Welcome!
Thank you for joining us today for the February 15, 2023, International Myeloma Foundation’s Regional Community Workshop –Midwest states.


Thank you for joining us today for the February 15, 2023, International Myeloma Foundation’s Regional Community Workshop –Midwest states.












At the close of the meeting a feedback survey will pop up.
This will also be emailed to you shortly after the workshop.

Please take a moment to complete this survey.
February 15, 2023, Agenda
5:30 – 5:35 PM Welcome and Announcements
Kelly Cox, Senior Director Regional Community Workshops

5:35 – 6:10 PM Myeloma 101 & Frontline Therapy

Rafat Abonour, MD - Indiana University College of Medicine
6:10 –

6:25 PM Q&A with Panel
6:25 –
6:35 PM Guided Meditation & Stretch Break
6:35 –
7:15 PM Relapsed Therapy & Clinical Trials
Jonathan Kaufman, MD – Winship Cancer Institute of Emory University
7:15 – 7:35 PM Life is a Canvas, You are the Artist
Daniel Verina, DNP, RN, ACNP-BC - Mount Sinai Hospital, IMF Nurse Leadership Board
7:35 – 8:00 PM Q&A with Panel



Harry and Edith Gladstein Professor of Cancer Research
Professor of Medicine, Pathology and Laboratory Medicine
Director, Multiple Myeloma, Waldenstrom's Disease and Amyloidosis Program
Indiana University School of Medicine


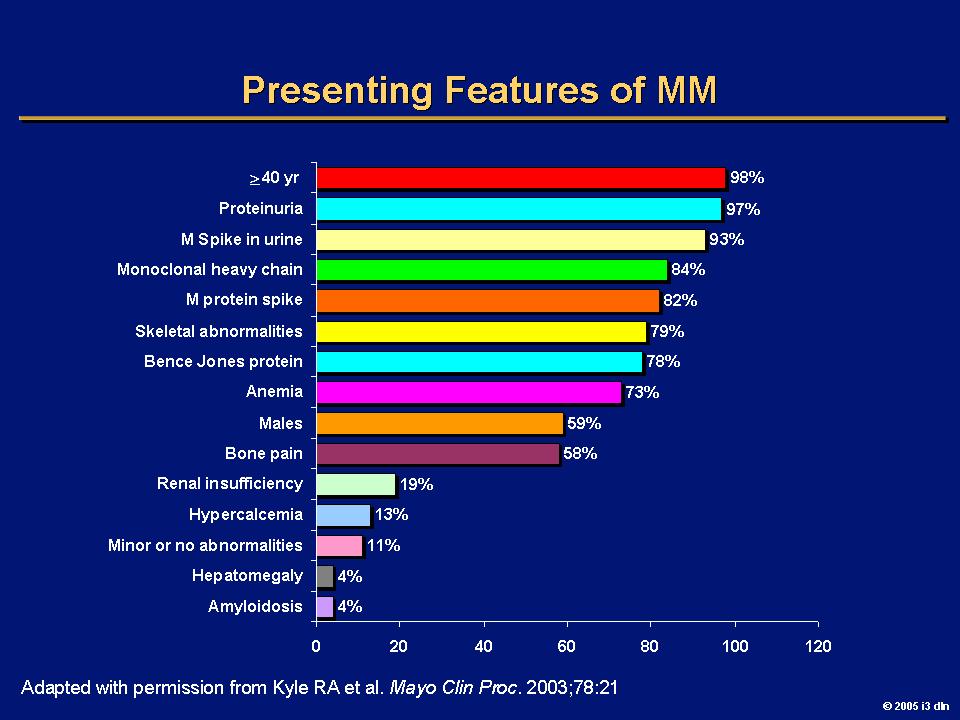
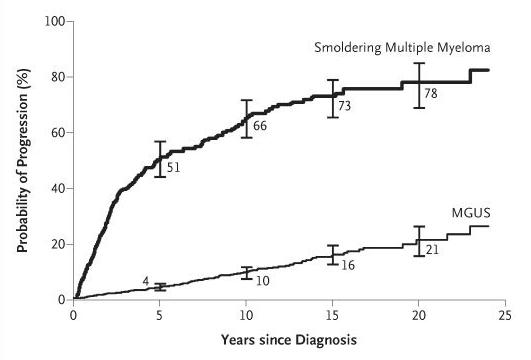
Myeloma is a disease where the immune system falls apart
Plasma cells become malignant
T cells become ineffective
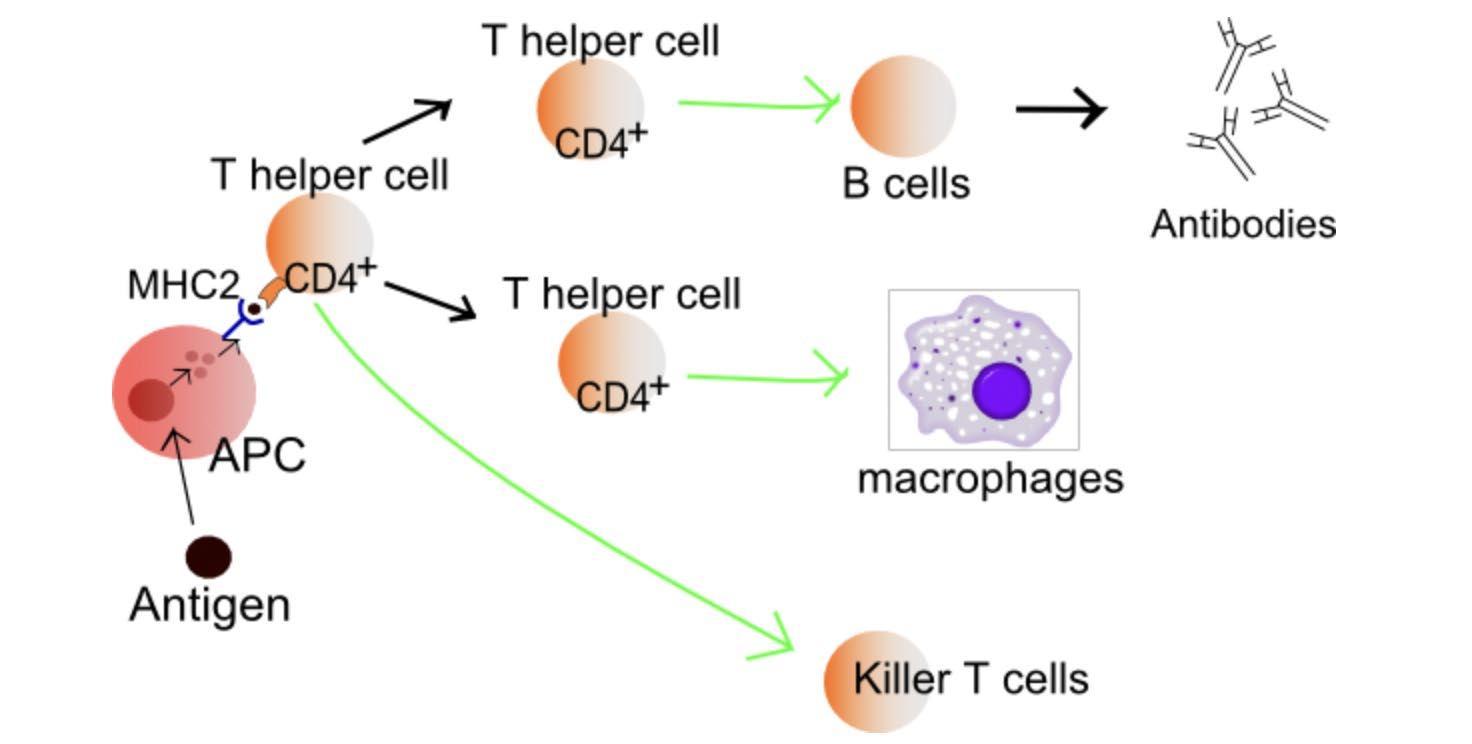

B cell
SURVEILLANCE
“RIGHT FIT”
Plasma cell
REPLICATION & MASSIVE ANTIBODY PRODUCTION


• Myeloma = too many plasma cells
• Each myeloma cell (clone) makes and releases one type of antibody (gamma globulin) into the circulation (monoclonal gammopathy)

Light Chain
Heavy Chain
Kappa releasing Lambda releasing Kappa, κ Lambda, λSerum Protein electrophoresis (sPEP)

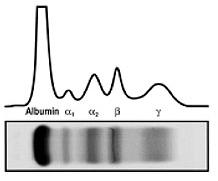
Normal profile
Monoclonal protein = M-spike
MAYO CLIN PROC. 2001;76:476-487
© 2001 MAYO FOUNDATION FOR MEDICAL EDUCATION AND RESEARCH


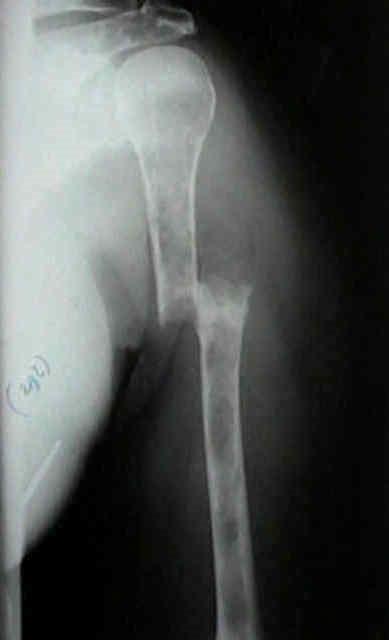
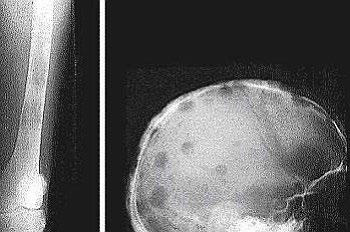




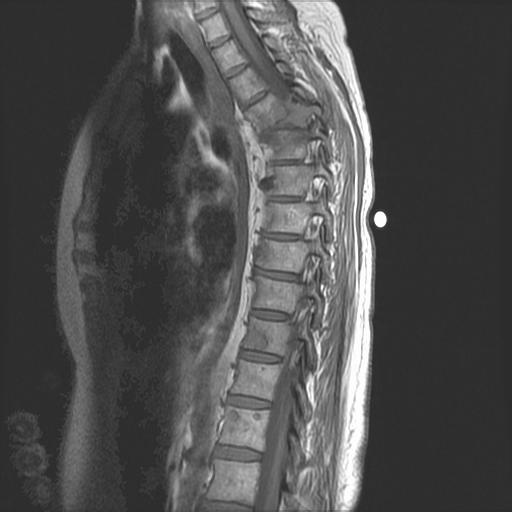

• > 3 focal lesions or SUV > 4.2 at diagnosis results in shorter PFS and OS[1]
• 65% of pts PET/CT negative 3 mos after ASCT with longer PFS and OS vs PET positive[1]
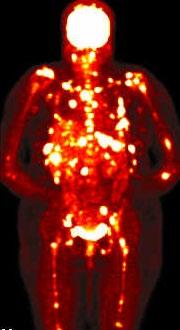
• Complete FDG suppression associated with durable disease control and prolonged OS[1]

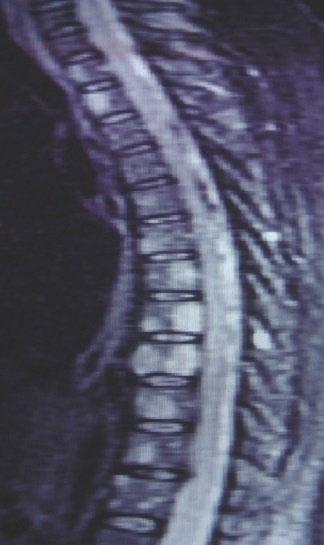
• Skeletal survey recommended in cases of plasmacytoma, extramedullary disease, suspected spinal cord compression as well as with new symptoms or progression[2]
• MRI and/or PET/CT indicated when symptomatic areas show no abnormality on radiograph[3]
 1. Zamagni E, et al. Blood. 2011;118:5989-5995.
2. Ludwig H, et al. Leukemia. 2014;28:981-992.
3. NCCN. Clinical practice guidelines in oncology: multiple myeloma. v.2.2014. 4. Boot M, et al. Novel prognostic modalities in multiple myeloma. 2013.
MRI FDG PET
Imaging Techniques[4]
1. Zamagni E, et al. Blood. 2011;118:5989-5995.
2. Ludwig H, et al. Leukemia. 2014;28:981-992.
3. NCCN. Clinical practice guidelines in oncology: multiple myeloma. v.2.2014. 4. Boot M, et al. Novel prognostic modalities in multiple myeloma. 2013.
MRI FDG PET
Imaging Techniques[4]
DNA
Conventional cytogenetic analysis (karyotyping)
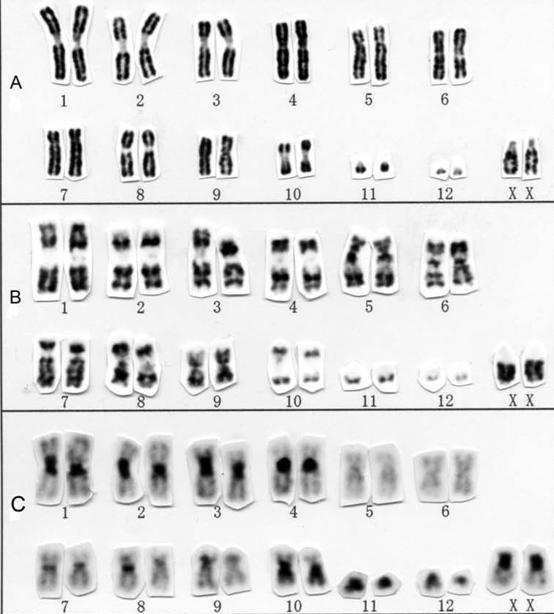
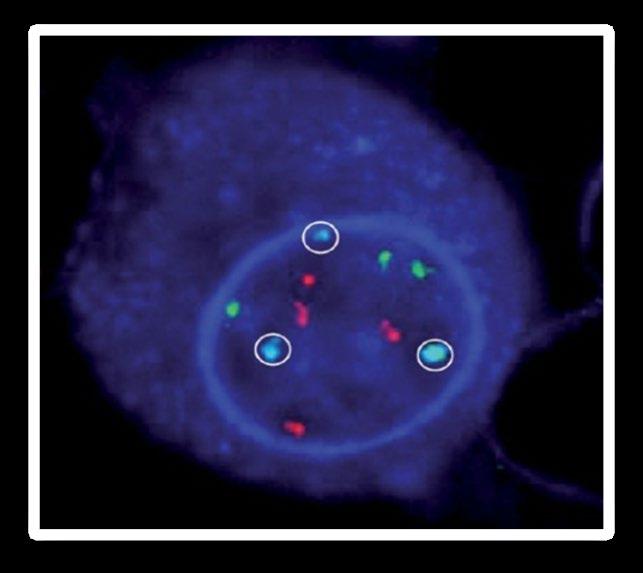
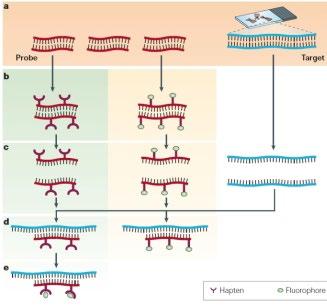
FISH (fluorescence in situ hybridization)
Advances
• Genetic expression profiling [GEP]

• Whole-genome/ whole-exome sequencing)
 Myeloma
Myeloma

•
•
•
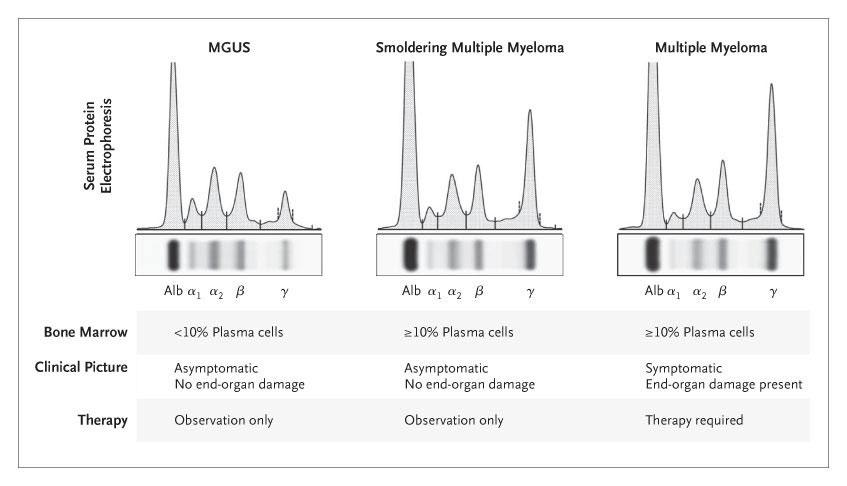

• At least one of the CRAB Criteria (evidence of end organ damage)
Hypercalcemia
Serum calcium >2.75 mmol/L (>11 mg/dL)
Renal Failure
Serum creatinine ≥ 2 mg/dL or creatinine clearance <40 mL per min

Anemia
Hemoglobin >20 g/L below the lower limit of normal, or a hemoglobin value <100 g/L
Bone
Lytic lesions, pathologic fractures, or severe
osteopenia
• ≥60% clonal bone marrow plasma cells
• Serum involved/uninvolved free light chain ratio ≥100
• >1 Focal bone lesion (≥5mm) on MRI
• Use your Clinical judgment when using these criteria
Rajkumar, et al. Lancet Oncology. 2014;15(12):e538-48.
•

•
•

• Symptom Control
• Ameliorate pain and other disease-related symptoms
• Prevent further organ damage
• Preserve and improve performance status and quality of life
• Disease Response and Survival
• Rapid cytoreduction to relieve symptoms
• Minimize treatment-related toxicity
• Prolong survival – Overall Survival

Bortezomib
Lenalidomide
Carfilzomib
Pomalidomide
Selinexor
Panobinostat
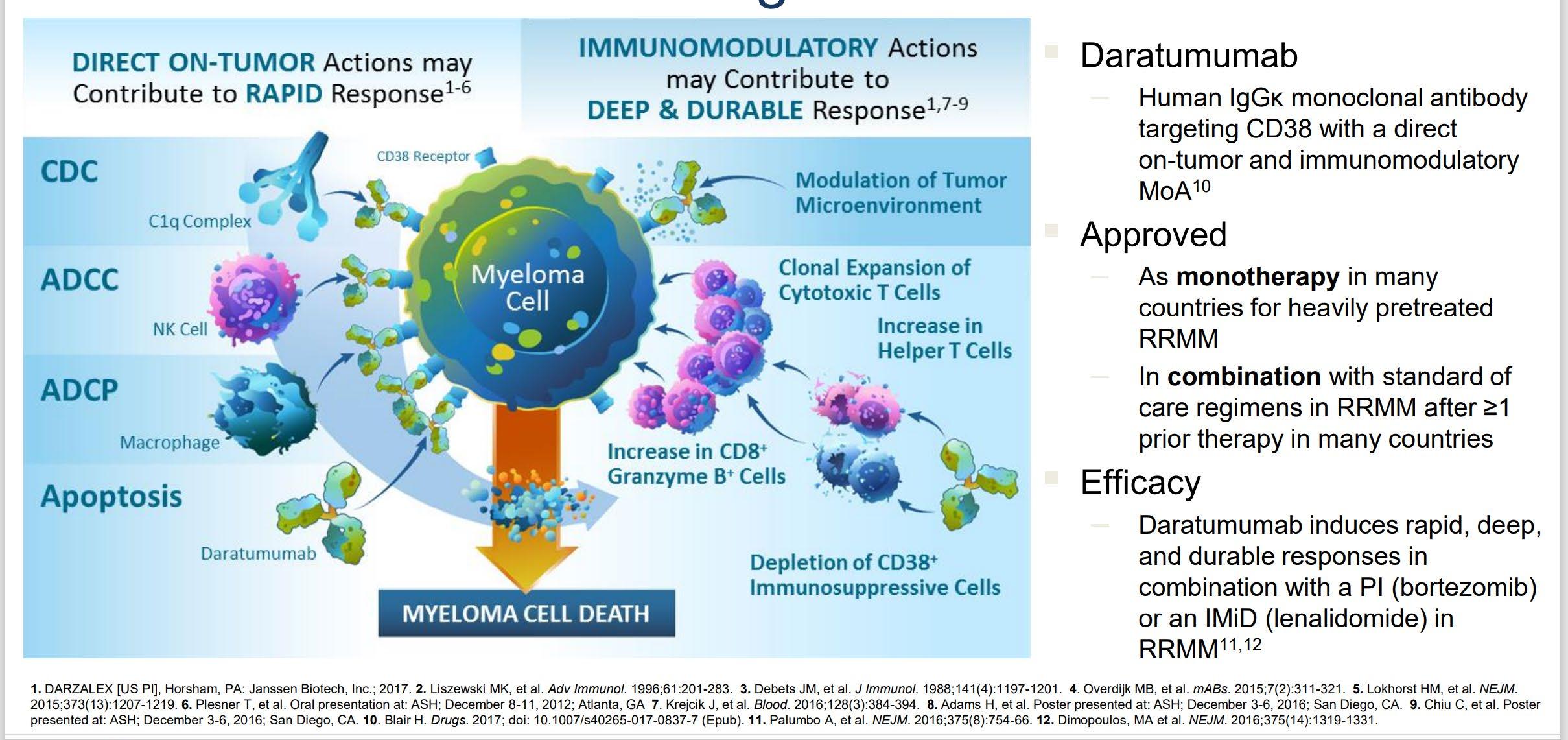
Daratumumab
Ixazomib
Elotuzumab
Isatuximab
Belantamab mafodotin
Melphalan flufenamide
Idecabtagene autoleucel
Daratumumab?
Carfilzomib?
Lenalidomide + PI
Bispecific/Trispecific Antibodies
Cell Modifying Agents
Venetoclax?
PD/PDL-1 Inhibition?
Multiple
ASCT, autologous stem cell transplant; CAR, chimeric antigen receptor; Cy, cyclophosphamide; d- daratumumab; D/dex, dexamethasone; isa, isatuximab; K, carfilzomib; M, melphalan; PD-L1, programmed death ligand-1; PI, proteasome inhibitor; Rev, lenalidomide; V, bortezomib
• Combination therapy continues to prove superiority in providing long remission and long survival.
• The patient is at good place as options are becoming more prevalent.
• Lenalidomide, bortezomib and dexamethasone continues to be an effective regimen.

• Daratumumab in combination with lenalidomide is an excellent option for “frail patient”
• But wait there are more options
Daratumumab (DARA) Plus Lenalidomide, Bortezomib, and Dexamethasone (RVd) in Patients (Pts) With Transplant-eligible
Newly Diagnosed Multiple Myeloma (NDMM):
Updated Analysis of GRIFFIN After 24 Months of Maintenance
Presented at the 63rd American Society of Hematology (ASH) Annual Meeting & Exposition; December 11-14, 2021; Atlanta, GA/Virtual
*Presenting author.
Additional information can be viewed by scanning the QR code or accessing this link: https://www.oncologysciencehub.com/ ASH2021/Daratumumab/Laubach The QR code is intended to provide scientific information for individual reference, and the information should not be altered or reproduced in any way.

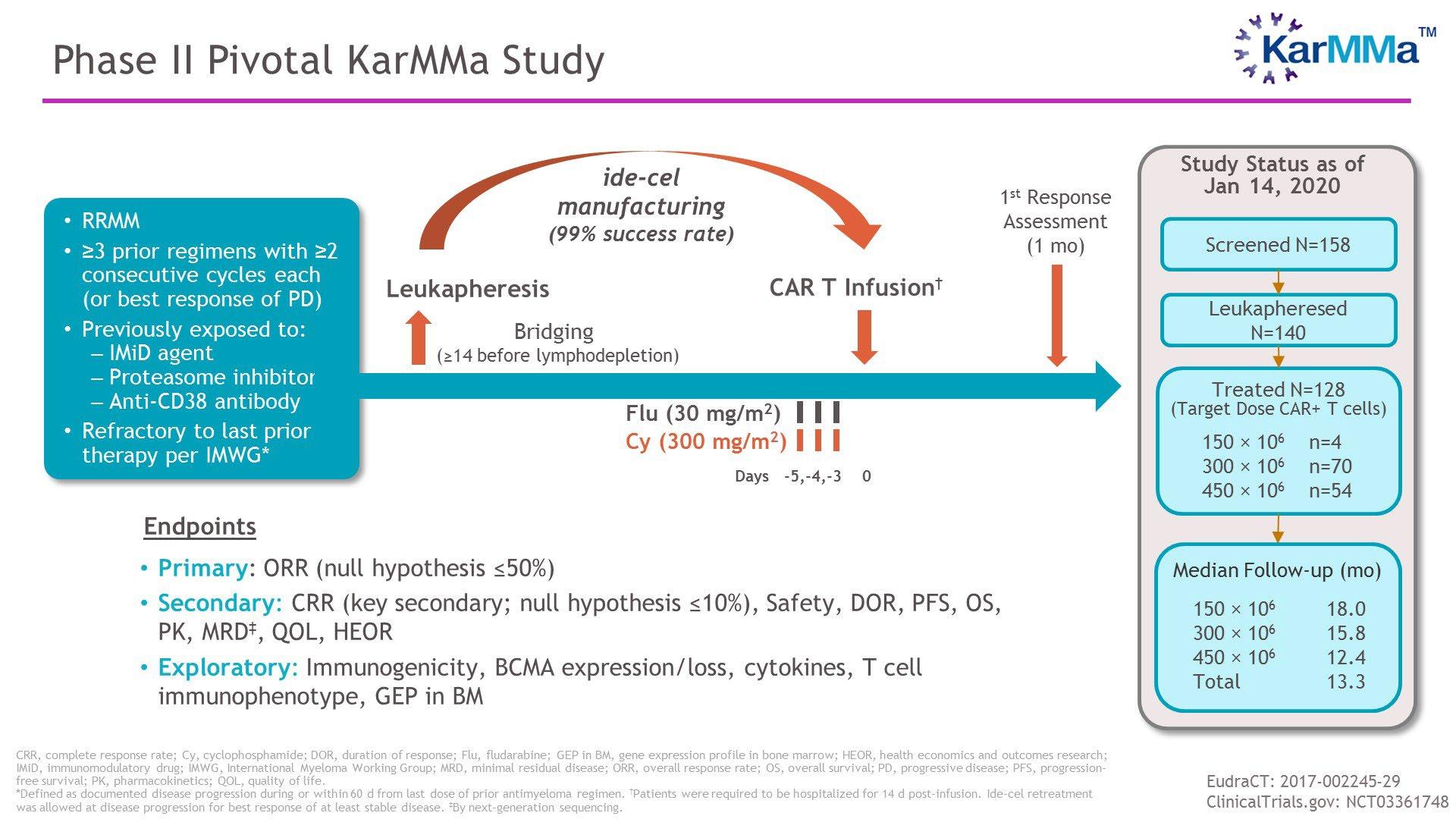
• Phase 2 study of D-RVd versus RVd in transplant-eligible NDMM, 35 sites in the United States with enrollment between December 2016 and April 2018

• Response rates for sCR and ≥CR were greater for D-RVd versus RVd at all time points, with the deepest responses occurring after 2 years of maintenance therapy


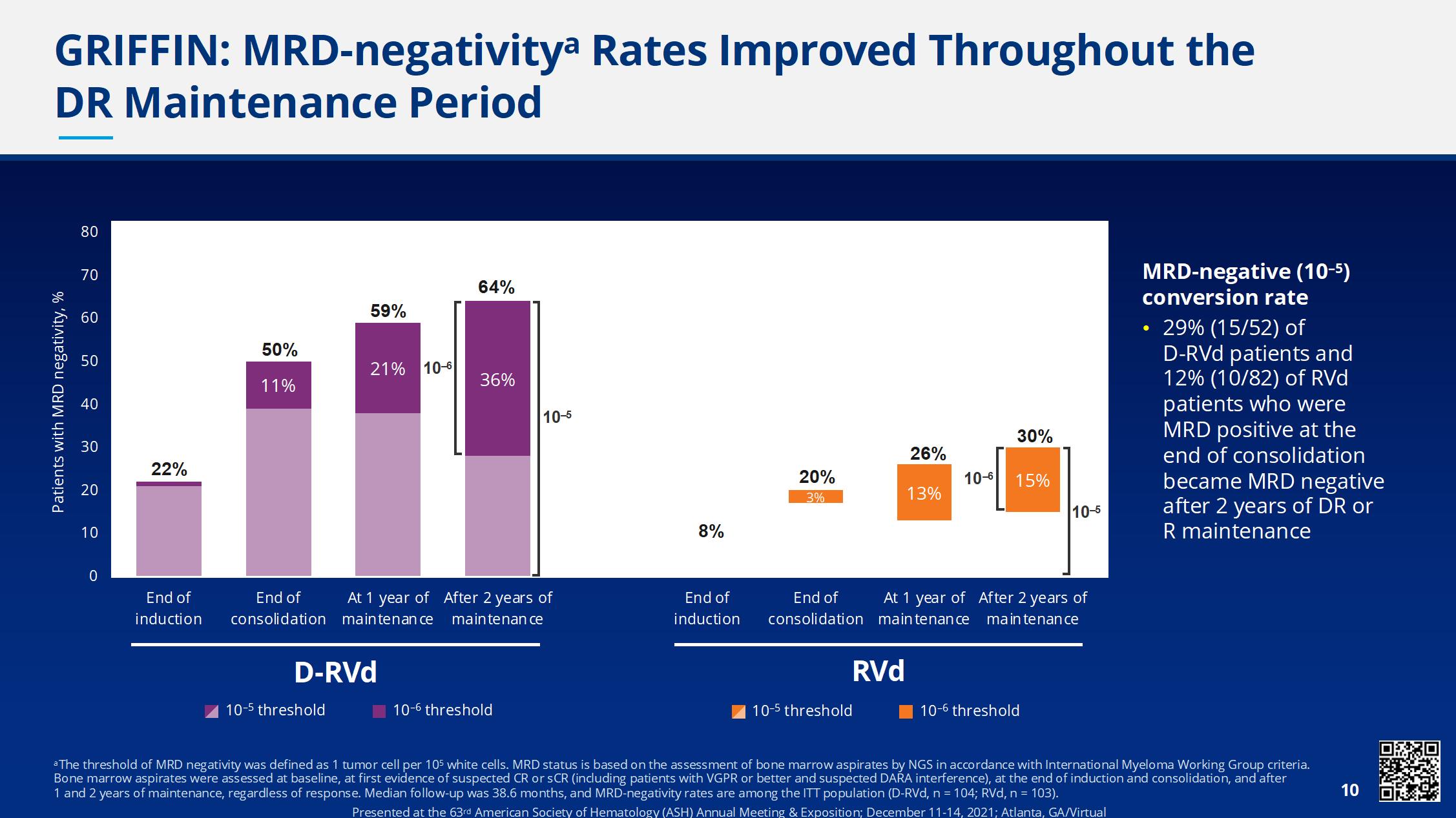
• Median follow-up: 38.6 months
• Median PFS was not reached in either group
• There is a positive trend toward improved PFS for DRVd/DR versus RVd/R
• The separation of the PFS curves begins beyond 1 year of maintenance and suggests a benefit of prolonged DR therapy

• 4 drugs produce a deeper response than 3
• Interestingly, it deepens at each step – post induction, transplant, consolidation and maintenance
• It is feasible to add dara to lenalidomide for maintenance
• The trend seems to improve PFS
• We are getting close – need a phase 3 study…

Daratumumab, Carfilzomib, Lenalidomide and Dexamethasone (Dara-KRd), Autologous Transplantation and MRD Response-Adapted Consolidation and Treatment Cessation-Final Primary Endpoint Analysis of the MASTER Trial
Luciano J. Costa1, Saurabh Chhabra2, Natalie S. Callander, MD3 , Eva Medvedova4, Bhagirathbhai Dholaria5, Rebecca Silbermann4, Kelly Godby1, Binod Dhakal2, Susan Bal1, Smith Giri1, Anita D’Souza2, Timothy Schmidt3, Aric
COMMIT- Academic Consortium to Overcome Multiple Myeloma through Innovative Trials
 Hall3, Pamela Hardwick1, Robert F. Cornell5, Parameswaran Hari2
1- University of Alabama at Birmingham; 2- Medical College of Wisconsin; 3- University of Wisconsin at Madison; 4- Oregon Health and Science University; 5- Vanderbilt University
Hall3, Pamela Hardwick1, Robert F. Cornell5, Parameswaran Hari2
1- University of Alabama at Birmingham; 2- Medical College of Wisconsin; 3- University of Wisconsin at Madison; 4- Oregon Health and Science University; 5- Vanderbilt University
Dara-KRd
• Daratumumab 16 mg/m2 days 1,8,15,22 (days 1,15 C 3-6; day 1 C >6)




• Carfilzomib (20) 56 mg/m2 Days 1,8,15
• Lenalidomide 25 mg Days 1-21
• Dexamethasone 40mg PO Days 1,8,15,22
MRD assessment by NGS
*24 and 72 weeks after completion of therapy
MASTER trial
”MRD-SURE” -Treatment-free observation and MRD surveillance*
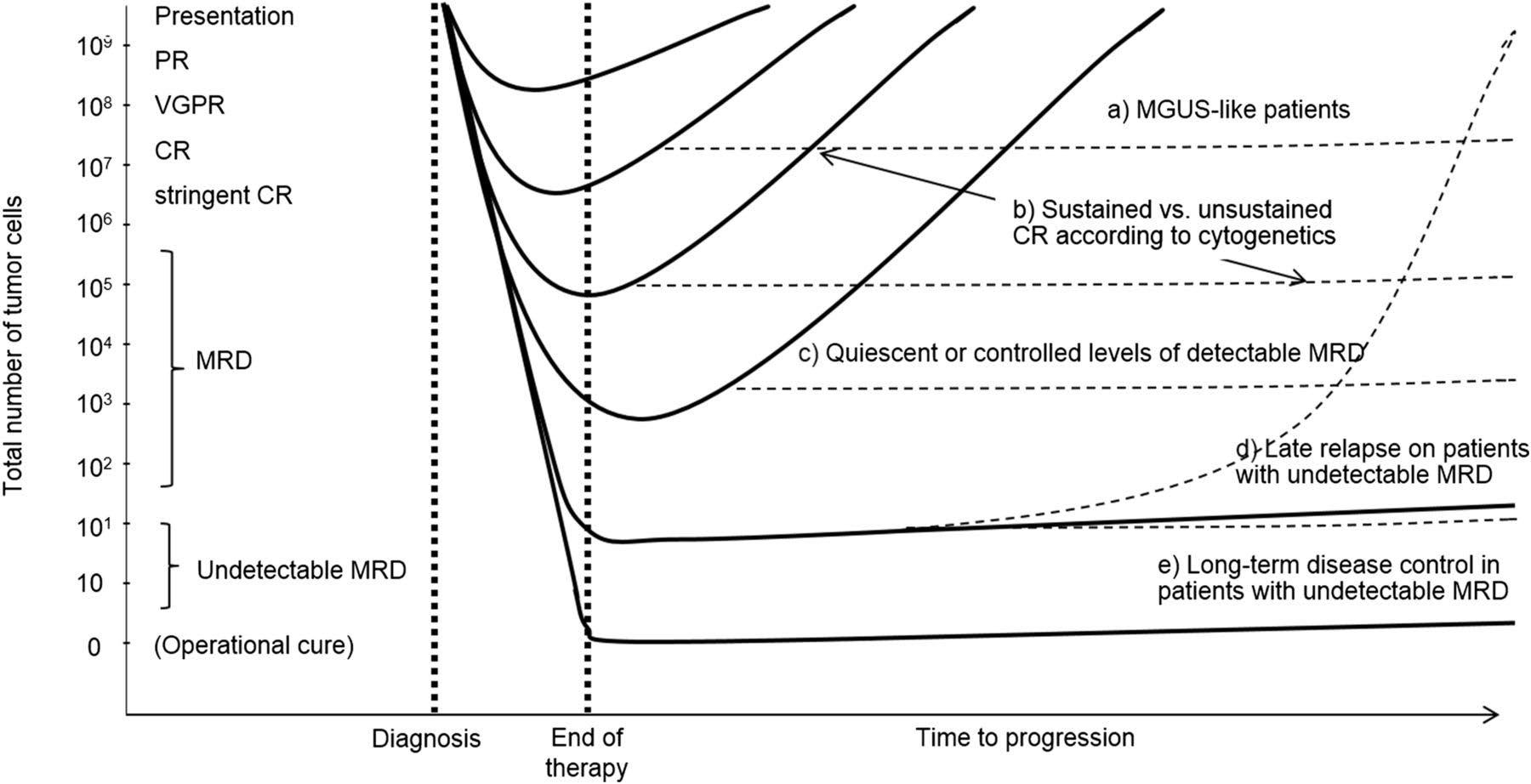
• NGS-MRD response-adapted therapy is feasible in ~96% of patients in multi center setting – 72% reaching MRD-SURE.
• Patients with standard and high-risk NDMM have similar depth of response and low risk of MRD resurgence or progression when treated with Dara-KRd/AHCT and MRDadapted treatment cessation.
• Quadruplet therapy and achievement of confirmed MRD (-) responses enables the exploration of treatment cessation and “MRD-SURE” as alternative to continuous therapy.
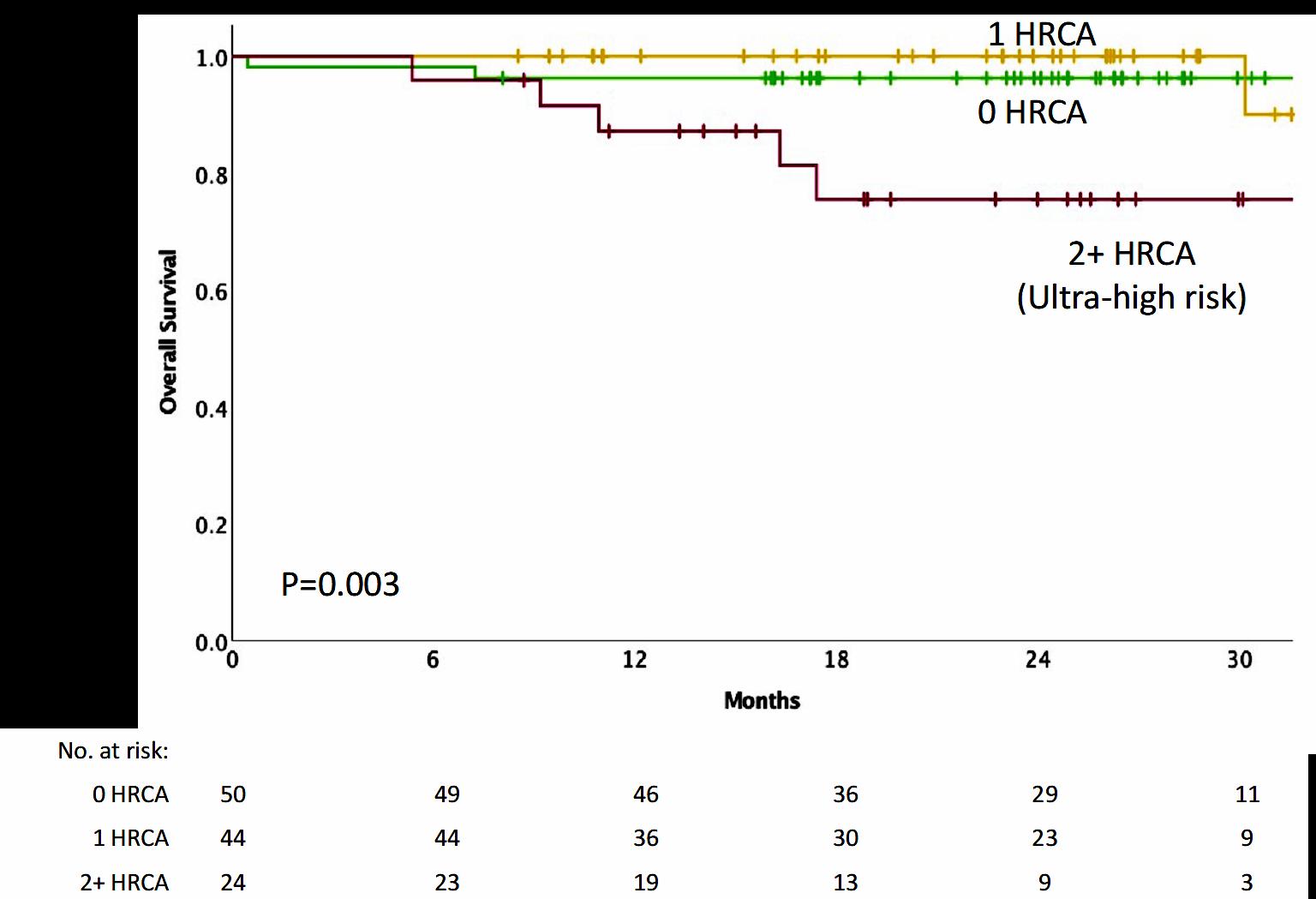
Effective novel consolidative strategies should be explored to clear MRD and improve outcomes in patients with ultra-high-risk MM

Hartmut Goldschmidt1,2, Elias K. Mai1, Eva Nievergall1, Roland Fenk3, Uta Bertsch1,2, Diana Tichy4, Britta Besemer5, Jan Dürig6, Roland Schroers7, Ivana von Metzler8, Mathias Hänel9, Christoph Mann10, Anne Marie Asemissen11, Bernhard Heilmeier12, Stefanie Huhn1, Katharina Kriegsmann1, Niels Weinhold1, Steffen Luntz13, Tobias A. W. Holderried14, Karolin Trautmann-Grill15, Deniz Gezer16, Maika Klaiber-Hakimi17, Martin Müller18, Cyrus Khandanpour19, Wolfgang Knauf20, Markus Munder21, Thomas Geer22, Hendrik Riesenberg23, Jörg Thomalla24, Martin Hoffmann25, Marc-Steffen Raab1, Hans J. Salwender26, Katja C. Weisel11 for the German-speaking Myeloma Multicenter Group (GMMG)
1Department of Internal Medicine V, University Hospital Heidelberg, Heidelberg, Germany; 2National Center for Tumor Diseases Heidelberg, Heidelberg, Germany;
3Department of Hematology, Oncology and Clinical Immunology, University Hospital Düsseldorf, Düsseldorf, Germany; 4Division of Biostatistics, German Cancer Research Center (DKFZ) Heidelberg, Heidelberg, Germany;


5Department of Internal Medicine II, University Hospital Tübingen, Tübingen, Germany; 6Department for Hematology and Stem Cell Transplantation, University Hospital Essen, Essen, Germany;
7Medical Clinic, University Hospital Bochum, Bochum, Germany; 8Department of Medicine, Hematology/Oncology, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany;
9Department of Internal Medicine III, Clinic Chemnitz, Chemnitz, Germany; 10Department for Hematology, Oncology and Immunology, University Hospital Gießen and Marburg, Marburg, Germany;
11Department of Oncology, Hematology and BMT, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; 12Clinic for Oncology and Hematology, Hospital Barmherzige Brueder Regensburg, Regensburg, Germany; 13Coordination Centre for Clinical Trails (KKS) Heidelberg, Heidelberg, Germany; 14Department of Oncology, Hematology, Immuno-Oncology and Rheumatology, University Hospital Bonn, Bonn, Germany;
15Department of Internal Medicine I, University Hospital Dresden, Dresden, Germany; 16Department of Hematology, Oncology, Hemostaseology, and Stem Cell Transplantation, Faculty of Medicine, RWTH Aachen University, Aachen, Germany; 17Clinic for Hematology, Oncology and Palliative Care, Marien Hospital Düsseldorf, Düsseldorf, Germany; 18Clinic for Hematology, Oncology and Immunology, Klinikum Siloah Hannover, Hannover, Germany; 19Medical Clinic A, University Hospital Münster, Münster, Germany; 20Center for Hematology and Oncology Bethanien, Frankfurt am Main, Germany; 21Department of Internal Medicine III, University Hospital Mainz, Mainz, Germany; 22Department of Internal Medicine III, Diakoneo Clinic Schwäbisch-Hall, Schwäbisch-Hall, Germany; 23Hematology/Oncology Center, Bielefeld, Germany; 24Hematology / Oncology Center, Koblenz, Germany; 25Medical Clinic A, Clinic Ludwigshafen, Ludwigshafen, Germany; 26Asklepios Tumorzentrum Hamburg, AK Altona and AK St. Georg, Hamburg, Germany
Induction phase (3 x 6-week cycles)
Maintenance phase (4-week cycles)
MRD (bone marrow aspirate)
Primary endpoint:
• MRD negativity at the end of induction treatment (NGF, sensitivity 10-5) stratified according to RISS
Secondary endpoints:
• CR after induction
• Safety Data cut-off:
• April 2021
ASCT, autologous stem cell transplant; CR, complete response; d, dexamethasone; HDT, high-dose therapy; Isa, isatuximab MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; NGF, next-generation flow; PD, progressive disease; R, lenalidomide; R-ISS, Revised International Staging System; Te, transplant eligible; V, bortezomib 1. ClinicalTrials.gov: NCT03617731

Low number of not assessable/missing† MRD status: Isa-RVd (10.6%) and RVd (15.2%)
Isa-RVd is the first regimen to demonstrate a rapid and statistically significant benefit from treatment by reaching a MRD negativity of 50.1% at the end of induction and to show superiority vs. RVd in a Phase 3 trial
*P value derived from stratified conditional logistic regression analysis †Missing NGF-MRD values were due to either patients’ loss to follow-up during induction therapy or to missing bone marrow samples or technical failures in measurement counted as non-responders, i.e. NGF-MRD positive CI, confidence interval; d, dexamethasone; Isa, isatuximab; ITT, intent-to-treat; MRD, minimal residual disease; NGF, next-generation flow; OR, odds ratio; R, lenalidomide; V, bortezomib

First primary endpoint, end of induction MRD negativity by NGF (10-5), was met in ITT analysis
Although the rates of CR after induction therapy did not differ between the Isa-RVd and RVd arms, there was a significant increase in ≥VGPR rates and ORR with Isa-RVd
*P values derived from Fisher’s exact test †Data adjusted per M-protein interference CR, complete response; d, dexamethasone; Isa, isatuximab; nCR; near-complete response; ORR, overall response rate; PR, partial response; R, lenalidomide; V, bortezomib; VGPR, very good partial response


A comparable number of patients discontinued induction therapy due to AEs in the
arm vs. RVd arm
*SOC considered as “Investigations” as defined by the CTCAE †Includes five episodes of febrile neutropenia during induction: Isa-VRd (n=3) vs. VRd (n=2) ‡Infusion-related reactions of CTCAE grade 2 or higher in the Isa-RVd arm were n=42 (12.7%) AE, adverse event; CTCAE Common Terminology Criteria for Adverse Events d, dexamethasone; Isa, isatuximab; NA, not applicable; PT, preferred term; R, lenalidomide; SOC, system organ class; V, bortezomib
• Adding a CD38 antibody to VRD or KRD is feasible with limited incremental toxicity
• Depth of response is improved, including MRD negativity
• MRD will be an even more important measure going forward
• There is a trend to improved PFS
• This may overcome the high-risk feature of some patients
• Ultra high-risk disease remains challenging to treat with current strategies
• What about transplant??

MASTER 2 The Dream Trial
Quadruplet x 6 cycles













Intensification
MRD (-) randomization MRD (+) randomization


Consolidation Arm M ← MRD2 →



AHCT AHCT ← MRD1x

Quadruplet X 3 cycles CD38MoAb-R X3 cycles
T-cell Redirecting therapy


• Open the Q&A window, allowing you to ask questions to the host and panelists. They can either reply to you via text in the Q&A window or answer your question live.



• If the host answers live, you may see a notification in the Q&A window.


• If the host replies via the Q&A box – you will see a reply in the Q&A window.



6:35 – 7:15 PM Relapsed Therapy & Clinical Trials

Jonathan Kaufman, MD – Winship Cancer Institute of Emory University
7:15 – 7:35 PM Life is a Canvas, You are the Artist
Daniel Verina, DNP, RN, ACNP-BC - Mount Sinai Hospital, IMF Nurse Leadership
Board Member

7:35 – 8:00 PM Q&A with Panel



 Jonathan Kaufman, MD
Jonathan Kaufman, MD


 Jonathan L. Kaufman, MD
Jonathan L. Kaufman, MD
• Biochemical progression:
• Progression of disease based on M-protein parameter increase only
• Timing of therapy institution/escalation dependent on numerous factors
• Pace of progression
• Original clinical presentation
• Standard- vs high-risk disease biology
• Patient/physician comfort level
• Clinical relapse:
• “Direct indicators of increasing disease and/or end organ dysfunction (CRAB features) related to the underlying clonal plasma cell proliferative disorder”
• Mandates immediate institution/escalation of therapy
Progression Clinical Relapse
↑ of ≥25% from nadir response value in one or more of the following:
1) Serum M-protein (absolute increase ≥0.5 g/dL, ≥1 g/dL if nadir ≥5 g/dL)
2) Urine M-protein (absolute increase ≥200 mg/ 24 hours)
3) Measurable by serum FLC testing only: difference between involved and uninvolved FLCs (absolute increase ≥10 mg/dL)
4) Non-secretory: bone marrow PC % (absolute increase ≥10%)
1) Development of new soft tissue plasmacytomas or bone lesions (osteoporotic fractures do not constitute progression)
2) Definite increase in the size of existing plasmacytomas or bone lesions. A definite increase is defined as a 50% (and ≥1 cm) increase as measured serially by the SPD of the measurable lesion
3) Hypercalcemia (>11 mg/dL);
4) Decrease in hemoglobin of ≥2 g/dL not related to therapy or other non-myelomarelated conditions
5) Rise in serum creatinine by 2 mg/dL or more from the start of the therapy and attributable to myeloma
6) Hyperviscosity related to serum paraprotein
≥50% increase in circulating plasma cells (minimum 200 cells/mcL) if this is the only disease measure available
NOT EVERY RELAPSE NEEDS TO BE TREATED WITH THE SAME UNRGENCY
WE CAN GAIN SOME MORE MILEAGE WITH THE SAME TREATMENT AS LONG AS THERE IS NO CLINICAL RELAPSE
UNDERSTANDING THE RELPASE IS THE KEY
KNEE JERK REACTIONS – RELAPSE ≠ CHANGE IN TREATMENT
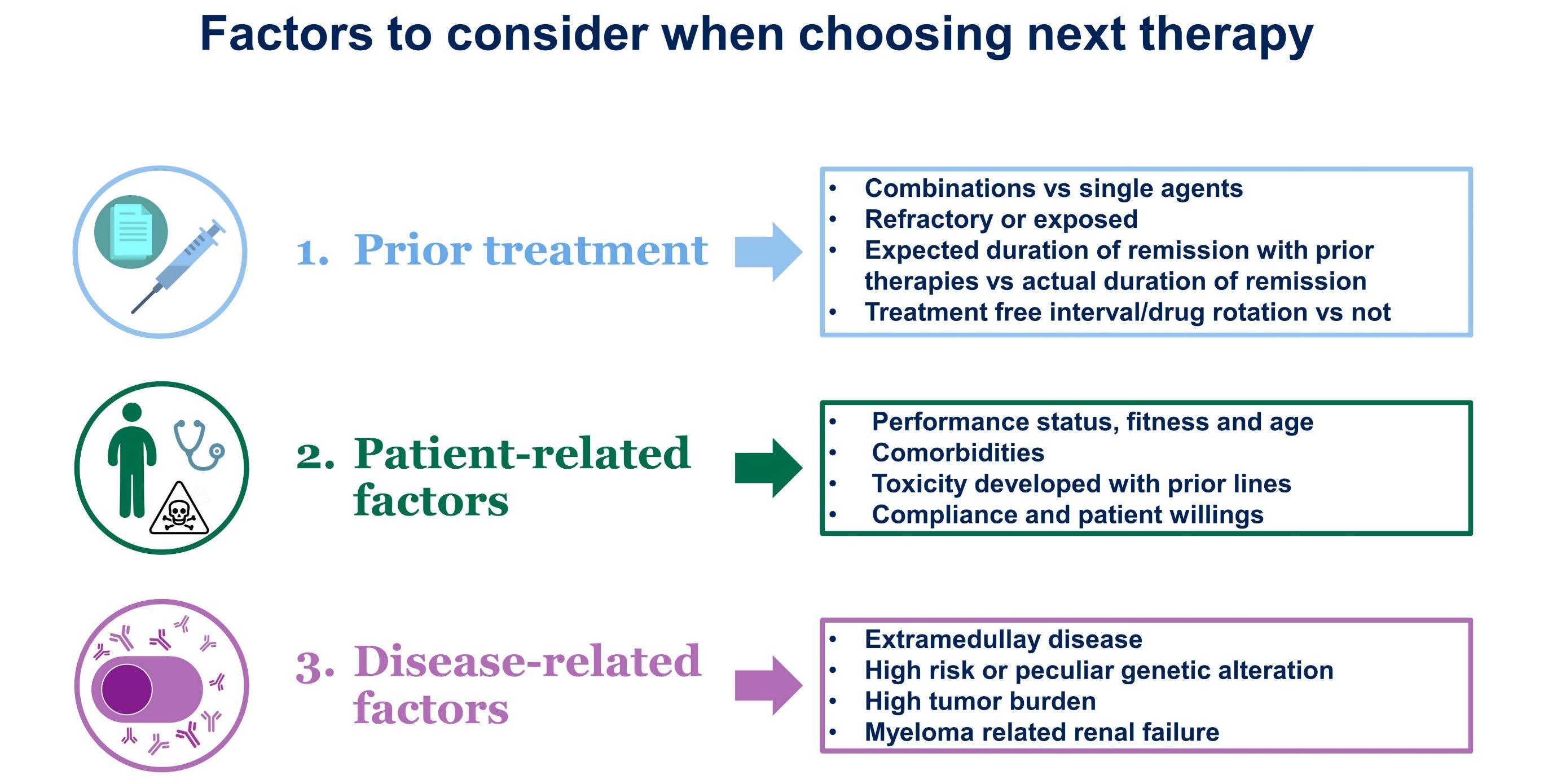

ONE SIZE DOES NOT FIT ALL
KNOWING ABOUT YOUR PRIOR TREATMENTS WILL HELP FORMULATE THE NEW PLAN
YOUR MYELOMA MAY BE MORE SENSITIVE TO A SPECIFIC THERAPY
IT WILL ALSO ALLOW FOR NOT CHOOSING TREATMENTS THAT YOU DID NOT TOLERATE WELL BEFORE
NOT EVERY PATIENT IS THE SAME
AGE, OTHER EXISTING ILLNESSES WILL ALLOW FOR CHOOSING OR ‘NOT CHOOSING’ CERTAIN TREATMENTS
SOME TREATMENTS MAY NOT BE SAFE FOR PATIENTS WITH HEART DISEASE, KIDNEY DISEASE ETC
DYNAMIC SCENARIO – MAY BE ABLE TO ADD OR PEEL OFF TREATMENTS BASED ON SPECIFIC PATIENT
PATIENT PREFERENCES – ORAL MEDICATIONS VS INTRAVENOUS OR SUBCUTANEOUS MEDICATIONS
MYELOMA IS NOT A SINGLE DISEASE
• HIGH RISK MYELOMAS NEED TO BE DEALT WITH AGGRESSIVELY WITH COMBINATION THERAPIES
CERTAIN GENETIC ALTERATIONS MAY ALLOW FOR USING OFFLABEL TREATMENTS
• SOME TREATMENTS WITH URGENT CHEMOTHERAPY ARE NECESSARY TO ‘PUT OUT THE FIRE’ AND TO PREVENT ORGAN DAMAGE FROM MYELOMA
HDACis: Panobinostat
Steroids: prednisone, dexamethasone
Conventional chemotherapeutic agents: melphalan, cyclophosphamide, doxorubicin, bendamustine, combos (DCEP, VDT-PACE)










Directly kills myeloma cells (Tumouricidal effects)



















Myeloma Cells



Apoptotic Myeloma Cells
Enhances the immune system (Immunomodulatory effects)






IFN, interferon; IL, interleukin; NK, natural killer; NKT, natural killer T cell; T, T cell.












Hayashi T, et al. Br J Haematol 2004;128:192–203; 2. Brissot E, et al. Leukemia 2015;29:2098–100; 3. Görgün G, et al. Blood 2010;116:3227–37; 3. Lu G, et al. Science 2014;343:305–9



















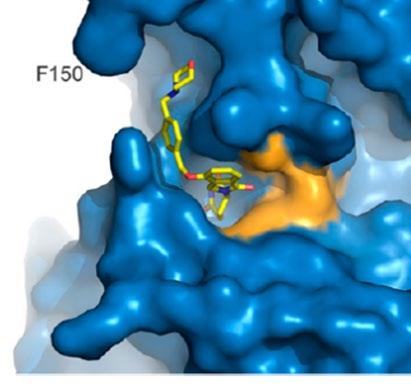
• IBER enhances in vitro immune stimulatory activity versus LEN and POM1
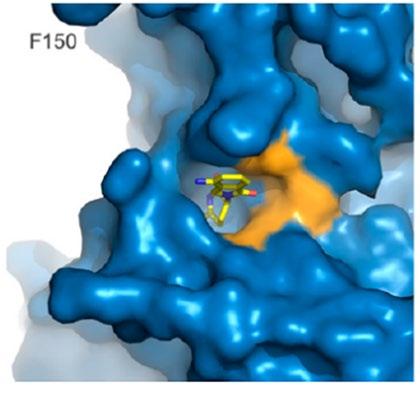
CRBN binding IC50
• CC-220: 0.06 uM
• Pomalidomide: 1.20 uM
• Lenalidomide: 1.50 uM
Aiolos degradation (5 hrs) EC50
• CC-220: 0.5 nM
• Pomalidomide: 22.0 nM
• Lenalidomide: 87.0 nM
DSMO, dimethylsulfoxide; EC50, half maximal effective concentration; IL, interleukin; PBMC, peripheral blood mononuclear cell.
Ikaros degradation (5 hrs) EC50
• CC-220: 1.0 nM
• Pomalidomide: 24.0 nM
• Lenalidomide: 67.0 nM



Teclistamab, a B-cell Maturation Antigen (BCMA) x CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma (RRMM): Updated Efficacy and Safety Results from MajesTEC-1
Ajay Nooka1, Philippe Moreau2, Saad Z Usmani3, Alfred L Garfall4, Niels WCJ van de Donk5, Jesús F San-Miguel6, Albert Oriol7, Ajai Chari8, Lionel Karlin9, Maria-Victoria Mateos10, Rakesh Popat11, Joaquín Martínez-López12, Surbhi Sidana13, Danielle Trancucci14, Raluca Verona15, Suzette Girgis15, Clarissa Uhlar15, Tara Stephenson15, Arnob Banerjee15, Amrita Krishnan16
1Winship Cancer Institute, Emory University, Atlanta, GA, USA; 2University Hospital Hôtel-Dieu, Nantes, France; 3Levine Cancer Institute/Atrium Health, Charlotte, NC, USA; 4Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 5Amsterdam University Medical Center, Vrije Universiteit Amsterdam, Amsterdam, Netherlands; 6Clínica Universidad de Navarra, CCUN, CIMA, CIBERONC, IDISNA, Pamplona, Spain; 7Institut Català d’Oncologia and Institut Josep Carreras and Hospital Germans Trias i Pujol, Badalona, Barcelona, Spain; 8Mount Sinai School of Medicine, New York, NY, USA; 9Service d’Hématologie Clinique, Centre Hospitalier Lyon Sud, Pierre-Bénite, France; 10University Hospital of Salamanca/IBSAL/CIC/CIBERONC, Salamanca, Spain; 11University College London Hospitals, NHS Foundation Trust, London, UK; 12Haematological Malignancies Clinical Research Unit, Hospital 12 de Octubre Universidad Complutense, CNIO, CIBERONC, Madrid, Spain; 13Stanford University School of Medicine, Stanford, CA, USA; 14Janssen Research & Development, Raritan, NJ, USA; 15Janssen Research & Development, Spring House, PA, USA; 16City of Hope Comprehensive Cancer Center, Duarte, CA, USA
https://www.congresshub.com/Oncology/ AM2021/Teclistamab/Nooka
Copies of this presentation obtained through Quick Response (QR) Code are for personal use only and may not be reproduced without permission from ASCO® or the author of this presentation

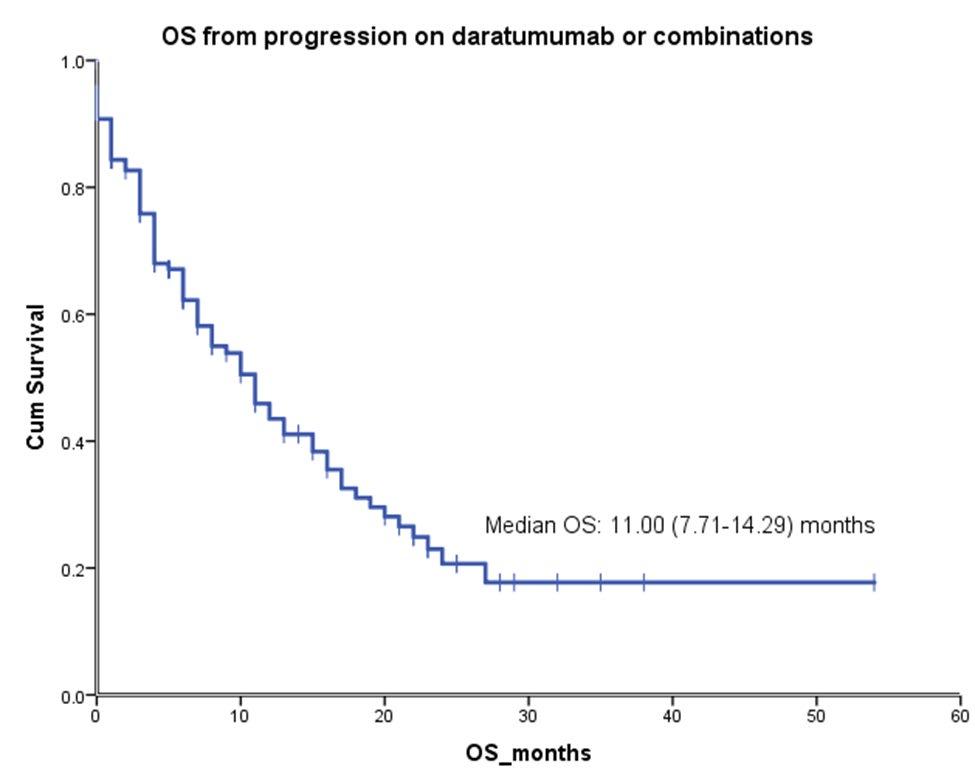
ORR of 63.0% (95% CI: 55.2–70.4) represents a substantial benefit for patients with triple-class exposed disease
• Median time to response (n=104)
• First response: 1.2 months (range: 0.2–5.5)
• Best response: 3.8 months (range: 1.1–16.8)
• MRD negativity rate at 10-5,b
• 26.7% in the overall treated (N=165) patient population
81.5% of MRD-evaluable patients (44 of 54) were MRD negative

• Almost half (46.2%) of patients with ≥CR were MRD negative
Analysis cutoff date: March 16, 2022. aPR or better, IRC assessed, per IMWG 2016 criteria; bAll MRD assessments were done by next-generation sequencing. ≥CR, CR or better; CR, complete response; IMWG, International Myeloma Working Group; IRC, independent review committee; ITT, intent-to-treat; MRD, minimal residual disease; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response
Analysis cutoff date: March 16, 2022.
CI confidence interval; CR complete response; DOR
Median DOR was 18.4 months (95% CI: 14.9–NE) and not mature with 71 patients (68.3%) censored
• An estimated 68.5% (95% CI: 57.7–77.1) of responders maintained their response for at least 12 months
duration of response; NE not estimable; VGPR very
• Several antimyeloma agents in the RRMM space
• Not every relapse needs to be treated right away but will need to be monitored closely
• Several factors – patient related, disease related, prior therapies need to be considered prior to choosing a plan
• Several drugs and combinations that are FDA approved and available
• More importantly, the new effective drugs are in pipeline and available as clinical trials – ‘a proactive patient’ always makes the best use of this option.
• ‘When myeloma comes back’ there is hope and promise – don’t panic
• We have a basic obligation and responsibility to provide the best possible cancer care to our patients. • Centers that do research provide better care for all their patients, not just those enrolled in research trials.
• Research enhances the quality of basic and continuing education.
• Learn a working definition for Clinical Research
•
To understand the different types of clinical research
•
To become familiar with the different designs of a clinical trial
• To learn the difference between an Phase 1 and Phase 3 trial
Research leads to:
• Improved screening techniques and understanding of the value and limitations of screening.
• Better treatment because of the rigor required to do research.
• Better trained staff.
• Increase in economic activity for the community. But most important, research leads to improvements in treatment.
Trials conducted with people who volunteer to take part. Each study answers scientific questions and tries to find better ways to prevent, screen for, diagnose, or treat a disease.
• Trials of tests and procedures that can be used to identify disease.
• Most people in these trials already have signs or symptoms of the disease
• Trials of the population of people with a particular disease and the characteristics they share
• Trials to study ways to reduce risk of developing a disease.
• May be done on healthy people.
• In cancer patients, may also be done on those that have had the disease and want to prevent recurrence
• Trials to improve the comfort and quality of life of patients.
Nausea –
–
Vomiting –
Fatigue –
Depression –
Effects of treatment on the patient
• Trials designed to answer specific questions about new treatments or approaches to treatment of disease
• Advance our understanding of a disease and its effects
Diagnosis –
–
Prevention –
Treatment –
Quality of Life
• Objective evaluation of new treatments and options
• Evidenced based medicine
Small number of people (20-100)
• Phase two is once the intervention is found to be safe it is tested for efficacy
• Several hundred people
• Randomized – One group receives the intervention and the second receives a control
• Only about one third of experimental drugs successfully complete both phase 1 and phase 2 studies
Phase three done to provide more thorough understanding of effectiveness, benefit, and range of possible adverse reaction
Several hundred to thousand people
• Patients are randomized to treatment assignments • When/where possible trial conducted in a double-blinded fashion • Evaluate superiority or equivalence
When completed may apply for FDA approval
• Compare on treatment to another • Monitor long term effectiveness
Impact on quality of life
• Cost effectiveness
Randomized Clinical Trials
Case series
Historical controls
Open trials
Retrospective reviews
• The Gold Standard
– Controlled comparison of two treatment options
– Done in a double-blind fashion
– Subjects are randomized to treatment assignment
– Significance of the outcome is evaluated using appropriate statistical methods
• A group or series of case reports involving patients who were given similar treatment.
• Detailed information about the individual patients reported: – age, gender, ethnic origin – on diagnosis, treatment, response to treatment, and follow-up after treatment.
Use of comparison from previous patients with the same disease
Limitations:
–
Bias with change of time
• Change in technology and supportive care
–
Retrospective analysis
–
Incomplete data
– Often difficult to compare
• A trial which is not blinded
•
A trial in which both the doctors and patients know what treatment is being given.
• Can be randomized or non-randomized
• a study based on the medical records of patients, looking backward in time at events that happened in the past.
Learn a working definition for Clinical Research
To understand the different types of clinical research
To become familiar with the different designs of a clinical trial
To learn the difference between an Phase 1 and Phase 3 trial






February 1, 2023
Mount Sinai Medical Center
New York, New York
Daniel Verina, DNP, RN, ACNP-BC

Myeloma treatment

Know your care team, Telehealth & Meeting Prep, & Shared Decision Making
Infection and Side Effect Management

• Rapid and effective disease control

• Durable disease control

• Minimize side effects
• Allow for good quality of life
• Improved overall survival
• Prevent disease- and treatmentrelated side effects
• Optimize symptom management
• Allow for good quality of life
DISCUSS GOALS AND PRIORITIES WITH YOUR HEALTHCARE TEAM
Transplant
Eligible Patients
Transplant
Consolidation
Maintenance
Initial Therapy
Transplant
Ineligible patients
Consolidation/ Maintenance/ Continued therapy

Treatment of Relapsed disease
Everyone
Supportive Care
A meta-analysis identified the most common patient-reported symptoms and impact on QOL, and were present at all stages of the disease. Symptoms resulted from both myeloma disease and treatment, including transplant, and were in these categories:
Physical
• Fatigue
• Constipation
• Pain
• Neuropathy

• Impaired Physical Functioning
• Sexual Dysfunction


Psychological

• Depression
• Anxiety
• Sleep Disturbance
• Decreased Cognitive Function
• Decreased Role & Social Function
Financial
• Financial burden (80%)
• Financial toxicity (43%)
• There are no black and white answers to deciding to undergo a transplant
• Undergoing transplant is a commitment for both you and your care partner
• Understanding the process will help bring to focus elements needed to decide if/when to undergo transplant

Clinical Experience Data from Research
Patient Preference
Adapted from Philippe Moreau, ASH 2015



&
Steroid Synergy
Steroids are a backbone and work in combination to enhance myeloma therapy
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over-the-counter or prescription medications

• Medications to prevent shingles, thrush, or other infections
Steroid Side Effects
• Irritability, mood swings, depression
• Difficulty sleeping (insomnia), fatigue

• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Increase in blood pressure, water retention
• Blurred vision, cataracts
• Flushing/sweating
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes

• Increase in blood sugar levels, diabetes
Rajkumar
SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR, Eastern Cooperative Oncology Group (2010) Lenalidomide plus highdose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 11(1):29–37.
T, Faiman B. Steroid-Associated Side Effects: A Symptom Management


Both viral and bacterial
– Up to a 3rd of patients on clinical trials has serious infections (requiring IV antibodies or hospitalization)
Increased risk of serious COVID complications despite history of vaccination
– Antibody levels

–
Tixagevimab co-packaged with cilgavimab (EVUSHELD)
–
Immediate treatment once diagnosed Nirmatrelvir with Ritonavi (Paxlovid)
• Start as soon as possible; must begin within 5 days of when symptoms start

7-10 fold increased risk of bacterial and viral infections for people with myeloma
care team
Good personal hygiene (skin, oral)
Environmental control (wash hands, avoid crowds and sick people, etc)
Growth factor (Neupogen [filgrastim])
Immunizations (NO live vaccines)
• Medications (antibacterial, antiviral)
Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
Manage stress
• Rest, relaxation, sleep hygiene
• Mental health / social engagement

• Complementary therapy
Maintain a healthy weight
• Nutrition
• Activity / exercise
Preventative health care
• Health screenings, vaccinations
• Prevent falls, injury, infection
• Stop smoking
• Dental care
Maintain renal health
• Myeloma management
• Hydration
• Avoid renally-toxic medications
– Dose adjust to renal function
• Diabetes management
Protect your bones
• Nutrition, Calcium + D supplement
• Weight-bearing activity / walking
• Bone strengthening agents
Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.
“An ounce of prevention is worth a pound of cure.” Benjamin Franklin
Care partner support is essential for the entire transplant and CAR T processes
• Sedated procedures; Education sessions
• Assistance with daily activities, managing medications and alerting the medical team of changes
• Continued support and assistance is often needed in the early days after returning home. Less assistance will be needed as time goes on.


Care partner can be one person or a rotation of many people.


Diarrhea may be caused by medications and supplements
• Laxatives, antacids with magnesium
• Antibiotics, antidepressants, others
• Milk thistle, aloe, cayenne, saw palmetto, ginseng
• Sugar substitutes in sugar free gum
Avoid caffeinated, carbonated, or heavily sugared beverages
Take anti-diarrheal medication
• Imodium®, Lomotil®, or Colestid if recommended
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
• Welchol® if recommended
Constipation may be caused by
• Opioid pain relievers, antidepressants, heart or blood pressure medications, others
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency
Increase fiber
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®

Fluid intake can help with both diarrhea and constipation, and good for kidneys. Discuss GI issues with health care providers to identify causes and make adjustments to medications and supplements.

Pain can significantly compromise quality of life

Sources of pain include bone disease, neuropathy and medical procedures
Management
• Prevent pain when possible
• Bone strengtheners to decrease fracture risk; anti viral to prevent shingles; sedation before procedures
• Interventions depends on source of pain
• Monitor serum calcium levels
• Imaging may be needed depending on type and location of pain (eg, MRI, PET-CT)
• May include medications (eg bone modifying agents), activity, surgical intervention, radiation therapy, etc

• Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)
Tell your health care provider about any new bone pain or chronic pain that is not adequately controlled
Peripheral neuropathy: damage to nerves in extremities (hands, feet, or limbs)
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness
Prevention / management:
• Bortezomib once-weekly or subcutaneous administration
• Massage area with cocoa butter regularly
• Supplements:
• B-complex vitamins (B1, B6, B12)
• Folic acid, and/or amino acids but do not take on day of Velcade® (bortezomib) infusion
• Safe environment: rugs, furnishings, shoes


If PN worsens, your HCP may:
• Change your treatment
• Prescribe oral or topical pain medication
• Suggest physical therapy
Report symptoms of peripheral neuropathy early to your health care provider; nerve damage from PN can be permanent if unaddressed
All can affect quality of life and relationships
• Fatigue is the most common reported symptom (98.8%)
Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression


• Anxiety reported in >35%


• Depression nearly 25% Financial concerns, disease progression, end-of-life, and change in social and sexual function were highlighted sources
Often, people do not share these symptoms with their provider. Talk to your provider about symptoms that are not well controlled or thoughts of self harm. Help is available.
Financial burden comes from
• Medical costs
• Premiums
• Co-payments

• Travel expenses
• Medical supplies

• Prescription costs
• Loss of income
• Time off work or loss of employment
• Caregiver time off work
Contact the Social Services department at your hospital or clinic to talk to a social worker for assistance.
Funding and assistance may be available
• Federal programs
• Pharmaceutical support
• Non-profit organizations
• Websites:
• Medicare.gov
• SSA.gov
• LLS.org
• Rxassist.org
• NeedyMeds.com
• HealthWellFoundation.org
• Company-specific website


You are central to the care team








Be empowered
• Ask questions, learn more
• Participate in decisions
Communicate with your team
• Understand the roles of each team member and who to contact for your needs

• Participate in support network







Come prepared:
• Bring a list of current medications, prescribed and over the counter
• Write down your questions and concerns. Prioritize them including financial issues

• Have there been any medical or life changes since your last visit?
• Current symptoms - how have they changed (improved, worsened, stable)? Keep a symptom diary. Bring it along
• Communicate effectively: your health care team can’t help if they don’t know
• Know the “next steps”, future appointments, medication changes, refills, etc
Check with your healthcare team –Is telemedicine an option?
Similar planning for “in-person” appointment PLUS:
• What is the process and what technology is needed?

• Plan your labs: are they needed in advance? Do you need an order?
• Plan your location: quiet, well-lit location with strong wi-fi is best
• Plan yourself: consider if you may need to show a body part and wear accessible clothing
• Collect recent vital signs (blood pressure, temp, heart rate) self-serve blood pressure cuff is available at many pharmacies and for purchase
Be empowered to be part of the treatment decision-making

• Ask for time to consider options (if needed/appropriate)
• Understand options; consider priorities
• Use reliable sources of information
• Use caution considering stories of personal experiences
• Consider your goals/values/preferences
• Express your goals/values/preferences; create a dialog


• My top priority is [goal/value]; additional [preferences] are also important.
• I think [treatment] may be a good choice given my priorities… What do you think?
• Arrive at a treatment decision together

























Thank you for joining us today for the February 15, 2023, International Myeloma Foundation’s Regional Community Workshop –Midwest states.


As follow up to today's workshop, we will have the speaker slides and a video replay available.

These will be provided to you shortly after the workshop concludes.

March 4, 2023* RETURN TO IN PERSON MEETINGS
IMF In-Person Regional Community Workshop (RCW) - San Diego
8:00AM Pacific Time
March 17, 2023
IMF Patient & Family Seminar 2023 - Boca Raton
8:00AM Eastern Time
Registration for these events can be found at myeloma.org under the “EVENTS” tab:













At the close of the meeting a feedback survey will pop up.

This will also be emailed to you shortly after the workshop.

Please take a moment to complete this survey.
