburn, ulcers, or gas • Increased risk of infections, heart disease • Increase in blood pressure, water retention Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR, Eastern Cooperative Oncology Group (2010) Lenalidomide plus high dose dexamethasone versus lenalidomide plus low dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open label randomised controlled trial. Lancet Oncol 11(1):29–37. King T, Faiman B. Steroid Associated Side Effects: A Symptom Management Update on Multiple Myeloma Treatment
. Clin J Oncol Nurs. 2017 Apr 1;21(2):240 249. doi: 10.1188/17.CJON.240 249. PMID: 28315528. Do not stop or adjust steroid doses without discussing it with your health care provider & 115
& MANAGEMENT

Increase fiber

Diarrhea may be caused by medications and supplements • Laxatives, antacids with magnesium • Antibiotics, antidepressants, others • Milk thistle, aloe, cayenne, saw palmetto, ginseng • Sugar substitutes in sugar free gum Avoid caffeinated, carbonated, or heavily sugared beverages Take anti-diarrheal medication • Imodium®, Lomotil®, or Colestid if recommended • Fiber binding agents – Metamucil®, Citrucel®, Benefiber® • Welchol® if recommended Smith LC, et al. CJON.2008;12(3)suppl:37-52. Faiman B. CJON. 2016;20(4):E100-E105. Fluid intake can help with both diarrhea and constipation, and good for kidneys. Discuss GI issues with health care providers to identify causes and make adjustments to medications and supplements. Constipation may be caused by • Opioid pain relievers, antidepressants, heart or blood pressure medications, others • Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency
• Fruits, vegetables, high fiber whole grain foods • Fiber binding agents – Metamucil®, Citrucel®, Benefiber® Physical GI SYMPTOMS: PREVENTION
116


Peripheral neuropathy: damage to nerves in extremities (hands, feet, or limbs) • Numbness • Tingling • Prickling sensations • Sensitivity to touch • Burning and/or cold sensation • Muscle weakness Prevention / management: • Bortezomib once-weekly or subcutaneous administration • Massage area with cocoa butter regularly • Supplements: • B complex vitamins (B1, B6, B12) • Folic acid, and/or amino acids but do not take on day of Velcade® (bortezomib) infusion • Safe environment: rugs, furnishings, shoes If PN worsens, your HCP may: • Change your treatment • Prescribe oral or topical pain medication • Suggest physical therapy PERIPHERAL NEUROPATHY MANAGEMENT 117Faiman B, et al. CJON. 2017;21(5)suppl:19 36. Tariman, et al. CJON.2008;12(3)suppl:29 36. Report symptoms of peripheral neuropathy early to your health care provider; nerve damage from PN can be permanent if unaddressed Physical
PAIN PREVENTION AND MANAGEMENT
Pain can significantly compromise quality of life

Sources of pain include bone disease, neuropathy and medical procedures Management
pain when possible
Tell your health care

Physical
• Prevent
• Bone strengtheners to decrease fracture risk; anti viral to prevent shingles; sedation before procedures • Interventions depends on source of pain • Monitor serum calcium levels • Imaging may be needed depending on type and location of pain (eg, MRI, PET-CT) • May include medications (eg bone modifying agents), activity, surgical intervention, radiation therapy, etc • Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)
Faiman B, et al. CJON. 2017;21(5)suppl:19 36.
provider about any new bone pain or chronic pain that is not adequately controlled 118
FATIGUE, ANXIETY & DEPRESSION
All can affect quality of life and relationships
Psychological
Fatigue is the most common reported symptom (98.8%)
Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression

• Anxiety reported in >35%
• Depression nearly 25%

Financial concerns, disease progression, end-of-life, and


change in social and sexual function were highlighted sources
Often, people do not share these symptoms with their provider. Talk to your provider about symptoms that are not well controlled or thoughts of self harm. Help is available.
•
Ramsenthaler, et al. 2016. https://doi.org/10.1111/ejh.12790. Catamero D et al. CJON. 2017; 21(5)suppl:7 18.
Physical
119
IMPORTANT WAYS TO SLOW THE SPREAD OF COVID-19







• Get a COVID-19 vaccine (and booster) as soon as you can • Wear a mask (N95 is most protective) that covers your nose and mouth • Stay 6 feet apart from others who don’t live with you • Avoid crowds and poorly ventilated indoor spaces • Test to prevent spread to others • Wash your hands often with soap and water. Use hand sanitizer if soap and water aren’t available CDC= Centers forDiseaseControl;FDA= Food andDrugAdministration. CDCwebsite.UnderstandingVariants.AccessedJanuary30,2022.https://www.cdc.gov/coronavirus/2019-ncov/variants/understanding-variants.html

Manage stress • Rest, relaxation, sleep hygiene • Mental health / social engagement • Complementary therapy Maintain a healthy weight • Nutrition • Activity / exercise Preventative health care • Health screenings, vaccinations • Prevent falls, injury, infection • Stop smoking • Dental care Maintain renal health • Myeloma management • Hydration • Avoid renally-toxic medications – Dose adjust to renal function • Diabetes management Protect your bones • Nutrition, Calcium + D supplement • Weight-bearing activity / walking • Bone strengthening agents Faiman B, et al. CJON. 2017;21(5)suppl:19 36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545 56. Brigle K, et al. CJON. 2017;21(5)suppl:60 76. Faiman B, et al. CJON. 2017;21(5)suppl:19 36. Faiman B, et al. CJON. 2011;15suppl:66 76. Miceli TS, et al. CJON. 2011;15(4)suppl:9 23. “An ounce of prevention is worth a pound of cure.” Benjamin Franklin 121 HEALTHFUL LIVING STRATEGIES: PREVENTION
KNOWLEDGE








REPUTABLE












IS POWER USE
SOURCES Download or order at myeloma.org Website: http://myeloma.org IMF InfoLine 1 800-452-CURE 9am to 4pm PST eNewsletter: Myeloma Minute IMF TV Teleconferences 122
YOU ARE NOT ALONE


Audience Q&A with Panel
• Open the Q&A window,allowing you to ask questions to the host and panelists. They can either reply to you via text in the Q&A windowor answer your question live.



• If the host answers live, you may see a notification in the Q&A window.




• If the host replies via the Q&A box – you will see a reply in the Q&A window.

124
Thank you for joining us today for the IMF Regional Community Workshop October

2022
22,
Thank you to our sponsors!







126
We want to hear from you!

Feedback Survey
At the close of the meeting a feedback survey will pop up.

This will also be emailed to you shortly after the workshop.
Please take a moment to complete this survey.

Workshop Video Replay & Slides


As follow up to today's workshop, we will have the speaker slides and a video replay available.
These will be provided to you shortly after the workshop concludes.
or order at























Website: http://myeloma.org eNewsletter: Myeloma Minute IMF Webinars Videos Download
myeloma.org







































































































































































































 R Vij et al. Br J Haem,
R Vij et al. Br J Haem,


 R Vij et al. Br J Haem,
R Vij et al. Br J Haem,































































































































































































































 INDIANA
INDIANA

 Hengeveld et al Bl Cancer J 2015 ; Maus, June, Clin Can Res 2013
Hengeveld et al Bl Cancer J 2015 ; Maus, June, Clin Can Res 2013





































































































































