

Thank you for joining us today!
March 4, 2023, International Myeloma Foundation’s Regional Community Workshop
San Diego




Thank you for joining us today!
March 4, 2023, International Myeloma Foundation’s Regional Community Workshop
San Diego











March 4, 2023, Agenda

9:00 – 9:15 AM Welcome and Announcements
Kelly Cox, Director of Support Groups, Senior Director Regional Community Workshops

9:15 – 10:15 AM Myeloma 101 & Frontline Therapy

Caitlin Costello, MD, University of California San Diego
10:15 – 10:30 AM Q&A with Panel
10:30 – 11:15 AM Maintenance Therapy
David Vesole, MD, PhD, FACP - MedStar Georgetown University Hospital
11:15 – 11:30 AM Q&A with Panel
11:30 – 12:15 PM Relapsed Therapy
David Vesole, MD, PhD, FACP - MedStar Georgetown University Hospital
12:15 – 12:30 AM Q&A with Panel 12:30 – 1:15 PM LUNCH BREAK
March 4, 2023, Agenda
1:15 – 2:00 PM Clinical Trials

David Vesole, MD, PhD, Medstar Georgetown University Hospital
2:00 –
2:15 PM Q&A
2:15 –

3:00 PM Life is a Canvas, You are the Artist
Sandra Rome, RN, MN, AOCN, CNS, Cedars-Sinai Medical Center, IMF Nurse Leadership Board
3:00 –
3:15 PM Q&A
3:15 –
3:30 PM Local Patient & Care Partner Panel
Tom Tucker, Patient Advocate and Helen Tucker, Care Partner
3:30 – 3:40 PM Q&A
3:40 – 3:55 PM Community Resource

3:55 – 4:00 PM Closing Remarks
4:00 PM Coffee / Network




. The IMF provides FREE resources to help both patients and families.
Established in 1990, the IMF’s InfoLine assists over 4600 callers annually and answers questions across a wide variety of topics including:
Frequent topics:

ü Newly Diagnosed MM/Myeloma 101
ü Treatment Options
ü Transplant
ü Maintenance
ü Side Effects
ü Relapsed/Refractory MM
ü Clinical Trials
ü Resources for Drug Access/Financial Support/Local Support
ü Referrals to Myeloma Specialists Within/Outside the US




ü Health Issues Related to Myeloma
ü Caregiver Support
Paul Hewitt, Missy Klepetar, and Deb











edge education to the myeloma community.
















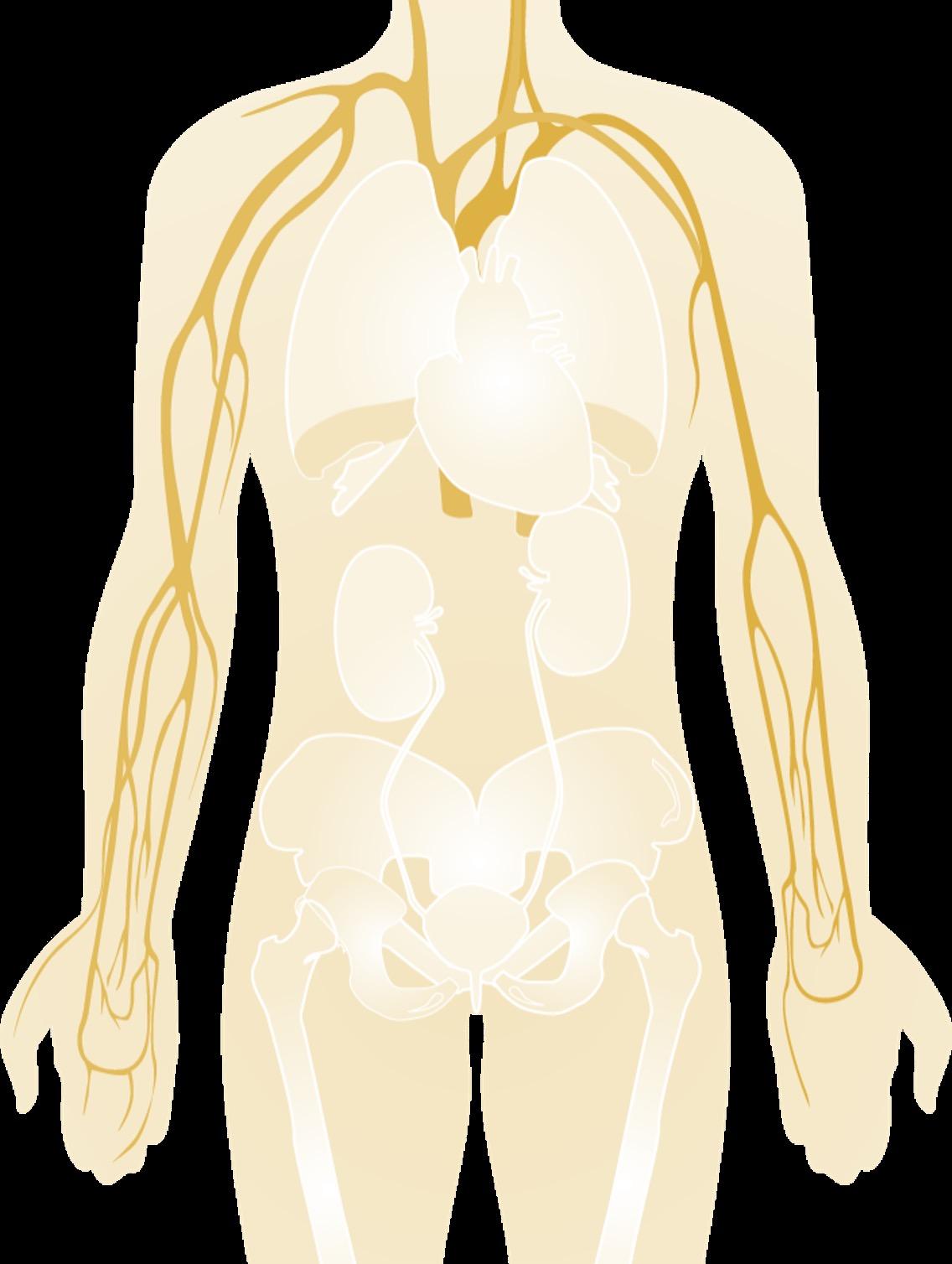
• The blood is an “organ” made up of both cells and liquid “plasma”

Think of wine (red/white/rose)
1. Red Cells – carry Oxygen…trucks
2. White Cells – immune system…army
3. Platelets – help with clotting…ambulance
All produced in the blood factory = Bone Marrow
Multiple Myeloma* is a blood cancer that starts in plasma cells from the center of bones (bone marrow).

This is where stem cells mature into red blood cells, white blood cells, and platelets
Myeloma cells are abnormal plasma cells that make an abnormal antibody called “M protein”
M = monoclonal (“identical” or cancerous)
* Myeloma is NOT a bone cancer or skin cancer (melanoma), it is a type of blood cancer.












Myeloma Cell JAK – STAT
MAPK
NFkB AKT SHP2 MEK MEK/MAPK
Angiogenesis Migration Growth

T Cells NFkB – IkB complex Protein kinases LFA1 FADD cFLIP Cell organelles Fibronectin cFLIP/FADD NFkB BindingSite IL-6 TNFα VLA4 VCAM1 NFkB NFkB – IkB complex Myeloma
NFkB








BM Stromal Cells









Increase in cytokine production and adhesion molecules










Dendritic Cells

Natural-Killer Cells TNFα

Inhibition of Anti-Myeloma Immunity
Monocytes








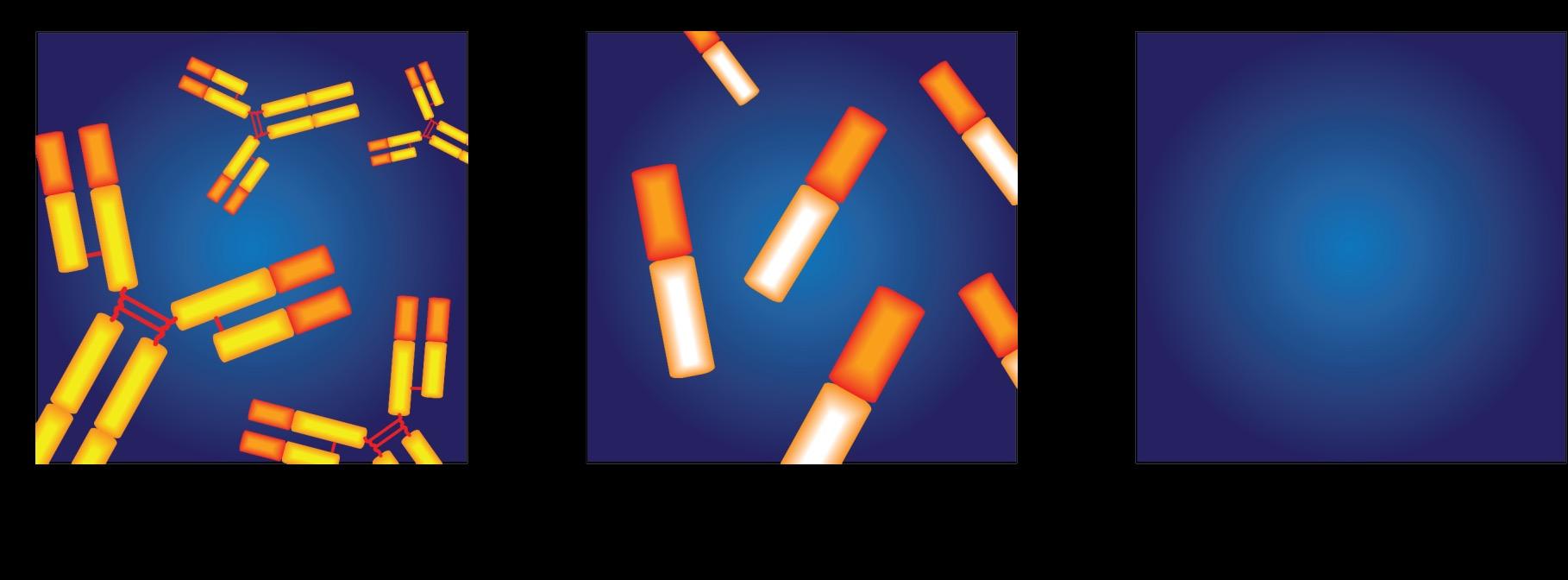
• Cancer of plasma cells
• Healthy plasma cells produce immunoglobulins G, A, M, D, and E
• Myeloma cells produce abnormal immunoglobulin
“paraprotein” or monoclonal protein (=M protein)

Bone marrow of patient with multiple myeloma

Image courtesy of American Society of Hematology
Kyle et al. Mayo Clin Proc. 2003;78:21-33;
1.8% of all cancers;



17% of hematologic malignancies in the United States
Most frequently diagnosed in ages 65 to 74 years (median, 69 years)
The average age of diagnosis of 4-5 years younger in African American and Hispanic patients
Approx 35,000 Estimated New Cases in 2022
Approx 13,000 Estimated Deaths in 2021
The Average Survival of patients with myeloma is IMPROVING!


MM Incidence Ø Higher incidence in AA vs White patients:
• 15.9 vs 7.5 cases per 100,000 per year
MM Mortality Ø Higher mortality in AA vs White patients:
• 5.6 vs 2.4 MM deaths per 100,000
Ø 5-year relative survival evolution from 1973 to 2005
The expected survival is nearly 10 years for all patients, but still less than 5 years in patients with high risk disease
MM Survival
• Survival for White patients increased significantly from 26.3% to 35%
• Survival for AA patients increased from 31% to 34.1%
• IgG+kappa
• IgG+lambda



• IgA+kappa
• IgA+lambda
• etc…
• 80% of myeloma cases
Jones protein
• 18% of all myeloma cases
• Renal failure more common in light chain multiple myeloma; creatinine >2 mg/dL in 1/3 of cases
protein present
• Less than 3% of cases of multiple myeloma
• Subtypes of MM are determined based on the kind of abnormal protein

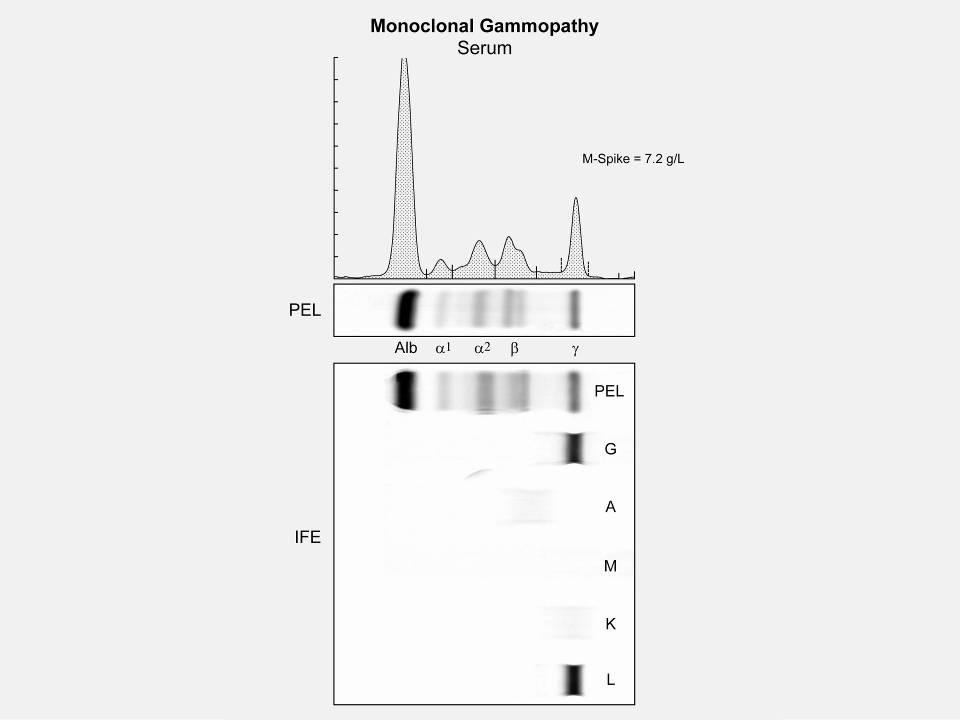 M spike in gamma region
M spike in gamma region
Premalignant Malignant
* In clinical trial (preferred) or offer treatment for those likely to progress within 2 years
1. Kyle RA, et al. N Engl J Med. 2007;356:2582-90.
2. International Myeloma Working Group. Br J Haematol. 2003;121:749-57.
3. Jagannath S, et al. Clin Lymphoma Myeloma Leuk. 2010;10(1):2843
4. Kyle RA, et al. Curr Hematol Malig Rep 2010;5(2):62-69.
5. Mateos M-V, et al. Blood. 2009;114:Abstract 614.
6. Durie BG, Salmon SE. Cancer. 1975;36:842-854.
7. Durie BG, et al. Leukemia. 2006;20(9):1467-1473.
8. Rajkumar SV, et al. Lancet Oncology 2014; 15:e538-e548.

Fatigue



Bone Pain
Kyle RA. Mayo Clin Proc. 2003;78:21-33.
Anemia
Clonal bone marrow ≥10% or bony/extramedullary plasmacytoma
AND any one or more Myeloma-Defining Events
C
R A B
alcium elevation
enal complications
nemia one disease
Clonal bone marrow ≥60%
BM, bone marrow; FLC, free light chain; MRI, magnetic resonance imaging; sFLC, serum free light chain. Rajkumar et al. Lancet Oncol 2014;15:e538-e548. Kyle et al. Leukemia 2010;24:1121-1127.
Not CRAB but now SLiM CRAB

• S (60% Plasmacytosis)
• Li (Light chains I/U >100)
• M (MRI 1 or more focal lesion)
• C (calcium elevation)
• R (renal insufficiency)
• A (anemia)
• B (bone disease)
Rajkumar SV, et al. Lancet Oncol. 2014;15:e538-e548.
Low Blood Counts
• May lead to anemia and infection
• Anemia is present in 60% at diagnosis
Decreased Kidney Function
• Occurs in over half of myeloma patients
Bone Damage
• Affects 85% of patients
• Leads to fractures
Bone Turnover
• Leads to high levels of calcium in blood (hypercalcemia)


Weakness
Fatigue
Infection
Weakness
Bone pain
Loss of Appetite & Weight loss
About 10% to 20% of patients with newly diagnosed myeloma will not have any symptoms.
Mutant Cell Corrupted DNA
Conventional cytogenetic analysis (karyotyping)



DNA

FISH (fluorescence in situ hybridization)

Advances
• Genetic expression profiling [GEP]
• Whole-genome/ whole-exome sequencing
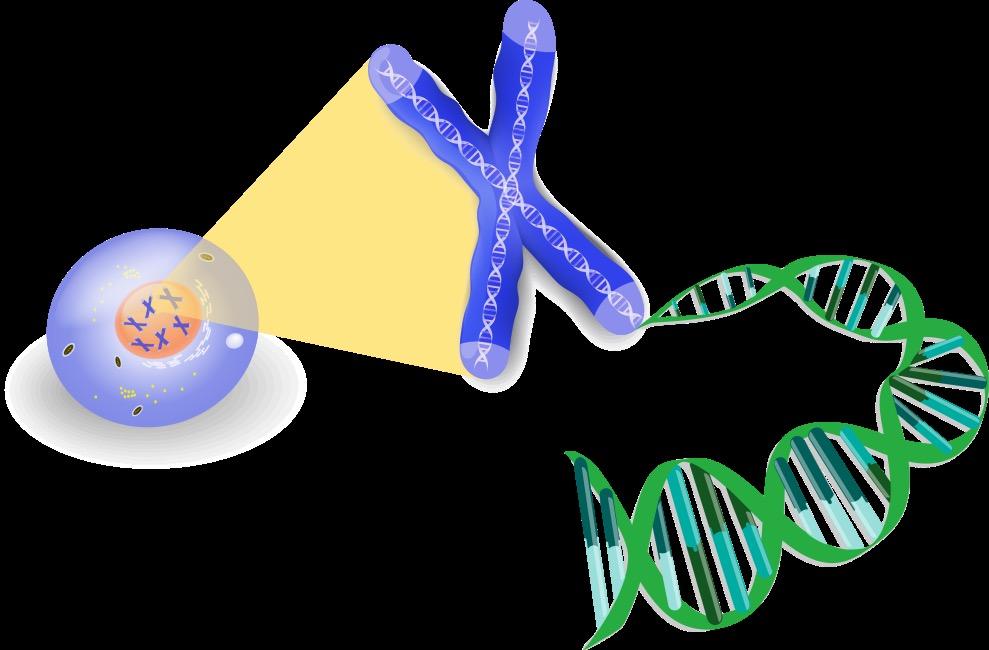
• Plasma cell next generation sequencing
Risk Category
Findings on Chromosome (FISH) Analysis Results in the Bone marrow
High Risk
FISH:
• Deletion 17th chromosome
• Gain of chromosome 1q
• Translocation 4 and 14
FISH:
Standard Risk
• Hyperdiploid: More than 1 pair of chromosomes (Trisomies)
• Translocation 11 and 14
• Translocation 6 and 14
• Translocation 14 and 16
• Translocation 14 and 20
NGS: p53 mutation (on chrom 17)

• Double Hit Myeloma: 2 high risk
genetic abnormalities
• Triple Hit Myeloma: 3 or more high risk genetic abnormalities
• Others
• Normal
Myeloma Stage:
Staging refers to the degree to which the cancer has progressed
Stage 1
β2-microglobulin under 3.6 mg/L
Stage 2
β2-microglobulin



























Between 3.5 & 5.4mg/L
Stage 3
β2-microglobulin over 5.5 mg/L
Normal
Lactate Dehydrogenase (LDH)
AND
NO High Risk
Cytogenetics (FISH)
NO High Risk
Cytogenetics (FISH)
HIGH Lactate Dehydrogenase (LDH)
AND/OR
High Risk Cytogenetics (FISH)
Deletion 17 chromosome
Translocation 4th and 14th
Translocation 14th and 16th
Translocation 14th and 20th
CBC Counts the number of red blood cells, white blood cells, and platelets

CoMP
Measures levels of albumin, calcium, and creatinine to assess kidney and liver functions, bone status ,and the extent of disease
Beta2 MicroG





Determines the level of a protein linked to MM and kidney function: USED FOR STAGE
LDH Lactate Dehydrogenase

















Determines the level of myeloma cell production and extent of MM : USED FOR STAGE
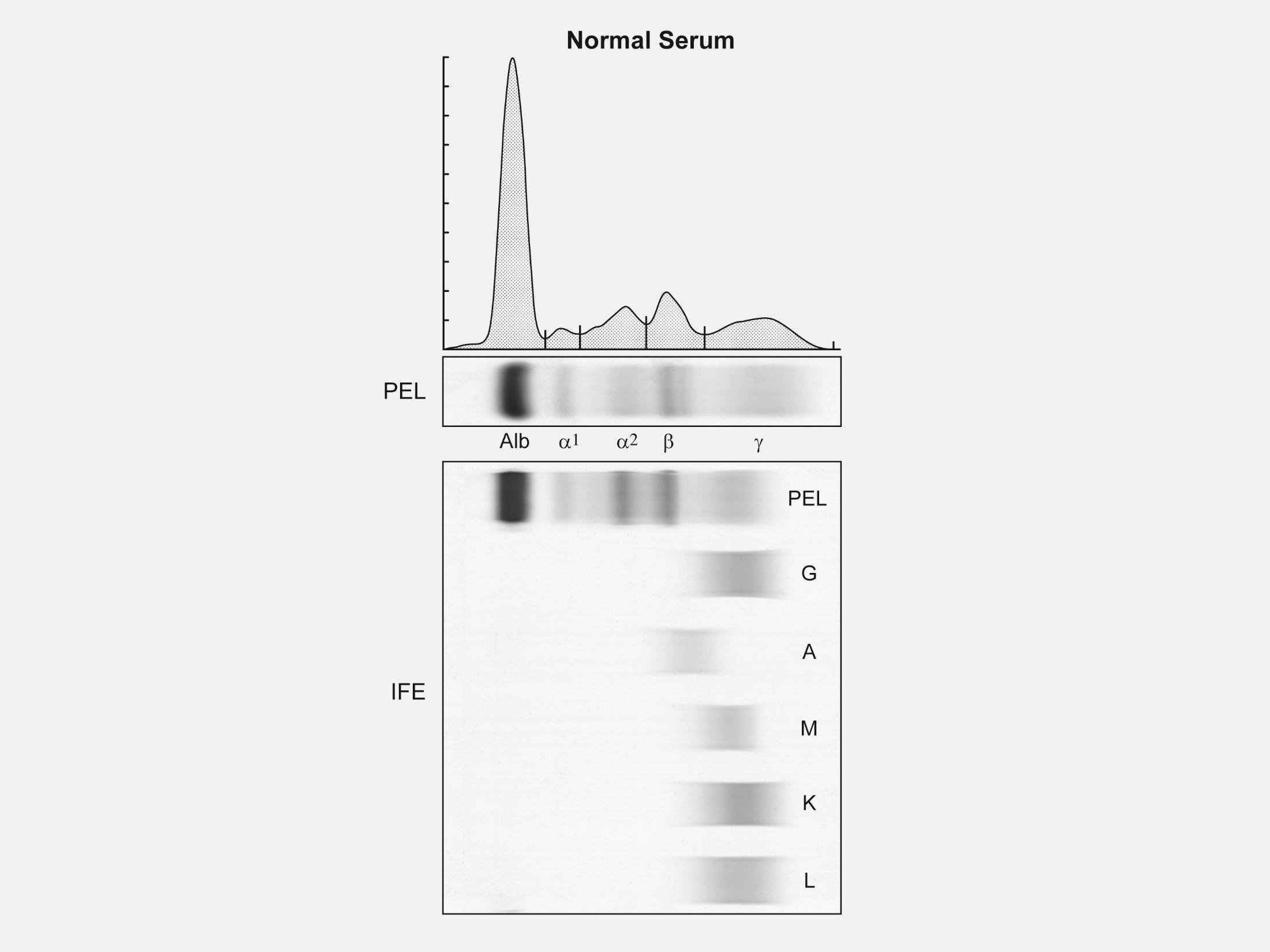
Serum Protein EP Detects the presence and level of M protein = how much myeloma
Immuno Fixation
Identifies the type of abnormal antibody proteins: IgG, IgA, IgM
Serum Free Light Chain Measures myeloma free light chains (kappa or lambda) in blood = how much myeloma
Urine Protein EP Detects Bence-Jones proteins (otherwise known as myeloma light chains) in urine (to determine if it’s present or not present)
24 hr Urine Analysis 24 hours of urine collected to test the presence and levels of Bence Jones protein in the urine = how much myeloma
Treatment Planning is the process of thinking about the treatment steps you can take with your doctor, based on your goals and preferences. Treatment decisions are based on:
The results of biomarker tests, cytogenetic (FISH) test, and the stage of multiple myeloma
Your values, goals, and preferences
Your age
Your health and symptoms (if you have kidney disease, heart disease, anemia, or other issues)
Your medical history and past treatments for multiple myeloma
Lifestyle Goals of Therapy
Age


Patient Preference
Myeloma Symptoms
You have the right to get a second opinion. Insurance providers may require second opinions.

A second opinion can help you:
Confirm your diagnosis
Give you more information about options
Talk to other experts
Introduce you to clinical trials
Help you learn which health care team you’d like to work with, and which facility
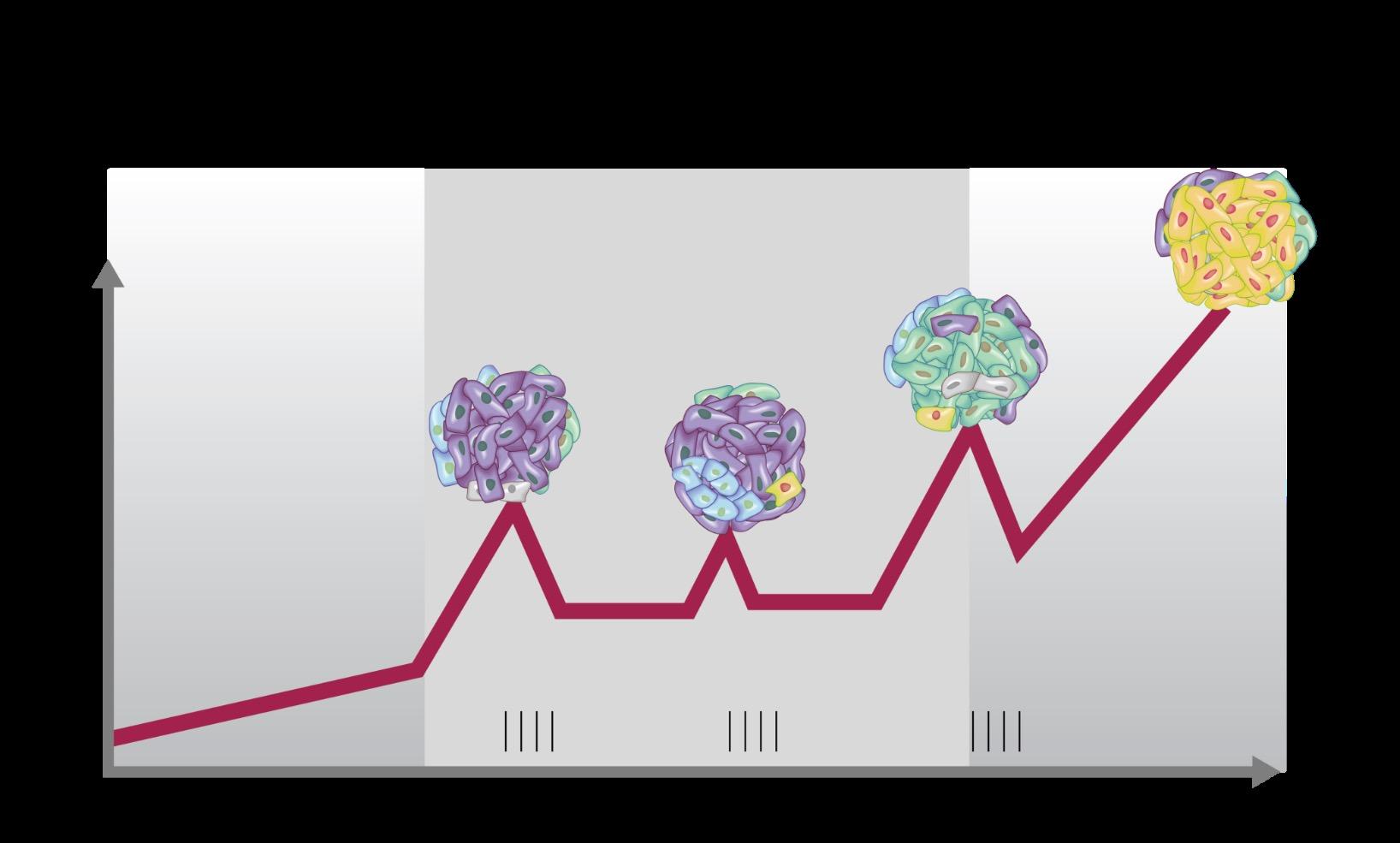
SYMPTOMATIC REFRACTORY RELAPSE
MGUS = monoclonal gammopathy of undetermined significance

Adapted from Dr. Brian Durie and Keats JJ, et al. Blood. 2012;120:1067-1076.
Clone 1.1
Clone 1.2
Clone 2.1 Clone 2.2
Steroids
Revlimid (lenalidomide)
Monoclonal Antibodies
Cytoxan (cyclophosphamide)

or Evomela (melphalan)
Decadron (dexamethasone)
Darzalex (daratumumab)
Sarclisa (isatuximab)
Empliciti (elotuzumab)
XPO1 Inhibitors Xpovio (selinexor)
Class
Drug Name
Abbreviation Administration
Peptide Drug Conjugate* Pepaxto (Melphalan Flufenamide) Melflufen Intravenous
BCMA Targeted Antibody Drug Conjugate (ADC)*
Blenrep (Belantamab mafodotin) B Intravenous
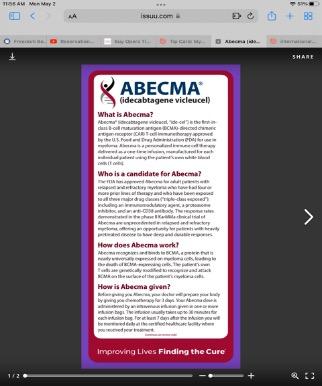
Abecma (idecabtagene vicleucel)
CAR T Cell therapy
Bispecific Antibodies
Carvycti (ciltacabtagene vicleucelel) Cilta-cel

Ide-cel Intravenous (IV) or subcutaneous injectionSC (under the skin)
Cytoxan (cyclophosphamide) C Oral or intravenous
??? MORE TO COME!!
* these agents are currently off the market but available through special programs
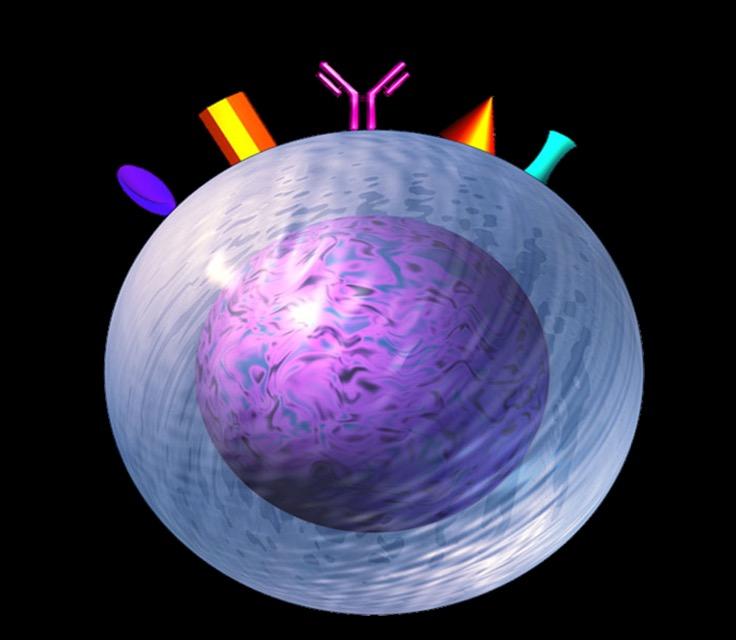
Antibody-dependent Cellular cytotoxicity (ADCC)
Effector cells:




Complement-dependent Cytotoxicity (CDC)
• Daratumumab
• SAR650984/Isatuximab (CD38)
• Lucatumumab or Dacetuzumab (CD40)
• Elotuzumab (CS1; SLAMF7)
• Daratumumab, SAR650984/Isatuximab (CD38)
• XmAbâ5592 (HM1.24)
• huN901-DM1 (CD56)
• nBT062-maytansinoid (CD138)
• Siltuximab (1339) (IL-6)
• BHQ880 (DKK1)
• RAP-011 (activin A)
• Daratumumab, SAR650984/Isatuximab (CD38)
Adapted from Tai & Anderson Bone Marrow Research 2011

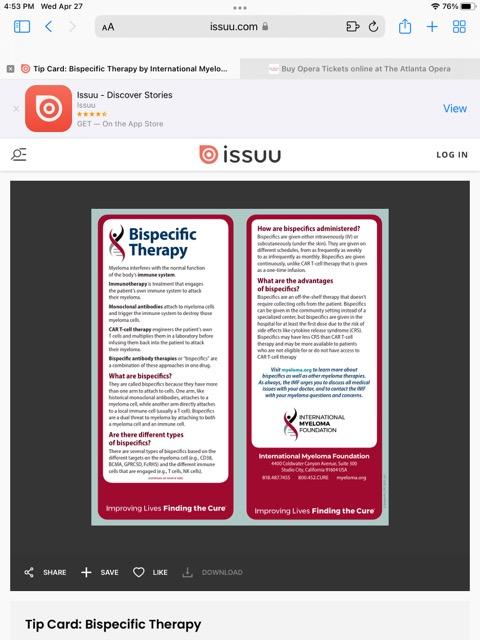
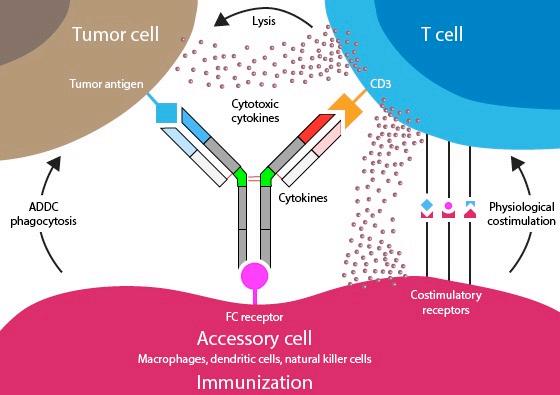
• Incorporates 2 antibody fragments to target and bind both tumor cells and T cells
• Brings target-expressing MM cells and T cells into close proximity, enabling T cells to induce tumor-cell death
Bispecific Molecule Targets Vary
“Off the Shelf” Advantage
• No manufacturing process, unlike CAR T-cell therapy (but like ADC/belantamab therapy)
• Thus, no delay between decision to treat and administration of drug
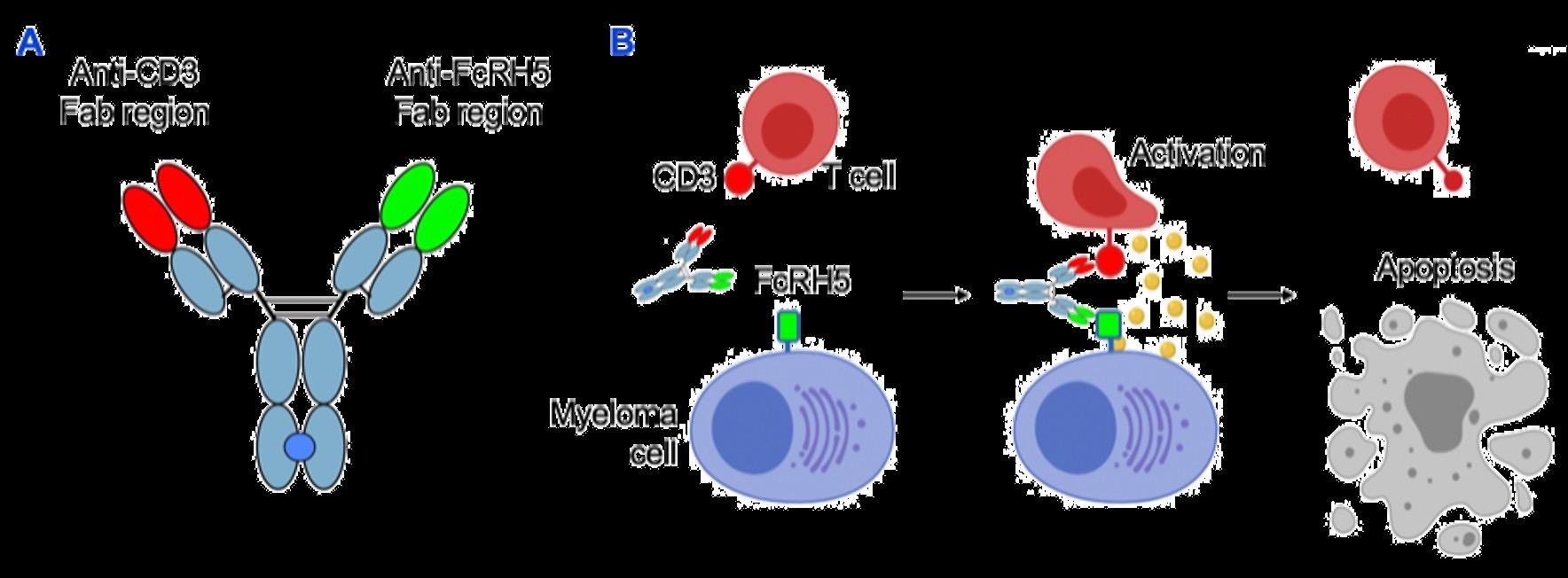
ADC = Antibody-Drug Conjugate; BCMA = B-Cell Maturation Antigen; CD3 = Cluster of Differentiation 3; FcRH5 = Fc receptor-homolog 5;

GPRC5D = G-protein coupled receptor family C group 5 member D
Image Source: Shah N, et al. Leukemia. 2020;34:985–1005. Creative Commons License: CC BY 4.0.
Barilà G, et al. Pharmaceuticals (Basel). 2021;14(1):40
Bi-Specific Antibodies
Talquetamab
CAR-T
Bi-Specific Antibodies
CAR-T
Antibody Drug
Elotuzumab
Bi-Specific Antibodies
Antibody Drug
Daratumumab and Darzalex Faspro
Isatuximab





























TAK-079
MOR202
Immune Therapies
Ide cel CAR T

Cilta cel CAR T
Teclistamab
Other Bi-Specific Antibodies
Other CAR-Ts
Induction
• Velcade/Revlimid/Dex:(VRD)
• Velcade/Thalomid/Dex:(VTD)
• Velcade/Cytoxan/Dex:(CyBorD)

• Darzalex/Revlimid/Dex:(DRD)
• Darzalex/Velcade/Melphalan/Dex
• Darzalex/Velcade/Thalidomide/Dex
• Kyprolis/Revimid/Dex(KRD)
• Darzalex/Velcade/Revlimid/Dex: Dara-RVD
• Ninlaro/Revimid/Dex(IRD)
• Clinical trials
Consolidation
Maintenance
• Stem Cell Transplant
• Continue Induction
• Clinical trial
Rescue
• Revlimid
• Velcade
• Ninlaro
• Observation
• Thalidomide
• Revlimid/Dara
• Clinical trial
Dara+Pomalyst+Dex
Kyprolis+Pomalyst+Dex
Cytoxan+Pomalyst+Dex
Ninlaro+Pomalyst+Dex
Elo+Pomalyst+Dex
Elo+Thaliomide+Dex
Dara+Kyprolis+Dex
Kyprolis+Revlimid+Dex
Elo+Revlimid+Dex
Dara+Revlimid+Dex
Dara+Velcade+Dex
Elo+Velcade+Dex
Cytoxan+Kyprolis+Thalidomide+dex
4 drug therapies of novel agents
Ninlaro+Cytoxan+Dex
Velcade+ Cytoxan+Dex
Velcade+ Pomalyst+Dex
Chemotherapy
Selinexor+Dex
Selinexor+Velcade
Selinexor+ Dara
Isatuximab(Sarclisa)+






Pomalyst+Dex
Darzalex Faspro (under skin)
Ide-cel CAR-T (FDA approved 3/26/2021)
Cilta-cel CAR-T (FDA approved 2/28/2022)
Teclistamab (FDA approved 10/25/2022)

Panobinostat
Idecabtagene
Elotuzumab Isatuximab
Ciltacabtagene

1. There is a longer time from symptoms to diagnosis among African Americans
2. African Americans are younger by about 5 years on average at diagnosis
3. MM and MGUS are more than 2x as common in African Americans
4. African Americans are less likely to receive the four T’s: Transplant, Triplets, Trials and CAR T
5. African Americans have biologic differences with more t(11;14) and less high-risk cytogenetics with deletion 17p
6. Survival outcomes in African Americans are HALF of what is seen in White Americans
7. African Americans can achieve equal or better outcomes when they receive therapy
The IMF has created the M-Power program:
The core vision of this initiative is to improve the short- and long-term outcomes of African American patients with myeloma.
We want to empower patients and communities to CHANGE the current course of myeloma…
Increase Awareness
Engage the community
Shorten the time to diagnosis
Educate providers about culturally sensitive care



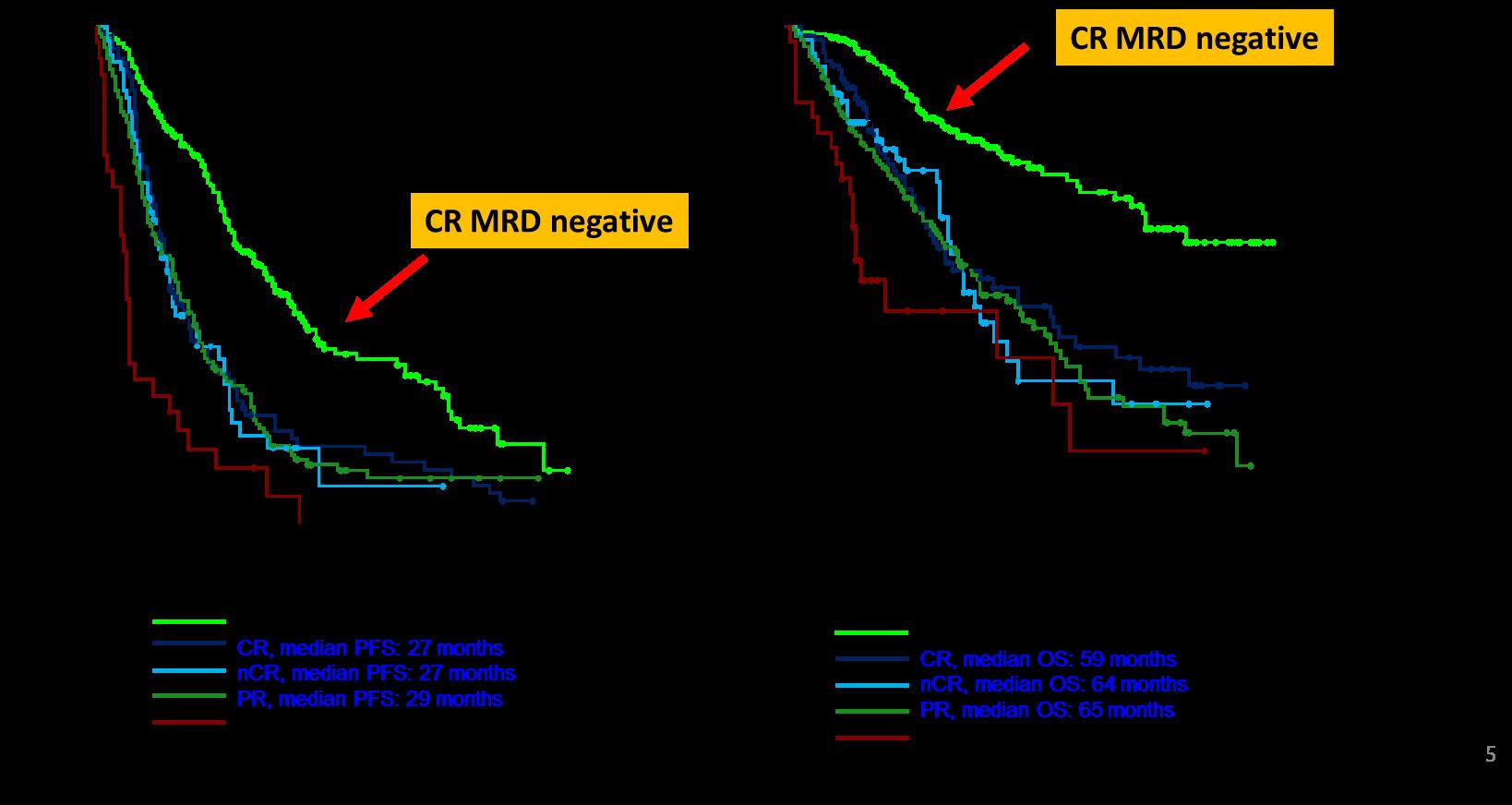
l Review the importance of DEPTH of response in early treatment of myeloma and the increasing use of MRD testing
l Discuss emerging approaches in transplant eligible patients, including quadruplet therapy and stem cell transplantation
l Outline the approach to a patient not going to transplant and how to optimize continuous therapy


Partial response


50% reduction in M protein
Very good partial response
90% reduction in M protein
immunofixation positive only


Complete remission
No M-protein
immunofixation negative




Minimal Residual Dis
Minimal Residual Dis
Next Generation Molecular testing

MRD refers to the persistence of residual tumor cells after treatment and is responsible for relapse1

Current techniques can detect MRD with a sensitivity of 10-6 for MM cells2
MINIMAL RESIDUAL DISEASE DETECTION
MR , minimal response; neg, negative; pos, positive; R, relapse
1. Adapted from Hauwel M, Matthes T. Swiss Med Wkly 2014:144:w13907
2. Biran N, et al. Curr Hematol Malig Rep 2014;9:368–78


Newly Diagnosed MM and Risk Stratified
Factors to be considered for ASCT
Age, performance status (PS), comorbidities (R-MCI score, HCT-Cl) and organ function
ASCT Eligible
ASCT Ineligible
1. Most patients will be given a combination of drugs to control the disease quickly
2. We don’t “save the best for last” because early therapies have a long term effect on survival
3. We seek a DEEP and DURABLE response
4. We mix and match from the 3 major classes of drugs and add steroids: Proteasome Inhibitors – most often botezomib (Velcade)
Immunomodulatory Drugs – lenalidomide (Revlimid)
Monoclonal Antibodies – daratumumab (Darzalex)
5. We decide early on whether or not someone will have a stem cell transplant
Induction
• Velcade/Revlimid/Dex:(VRD)
• Velcade/Thalomid/Dex:(VTD)
• Velcade/Cytoxan/Dex:(CyBorD)

• Darzalex/Revlimid/Dex:(DRD)
• Darzalex/Velcade/Melphalan/Dex
• Darzalex/Velcade/Thalidomide/Dex
• Kyprolis/Revimid/Dex(KRD)
• Darzalex/Velcade/Revlimid/Dex: Dara-RVD
• Ninlaro/Revimid/Dex(IRD)
• Clinical trials
Consolidation
Maintenance
• Stem Cell Transplant
• Continue Induction
• Clinical trial
Rescue
• Revlimid
• Velcade
• Ninlaro
• Observation
• Thalidomide
• Revlimid/Dara
• Clinical trial
Dara+Pomalyst+Dex
Kyprolis+Pomalyst+Dex
Cytoxan+Pomalyst+Dex
Ninlaro+Pomalyst+Dex
Elo+Pomalyst+Dex
Elo+Thaliomide+Dex
Dara+Kyprolis+Dex
Kyprolis+Revlimid+Dex
Elo+Revlimid+Dex
Dara+Revlimid+Dex
Dara+Velcade+Dex
Elo+Velcade+Dex
Cytoxan+Kyprolis+Thalidomide+dex
4 drug therapies of novel agents
Ninlaro+Cytoxan+Dex
Velcade+ Cytoxan+Dex
Velcade+ Pomalyst+Dex
Chemotherapy
Selinexor+Dex
Selinexor+Velcade
Selinexor+ Dara
Isatuximab(Sarclisa)+






Pomalyst+Dex
Darzalex Faspro (under skin)
Ide-cel CAR-T (FDA approved 3/26/2021)
Cilta-cel CAR-T (FDA approved 2/28/2022)
Teclistamab (FDA approved 10/25/2022)

maintenance 13 cycles (10-15 mg/d)

Median follow up 89.8 months
Median PFS 47.3 months (Transplantation, arm B)

Median PFS 35 months (RVD alone, arm A)
HR (95CI) 0.70 [0.59;0.83]
30% reduction in the risk of progression or death in patients receiving transplant
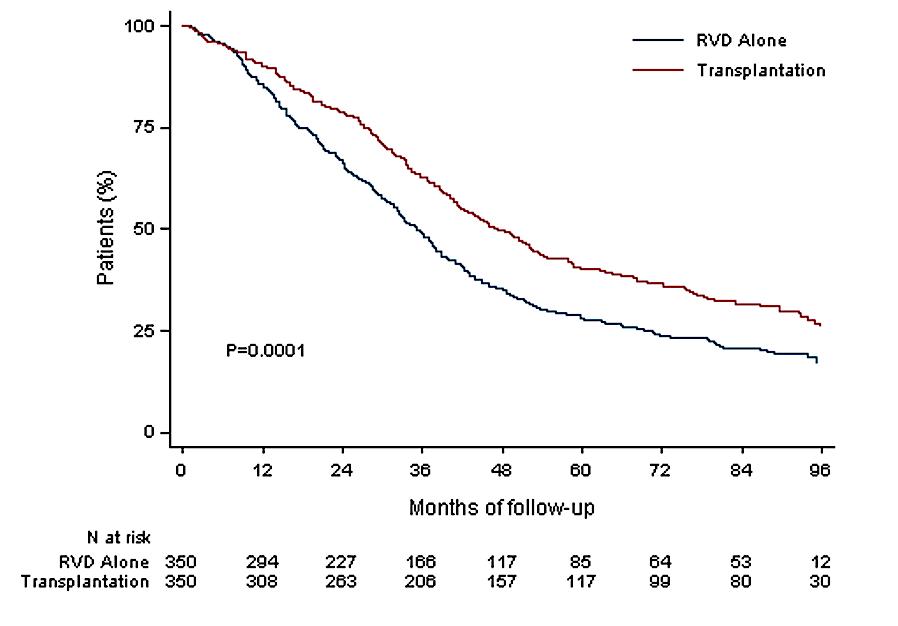
Median follow up 89.8 months
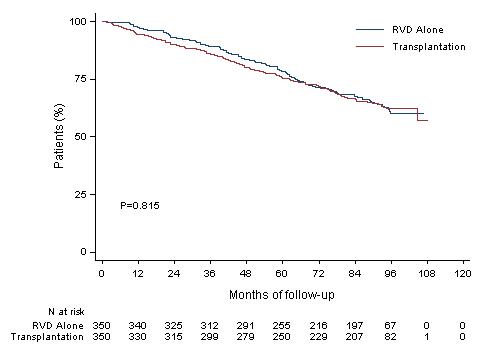
HR (95CI) 1.03 [0.8;1.32]
8y-OS 62.2% (Transplantation, arm B)

8y-OS 60.2% (RVD alone, arm A)
More than 60% of the patients in the two arms are alive after 8 years of follow -up
-Patients aged 18-65 yrs with symptomatic newly diagnosed MM following 1 cycle of RVD -56 sites within the United States from 2010 to 2018
End Points of Study and Follow-up
• Primary end point: progression-free survival (time to next relapse)
• Secondary end points included:
• Response rates, overall survival, quality of life, and adverse events
• Follow-up on participant status : median of 6 years

Data cut off:12/12/21
*p-value adjusted using Bonferroni’s correction to control overall family-wise error rate for secondary outcomes

• ASCT remains very relevant and important in prolonging PFS in younger and eligible patients • BUT it may not be mandatory in all eligible patients upfront • As with other agents, we INDIVIDUALIZE the sequencing patterns
• ASCT does carry genuine toxicity, short term and long term
• We may become callous to these toxicities
• Maintenance therapy remains an important part of myeloma therapy
Daratumumab (DARA) + Lenalidomide, Bortezomib, and Dexamethasone (RVd) in Patients With Transplant-eligible Newly Diagnosed Multiple Myeloma (NDMM): Final Analysis
Douglas W. Sborov,1 Jacob Laubach,2 Jonathan L. Kaufman,3 Brandi Reeves,4 Cesar Rodriguez,5
Ajai Chari,5 Rebecca Silbermann,6 Luciano J. Costa,7 Larry D. Anderson Jr.,8 Nitya Nathwani,9
Nina Shah,10 Naresh Bumma,11 Sarah A. Holstein,12 Caitlin Costello,13 Andrzej Jakubowiak,14
Robert Z. Orlowski,15 Kenneth H. Shain,16 Andrew J. Cowan,17 Huiling Pei,18 Annelore Cortoos,19
Sharmila Patel,19 Thomas S. Lin,19 Paul Richardson,2 Saad Z. Usmani,20 Peter M. Voorhees21
1Huntsman Cancer Institute, University of Utah School of Medicine, Salt Lake City, UT, USA; 2Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA; 3Winship Cancer Institute, Emory University, Atlanta, GA, USA; 4University of North Carolina – Chapel Hill, Chapel Hill, NC, USA; 5Icahn School of Medicine at Mount Sinai, New York, NY, USA; 6Knight Cancer Institute, Oregon Health & Science University, Portland, OR, USA; 7University of Alabama at Birmingham, Birmingham, AL, USA; 8Department of Internal Medicine, Division of Hematology/Oncology, UT Southwestern Medical Center, Dallas, TX, USA; 9Judy and Bernard Briskin Center for Multiple Myeloma Research, City of Hope Comprehensive Cancer Center, Duarte, CA, USA; 10Department of Medicine, University of California San Francisco, San Francisco, CA, USA; 11Division of Hematology, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA; 12University of Nebraska Medical Center, Division of Oncology and Hematology Department of Internal Medicine, Omaha, NE, USA; 13Moores Cancer Center, University of California San Diego, La Jolla, CA, USA; 14University of Chicago Medical Center, Chicago, IL, USA; 15Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 16Department of Malignant Hematology, H. Lee Moffitt Cancer Center, Tampa, FL, USA; 17Division of Medical Oncology, University of Washington, Seattle, WA, USA; 18Janssen Research & Development, LLC, Titusville, NJ, USA; 19Janssen Scientific Affairs, LLC, Horsham, PA, USA; 20Memorial Sloan Kettering Cancer Center, New York, NY, USA; 21Levine Cancer Institute, Atrium Health, Charlotte, NC, USA
Scan the QR code.

https://www.congresshub.com/Oncology/IMS20
22/Daratumumab/Sborov
The QR code is intended to provide scientific information for individual reference, and the information should not be altered or reproduced in any way.
sCR, P = 0.0079a

≥CR, P = 0.0005a
• Rates of ≥CR improved over time and the deepest responses occurred at the end of study maintenance
• At all timepoints, response rates for D-RVd were consistently higher versus RVd
PR, partial response; SD/PD/NE, stable disease/progressive disease/not evaluable. a P value was calculated using the Cochran–Mantel–Haenszel chi-squre test. b Response rates are from the primary analysis cutoff (median follow-up: 13.5 months), and the response-evaluable population included 196 patients (D-RVd, n = 99; RVd, n = 97). cResponse rates for the maintenance phase were evaluated at the time of final analysis (median follow-up: 49.6 months), and the response-evaluable population included 198 patients (D-RVd, n = 100; RVd, n = 98).
• Median follow-up: 49.6 months

• Median PFS was not reached in either group
• PFS was longer for D-RVd/DR versus RVd/R, with a clinically meaningful 55% reduction in the risk of disease progression or death
• The separation of the PFS curves occurred beyond 1 year of maintenance
a HR and 95% CI from a Cox proportional hazards model with treatment as the sole explanatory variable and stratified with ISS staging (I, II, III) and baseline creatinine clearance (CrCl [30-50 mL/min or >50 mL/min]) at randomization. A hazard ratio <1 indicates an advantage for D-RVd. P-value is based on the log-rank test stratified with ISS staging and baseline creatinine clearance at randomization.

Myeloma to evaluate PAd vs VCD induction prior to HDT followed by maintenance – final analysis on induction therapy

Mathias Haenel2, Igor W. Blau2, Dirk Hose1, Anna Jauch1, Baerbel Schurich1, Kai Neben2, Anja Seckinger1, Barbara Zeis2, Christian Gerecke2, Ingo G. H. Schmidt-Wolf2, Katja Weisel2, Christof Scheid2, Hans Salwender2 Germany, 2GMMG, Germany, 3Division of Biostatistics, German Cancer Research Center Heidelberg, Germany
Induction phase (3 x 6-week cycles)
In the PAd group 91.2% and in the VCD group 96.0% of the patients completed three planned induction cycles. Applied total bortezomib dose over all three cycles was comparable in both, PAd and VCD arms. Response rates were similar in both induction regimens (PAd vs. VCD) with 34.3% vs. 37.0% of patients achieving VGPR or better. Non-inferiority of VCD compared to PAd was shown (one-sided p=0.0013). Similar results were obtained in the PP analysis. CR rates were 4.4% and 8.4% (PAd vs. VCD) and 21.1% and 22.3% (PAd vs. VCD) for near complete response (nCR) or better.
Partial response (PR) or better was reached in 72.1% vs. 78.1% of the patients (PAd vs. VCD) (figure 3).
Maintenance phase (4-week cycles)
Isa (IV) 10 mg/kg Cycle 1 Cycle 2–3
equally distributed for ISS and Durie-Salmon disease stage, cytogenetic abnormalities deletion (17p), translocation t(4;14) and significant differences in patient age and distribution of WHO
characteristics
Bor (SC) 1.3 mg/m² Len (PO) 25 mg
The proportion of patients with any adverse event was comparable in PAd vs. VCD (61.3% vs. 64.0%, p=0.58), but more serious adverse events (SAEs) were observed during PAd induction (32.7% vs. 24.0%, p=0.04). VCD led to a significantly higher proportion of leukocytopenia and neutropenia CTCAE grade 3 and 4 (PAd 11.3% vs. VCD 35.2%, p=<0.001). The number of infections (≥ CTCAE grade 2) and infection-related SAE was similar (PAd 24.6% vs. VCD 22.4% for AE, p=0.60 and PAd 12.9% vs. VCD 10.8% for SAE, p=0.49). Compared to the infection rate (AE ≥ CTCAE grade 2) of 49% during PAD (dexamethasone 40 mg on days 1-4, 9-12, 17-20) in the HOVON65/GMMG-HD4trial, a reduction in MM5 during induction was observed. Preliminary data (412 patients) of numbers of collected CD34+ stem cells were comparable (PAd median 9.8x106 vs. VCD median 9.4x106 kg bodyweight, p=0.15). In the PAd arm more deaths were observed compared to the VCD arm (5 vs 1).
Dex (PO) 20 mg
Isa (IV) 10 mg/kg: Cycle 1 Cycle 2–3 Cycle 4+ Len (PO) 10 mg increased to 15 mg after 3 months

Dex (PO) 20 mg: first cycle
Days 1–28
ASCT, autologous stem cell transplant; D, day; d/Dex, dexamethasone; HDT, high-dose therapy; Isa, isatuximab; IV, intravenous; NDMM, newly diagnosed multiple myeloma; PD, progressive disease; PO, oral; R/Len, lenalidomide; SC, subcutaneous; Te, transplant eligible; V/Bor, bortezomib; RVd is off label use in some countries according to the lenalidomide summary of product characteristics. 1. ClinicalTrials.gov: NCT03617731

Myeloma to evaluate PAd vs VCD induction prior to HDT followed by maintenance – final analysis on induction therapy

Mathias Haenel2, Igor W. Blau2, Dirk Hose1, Anna Jauch1, Baerbel Schurich1, Kai Neben2, Anja Seckinger1, Barbara Zeis2, Christian Gerecke2, Ingo G. H. Schmidt-Wolf2, Katja Weisel2, Christof Scheid2, Hans Salwender2 Germany, 2GMMG, Germany, 3Division of Biostatistics, German Cancer Research Center Heidelberg, Germany
Randomized (n = 504)
Patients with MRD negativity at the end of induction therapy
OR 1.83 (95% CI 1.34–2.51)
Patients not receiving allocated intervention due to:
- non-compliance (n = 1)

withdrawal of consent (n = 2)
In the PAd group 91.2% and in the VCD group 96.0% of the patients completed three planned induction cycles. Applied total bortezomib dose over all three cycles was comparable in both, PAd and VCD arms. Response rates were similar in both induction regimens (PAd vs. VCD) with 34.3% vs. 37.0% of patients achieving VGPR or better. Non-inferiority of VCD compared to PAd was shown (one-sided p=0.0013). Similar results were obtained in the PP analysis. CR rates were 4.4% and 8.4% (PAd vs. VCD) and 21.1% and 22.3% (PAd vs. VCD) for near complete response (nCR) or better. Partial response (PR) or better was reached in 72.1% vs. 78.1% of the patients (PAd vs. VCD) (figure 3).
VCD (n = 251) Received allocated intervention (n = 249) 20
One patient excluded from ITT (due to unconfirmed diagnosis of multiple myeloma requiring systemic therapy) received VCD therapy and was included in safety analysis

= 234 30
1) 10
Excluded from PP analysis
= PAd VCD Percent (%) 0
and (n CR nCR PR MR SD PD
- incomplete induction therapy (n = 5)
- missing response assessment (n = 3)
equally distributed for ISS and Durie-Salmon disease stage, cytogenetic abnormalities deletion (17p), translocation t(4;14) and significant differences in patient age and distribution of WHO
= 244 40 Response rates (ITT) VGPR missing PAd VCD P value patients % in PAd arm no of patients % in VCD arm 58.6 / 41.4 153 / 98 61.0 / 39.0 0.65 59.4 (37 - 70) 58.7 (33 - 70) 0.04 10.8 / 88.2 30 221 12.0 / 88.0 0.78 39.4 / 31.9 28.7 94 82 / 75 37.5 / 32.7 29.9 0.91 85.7 / 11.9 / 2.4 230 / 21 0 91.6 / 8.4 0.0 0.01 18.4 44 17.5 0.82 15.9 31 12.3 0.31
- one patient not ITT not PP but Safety (see above)
n = 240 Characteristic PAd VCD P value No of patients % in PAd arm No of patients % in VCD arm AE ≥ 3º (or ≥ 2º for infections, cardiac disorders, PNP and thromboembolic events) 152 61.3 160 64.0 0.58 Any SAE 81 32.7 60 24.0 0.04 Leukocyto-/Neutropenia ≥ 3º 28 11.3 88 35.2 <0.01 AE Infections and Infestations ≥ 2º 61 24.6 56 22.4 0.60
Low number of not assessable/missing† MRD status: Isa-RVd (10.6%) and RVd (15.2%)
The proportion of patients with any adverse event was comparable in PAd vs. VCD (61.3% vs. 64.0%, p=0.58), but more serious adverse events (SAEs) were observed during PAd induction (32.7% vs. 24.0%, p=0.04). VCD led to a significantly higher proportion of leukocytopenia and neutropenia CTCAE grade 3 and 4 (PAd 11.3% vs. VCD 35.2%, p=<0.001). The number of infections (≥ CTCAE grade 2) and infection-related SAE was similar (PAd 24.6% vs. VCD 22.4% for AE, p=0.60 and PAd 12.9% vs. VCD 10.8% for SAE, p=0.49). Compared to the infection rate (AE ≥ CTCAE grade 2) of 49% during PAD (dexamethasone 40 mg on days 1-4, 9-12, 17-20) in the HOVON65/GMMG-HD4trial, a reduction in MM5 during induction was observed. Preliminary data (412 patients) of numbers of collected CD34+ stem cells were comparable (PAd median 9.8x106 vs. VCD median 9.4x106 kg bodyweight, p=0.15). In the PAd arm more deaths were observed compared to the VCD arm (5 vs 1).
Isa-RVd is the first regimen to demonstrate a rapid and statistically significant benefit from treatment by reaching a MRD negativity of 50.1% at the end of induction and to show superiority vs. RVd in a Phase 3 trial
*P value derived from stratified conditional logistic regression analysis
n = 249 HD7 66
First primary endpoint, end of induction MRD negativity by NGF (10-5), was met in ITT analysis





Dara-KRd
• Daratumumab 16 mg/m2 days 1,8,15,22 (days 1,15 C 3-6; day 1 C >6)


• Carfilzomib (20) 56 mg/m2 Days 1,8,15
• Lenalidomide 25 mg Days 1-21
• Dexamethasone 40mg PO Days 1,8,15,22
MRD assessment by NGS
”MRD-SURE” -Treatment-free observation and MRD surveillance*
We are transitioning to quadruplets in frontline eligible patients
• BUT the optimal length of a quadruplet is still to be determined!
Transplant still has a role in MM even with long term use of novel agents
Consolidation therapy may deepen responses and should be considered in patients who have not achieved VGPR
MRD guided discontinuation may be possible in lower risk groups but not high risk patients



Recommendation
Patients should be referred to a transplant center to determine transplant eligibility
Evidence Rating
Type: Evidence based
Evidence quality: Intermediate, benefit outweighs harm
Strength of recommendation: Moderate
Chronologic age and renal function should not be the sole criteria used to determine eligibility for SCT.
Type: Evidence based
Evidence quality: Intermediate, benefit outweighs harm
Strength of recommendation: Moderate


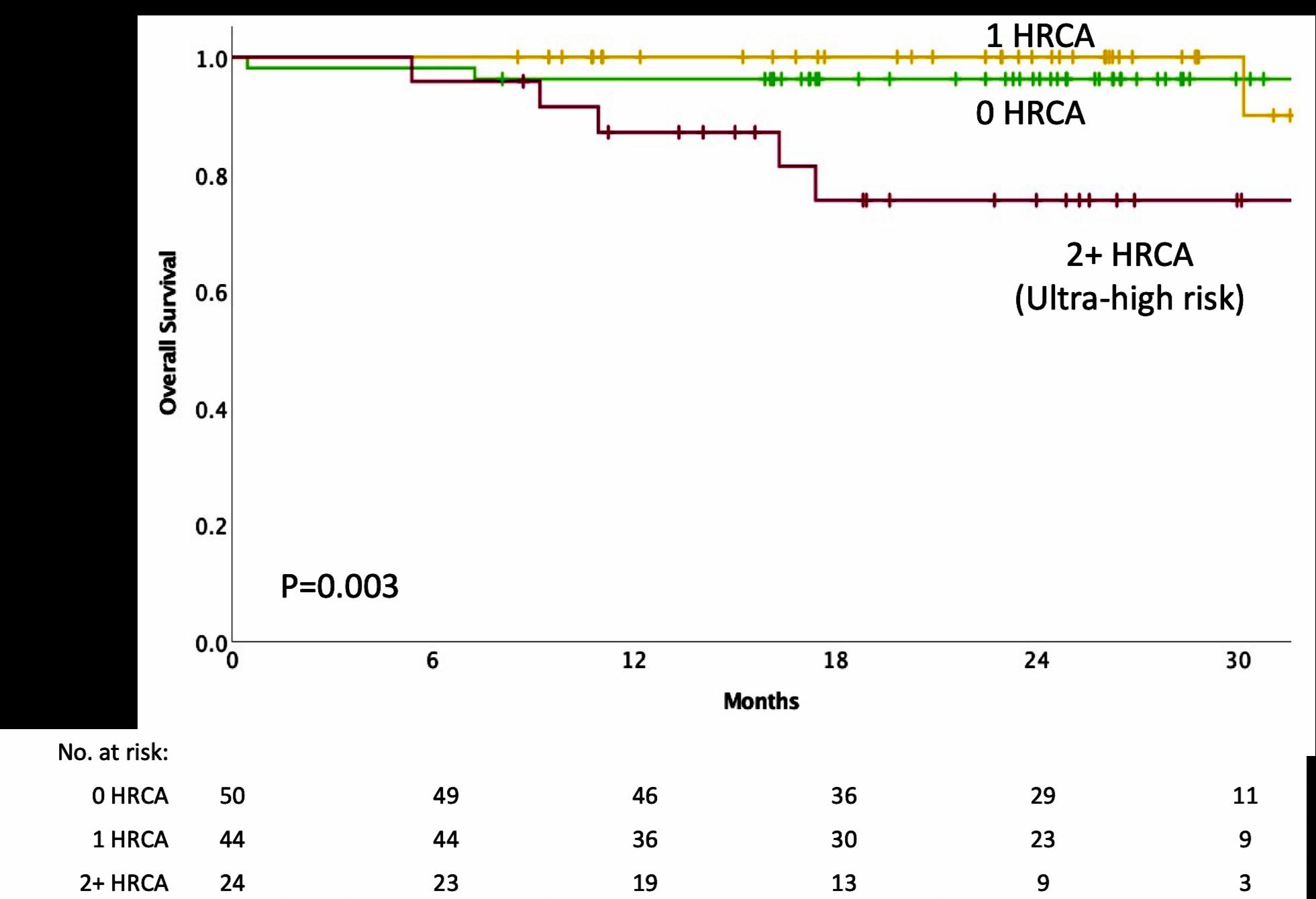
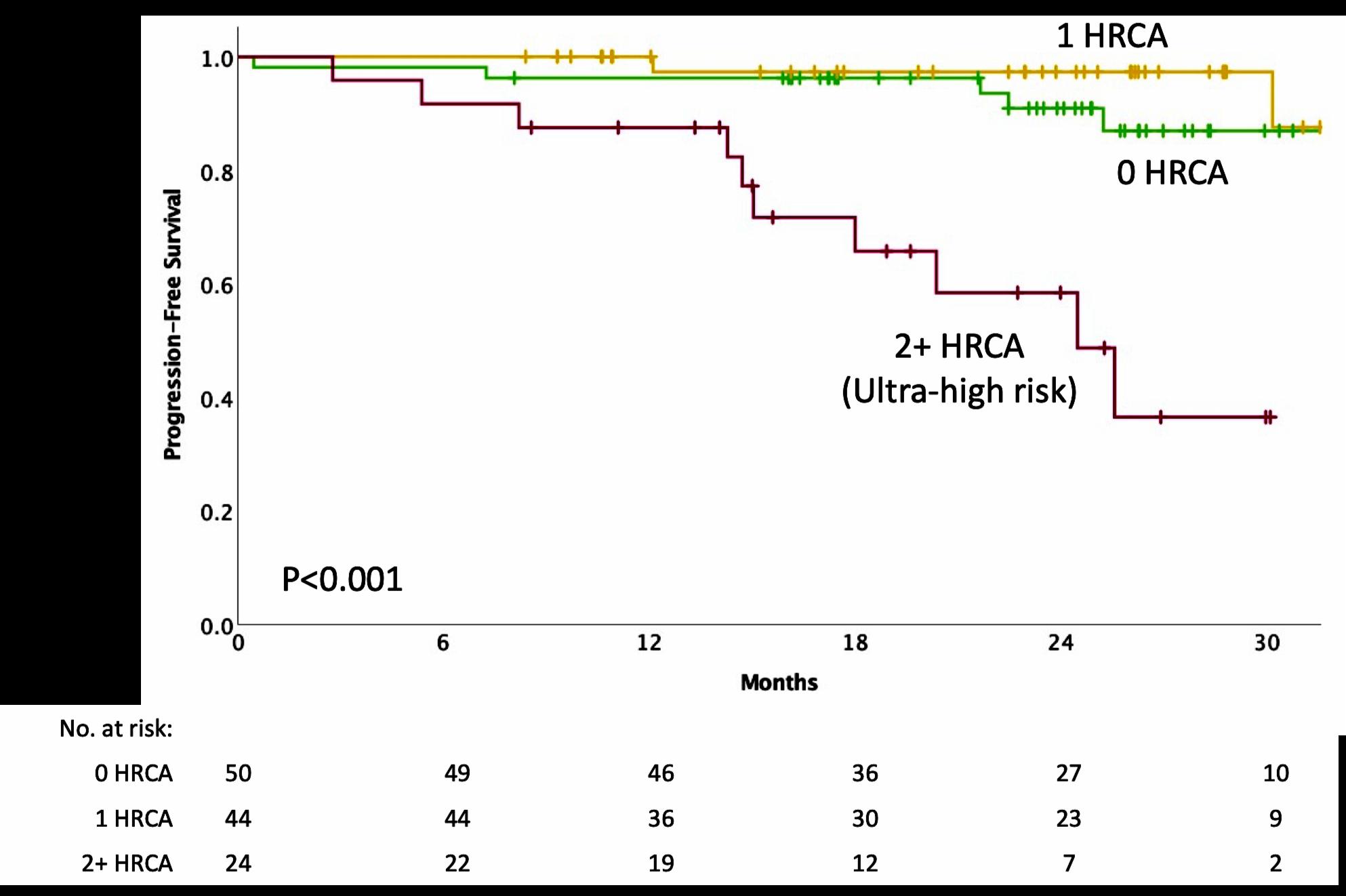
IMWG score of 1 = Intermediate-Fit
3-year OS was 76% (HR=1.61; 95% CI 1.02-2.56; p=.042)
Toxicities 16.7% (HR 1.23, 95% CI 0.89-1.71; p=.217) and
Discontinuation 20.8% (HR=1.41; 95% CI 1.00-2.01; p=.052).
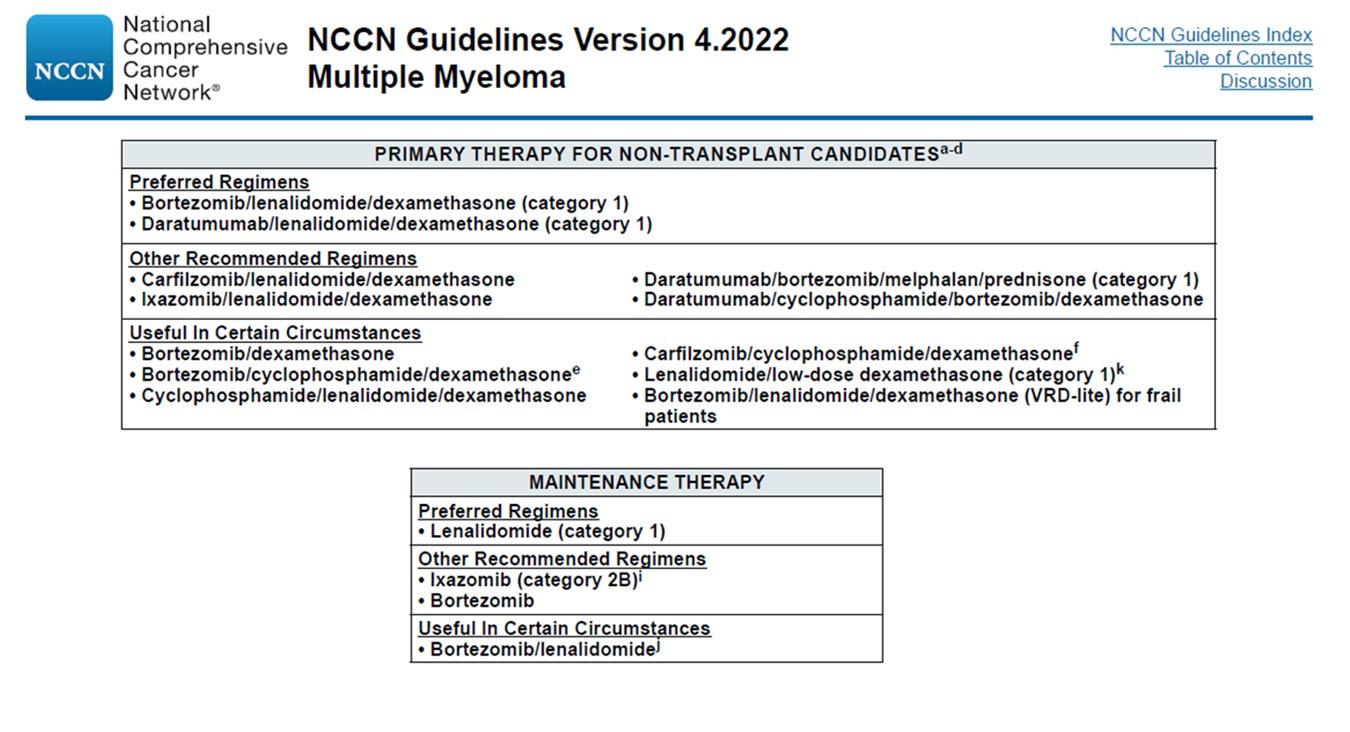
‒ Patients were enrolled in MAIA from March 2015 through January 2017
Key eligibility criteria
• TIE NDMM
• ECOG PS score 0-2
• CrCl
≥30 mL/min
1:1 randomisation
D: 16 mg/kg IV
TIE,
aOn
R: 25 mg PO Days 1-21 until PD
da: 40 mgb PO or IV Days 1, 8, 15, 22 until PD

QW Cycles 1-2, Q2W Cycles 3-6, then Q4W thereafter until PD
Rd
R: 25 mg PO Days 1-21 until PD
d: 40 mg PO Days 1, 8, 15, 22 until PD
D -Rd Cycles: 28 days
End-oftreatment visit (30 days after last dose)
Primary endpoint
• PFS
Longterm follow-up
Key secondary endpoints
• OS
• PFS2
• ORR
• CR/sCR rate
• MRD (NGS; 10–5)
MAIA is a multicentre, randomised, open-label, active-controlled, phase 3 study of D-Rd versus Rd alone in patients with NDMM who are transplant ineligible
transplant-ineligible; ECOG PS, Eastern Cooperative Oncology Group performance status; CrCl, creatinine clearance; IV, intravenous; QW, once weekly; Q2W, once every 2 weeks; Q4W, once every 4 weeks; PD, progressive disease; PO, oral; ORR, overall response rate; CR, complete response; sCR, stringent complete response; MRD, minimal residual disease; NGS, next-generation sequencing; BMI, body mass index.
days when DARA is administered, dexamethasone will be administered to patients in the D-Rd arm and will serve as the treatment dose of steroid for that day, as well as the required pre-infusion medication. bFor patients >75 years of age or with BMI <18.5 kg/m2 , dexamethasone was administered at a dose of 20 mg QW.
Demographics and baseline characteristics were well balanced between arms

Median
Primary:
Update: 56.2 months
• D-Rd induced deeper responses with significantly higher rates of ≥CR and ≥VGPR, compared with Rd
• With >28 months of additional follow-up, responses deepened with continued DARA therapy

• D-Rd continued to demonstrate a significant PFS benefit, with median PFS not reached with D-Rd
• These data provide a new PFS benchmark in patients with NDMM who are transplant ineligible

D -Rd demonstrated a significant benefit in OS, with a 32% reduction in the risk of death, in patients with NDMM who are transplant ineligible

• Although ASCT remains the standard of care, use is likely to decline in patients who are 65-75 or with significant comorbidities
• Continuous therapy has resulted in better outcomes

• The balance of toxicity and efficacy is particularly important in this population
• ESPECIALLY with dexamethasone
• Most common approach is to select 2 agents from the 3 Novel Classes (PIs, IMiDs and MoAbs)
• Most will use DRD in standard risk patients
• Some may favor VRD in certain high risk patients
• DRD is more easily delivered and feasible
• D-VRD may well be a future standard of care even in these patients
Panobinostat
Idecabtagene
Elotuzumab Isatuximab
Ciltacabtagene





Director, Myeloma Program
MedStar Georgetown University Hospital
Professor of Medicine
Georgetown University School of Medicine
Co-Chief, Myeloma Division
Director, Myeloma Research
John Theurer Cancer Center at Hackensack UMC
Professor of Medicine
Hackensack Meridian School of Medicine
David.Vesole@hmhn.org
• Induction: Intense and short term therapy with goal to achieve rapid remission

• Consolidation: Intense and shorter term therapy with goal of deep remission
• Maintenance: Less intense longer term therapy with goal of better PFS and OS
What does the Ideal Maintenance therapy look like? • Deepen remission • Prolong remission
• Easy to administer
Minimal toxicity


 McCarthy et al. J Clin Oncol. 2017, 35:3279-3289.
McCarthy et al. J Clin Oncol. 2017, 35:3279-3289.



3 randomized trials: 1,209 patients:
Median follow up 6.6 years
• PFS 52.8 months for lenalidomide vs 23.5 in placebo

Median overall survival: 86 months v. not reached: P = 0.001
Benefit for ≤ PR as well as VGPR/CR patients
29% discontinuation rate with lenalidomide
Second primary malignancy rate higher at 6.1% vs 2.8% in placebo after PD

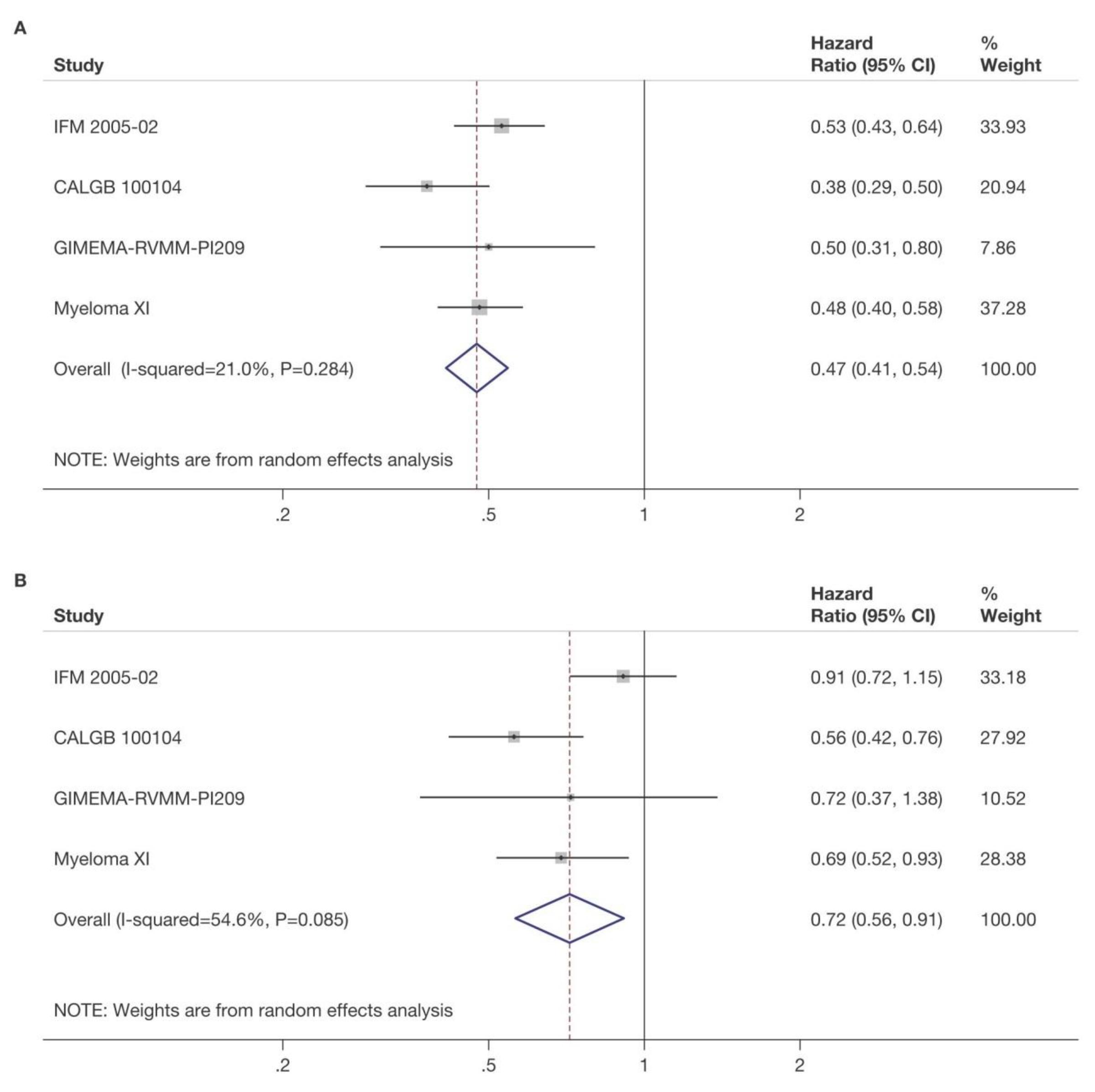



4x KCd
K: 36^ mg/m2 d 1-2,8-9,15-16
C: 300 mg/m2 d 1,8,15
d: 20 mg. d 1-2,8-9,15-16,22-23
4x KRd
K: 36^ mg/m2 d 1-2,8-9,15-16
R: 25 mg d 1-21
d: 20 mg. d 1-2,8-9,15-16,22-23
Intensification with high-dose melphalan followed by autologous stem-cell reinfusion MOBILIZATION
4x KRd
K: 36^ mg/m2 d 1-2,8-9,15-16
R: 25 mg d 1-21
d: 20 mg. d 1-2,8-9,15-16,22-23
4x KRd
K: 36 mg/m2 d 1-2,8-9,15-16
R: 25 mg d 1-21
d: 20 mg. d 1-2,8-9,15-16,22-23
4x KCd
K: 36 mg/m2 d 1-2,8-9,15-16
C: 300 mg/m2 d 1,8,15
d: 20 mg. d 1-2,8-9,15-16,22-23
4x KRd
K: 36 mg/m2 d 1-2,8-9,15-16
R: 25 mg d 1-21
d: 20 mg. d 1-2,8-9,15-16,22-23
R: 10 mg days 1-21, until progression or intolerance
K: 36 mg/m2 d 1, 2, 15, 16 up to 2 years*
R: 10 mg days 1-21, until progression or intolerance
K: 36 mg/m2 d 1-2,8-9,15-16
R: 25 mg d 1-21
d: 20 mg. d 1-2,8-9,15-16,22-23
^20 mg/m2 on days 1-2, cycle 1 only. *Carfilzomib 70 mg/m2 days 1, 15 every 28 days up to 2 years for patients that have started the maintenance treatment from 6 months before the approval of Amendment 5.0 onwards.
NDMM, newly diagnosed multiple myeloma, R1, first randomization (induction/consolidation treatment); R2, second randomization (maintenance treatment); ASCT, autologous stem-cell transplantation; K, carfilzomib; R, lenalidomide; C, cyclophosphamide; d, dexamethasone; KCd_ASCT, KCd induction-ASCT-KCd consolidation; KRd_ASCT, KRd induction-ASCT-KRd consolidation; KRd12, 12 cycles of KRd.
KRd_ASCT vs. KRd12 vs. KCd_ASCT KR vs. R
Median follow-up from Random 1: 51 months (IQR 46‒55)
Median follow-up from Random 2: 37 months (IQR 33‒42)
KRd_ASCT vs. KCd_ASCT: HR 0.54, 95% CI 0.38-0.78, p<0.001
KRd_ASCT vs. KRd12: HR 0.61, 95% CI 0.43-0.88, p=0.0084
KRd12 vs. KCd_ASCT: HR 0.88, 95% CI 0.64-1.22, p=0.45
KR vs. R: HR 0.64, 95% CI 0.44-0.94, p=0.02294
3-year PFS reported in the figure. Random 1, first randomization (induction/consolidation treatment); ASCT, autologous stem-cell trasplantation; K, carfilzomib; R, lenalidomide; C, cyclophosphamide; d, dexamethasone; KCd_ASCT, KCd induction-ASCT-KCd consolidation; KRd_ASCT, KRd induction-ASCT-KRd consolidation; KRd12, 12 cycles of KRd; Random 2, second randomization (maintenance treatment); p, p-value; HR, hazard ratio; CI, confidence interval
• It appears that dual maintenance therapy prolongs PFS
• This occurs in both standard risk AND high risk patients
• It further opens the door to other dual maintenance strategies currently being used and explored:
• Lenalidomide + Bortezomib
• Lenalidomide + Ixazomib
• Lenalidomide + Daratumumab
• Others??

• Recall that GRIFFIN gave Dara for 2 years with lenalidomide in maintenance
• Responses continued to deepen with Dara
• CASSIOPEIA randomized pts to no maintenance vs dara q 8 weeks
• Overall there was a benefit to having dara maintenance vs placebo
• However, if dara had been given at induction, that benefit did not seem to continue (ie If you had dara upfront, it didn’t add more to maintenance)
• Two large international trials comparing daratumumab + lenalidomide versus lenalidomide alone: Perseus and Auriga; others in planning stages




Defining
lenalidomide
stem cell transplant –
from the Myeloma XI trial. Charlotte Pawlyn1,2, Tom Menzies3, Faith Davies4, Ruth de Tute5, Rowena Henderson3, Gordon Cook3,6 , Matthew Jenner7, John Jones8, Martin Kaiser1,2, Mark Drayson9, Roger Owen8, David Cairns3 , Gareth Morgan4, Graham Jackson10

1) The Institute of Cancer Research, London, UK; 2) The Royal Marsden Hospital, London, UK; 3) Clinical Trials Research Unit, Leeds Institute of Clinical Trials Research, University of Leeds, Leeds, UK; 4) Perlmutter Cancer Center, NYU Langone Health, New York, US; 5) HMDS, Leeds Cancer Centre, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom; 6) Leeds Cancer Centre, Leeds Teaching Hospitals NHS Trust, Leeds, UK; 7) University Hospital Southampton NHS Foundation Trust, Southampton, UK; 8) Kings College Hospital NHS Foundation Trust, London, UK; 9) Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK; 10) Department of Haematology, University of Newcastle, Newcastle-upon-Tyne, UK
On behalf of the Myeloma XI Trial Management Group and NCRI Haem-Onc Clinical Studies Group

the optimum duration of
data
• Open-label, randomized phase III study with 3 randomizations: induction (allocation by ASCT eligibility), intensification (allocation by response to induction therapy), and maintenance treatment
• Current analysis: maintenance with lenalidomide monotherapy vs observation following ASCT
Patients with newly diagnosed, symptomatic, transplant-eligible MM completing Myeloma XI induction protocols* and ASCT; responded to lenalidomide; without PD (N = 1248)
Lenalidomide 10 mg/day on Days 1-21/28 (n = 730) Observation (n = 518)
Continued until disease progression
• Median follow-up: 44.7 mo (IQR: 32.4-62.7); median duration of lenalidomide therapy: 28 cycles (range: 1-96) with 45% of patients (330/730) still on therapy
• Endpoints: Overall PFS, PFS2; landmark PFS by genetic risk subgroups and MRD status
*Cyclophosphamide/thalidomide/dexamethasone or cyclophosphamide/lenalidomide/dexamethasone or carfilzomib/cyclophosphamide/lenalidomide/dexamethasone.
Pawlyn. ASH 2022. Abstr 570.
Pawlyn. ASH 2022. Abstr 570. Reproduced with permission.
Pawlyn. ASH 2022. Abstr 570. Reproduced with permission.
• Long-term results from Myeloma XI phase III trial demonstrated ongoing PFS improvement with continuing lenalidomide maintenance beyond 4-5 yr in patients with newly diagnosed MM after response to protocol-specified induction and ASCT
• Continuing lenalidomide maintenance for ≥3 yr benefited patients with sustained MRD negativity, but benefit of additional lenalidomide maintenance is unclear
• In MRD-positive patients, results support continuing lenalidomide until disease progression
• No evidence of cumulative hematologic toxicity observed with long -term lenalidomide maintenance
• Investigators concluded that additional data from ongoing studies needed to determine optimal duration of lenalidomide maintenance



 David Vesole, MD, PhD, FACP
David Vesole, MD, PhD, FACP

Director, Myeloma Program
MedStar Georgetown University Hospital
Professor of Medicine
Georgetown University School of Medicine
Co-Chief, Myeloma Division
Director, Myeloma Research
John Theurer Cancer Center at Hackensack UMC
Professor of Medicine
Hackensack Meridian School of Medicine
David.Vesole@hmhn.org
• Most patients with MM have multiple distinct subclonal populations as a result of the expansion of genetically different myeloma cells; this causes intratumoral heterogeneity
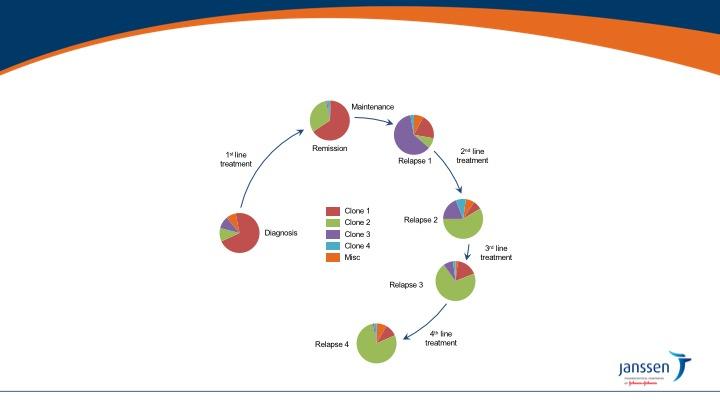
• MM is clonally heterogeneous at diagnosis and throughout treament
• The genomic heterogeneity of MM contributes to treatment resistance and relapse3
• Wide variety of mutations found within a single patient may result in treatment resistance and refractory disease
• Furthermore, subclones continually mutate over time, including after treatment, which may contribute to resistance and result in disease progression1,5
Definitions: What is relapsed/refractory disease and a line of therapy?

• Relapsed: recurrence (reappearance of disease) after a response to therapy
• Refractory: progression despite ongoing therapy

• Progression: change in M protein/light chain values
• Line of therapy: change in treatment due to either progression of disease or unmanageable side effects
• Note: initial (or induction) therapy + stem cell transplant + consolidation/ maintenance therapy = 1 line of therapy
Timing of therapy
Biochemical
• Patients with asymptomatic rise in blood or urine M protein, free light chains, or plasma cells
initiation/escalation dependent on many factors
Requires immediate
Clinical
• Based on direct indicators of increasing disease and/or end-organ dysfunction
initiation/escalation of therapy
• At biochemical relapse?
• When myeloma protein starts rising!
• When involved free light chain starts rising!
• At Clinical relapse?
• CRAB criteria (Anemia, kidney failure, high calcium or new bone disease)
• Extramedullary disease (myeloma growing at tumors outsides the bone)
Choices are broadest and guided by
Disease biology
Nature of relapse
Patient preference
Factors to consider
Prior autologous stem cell transplant
Prior therapies
Aggressiveness of relapse
Comorbidities
Psychosocial issues
Access to care
IMiDs Proteasome inhibitors Chemotherapy
anthracyclines Chemotherapy
alkylators Steroids
Novel mechanisms of action
Monoclonal antibodies
Cellular therapy
Thalomid (thalidomide)
Velcade (bortezomib) Adriamycin
Cytoxan (cyclophosphamide) Dexamethasone
XPOVIO (selinexor)
Empliciti (elotuzumab)
Revlimid (lenalidomide)
Kyprolis (carfilzomib)
Doxil (liposomal doxorubicin)
Pomalyst (pomalidomide)
Ninlaro (ixazomib)
Bendamustine Prednisone
Melphalan
Venclexta (venetoclax)*
Farydak (Panobinostat)†
Pepaxto (melflufen)†
Darzalex (daratumumab)
Sarclisa (isatuximab)
Blenrep (belantamab mafodotin)‡
Tecvayli (teclistamab)§
Abecma (idecabtagene vicleucel)
Carvykti (ciltacabtagene autoleucel)
*Not yet FDA-approved for patients with multiple myeloma; †Withdrawn from the US market in 2021; ‡
Antibody-drug conjugate; §Bispecific antibody
GREAT NEWS WE HAVE OPTIONS
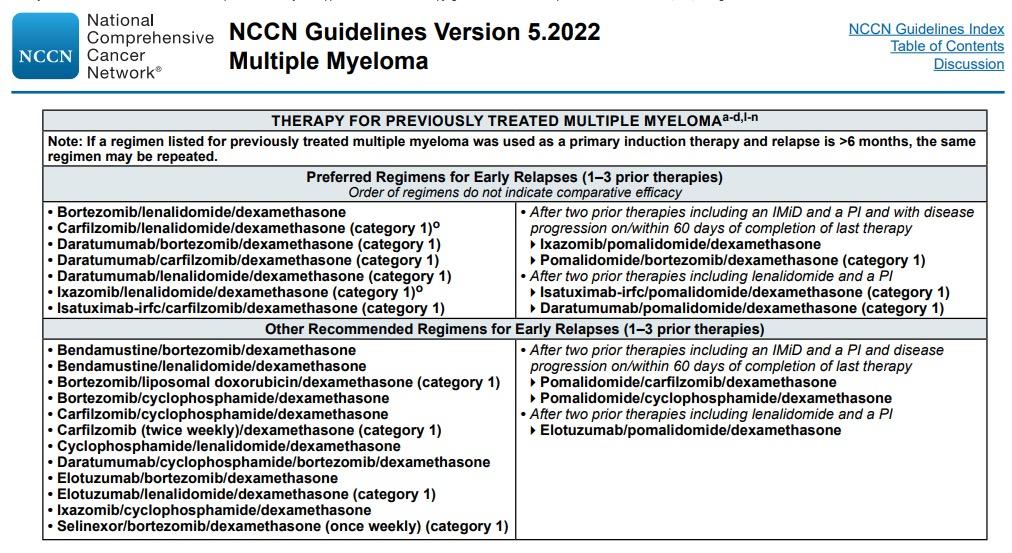
Preferred options: DRd (or KRd)
Not Refractory to Lenalidomide
Refractory to Lenalidomide
Alternatives: DVD, Kd, DaraKd, IsaKd, IRd, Erd
When Dara, Isa, K not available: Rd, Vd, VTD, VCD, VMP
Preferred options: PVd DaraKd IsaKd
DKd,daratumumab/carfilzomib/dexamethasone; DPd, daratumumab/pomalidomide/dexamethasone; DRd, daratumumab/lenalidomide/ dexamethasone; DVd, daratumumab/bortezomib/dexamethasone; Elo–Rd, elotuzumab/lenalidomide/dexamethasone; Ipd, ixazomib/pomalidomide/dexamethasone; Ird, ixazomib/lenalidomide/dexamethasone; Isa–Kd, isatuximab/carfilzomib/dexamethasone; Kd, carfilzomib/dexamethasone; KPd, carfilzomib/pomalidomide/dexamethasone; KRd, carfilzomib/lenalidomide/ dexamethasone; PVd, pomalidomide/bortezomib/dexamethasone; Rd, lenalidomide/dexamethasone; SVd, selinexor/bortezomib/dexamethasone; VCd, bortezomib/cyclophosphamide/dexamethasone; Vd, bortezomib/dexamethasone; VMP, bortezomib/melphalan /prednisone; VTd, bortezomib/thalidomide/dexamethasone.
Second options
DaraVd;Kd
Other options: KPd; DaraPd; Ipd
When Dara, isa, K or P not available: VCD, Vd, VMP
Moreau P, et al. IMWG Recommendations for RRMM. Lancet Oncol. 2021;22(3):e105-e118.
Key eligibility criteria
• RRMM
• ≥1 prior line of therapy
• Prior lenalidomide exposure, but not refractory
• Creatinine clearance
≥30 mL/min
DRd (n = 286)

Daratumumab 16 mg/kg IV
• Qw in Cycles 1 to 2, q2w in Cycles 3 to 6, then q4w until PD
R 25 mg PO
• Days 1 to 21 of each cycle until PD
d 40 mg PO
• 40 mg weekly until PD
Rd (n = 283)
R 25 mg PO
• Days 1 to 21 of each cycle until PD
d 40 mg PO
• 40 mg weekly until PD
Primary endpoint
• PFS
Secondary endpoints



• TTP
• OS
• ORR, VGPR, CR
• MRD
• Time to response
• Duration of response
Stratification factors

• No. of prior lines of therapy
• ISS stage at study entry
• Prior lenalidomide
Cycles: 28 days
Statistical analyses
• Primary analysis: ~177 PFS events
Pre-medication for the DRd treatment group consisted of dexamethasone 20 mg,a acetaminophen, and an antihistamine
ISS, international staging system; DRd, daratumumab/lenalidomide/dexamethasone; IV, intravenous; qw, weekly; q2w, every 2 weeks; q4w, every 4 weeks; PD, progressive disease; R, lenalidomide; PO, oral; d, dexamethasone; Rd, lenalidomide/dexamethasone; PFS, progression-free survival; TTP, time to progression; OS, overall survival; ORR, overall response rate; VGPR, very good partial response; CR, complete response; MRD, minimal residual disease.
a On daratumumab dosing days, dexamethasone 20 mg was administered as pre-medication on Day 1 and Day 2.
Multicenter, randomized (1:1), open-label, active-controlled, phase 3 study
not reached
17.5 months
Median follow-up: 32.9 months (range, 0 - 40.0 months)
56% reduction in risk of progression/death for DRd versus Rd
updated analysis: PFS (time without relapse is much better with 3 drugs....)



Drug Formulation Approval
Velcade (bortezomib)
Kyprolis (carfilzomib)

Ninlaro (ixazomib)
Revlimid (lenalidomide)*
Pomalyst (pomalidomide)*





XPOVIO (selinexor)
• IV infusion
• SC injection
• IV infusion
• Weekly dosing
Once-weekly pill
• For relapsed/refractory myeloma
• For relapsed/refractory myeloma as a single agent, as a doublet with dexamethasone, and as a triplet with Revlimid or Darzalex plus dexamethasone
• For relapsed/refractory myeloma as a triplet with Revlimid and dexamethasone
Once-daily pill
• For relapsed/refractory myeloma in combination with dexamethasone
Once-daily pill
• For relapsed/refractory myeloma in combination with dexamethasone
Once-weekly pill
• For relapsed/refractory myeloma as a triplet with Velcade and dexamethasone
*Black box warnings: embryo-fetal toxicity; hematologic toxicity (Revlimid); venous and arterial thromboembolism
IV, intravenous; SC, subcutaneous
OPTIMISMM
• Velcade-Pomalystdex (VPd) vs Vd Regimens compared
• Kyprolis-Revlimiddex (KRd) vs Rd
• Ninlaro-Rd (IRd) vs Rd
• XPOVIO-Velcade-dex (XPO-Vd) vs Vd










Median progression-free survival favored
• VPd: 11 vs 7 months
• KRd: 26 vs 17 months
• IRd: 21 vs 15 months
• XPO-Vd: 14 vs 9 months
Clinical considerations
• Consider for relapse on Revlimid
• VPd associated with more low blood counts, infections, and neuropathy than Pd

• KRd associated with more upper respiratory infections and high blood pressure than Rd
• IRd an oral regimen
• Gastrointestinal toxicities and rashes
• Lower incidence of peripheral neuropathy



• XPO-Vd associated with low platelet counts and fatigue with triplet, but less neuropathy than the Vd

Velcade
• Risk of peripheral neuropathy (PN; numbness, tingling, burning sensations and/or pain due to nerve damage)
- Avoid in patients with severe existing PN
- Reduced with subcutaneous once-weekly dosing



• High risk of shingles
- Use appropriate vaccination
• No dose adjustment for kidney issues; adjust for liver issues
Kyprolis
• Less PN than Velcade
• High risk of shingles
- Use appropriate vaccination
• Monitor for heart, lung, and kidney side effects
- Use with caution in older patients with cardiovascular risk factors
• High blood pressure
• No dose adjustment for kidney issues; adjust for liver issues

Ninlaro
• Less PN than Velcade
• High risk of shingles

- Use appropriate vaccination
• Monitor for rashes and gastrointestinal (GI) side effects
- GI effects occur early
• Needs to be taken at least 1 hour before or 2 hours after a meal

Revlimid*
• Rash
- Consider antihistamines


• Diarrhea
- Consider bile acid sequestrants
• Risk of blood clots
• Risk of second primary malignancies
• Dose adjustment based on kidney function
Pomalyst*
• Low blood counts

• Less rash than Revlimid
• Risk of second primary malignancies
• Risk of blood clots
Gastrointestinal Low Na Sodium 22.990
Begin prophylactic anti-nausea medications. Consult with your doctor if nausea, vomiting, or diarrhea occur or persist.
Fatigue
blood counts (cytopenias)







Drug Formulation
Approval
Darzalex (daratumumab)

SC once a week for first 8 weeks, then every 2 weeks for 4 months, then monthly
• For relapsed/refractory myeloma as a single agent and as a triplet with Revlimid or Velcade or Kyprolis or Pomalyst plus dexamethasone
Empliciti (elotuzumab)


IV once a week for first 8 weeks, then every 2 weeks (or every 4 weeks with pom)
• For relapsed/refractory myeloma as a triplet with Revlimid or Pomalyst and dexamethasone
Sarclisa (isatuximab)
IV once a week for first 4 weeks, then every 2 weeks
• For relapsed/refractory myeloma as a triplet with Pomalyst or Kyprolis and dexamethasone

Regimens compared
• Darzalex-Revlimiddex (DRd) vs Rd
• Darzalex-Velcade-dex (DVd) vs Vd




• Darzalex-Kyprolis-dex (DKd) vs Kd
• Darzalex-Pomalystdex (DPd) vs Pd
Median progressionfree survival favored
• DRd: 45 vs 18 months
• DVd: 17 vs 7 months
• DKd: 29 vs 15 months
• DPd: 12 vs 7 months
• Consider for relapses from Revlimid or Velcade maintenance
Clinical consider-ations
• DRd associated with more upper respiratory infections, low blood white blood cell counts, and diarrhea
• Consider for patients who are Revlimid - refractory without significant neuropathy
• DVd associated with more low blood cell counts
• Consider for younger, fit patients who are doublerefractory to Revlimid and Velcade
• DKd associated with more respiratory infections
• Sever side effects (possibly fatal) in intermediate fit patients 65 and older
• Consider in patients who are double - refractory to Revlimid and a proteasome inhibitor (Velcade, Kyprolis, Ninlaro)
• Severe low white blood cell counts










ELOQUENT-2












ELOQUENT-3 ICARIA-MM IKEMA
Regimens compared
• Empliciti-Revlimiddex vs Rd
• EmplicitiPomalyst-dex vs Pd
Median progressionfree survival favored
• Empliciti-Rd: 19 vs 15 months
• Empliciti-Pd: 10 vs 5 mos
• Sarclisa-Pomalyst-dex vs Pd
• Sarclisa-Kyprolis-dex vs Kd
Clinical considerations
• Consider for non - Revlimid refractory, frailer patients
• Overall survival benefit with Empliciti- Rd

• Empliciti- Rd associated with more infections
• Sarclisa-Pd: 12 vs 7 mos
• Sarclisa-Kd: 41 vs 19 mos
• Consider for patients refractory to Revlimid and a proteasome inhibitor (Velcade, Kyprolis, Ninlaro)
• Consider for patients refractory to Revlimid and a proteasome inhibitor (Velcade, Kyprolis, Ninlaro)
• Sarclisa - Pd associated with severe low white blood cell counts, more dose reductions, upper respiratory infections, and diarrhea
• Consider for patients refractory to Revlimid and Velcade
• Sarclisa - Kd associated with higher MRD negativity rates
• Sarclisa - Kd associated with severe respiratory infections
Refractory to IMiD, PI, Anti-CD38
Refractory to IMiD, PI, Anti-CD38, Alkylators, and Anti-BCMA
Existing drugs:
Combinations with Cyclophosphamide
that do not have IMiD, PI, Anti CD38 (e.g., KCd)
Anti BCMA strategy
Anti-BCMA
Bispecific
BCMA CAR-Ts
Elotuzumab
Selinexor
Venetoclax
Bendamustine
VDT PACE
New Drugs: Iberdomide, Mezigdomide
New bispecifics (Cevostamab, Talquetamab)
New CAR-Ts
New Monoclonals
New ADCs
Class
Drug

Formulation
Approval
Nuclear export inhibitor
XPOVIO (selinexor)
Antibody-drug conjugate
Blenrep (belantamab mafodotin)*
Twice-weekly pill
• For relapsed/refractory myeloma in combination dexamethasone (after at least 4 prior therapies disease is refractory to at least 2 PIs, at least 2 IMiDs, and an anti-CD38 mAb
2.5 mg/kg IV over approximately 30 minutes once every 3 weeks
• For relapsed/refractory myeloma (after at least including an anti-CD38 mAb, a PI, and an IMiD
Chimeric antigen receptor (CAR) T cell
Abecma (idecabtagene vicleucel)†
300 to 460 × 106 genetically modified autologous CAR T cells in one or more infusion bags
• For relapsed/refractory myeloma (after 4 or more prior lines of therapy, including an IMiD, a PI, and anti-CD38 mAb
CAR T cell
Bispecific antibody



Carvykti (ciltacabtagene autoleucel)‡
0.5 to 1.0 × 106 genetically modified autologous CAR T cells/kg of body weight
Tecvayli (Teclistamab) ‡ Step up dosing then weekly SQ
IMiD, immunomodulatory agent; PI, proteasome inhibitor; mAb, monoclonal antibody
• For relapsed/refractory myeloma (after 4 or more prior lines of therapy, including a PI, an IMiD, and anti-CD38 mAb
For relapsed/refractory myeloma (after 4 or more therapy, including a PI, an IMiD, and an anti-CD38
†Black box warning: cytokine release syndrome; neurologic toxicities; hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS); prolonged cytopenia
‡Black box warning: cytokine release syndrome; neurologic toxicities; Parkinsonism and Guillain-Barré syndrome; hemophagocytic lymphohistiocytosis/ macrophage activation syndrome (HLH/MAS); prolonged cytopenia
Abecma and Carvykti are available only through a restricted distribution program
Previous therapies to which the disease was refractory, n (%)
Additional analyses showed clinical benefit with XPOVIO regardless of patient age and kidney function.2,3
Bispecific antibodies
• Elranatamab, talquetamab, cevostamab, and others
• Target BCMA, GPRC5D, or FcRH5 on myeloma cells and CD3 on T cells
• Redirects T cells to myeloma cells
Cereblon E3 ligase modulators (CELMoDs)
• Iberdomide
• Targets cereblon
• Enhances tumoricidal and immune-stimulatory effects compared with immunomodulatory agents
Small molecule inhibitors
• Venetoclax
• Targets Bcl-2
• Induces multiple myeloma cell apoptosis



Conjugates

Cho. Front Immunol. 2018;10:1821.

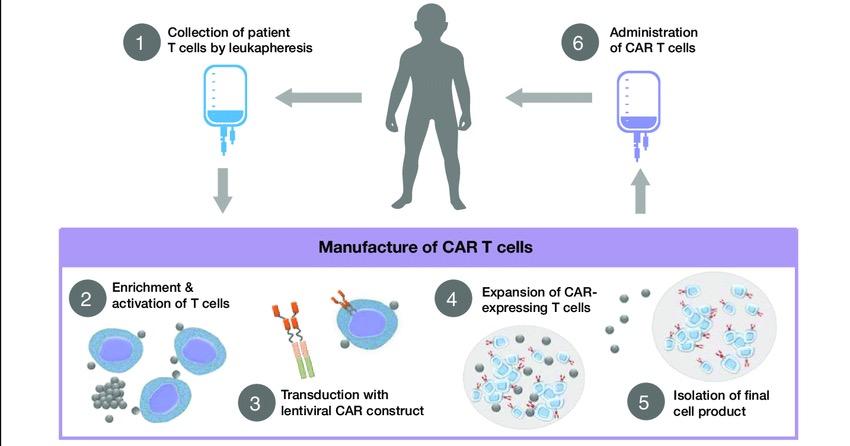
Collect patient’s white blood cells
Isolate and activate T cells Engineer T cells with CAR gene
Tumor cell
Targeting element (eg, CD19, BCMA, CD20)
Spacer
Transmembrane domain
Expand CAR T cells Infuse same patient with CAR T cells
CD19
Viral vector with CAR DNA
CARengineered T cell

Costimulatory domain (eg, CD28 or 4-1BB)
CD3� (essential signaling domain)
Median manufacturing time: 17-28 days
Patients undergo lymphodepleting (and possibly salvage/bridging) therapy



3

Abecma (idecabtagene vicleucel)*
Carvykti (ciltacabtagene autoleucel)
300 to 460 × 106 genetically modified autologous CAR T cells in one or more infusion bags
0.5 to 1.0 × 106 genetically modified autologous CAR T cells/kg of body weight
IMiD, immunomodulatory agent; PI, proteasome inhibitor; mAb, monoclonal antibody
• For relapsed/refractory myeloma (after 4 or more prior lines of therapy, including an IMiD, a PI, and an anti-CD38 mAb)
• For relapsed/refractory myeloma (after 4 or more prior lines of therapy, including a PI, an IMiD, and an anti-CD38 mAb)
*Black box warning: cytokine release syndrome; neurologic toxicities; hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS); prolonged cytopenia
†Black box warning: cytokine release syndrome; neurologic toxicities; Parkinsonism and Guillain-Barré syndrome; HLH/MAS; prolonged cytopenia

Abecma and Carvykti are available only through a restricted distribution program.

CR or sCR and MRD NE CR or sCR and MRD-
ORR, overall response rate; PR, partial response; VGPR, very good partial response; CR, complete response; sCR, stringent complete response; MRD, minimal residual disease; PFS, progression-free survival
KarMMa Trial. Munshi NC et al. N Engl J Med. 2021;384:705.
CARTITUDE-1 Trial. Berdeja JG et al. Lancet. 2021;398:314; Martin T et al. J Clin Oncol. June 4, 2022 [Epub ahead of print].
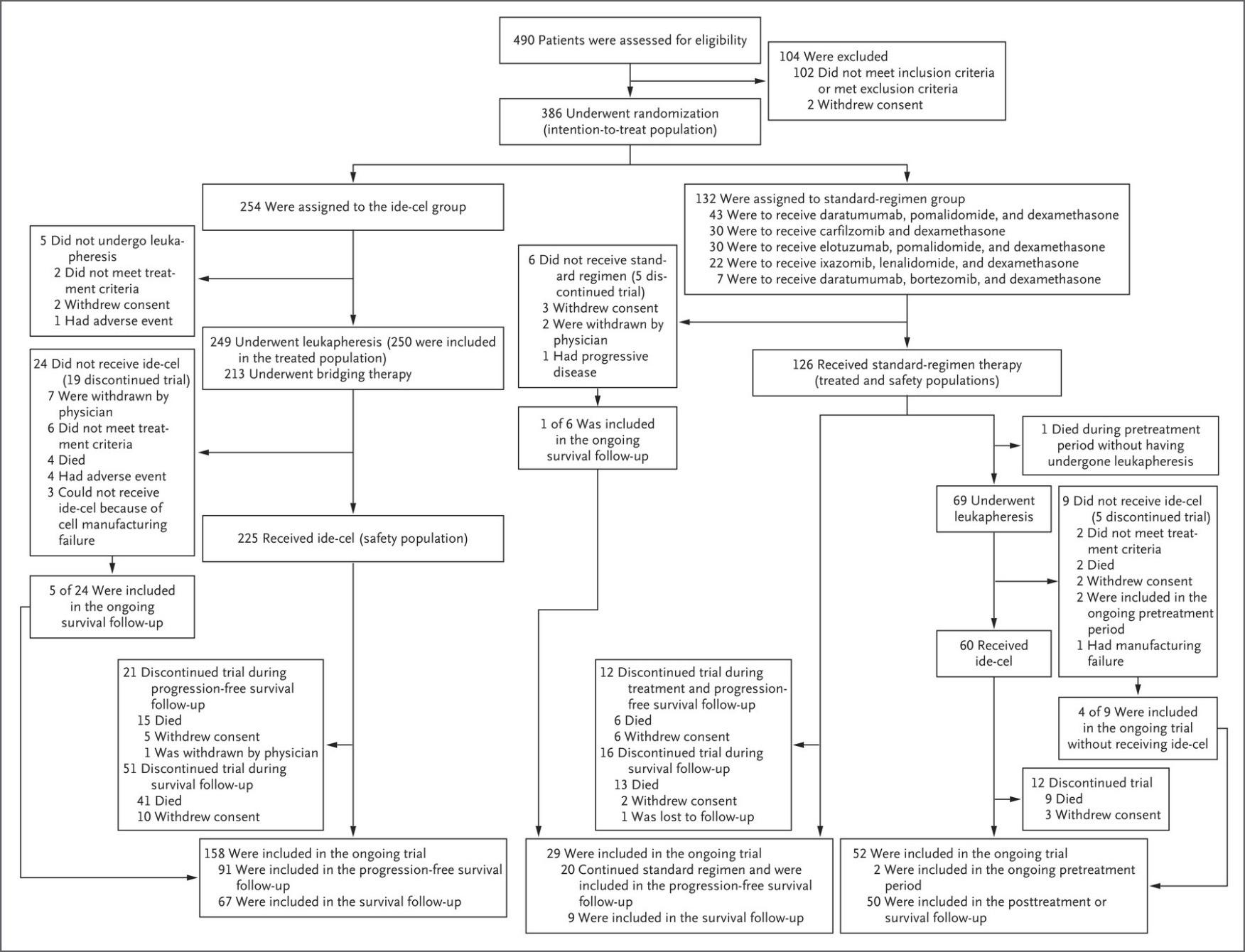
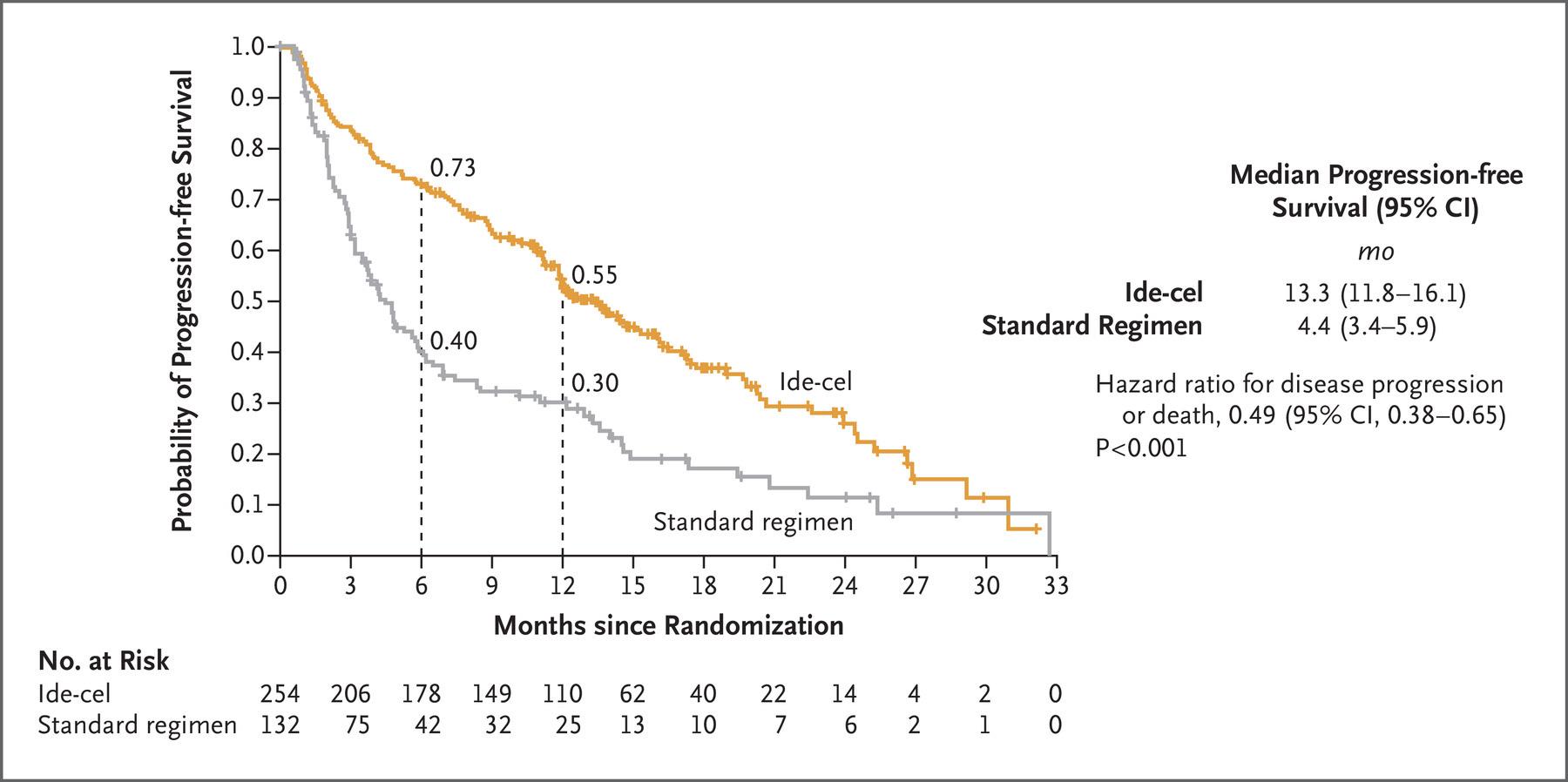

• Relapsed/refractory multiple myeloma who received 1 to 3 prior lines of therapy
• Randomly assigned to cilta-cel, pomalidomide (Pomalyst), bortezomib (Velcade), and dexamethasone (PVd); or daratumumab (Darzalex), pomalidomide, and dexamethasone (DPd)
• primary endpoint of the study is PFS; safety, overall survival, minimal residual disease negative rate and overall response rate are secondary endpoints
• Cilta-cel Meets PFS End Point in Relapsed/Lenalidomide-Refractory
Multiple Myeloma: January 27, 2023.
Nausea Vomiting Anorexia
Fatigue
Headache
Myalgia Arthralgia
Rigors
High Fevers
Hypotension
Tachycardia
Neurologic Change
Dyspnea
Tachypnea
Hypoxia
Onset 1-9 days after CAR T-cell infusion
Duration 5-11 days
Symptoms
• Fever
• Difficulty breathing
• Dizziness
• Nausea
• Headache
• Rapid heartbeat
• Low blood pressure
Management

• Actemra (tocilizumab)

• Corticosteroids
• Supportive care
2-9 days after CAR T-cell infusion
3-17 days
• Headache
• Confusion
• Language disturbance

• Seizures
• Delirium
• Cerebral edema

• Antiseizure medications
• Corticosteroids
*Based on the ASTCT consensus; †Based on vasopressor; ‡For adults and children >12 years;
§For children ≤12 years; ‖Only when concurrent with CRS
Xiao X et al. J Exp Clin Cancer Res. 2021;40(1):367. Lee DW et al. Biol Blood Marrow Transplant. 2019;25:625; Shah N et al. J Immunother Cancer. 2020;8:e000734.
Survey of 20 centers. Responses from 17 centers.
• BCMA cell therapy has demonstrated unparalleled efficacy, but it is not readily available to all patients
• Allogeneic CAR T-cell therapy has the potential to make CAR T-cell therapy accessible to all eligible patients on demand and supports redosing
• ALLO-715 is an allogeneic anti-BCMA CAR T-cell product that uses gene editing specifically designed to:
• Disrupt the TCRα constant gene, thereby leading to a reduced risk of GVHD
• Edit the CD52 gene to allow the use of ALLO-647 (a humanized anti-CD52 mAb) to selectively deplete host T-cells while simultaneously protecting donor cells
Knockout of CD52 KO for selective lymphodepletion with ALLO-647
Knockout of TRAC to minimize risk of GVHD
multiple myeloma: phase 1
UNIVERSAL trial interim results

Cellular therapies CAR T-cell therapy
Patient’s cells collected
Types of cells collected T
Collected cells are genetically engineered in a lab
Patient given chemotherapy before cells are infused back into patient Yes,
When in the course of myeloma is this usually done?
Side effects of treatment
After multiple relapses As part of initial treatment
Cytokine release syndrome; confusion
*An immune cell that is the “business end” of the system, in charge of maintaining order and removing cells.
†Precursor cells that give rise to many types of blood cells. We actually collect CD34+ve cells.
Fatigue, nausea, diarrhea


Bispecific antibodies are also referred to as dual specific antibodies, bifunctional antibodies, or T-cell engaging antibodies
Bispecific antibodies can target two cell surface molecules at the same time (one on the myeloma cell and one on a T cell)


Many different bispecific antibodies are in clinical development; none are approved for use in myeloma
Availability is off-the-shelf, allowing for immediate treatment
Examples:
• Elranatamab
• Teclistamab
• TNB-303B (ABBV-383)
• REGN5458
• Cevostamab
• Talquetamab
Drug Formulation
Approval
Tecvayli (teclistamab)*
Step-up dosing† the first week then once weekly thereafter by subcutaneous injection

• For relapsed/ refractory myeloma (after 4 or more prior lines of therapy, including an IMiD, a PI, and an anti-CD38 mAb)

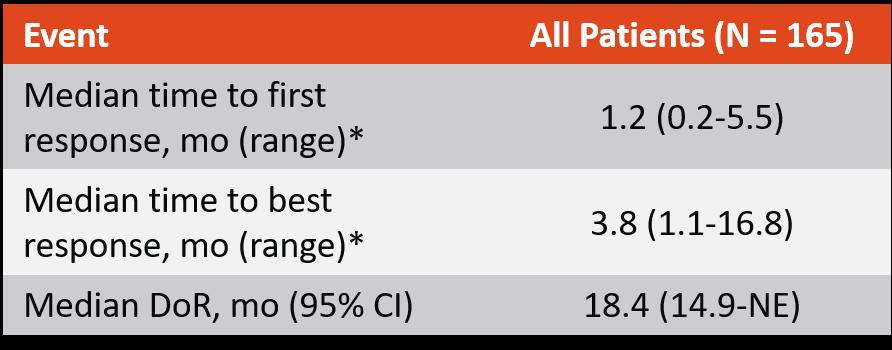
Median duration of response 18.4 months
IMiD, immunomodulatory agent; PI, proteasome inhibitor; mAb, monoclonal antibody
*Black box warning: cytokine release syndrome; neurologic toxicities
†Patients are hospitalized for 48 hours after administration of all step-up doses.
Tecvayli is available only through a restricted distribution program.
CR or better median DOR not reached (95% CI: 16.2–NE)
• Overall median DOR of 18.4 months (95% CI: 14.9–NE), and was not yet mature with data from 71 patients (68.3%) censored
• 12-month event-free rate:
• Overall:
Overall median DOR 18.4 months (95% CI: 14.9–NE)
• Patients with CR or better:
68.5% (95% CI: 57.7–77.1)
80.1% (95% CI: 67.6–88.2)
Side Effects
• Cytokine release syndrome
• Injection-related reactions
• Injection-site reaction
• Infections
• Neutropenia
• Anemia
• Thrombocytopenia

• Neurotoxicity
• Available only through a Risk Evaluation and Mitigation Strategies (REMS) due to the risk of cytokine release syndrome
• Patients will receive step-up dosing and will be monitored in an inpatient setting
• Cytokine release syndrome is managed in the same fashion as CAR T


• Injection reactions are managed with oral antihistamines and topical steroids
• Infection prevention!
• COVID precautions

*Based on a recent sampling
Duration of Response (n = 39)
On Treatment as of April 6, 2022 In follow-up as of April 6, 2022 Dose level (mg/kg) 1.5 QW 1.5 QW 1.5 QW 3.0 QW 3.0 QW Penta-ref; BCMA-ex 3.0 QW CD-38-ex 1.5 QW Penta-ref 1.5 QW Penta-ref 1.5 QW Penta-ref 3.0 Q2W Penta-ref; BCMA-ex 1.5 QW 1.5 QW Triple-ref 3.0 QW CD-38-ex 1.5 QW 3.0 QW Triple-ref 1.5 QW CD-38-ref 3.0 Q2W Triple-ref 3.0 Q2W Penta-ref 3.0 Q2W Triple-ref 1.5 Q2W Triple-ref 3.0 Q2W 1.5 QW Penta-ref; BCMA-ex 1.5 QW Penta-ref; BCMA-ex 1.5 QW 3.0 Q2W Triple-ref 3.0 Q2W CD-38-ref 1.5 QW Triple-ref 3.0 Q2W Penta-ref 3.0 Q2W Penta-ref 3.0 Q2W Penta-ref 3.0 Q2W 3.0 Q2W CD-38-ex 3.0 Q2W 3.0 Q2W CD-38-ex 3.0 Q2W Triple-ref 3.0 Q2W CD-38-ex 3.0 Q2W 3.0 Q2W CD-38-ref 1.5 Q2W Triple-ref
sCR CR VGPR PR MR SD PD
D/C-PD D/C-AE D/C-Other Death
*Response evaluable patients.
Rodriguez-Otero. EHA 2022. Abstr S188.
§ Responses were durable and deepened over time
§ At median follow-up of 8.6 mo (range: 0.3-19.6), 66.7% of responders were alive and continuing on treatment
Phase 1b study (MajesTEC-2) in RRMM with 1–3 prior lines of therapy (including an IMiD and a PI).
32 patients—who had received at least 2 prior lines of therapy—received treatment with the triplet with Tecvayli at 2 different doses (0.72 mg/kg and 1.5 mg/kg) subcutaneously.
IMiD, immunomodulatory drug; PI, proteasome inhibitor
Data from this trial was recently used to submit a Biologics License Application to the U.S. Food and Drug Administration for the treatment of patients with relapsed or refractory multiple myeloma.
288 patients—with no prior T-cell redirecting therapies—received treatment with talquetamab at 2 different doses (0.4 mg/kg every week and 0.8 mg/kg every other week) subcutaneously. IMiD, immunomodulatory drug; PI, proteasome inhibitor
Data from this trial was recently used to submit a Biologics License Application to the U.S. Food and Drug Administration for the treatment of patients with relapsed or refractory multiple myeloma.
 Phase 1/2 study (MonumenTAL-1) in RRMM
Phase 1/2 study (MonumenTAL-1) in RRMM

At the time of this presentation, no patients who achieved an sCR have relapsed!
• Viruses: CMV, EBV
• PCP/PJP
• Ongoing discussions regarding prophylactic measures
- IVIG
- Anti-infectives
Off target effects (with GPRC5D targeted agents)
Cytokine release syndrome (CRS)
Infections
• Usually occurs within first 1–2 weeks

• Frequency (all grade and grade 3–

5) higher with CAR T
Cytopenias
Neurotoxicity (ICANS)

ICANS, immune effector cell-associated neurotoxicity syndrome; CMV, cytomegalovirus; EBV, Epstein-Barr virus; PCP/PJP, pneumocystis pneumonia/pneumocystis jiroveci pneumonia
Cytokeratin changes/rash
Dysgeusia

CAR T and bispecific antibodies are very active even in heavily pretreated patients.
Side effects of CAR T cells and bispecific antibodies include cytokine release syndrome, confusion, and low blood counts, all of which are treatable.
Abecma and Carvykti are only the first-generation CAR T cells and target the same protein. Different CAR Ts and different targets are on the way.
Bispecific antibodies represent an “off-the-shelf” immunotherapy; Tecvayli was approved in October 2022.
Several additional bispecific antibodies are under clinical evaluation.
Panobinostat Daratumumab Ixazomib
Elotuzumab Isatuximab
Idecabtagene
Ciltacabtagene


March 4, 2023, Agenda
1:15 – 2:00 PM Clinical Trials

David Vesole, MD, PhD, Medstar Georgetown University Hospital
2:00 –
2:15 PM Q&A
2:15 –

3:00 PM Life is a Canvas, You are the Artist
Sandra Rome, RN, MN, AOCN, CNS, Cedars-Sinai Medical Center, IMF Nurse Leadership Board
3:00 –
3:15 PM Q&A
3:15 –
3:30 PM Local Patient & Care Partner Panel
Tom Tucker, Patient Advocate and Helen Tucker, Care Partner
3:30 – 3:40 PM Q&A
3:40 – 3:55 PM Community Resource

3:55 – 4:00 PM Closing Remarks
4:00 PM Coffee / Network

 David
David
 MD, PhD, FACP
MD, PhD, FACP
Director, Myeloma Program
MedStar Georgetown University Hospital
Professor of Medicine
Georgetown University School of Medicine
Co-Chief, Myeloma Division
Director, Myeloma Research
John Theurer Cancer Center at Hackensack UMC
Professor of Medicine
Hackensack Meridian School of Medicine
David.Vesole@hmhn.org

• Clinical trials translate results of basic scientific research into better ways to prevent, diagnose, or treat cancer
• The more people that take part, the faster we can:
• Answer critical research questions
• Find better treatments and ways to prevent cancer

Do Many People Participate in Cancer Clinical Trials?
Only 3 percent of U.S. adults with cancer participate in clinical trials
• Cancer clinical trials are
– Carefully controlled research studies
– Conducted by doctors to improve the care and treatment of cancer patients
• The aim of a clinical trial is to
– Study a new therapy or a new use for an already approved therapy
– Compare a new treatment with a standard treatment to find out which one works better and/or has fewer side effects
• Each cancer clinical trial has a written detailed study design called a protocol that includes:
• Why the clinical trial is needed
• Purpose of the clinical trial
• What drug or drug(s) are being tested, with a treatment and follow-up schedule
• Safety measures throughout the clinical trial program
• How outcomes will be measured
• Who is eligible for the clinical trial
• How the clinical trial will be organized, one site or multiple sites
• If the clinical trial is a multi-site trial, all participating physicians must follow the same protocol
Identify a target for therapy in the laboratory
Confirm the anticancer activity in laboratory and animal studies
Clinical trials (human studies) to determine safety, dosing and effectiveness
The whole process costs millions of dollars and years of effort!
How How do clinical trials work?
Phase I investigates for safety and side effects, dosage and best way to give treatment–includes 20 or more people



Phase II determines effectiveness and safety–typically includes fewer than 100 (may include up to 300) people
Phase III looks at effectiveness, side effects and safety in comparison with other treatments–includes 100s to 1000s of people
Phase IV gathers more information after FDA approval & drug is on market
Possible benefits:
•Patients will receive, at a minimum, the best standard treatment
•If the new treatment or intervention is proven to work, patients may be among the first to benefit
•Patients have a chance to help others and improve cancer care
Possible risks:
• New treatments or interventions under study are not always better than, or even as good as, standard care
• Even if a new treatment has benefits, it may not work for every patient
• Health insurance and managed care providers do not always cover clinical trials
Patients may:
• Be unaware of clinical trials
• Lack access to trials
• Fear, distrust, or be suspicious of research
• Have practical or personal obstacles
• Face insurance or cost problems
• Be unwilling to go against their physicians’ wishes
• Be unwilling to “lose control” of a person’s care
• Believe that standard therapy is best


I may get a sugar pill (placebo) instead of real therapy.
No placebos are given alone every patient receives treatment.
I’ll be treated like a guinea pig.
Clinical studies are for people with no other options.
Most patients receive care that exceeds expectations.

Many involve an adjustment to a standard of care that may improve outcome or quality of life
The more people who participate, helps to speed drug development.



• Discuss whether or not you are eligible for a clinical trial with your physician
• Work with your physician to determine the best trial for you
• Meet with the clinical research nurse or trials coordinator to discuss the trial
• Carefully review the provided “Informed Consent”
• Describes the study and any potential safety concerns related to the experimental medication

• SO many areas being studied right now including…
• CAR T Cell therapy (used earlier in myeloma, new targets, faster manufacturing, even allo CAR T!)
• Novel Bispecific Therapies (with new targets)
• New molecules – CelMods, Modakafusp…
And MANY more!





March 4, 2023
Sandra Rome, RN, MN, AOCN, CNS
Cedars-Sinai Medical Center
Los Angeles, California
COLOR WHEEL OF TREATMENT
Myeloma treatment
Infection and Side Effect Management
Know your care team, Telehealth & Meeting Prep, & Shared Decision Making



• Rapid and effective disease control

• Durable disease control
• Minimize side effects
• Allow for good quality of life
• Improved overall survival
• Prevent disease- and treatmentrelated side effects
• Optimize symptom management

• Allow for good quality of life
DISCUSS GOALS AND PRIORITIES WITH YOUR HEALTHCARE TEAM
Transplant
Eligible Patients
Transplant
Consolidation
Maintenance
Initial Therapy
Transplant
Ineligible patients
Consolidation/ Maintenance/ Continued therapy

Treatment of Relapsed disease
Everyone
Supportive Care
• There are no black and white answers to deciding to undergo a transplant
• Undergoing transplant is a commitment for both you and your care partner
• Understanding the process will help bring to focus elements needed to decide if/when to undergo transplant

Clinical Experience Data from Research
Patient Preference
Adapted from Philippe Moreau, ASH 2015




A meta-analysis identified the most common patient-reported symptoms and impact on QOL, and were present at all stages of the disease. Symptoms resulted from both myeloma disease and treatment, including transplant, and were in these categories:
Physical
• Fatigue
• Constipation
• Pain
• Neuropathy

• Impaired Physical Functioning
• Sexual Dysfunction

Psychological
• Depression
• Anxiety
• Sleep Disturbance

• Decreased Cognitive Function
• Decreased Role & Social Function

Financial
• Financial burden (80%)
• Financial toxicity (43%)
&
Steroid Synergy
Steroids are a backbone and work in combination to enhance myeloma therapy
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over-the-counter or prescription medications

• Medications to prevent shingles, thrush, or other infections
Steroid Side Effects
• Irritability, mood swings, depression

• Difficulty sleeping (insomnia), fatigue
• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Increase in blood pressure, water retention
• Blurred vision, cataracts
• Flushing/sweating
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes

• Increase in blood sugar levels, diabetes
Rajkumar
SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR, Eastern Cooperative Oncology Group (2010) Lenalidomide plus highdose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 11(1):29–37.
Faiman B. Steroid-Associated Side Effects: A Symptom Management
Both viral and bacterial
– Up to a 3rd of patients on clinical trials has serious infections (requiring IV antibodies or hospitalization)
Increased risk of serious COVID complications despite history of vaccination
– Antibody levels
–
Tixagevimab co-packaged with cilgavimab (EVUSHELD)
–
Immediate treatment once diagnosed Nirmatrelvir with Ritonavi (Paxlovid)
• Start as soon as possible; must begin within 5 days of when symptoms start

7-10 fold increased risk of bacterial and viral infections for people with myeloma
care team
Good personal hygiene (skin, oral)
Environmental control (wash hands, avoid crowds and sick people, etc)
Growth factor (Neupogen [filgrastim])

Immunizations (NO live vaccines)
• Medications (antibacterial, antiviral)
Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
Manage stress
• Rest, relaxation, sleep hygiene
• Mental health / social engagement
• Complementary therapy
Maintain a healthy weight
• Nutrition
• Activity / exercise

Preventative health care
• Health screenings, vaccinations
• Prevent falls, injury, infection
• Stop smoking
• Dental care
Maintain renal health
• Myeloma management
• Hydration
• Avoid renally-toxic medications
– Dose adjust to renal function
• Diabetes management
Protect your bones
• Nutrition, Calcium + D supplement
• Weight-bearing activity / walking
• Bone strengthening agents
Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.
“An ounce of prevention is worth a pound of cure.” Benjamin Franklin
Diarrhea may be caused by medications and supplements
• Laxatives, antacids with magnesium
• Antibiotics, antidepressants, others
• Milk thistle, aloe, cayenne, saw palmetto, ginseng
• Sugar substitutes in sugar free gum
Avoid caffeinated, carbonated, or heavily sugared beverages
Take anti-diarrheal medication
• Imodium®, Lomotil®, or Colestid if recommended
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
• Welchol® if recommended

Constipation may be caused by
• Opioid pain relievers, antidepressants, heart or blood pressure medications, others
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency
Increase fiber
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
Fluid intake can help with both diarrhea and constipation, and good for kidneys. Discuss GI issues with health care providers to identify causes and make adjustments to medications and supplements.

Pain can significantly compromise quality of life
Sources of pain include bone disease, neuropathy and medical procedures
Management
• Prevent pain when possible
• Bone strengtheners to decrease fracture risk; anti viral to prevent shingles; sedation before procedures
• Interventions depends on source of pain
• Monitor serum calcium levels
• Imaging may be needed depending on type and location of pain (eg, MRI, PET-CT)
• May include medications (eg bone modifying agents), activity, surgical intervention, radiation therapy, etc



• Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)
Tell your health care provider about any new bone pain or chronic pain that is not adequately controlled
Peripheral neuropathy: damage to nerves in extremities (hands, feet, or limbs)
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness
Prevention / management:
• Bortezomib once-weekly or subcutaneous administration
• Massage area with cocoa butter regularly
• Supplements:
• B-complex vitamins (B1, B6, B12)
• Folic acid, and/or amino acids but do not take on day of Velcade® (bortezomib) infusion
• Safe environment: rugs, furnishings, shoes

If PN worsens, your HCP may:
• Change your treatment
• Prescribe oral or topical pain medication
• Suggest physical therapy
Report symptoms of peripheral neuropathy early to your health care provider; nerve damage from PN can be permanent if unaddressed
All can affect quality of life and relationships
• Fatigue is the most common reported symptom (98.8%)
Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression


• Anxiety reported in >35%


• Depression nearly 25% Financial concerns, disease progression, end-of-life, and change in social and sexual function were highlighted sources
Often, people do not share these symptoms with their provider. Talk to your provider about symptoms that are not well controlled or thoughts of self harm. Help is available.
Financial burden comes from
• Medical costs
• Premiums
• Co-payments

• Travel expenses
• Medical supplies

• Prescription costs
• Loss of income
• Time off work or loss of employment
• Caregiver time off work
Contact the Social Services department at your hospital or clinic to talk to a social worker for assistance.
Funding and assistance may be available
• Federal programs
• Pharmaceutical support
• Non-profit organizations
• Websites:
• Medicare.gov
• SSA.gov
• LLS.org
• Rxassist.org
• NeedyMeds.com
• HealthWellFoundation.org
• Company-specific website
Care partner support is essential for the entire transplant and CAR T processes particularly
• Sedated procedures; Education sessions
• Assistance with daily activities, managing medications and alerting the medical team of changes
• Continued support and assistance is often needed in the early days after returning home. Less assistance will be needed as time goes on.

Care partner can be one person or a rotation of many people.




You are central to the care team








Be empowered
• Ask questions, learn more

• Participate in decisions
Communicate with your team
• Understand the roles of each team member and who to contact for your needs
• Participate in support network








Come prepared:
• Bring a list of current medications, prescribed and over the counter
• Write down your questions and concerns. Prioritize them including financial issues
• Have there been any medical or life changes since your last visit?
• Current symptoms - how have they changed (improved, worsened, stable)? Keep a symptom diary. Bring it along
• Communicate effectively: your health care team can’t help if they don’t know
• Know the “next steps”, future appointments, medication changes, refills, etc
Check with your healthcare team –Is telemedicine an option?
Similar planning for “in-person” appointment PLUS:
• What is the process and what technology is needed?


• Plan your labs: are they needed in advance? Do you need an order?
• Plan your location: quiet, well-lit location with strong wi-fi is best
• Plan yourself: consider if you may need to show a body part and wear accessible clothing
• Collect recent vital signs (blood pressure, temp, heart rate) self-serve blood pressure cuff is available at many pharmacies and for purchase
Be empowered to be part of the treatment decision-making

• Ask for time to consider options (if needed/appropriate)
• Understand options; consider priorities
• Use reliable sources of information
• Use caution considering stories of personal experiences
• Consider your goals/values/preferences
• Express your goals/values/preferences; create a dialog


• My top priority is [goal/value]; additional [preferences] are also important.
• I think [treatment] may be a good choice given my priorities… What do you think?
• Arrive at a treatment decision together
Philippe Moreau. ASH 2015.






























• Born in Yosemite in 1947

• Father worked for National Park Service
• Graduated Eighth Grade from Yosemite Elementary School 1960
• Moved to San Diego
• Graduated Mission Bay High School 1964
• Member of First Entering Freshman Class at UC San Diego 1964
• Cooperative Physics Trainee at Navy Electronics Laboratory while in college
• Graduated BA Physics from UC San Diego in 1969

• Co-op program included one year off, working full time
• My assignments included at sea time on USS Baya, NEL’s research submarine

• Completed Navy OCS, Nuclear Power Training, Submarine School, reported to USS Plunger in Pearl Harbor April 1971

• Co-op program cancelled when I was returning to complete Senior Year
• Applied for Navy OCS, picked up by NUPOC Program
• ADM Rickover accepted me, after explaining I was a Draft Dodger
• Plunger reassigned to Mare Island Naval Shipyard for SUBSAFE, Refueling Overhaul
September 1971
• Met Army Nurse, Helen Shriver, at Pearl Harbor Officers Club, married her in February 1972

• Plunger 18 Month Overhaul took 27 months
• Had to extend Active Duty for 10 months to complete Submarine Qualification
• Returned to Navy Lab in 1975 after a quarter at UC San Diego Grad School
• Affiliated with Naval Reserve after Release from Active Duty

• Worked Developmental Undersea Surveillance Projects, including significant sea time
• Completed MS, Applied Physics in 1978
• Was Navy Scientific Diver
• Settled in San Diego
• Enjoyed 3 children: Rick born 1973, Kathy born 1975, Mike born 1978

• Helen became School Nurse
• Moved to private sector in 1985
• Advanced Digital Systems, Titan Corp (1985 – 1998)
• Cubic Transportation Systems (1998 -2001)
• Daughter Kathy killed in bicycle accident in 1988
• Retired from Naval Reserve in 1995 after 21 years, 3 Command Tours.

• Diagnosed with Myeloma in October 2006 because of abnormal blood tests in routine physical
• 70% Plasma Cells in Bone Marrow
• Started Induction Therapy with Thalidomide
• Referred to Kaiser LA Bone Marrow Transplant Dept
• Dr. Firoozeh Sahebi recruited me for ASCT Clinical Trial at City of Hope
• Randomized to Tandem Transplant Arm
• ASCTs at City of Hope in April, June 2007

• Bone Marrow Biopsies showed
• Cubic laid me off in 2001
• Returned to Navy Lab
• Picked up 6 months Sick Leave
• Worked up to Autonomous Distributed Networked Systems Branch Head
• 30% Plasma Cells after Induction Therapy
• 15% after First ASCT
• 5% after Second ASCT
• 0% in September 2007
• Cognitive Issues after ASCTs resulted in retiring from Navy Lab in January 2008

• Randomized to No Maintenance Therapy Arm of Clinical Trial
• Relapsed Spring 2013
• Detectable M-Protein, Increasing IgA, Lambda in labs
• Bone Marrow Biopsy 30% Plasma Cells
• Returned to treatment with 10 mg Revlimid® in August 2013
• Disease responded to Revlimid®
• IgA, Lambda in normal range, M-Protein below quantifiable level
• Relapsed late 2010

• Detectable M-Protein, Increasing IgA, Lambda in labs
• Started treatment with 10 mg Revlimid® in January 2011
• Discontinued Revlimid® due to low blood counts (WBC, Platelets)
November 2011
• IgA, Lambda levels back in normal range, no quantifiable M-Protein
• Discontinued Revlimid® November 2015 because of low neutrophils
• Slow Increase in IgA, Lambda in early 2016
• Tried 5 mg Revlimid®, but didn’t control


• Started Velcade® in June 2016
• IgA slowly increasing out of normal range in August 2018
• Bone Marrow Biopsy showed 5% Plasma Cells
• Continued Sub-Q Velcade® based on Bone Marrow Biopsy results
• Added 2 mg Pomalyst® in June 2021 due to rising IgA
• Filgrastim support added October 2021 because of neutropenia
• Discontinued Velcade® in December 2019 due to increasing Peripheral Neuropathy, Stable Disease
• Changed to Darzalex®/Dexamethasone in August 2020 due to rising IgA
• Increased Filgrastim to twice weekly in April 2022 because of low WBC, neutrophil levels

I am incredibly blessed to be a 16 year myeloma survivor
• No such thing as an average myeloma patient
• Some have aggressive disease, others less aggressive
• Patients range across all walks of life, economic levels
• Very conscious that my myeloma has been relatively indolent, allowing treatments to keep me going
• For me, myeloma has been a chronic disease, rather than the near-term terminal disease that was the expectation when I was diagnosed in 2006
• Looking forward to new treatments becoming available to allow me to extend my survival
• Also looking forward to effective treatments for those who have a more aggressive myeloma
• Appreciate having Helen as my partner and caregiver
• Celebrated 50th Anniversary in February 2022






For Myeloma Action Month (MAM), let’s join together to increase awareness of myeloma AND support groups all while nurturing ourselves!
The IMF invites support group members to take action through mindfulness, movement and self enrichment. Support Group Members will work individually but together as a group to record the actions accomplished.

ITS AS EASY AS 1-2-3!
1. Sign up: Here
2. Take Action: complete as little or as many activities as you’d like each day
3. Log your activities: Here


How to earn points:
1 point per 15 minutes of each activity completed
•Mindfulness
•Movement
•Self Enrichment
•Share & Educate
How, what and when you do it is up to you!












Patient and Family Webinars:


Boca Raton, FL – March 17 & 18, 2023 - with local Myeloma expert Dr. James Hoffman, and a presentation from Professor Sigurður Kristinsson from Iceland.
Virtual PFW – May 17, 2023 – keep an eye on our website for registration for this virtual program. It will air LIVE at 5:30PM PST
Los Angeles – August 18 & 19, 2023 – details to be announced, this event will have an international myeloma expert presenting!
Thank you for attending today's program!
March 4, 2023, International Myeloma Foundation’s Regional Community Workshop –San Diego.
A recording of todays program will be made available on our website in the coming weeks.
