

Thank you for joining us today for the May 18th, 2024, International Myeloma Foundation’s Regional Community Workshop –
Salt Lake City
Welcome!
Thank you to our sponsors!








IMF Regional Community Workshop
May 18th, 2024 - Agenda

9:00 – 9:05 AM Welcome & Introductions, Robin Tuohy
9:05 – 9:15 AM IMF’s Vision, Yelak Biru
9:15 – 9:45 AM Myeloma 101, Peter Forsberg, MD
9:45 – 9:55 AM Q&A
9:55 – 10:30 AM Life is a Canvas, You are the Artist – Mary Steinbach, DNP, APRN
10:30 – 10:40 AM Q&A
10:40 – 11:00 AM Coffee Break
11:00 – 11:35 AM Frontline Therapy, Doug Sborov, MD, MS
11:35 – 11:45 AM Q&A
11:45 AM – 12:40 PM LUNCH
IMF Regional Community Workshop
May 18th, 2024 – Agenda after lunch

12:40 – 12:50 PM Engaging & Partnering with the IMF, Sylvia Dsouza
12:50 – 1:10 PM Local Patient & Care Partner Panel, John and Ann Bailey
1:10 – 1:20 PM Q&A
1:20 – 1:40 PM Maintenance Therapy, Doug Sborov, MD, MS
1:40 – 1:50 PM
1:50 – 2:30 PM Relapsed Therapies & Clinical Trials, Peter Forsberg, MD 2:30 – 2:45 PM
Closing Remarks/Coffee & Networking
Q&A
Q&A 2:45 – 3:00 PM
Multiple Myeloma affects patients and families.
The IMF provides FREE resources to help both patients and families.
Established in 1990, the IMF’s InfoLine assists over 4600 callers annually and answers questions across a wide variety of topics including:

Frequent topics:
Treatment questions along the spectrum of care
Clinical Trial access and understanding
Side effect management and health issues
Financial resources for myeloma-related expenses
Myeloma Specialist Referral contact information
Support group information
Caregiver Support




6 Contact the InfoLine: 800-452-CURE (2873) InfoLine@myeloma.org
Paul Hewitt, Missy Klepetar, & Teresa Miceli
Educational Publications

A core mission of the IMF is to provide thorough and cutting-edge education to the















New publications




The IMF Support Group Team is Here For You! 8 Shared Experiences Help to Better Understand the Myeloma Journey • Support Groups Empower Patients & Care Partners with information, insight, & hope • The IMF provides educational support to a network of over 150 myeloma specific groups We are happy to help connect you with an existing support group or help form a new one! We assist with virtual, in-person, and hybrid options for meetings. Reach out to us at SGTeam@myeloma.org Support.myeloma.org
Local Support Groups: You Are Not Alone!


Huntsman’s Multiple Myeloma
Patient Education Group
Meets Virtually on the 2nd Tuesday of each month at 12pm Mountain Time

9
IMF – Special Interest Virtual Groups

Special interest groups are designed as a supplemental support for specific populations of patients, in addition to their local Support Groups
Las Voces de Mieloma
Designed for Spanish speaking patients only
Living Solo & Strong with Myeloma
Designed for patients without a care partner

Starting on June 13, 2024!
Care Partners Only
Designed to address the needs of care partners only

Smolder Bolder
Created for people living with Smoldering Multiple Myeloma
MM Families

High Risk Multiple Myeloma
Designed to address the needs of the high-risk MM population
MGUS 4 Us
Created for people living with MGUS
For patients/care partners with young children

10

IMPACT
VIDEO
11


Vision

A world where every myeloma patient can live life to the fullest, unburdened by the disease.

Our Four Pillars

The potential of coordinated, consolidated and global SUPPORT to patients and their partners is unparalleled

The need for informed and global ADVOCACY is critical to both improving lives and finding the cure

Raise the Bar
Examine the why of all our actions to ensure they are purpose-driven, meaningful, and effective.
Mission

Improving the quality of life of myeloma patients while working toward prevention and a cure!

Myeloma specific EDUCATION historically was not patient directed nor of high caliber – theright knowledge is power

The medical and RESEARCH world can often exist in silos due to institutional demands and limitations
Strategy

Broaden our Reach
Address unmet patient needs by expanding our reach to diverse & underserved populations in everything we do.

Innovate Every Step of The Way
Provide those who need it most with what they need the most, throughout their myeloma journeys.
12 8
Myeloma 101
Peter Forsberg, MD
Colorado Blood Institute

Denver, CO
Multiple Myeloma 101
IMF Salt Lake City Regional Community Workshop
Peter Forsberg MD Co-Director of Plasma Cell Disorders
Colorado Blood Cancer Institute


Disclosures
•Consultant - BMS, GSK, Karyopharm, Sanofi
•Research Support - Karyopharm, Genentech, BMS, Janssen


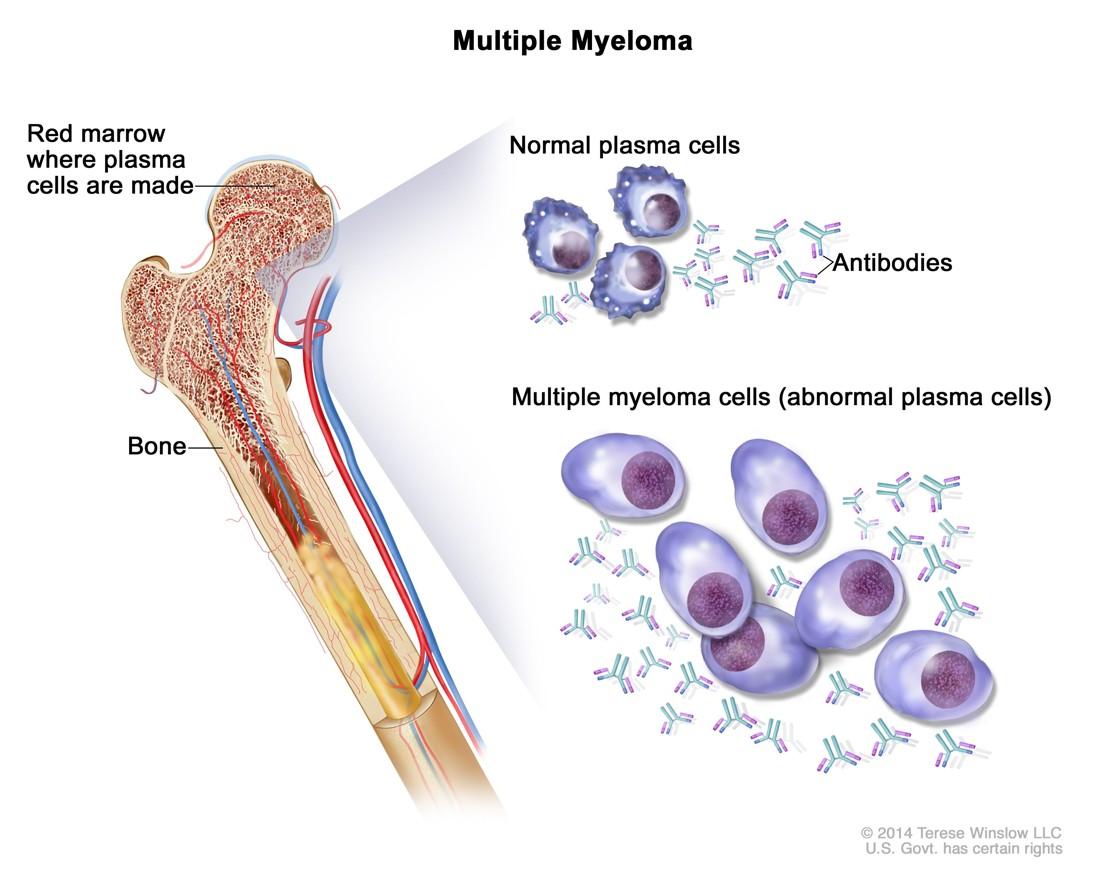
What is Multiple Myeloma?
•Cancer arising from Plasma Cells
•Cancer is any condition where cells grow and survive in uncontrolled way
•Plasma cells are antibody producing immune cells in bone marrow
•When antibody is coming from cells that are very similar it is atypical (monoclonal)

https://www.cancer.gov/publications/dictionaries/cancer-terms/def/plasma-cellmyeloma.

Plasma Cells are fully developed B-cells



At diagnosis myeloma is usually causing problem

Multiple Myeloma Cells









BoneRelated Signs and Symptoms
1. Kyle RA, et al. Mayo Clin Proc. 2003;78(1):21-33. 2. Ropper AH, et al. N Engl J Med. 1998;338(22):1601-1607. 3 Kissel JT, et al. Neuromuscul Disord. 1996;6:3-18. 4. Bladé J, et al. Hematol Oncol Clin N Am. 2007;21(6):1231-1246. 5. McGuire TR. Multiple myeloma. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York, NY: McGraw-Hill; 2008:2295-2307.

1 M protein Bone Pain1 Neuropathy2,3 Hypercalcemia1 Immunodeficiency Anemia1 Lytic Lesions1 Infection4,5 Marrow
n
Renal Impairment
Infiltratio
Signs/Symptoms at MM Presentation
•Anemia – 73%
•Bone pain – 58%
• Predominantly involves the central skeleton (back, neck, shoulders, pelvis, hip), rather than the extremities
•Elevated creatinine – 48%
• Cast nephropathy & Hypercalcemia most common causes
•Fatigue/generalized weakness – 32%
•Hypercalcemia – 28%
•Weight loss – 24%
•Infection


Kyle RA. Mayo Clin Proc 2003;78:21
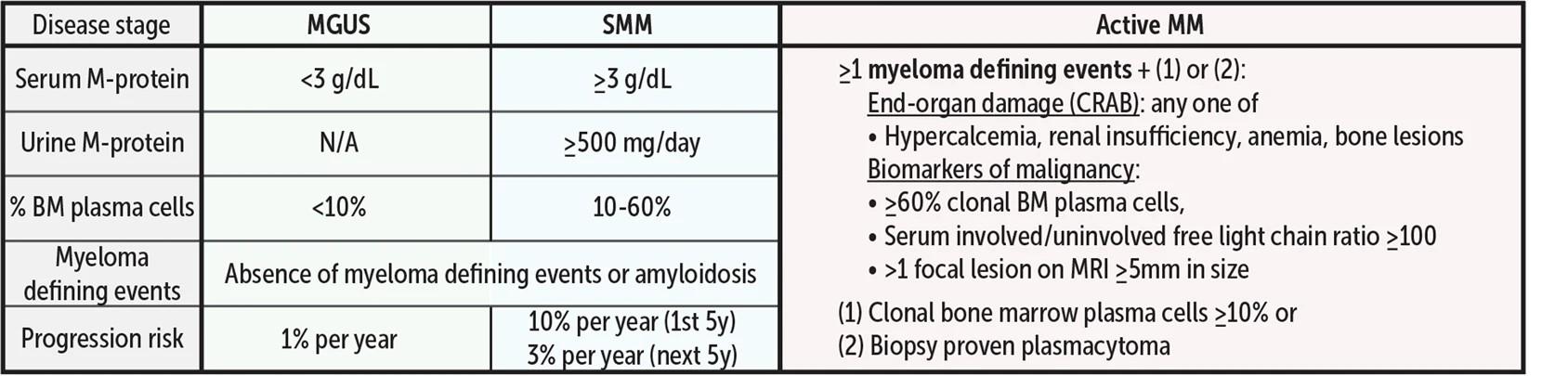
What are Smoldering MM and MGUS?
•Asymptomatic plasma cell disorders
•With smoldering there is higher disease burden and higher risk of developing MM (and more monitoring is needed)


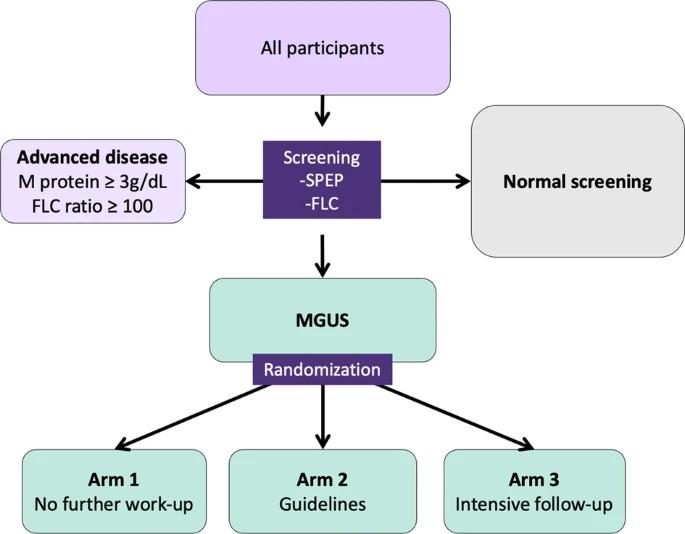
Do people need screening MGUS/Smoldering MM?
•Currently not recommended routinely, but we’re learning more
•iStopMM Trial: First population-based prospective screening study for monoclonal gammopathies with a randomized controlled trial of follow-up strategies
•All Icelandic residents over 40 (n=148,711) invited to enroll
•Over 15 months total of 80,759 (54.3%) consented to participate
•After enrollment serum collected for screening SPEP and sFLC
•Enrolled patients with monoclonal gammopathies randomized to 3 different monitoring arms


Rognvaldsson et al. Blood Cancer Journal 2021 11; 94
iStopMM
•Already yielding very useful information
• Prevalence of MGUS, smoldering myeloma
• Risk calculator for smoldering to guide BM biopsy
• Reference ranges for light chain levels in people with kidney issues, at different ages
•Long term data will help explain if screening for monoclonal gammopathies in asymptomatic people may improve survival


What are these labs? Part 1: Antibodies
•Antibodies are part of the immune response
•They attach to something foreign (like bacteria and viruses) and activate an immune response
•In MM one atypical antibody (or the light chain fragment of an antibody or some of both) is produced by myeloma cells
•Measuring the MM related antibody is how we track MM


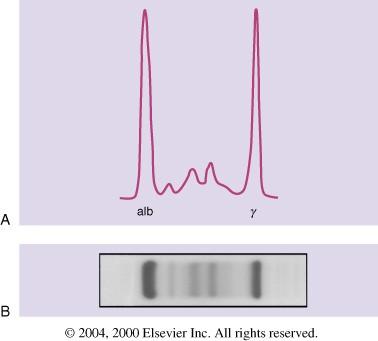
What are all these labs? Part 2:
The SPEP
•Serum protein electrophoresis
•Serum is the part of blood with cell removed
•Put on a gel and an electric current is applied
•Proteins move based on size/charge
•Most antibodies end up in the gamma region



Medicine, 22nd Edition, 2004
Kyle RA and
Rajkumar
SV. Cecil Textbook of
What are all these labs? Part 3:
The monoclonal protein
•In patients with MM the abnormal antibody all moves into one place in the gamma region and creates a band or spike (m-spike/m-protein)
•SPEP allow detection and measurement of this monoclonal spike


2004
Kyle RA and Rajkumar SV. Cecil Textbook of Medicine, 22nd Edition,
What are these labs? Part 4:The Immunofixation electrophoresis (IFE)
•Complimentary test to the SPEP
•Tells what type of antibody spike is present (IgG vs IgA or IgM, kappa or lambda)
•More sensitive than an SPEP for small m-proteins
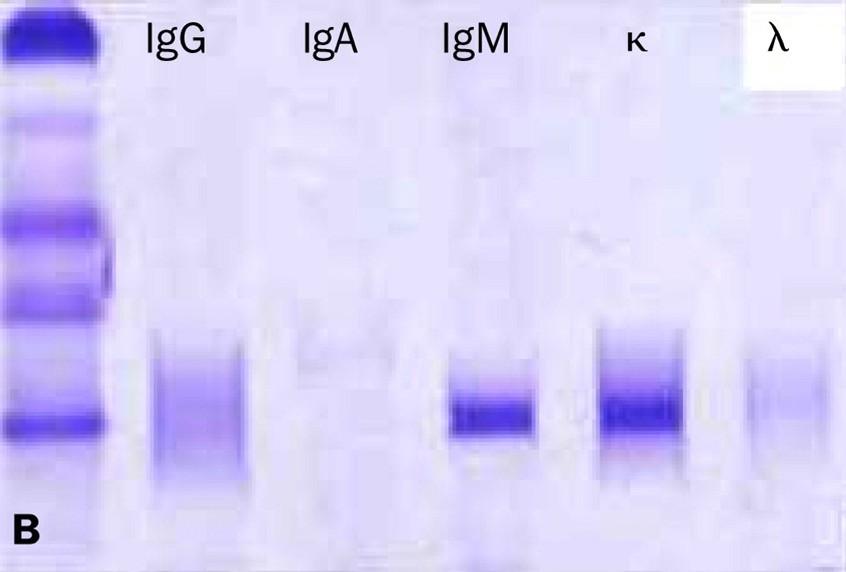
kappa M-protein



IgM
IFE Copyright © 2003 Elsevier Ltd Terms and Conditions The Lancet Oncology 2003 4, 679-685DOI: (10.1016/S1470-2045(03)01246-4)
What are these labs? Part 5:
Serum Free light chains
•Light chains are a fragment of an intact antibody
•Free light chain tests detect light chains that are separate from a full antibody
•Two different types are produced- kappa/lambda
•Serum FLC tests have reduced need for urine monitoring


exposed surface hidden surface hinge region
Previously hidden surface
carbohydrate
Kappa
Lambda
Intact Immunoglobulin Free Light Chain
Bradwell, Serum free light chain assay
What is MRD?
•Measurable (or minimal) residual disease
•Any approach to detect residual disease beyond historical techniques
• Several different methods
• Different techniques used in different diseases
•In myeloma flow cytometry and next generation sequencing are most commonly used
•Can detect presence of myeloma cells in the bone marrow at level of 1 in 100,000 (10 -5) or 1,000,000 (10-6) cells


How rare/common is multiple myeloma?
Accounts for ~10% of all hematologic malignancies,~2% of all malignancies, lifetime risk 0.8%
The amount of myeloma in the population is changing:
• 20,180 patients diagnosed in 2010; 26,850 in 2015; 34,470 estimated in 2022
• 159,787 patients living with MM in the USA in 2019, up from 61,000 in 2010

https://seer.cancer.gov/statfacts/html/mulmy.html

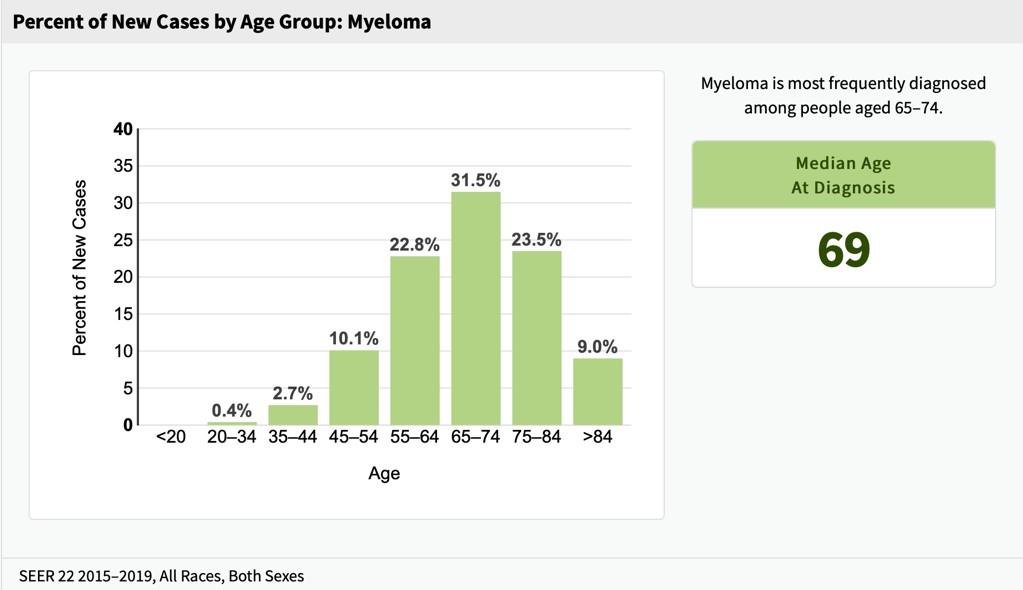
Who gets multiple myeloma?


2014 IMWG Active Myeloma Criteria: Myeloma-Defining Events
Clonal bone marrow ≥10% or bony/extramedullary plasmacytoma AND any one or more Myeloma-Defining Events
alcium elevation
enal complications nemia one disease



A B
R
C
Clonal bone marrow ≥60% BM FLC MRI sFLC ratio >100 >1 focal lesion by MRI BM, bone marrow; FLC, free light chain; MRI, magnetic resonance imaging; sFLC, serum free light chain. Rajkumar et al. Lancet Oncol. 2014;15:e538-e548. Kyle et al. Leukemia 2010;24:1121-1127. Now SLiM CRAB S (60% Plasmacytosis) Li (Light chains I/U >100) M (MRI 1 or more focal lesion) C (calcium elevation) R (renal insufficiency) A (anemia) B (bone disease)
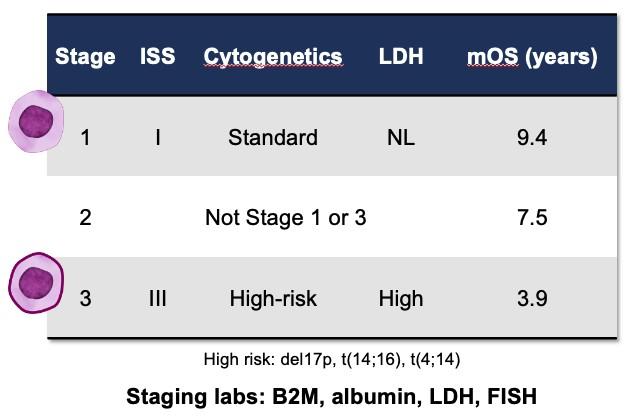
What is my Stage?
•Stage in myeloma is not anatomically based

•Staging is a form of risk-stratification using lab and genetic features Palumbo A, et al. JCO 2015;33(26):2863-2869.
The Revised-ISS

Stage 1 ALB > 3.5 and 2M < 3.5 + Absence of high risk CA AND LDH wnl 28% Stage 2 Neither stage 1 or 3 62% Stage 3 2M > 5.5 + High risk CA OR LDH > ULN 10% 2M=serum 2 microglobulin in mg/dL; ALB=serum albumin in g/dL, CA = cytogenetic abnormalities (del 17p, t(4;14),t(14;16))
What should I expect after my diagnosis with MM?
•Expectations continue to evolve in very broadly positive ways
•I struggle greatly to give “life expectancy” predictions
• Our measurements haven’t caught up with our current treatments
•Innovation has led to more options that allow us to tailor the right approach to the right patient
•Risk stratification and discussion around characteristics of an individuals myeloma remains very important
•What is clear is patients with myeloma are living LONGER and living BETTER and that progress should continue


How did we treat myeloma in the past?
Oral melphalan and prednisone
High-dose dexamethasone
Bisphosphonates
High-dose therapy with autologous stem cell support
1962 1984 1986 1996 1990s

Barlogie B et al. N Engl J Med. 1984;310:1353; Berenson JR et al. N Engl J Med. 1996;334:488; Alexanian R et al. Ann Intern Med. 1986;105:8; Bergsagel D. Cancer Chemother Rep. 1962;21:87; Salmon SE et al. Cancer Chemother Rep. 1967;51:179; Rousselot P et al. Cancer Res. 1999;59:1041;McElwainTJ, Powles RL. Lancet. 1983;2:822

VAD
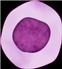
MM Therapy, the Modern Era


Thalidomide 1999 2003 Bortezomib 2006 Lenalidomide 2007 Liposomal Doxorubicin 2012 Carfilzomib 2013 Pomalidomide 2015 Panobinostat Daratumumab Elotuzumab Ixazomib 2019 Selinexor 2020 Belantamab mafadotin Isatuximab 2021 Ide-cel Melflufen 2022 Cilta-cel Teclistimab Early 2000-2013:
targeted therapy 2015-2021: Expansion of Monoclonal Antibodies 2021+: Dawn of T-cell mediated immunotherapies 2023 Talquetamab Elranatamab
First wave of
Myeloma Therapies in 2010
IMiDs
Proteasome inhibitors
Conventional chemotherap
Other stuff
Lenalidomid e (Revlimid)
Bortezomib (Velcade)
Clarithromyc in (Biaxin)
Thalidomide


y
e
Melphalan Cytoxan Bendamustin
Doxil
Myeloma Therapies in 2015
IMiDs
Lenalidomid e (Revlimid)
Proteasome inhibitors
Monoclonal antibodies
Conventional chemotherap
Pomalidomi de (Pomalyst)
Bortezomib (Velcade)
Daratumum ab (Darzylex)
Ixazomib (Ninlaro)
Thalidomide

Carfilzomib (Kyprolis)
Elotuzumab (Empliciti)
Clarithromy cin (Biaxin)
Panobinost at (Farydak)

y Melphalan
Bendamusti ne Doxil
Cytoxan
Other stuff
Myeloma Therapies in 2024
IMiDs/ Cereblon Modifiers
Lenalidomid e (Revlimid)
Bortezomib (Velcade)
Pomalidomi de (Pomalyst)
Thalidomid e Proteasom e inhibitors
Ixazomib (Ninlaro)
Monoclona l antibodies
Daratumum ab (Darzylex)
CAR-Ts and Bispecifics
Ida-Cel (Abecma)
Convention al chemother apy
Melphalan
Other stuff
Elotuzumab (Empliciti)
Carfilzomib (Kyprolis)
Isatuximab (Sarclisa)
Cilta-Cel (Karvykti) Teclistama b (Tecvayli) Elranatam ab (Elrexfio) Talquetam ab (Talvey)

Melflufan
Belantama b
Mafadotin (Blenrep)
Selinexor (Xpovio) Venetoclax (t11;14) Panobinosta t (Farydak)

Cytoxan Bendamusti ne Doxil
Some basic principals of therapy
•Many of our treatments work best in combinations
• 3 to 4 active therapies given together, generally includes a steroid
•What is needed to achieve control of myeloma may be different than what is needed to maintain control of MM
•Some amount of treatment is often continued in an open ended way
•Treatment goals in different patients may differ
•Still a role for autologous transplant
•Options in relapsed myeloma are evolving quickly
• Many approved therapies/combinations
• Expanding role of new immunotherapies (CAR-T and bispecifics)



Questions?

LIFE IS A CANVAS, YOU ARE THE ARTIST
Salt Lake City, UT May 18, 2024
Mary Steinbach, DNP, APRN
Huntsman Cancer Institute-University of Utah

Patient Education Slides 2021
OBJECTIVES
COLOR WHEEL OF TREATMENT
Myeloma and treatment side effects & symptom management

FRAMING YOUR CARE
Know your care team, Telehealth & Meeting Prep, & Shared Decision Making

LIVE LIFE IN COLOR
Healthful Living, infection prevention, renal and bone health

42
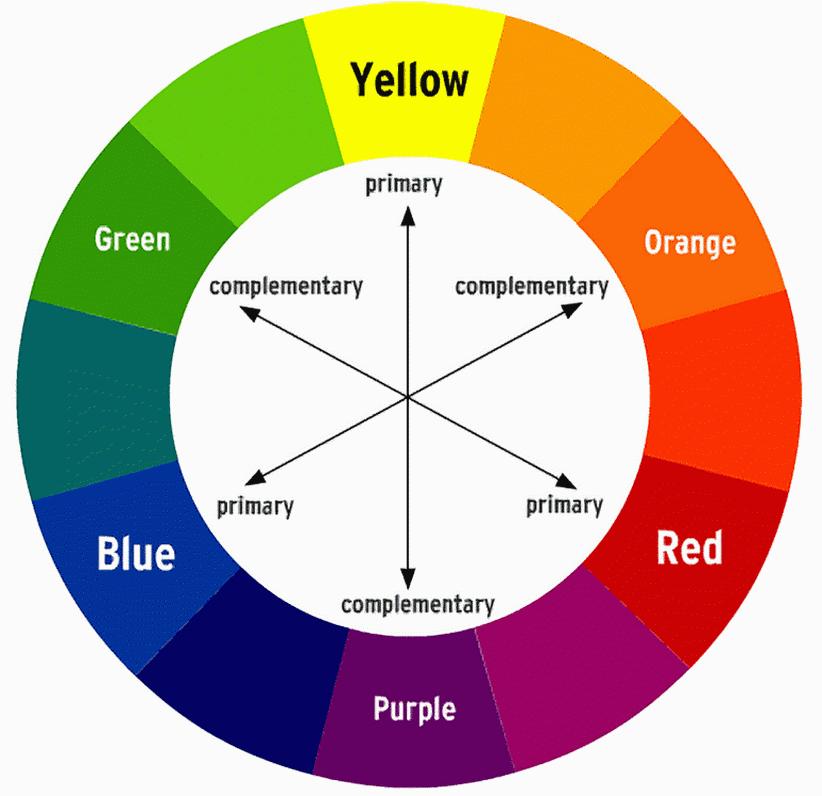
COLOR WHEEL OF TREATMENT
Treatment options, side effects, symptom management, & supportive care

43
GALLERY OF GOALS
MYELOMA TREATMENT

•Rapid and effective disease control
•Durable disease control
•Minimize side effects
•Allow for good quality of life
•Improved overall survival


SUPPORTIVE THERAPIES
•Prevent disease- and treatment-related side effects
•Optimize symptom management
•Allow for good quality of life

DISCUSS GOALS AND PRIORITIES WITH
YOUR HEALTHCARE TEAM

44
Maintenance
Color Wheel of Treatment Options
Velcade® (bortezomib)
Darzalex® (daratumumab)
Thalomid® (thalidomide)
Revlimid® (lenalidomide)
Velcade® (bortezomib)
Kyprolis® (carfilzomib)
Ninlaro® (ixazomib)
Darzalex® (daratumumab)
Empliciti® (elotuzumab)
Sarclissa® (Isatuximab)
Revlimid® (lenalidomide)
Thalomid® (thalidomide)
Revlimid® (lenalidomide)
Pomalyst® (pomalidomide)
CelMods
• Iberdomide
• Mezigdomide
Neuropathy
Carfilzomib: Cardiac

Immunotherapies
SoluMedrol®
Dexamethasone
Prednisone
Prednisolone
SoluMedrol®
Elrexfio™ (elranatamab)
Tecvayli® (teclistamab)
Talvey™ (talquetamab)
Melphalan + ASCT
Abecma® (Idecabtagene Vicleucel)
Carvykti® (ciltacabtagene autoleucel)
Blenrep®* (Belantamab mafodotin)
Infusion reaction
Blenrep: Keratopathy
Other CAR-T
Xpovio® (Selinexor)
Doxil (liposomal doxorubicin)
Venclexta® (venetoclax)
Infection risk
CAR-T: CRS and neurotoxicity
Myelosuppression, GI
Selinexor: Low sodium

-Mibs -MAbs -Mides Steroids Alkylators Bispecific Antibody Cellular Therapies Others
Dexamethasone Prednisone Prednisolone
Melphalan Cyclophosphamide
+ ASCT
Melphalan
Melphalan Cyclophosphamide Bendamustine
DVT/PE
steroid slide
Infusion reaction
See
Myelosuppression
Frontline Relapse Pending FDA Approval
45 ASCT = autologous stem cell transplant; CAR-T cell therapy; CRS = cytokine release syndrome; DVT = deep vein thrombosis; PE = pulmonary embolism *Withdrawn from FDA but still available in certain situations IMF Nurse Leadership Board ONS Symposia 2022; NCCN Guidelines. Multiple Myeloma. V3.2024. Accessed March 25, 2024.
Noted Side effects
COMBINATIONS: MIX, MATCH, BLEND FOR DEPTH


Myeloma Treatment Common Combinations
Velcade® (bortezomib)
DVRd, VRd, Vd
Lenalidomide DVRd, VRd, Rd
Kyprolis® (carfilzomib) KRd, Kd, DKd, Isa-Kd
Pomalyst® (pomalidomide) Pd, DPd, EPd, PCd, Isa-Pd
Darzalex® (daratumumab) DVRd, DRd, DVd, DPd, DVMP, DKd
Ninlaro®(ixazomib) IRd
Empliciti® (elotuzumab) ERd, EPd
Xpovio® (Selinexor) XVd, XPd, XKd
Sarclisa® (Isatuximab) Isa-Kd, Isa-Pd
Blenrep® (Belantamab mafodotin) Bela-d
Abecma® (Idecabtagene Vicleucel) --
Carvykti® (ciltacabtagene autoleucel) --
Elrexfio™ (elranatamab) --
Tecvayli® (teclistamab) --
Talvey™ (talquetamab) --
Venclexta® (venetoclax) Vd + ven
New agents or regimens in clinical trials are possible options
ASCT = autologous stem cell transplant; Bela = belantamab; C = cyclophosphamide; D = daratumumab; d = dexamethasone; E = elotuzumab; Isa = isatuximab; I = ixazomib; K = carfilzomib; M = melphalan; P = pomalidomide; R = lenalidomide; V = bortezomib; ven = venetoclax.
NCCN Guidelines. Multiple Myeloma. V3.2021. Accessed February 1, 2020.

Prescribing information
each
table.
V M R D Dex ASCT
for
drug listed in the
STEM CELL TRANSPLANT
ELIGIBILITY
Measuring Treatment Response
Determining Transplant
Eligibility
Insurance Authorization
Collecting Stem Cells

TRANSPLANT
High Dose
Chemotherapy Stem Cell Infusion
Supportive Care Engraftment
P H A S E 1 P H A S E 2 P H A S E 3
Duration: Approximately 2 weeks
Location: Transplant Center

Duration: Approximately 3-4 weeks
Location: Transplant Center

POST-TRANSPLANT
Restrengthening
Appetite recovery “Day 100” assessment
Begin maintenance therapy
Duration: Approximately 10-12 weeks
Location: HOME
Upfront stem cell transplant remains the standard of care for eligible patients

Miceli T, et al. Clin J Oncol Nurs. 2013;17(6)suppl:13-24. NCCN Guidelines. Multiple Myeloma. V3.2024. Accessed March 25, 2024. 47
CAR T-cell tHERAPY
Ask for a referral to CAR Tcell center as soon as it is possible as next treatment option (ie, before relapse)


No driving for 8 weeks
“One & Done” with continued monitoring
T-Cell Collection
Manufacturing takes ≈ 4 to 6 weeks
Bridging therapy may be needed
• Away from home
• Often some hospital stay
• Care Partner needed
• Side effect management
• CRS, ICANS
• Low blood counts
• Fatigue and fever
• Some patients need ongoing transfusion support

CAR = chimeric antigen receptor; CRS = cytokine release syndrome; ICANS = Immune Effector Cell-Associated Neurotoxicity Syndrome
48
BISPECIFIC ANTIBODIES
•Different bispecific antibodies have differences in efficacy, side effects
• Available after 4 prior lines of therapy (or clinical trial)
• About 7 in 10 patients respond
• Off-the-shelf treatment; no waiting for engineering cells
• CRS and neurotoxicity
• Risk of infection
•BCMA target: greater potential for infection
• Tecvayli® (teclistamab)
• Elrexfio™ (elranatamab)
•GPRC5D target: potential for skin and nail side effects, GI issues of taste change, anorexia and weight loss
• Talvey™ (talquetamab)

BISPECIFIC ANTIBODIES





MM cell death Target CD3 Cytotoxic cytokines Bispecific antibody T cell MM cell BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; GPRC5D = G protein–coupled receptor, class C, group 5, member D; MM = multiple myeloma; scFV = single chain fragment variable. Shah N, et al. Leukemia. 2020;34(4):985-1005. Yu B, et al. J Hematol Oncol. 2020;13:125.
49

SYMPTOM MANAGEMENT

50
PATIENT-REPORTED SYMPTOMS
A meta-analysis identified the most common patient-reported symptoms and impact on QOL, and were present at all stages of the disease.
Symptoms resulted from both myeloma disease and treatment, including transplant, and were in these categories:
Physical

• Fatigue
• Constipation
• Pain
• Neuropathy
• Impaired Physical Functioning
• Sexual Dysfunction

Psychologica
l
• Depression
• Anxiety
• Sleep Disturbance
• Decreased Cognitive Function
• Decreased Role & Social Function
Financial


• Financial burden (80%)
• Financial toxicity (43%)

Ramsenthaler, et al. 2016. https://doi.org/10.1111/ejh.12790. 51
Immunotherapy Side Effects
Cytokine Release Syndrome (CRS)

Immune effector cell–
associated neurotoxicity syndrome (ICANS)

Neuro Toxicity

Infection

52 CRS = cytokine release syndrome; ICANS = immune effector cell–associated neurotoxicity syndrome.
Physical
Immunotherapies: UNIQUE SIDE EFFECTS

CRS IS A COMMON BUT USUALLY MILD SIDE EFFECT WITH CAR T

CRS = cytokine release syndrome. Oluwole OO, Davila ML. J Leukoc Biol. 2016;100:1265-1272. June CH, et al. Science. 2018;359:1361-1365. Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Brudno JN, Kochenderfer JN. Blood Rev. 2019:34:45-55. Shimabukuro-Vornhagen, et al. J Immunother Cancer. 2018;6:56. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.

CRS Fever
Headache Nausea / vomiting Shortness of Breath Diarrhea Weakness Confusion
Fatigue
53 Physical
Immunotherapies: UNIQUE SIDE EFFECTS




Headache Confusion Altered wakefulness Hallucinations Ataxia Apraxia Facial nerve palsy Tremors Seizures Encephalopathy Neurotoxicity CRS = cytokine release syndrome; ICANS = immune effector cell–associated neurotoxicity syndrome; ICE = Immune Effector Cell Encephalopathy screening tool; MRI = magnetic resonance imaging. Brudno JN, Kochenderfer
Blood.
JN.
2016;127(26):3321-3330. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.
NEUROTOXICITY IS A RARE
SERIOUS SIDE EFFECT OF CAR T 54 Physical
BUT

INFECTION CAN BE SERIOUS FOR PEOPLE WITH MYELOMA
[P]reventing infections is paramount.
Infection remains the leading cause of death in patients with multiple myeloma. Several factors account for this infection risk, including the overall state of immunosuppression from multiple myeloma, treatment, age, and comorbidities (e.g., renal failure and frailty).
IMWG Consensus guidelines and recommendations for infection prevention in multiple myeloma; Lancet Haematol.2022;9(2):143–161.

Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
As recommended by your healthcare team: Infection Prevention Tips
Good personal hygiene (skin, oral)
Environmental control (avoid crowds and sick people; use a high-quality mask when close contact is unavoidable)
Immunizations: Flu, COVID, RSV & and pneumococcal vaccinations; avoid live vaccines
Preventative and/or supportive medications (next slide)
IMWG = International Myeloma Working Group; HCP = healthcare provider. Raje NS, et al. Lancet Haematol.2022;9(2):143–161. IMF Nurse Leadership Board ONS Symposia 2023.

Physical 55
MEDICATIONS CAN REDUCE INFECTION RISK
Type of Infection Risk
Viral: Herpes Simplex (HSV/VZV); CMV
Bacterial: blood, pneumonia, and urinary tract infection
PJP (P. jirovecii pneumonia)
Fungal infections
COVID-19 and Influenza

Medication Recommendation(s) for Healthcare Team
Consideration
Acyclovir prophylaxis
Consider prophylaxis with levofloxacin
Consider prophylaxis with trimethoprim-sulfamethoxazole
Consider prophylaxis with fluconazole
Antiviral therapy if exposed or positive for covid per institution recommendations
IgG < 400 mg/dL (general infection risk) IVIg recommended
ANC < 1000 cells/μL (general infection risk)
Consider GCSF 2 or 3 times/wk (or as frequently as needed) to maintain ANC > 1000 cells/μL and maintain treatment dose intensity
Some people receiving BCMA-targeting therapies have experienced infections that are less common like CMV, PJP and fungal infections
ANC = absolute neutrophil count; BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; CMV, cytomegalovirus; GCSF = granulocyte colony-stimulating factor; HSV = herpes simplex virus; IVIg = intravenous immunoglobulin; PJP = Pneumocystis jirovecii pneumonia; VZV = varicella zoster virus.
Raje NS, et al. Lancet Haematol.2022;9(2):143–161.

Physical
56
MANAGEMENT OF ORAL SIDE EFFECTS
Dry Mouth
OTC dry mouth rinse, gel, spray are recommended. Advise patients to avoid hot beverages. Initiate antifungal therapy for oral thrush
Dexamethasone oral solutions “swish and spit” have been tried but with no proven benefit yet. Sour citrus or candies before meals are also recommended. Taste Changes
Dental Care
Attention to oral hygiene.
Regular dental cleaning and evaluation. Close monitoring for ONJ, oral cancer and dental caries
ONJ = osteonecrosis of the jaw; OTC = over the counter.

Dysphagia
Weight Management
Dietary modifications with small bites, eating upright, and sips with food can help manage symptoms.
Some medications lead to weight gain, others to weight loss.
Dry mouth leads to taste changes which can lead to anorexia.
Meet with a Nutritionist
Consider diet changes, supplements
Monitor weight
Education and emotional support are key strategies to manage oral toxicities.
Catamero D, Purcell K, Ray C, et al. Presented at the 20th International Myeloma Society (IMS) Annual Meeting Nurse Symposium; September 27–30, 2023; Athens, Greece.

Physical 57
GI SYMPTOMS: PREVENTION & MANAGEMENT
Diarrhea may be caused by medications and supplements
Laxatives, antacids with magnesium
Antibiotics, antidepressants, others
Milk thistle, aloe, cayenne, saw palmetto, ginseng
Sugar substitutes in sugar free gum
Avoid caffeinated, carbonated, or heavily sugared beverages
Take anti-diarrheal medication
Imodium®, Lomotil®, or Colestid if recommended
Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
Welchol® if recommended

Constipation may be caused by
• Opioid pain relievers, antidepressants, heart or blood pressure medications, others
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency
Increase fiber
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil®, Citrucel®, Benefiber®

Fluid intake can help with both diarrhea and constipation, and good for kidneys.
Discuss GI issues with health care providers to identify causes and make adjustments to medications and supplements.

Smith LC, et al. CJON.2008;12(3)suppl:37-52. Faiman B. CJON. 2016;20(4):E100-E105.
Physical
58

Management of Skin and Nail Side Effects
Possible side effect to some treatments and supportive care medications

Skin Rash:
•Prevent dry skin; apply lotion
•Report changes to your care team
•Medication interruption or alternative, as needed
•Steroids:
• Topical for grades 1-2,
• Systemic and topical for Grade 3
•Antihistamines, as needed
Nail Changes:

•Keep your nails short and clean. Watch for “catching and tearing”
•Apply a heavy moisturizer like Vaseline or salve. Wear cotton hand coverings to bed
•A nail hardener may help with thinning
•Tell the team if you have signs of a fungal infection, like thickened or discolored nails
 Photos: Mount Sinai Hospital, NY, NY
Nurse Leadership Board
Photos: Mount Sinai Hospital, NY, NY
Nurse Leadership Board
Physical
The Bright Dark Side to Steroids

Steroid Synergy
Steroids are a backbone and work in combination to enhance myeloma therapy
Managing Steroid Side Effects
Consistent schedule (AM vs. PM)
Take with food
Stomach discomfort: Over-the-counter or prescription medications
Medications to prevent shingles, thrush, or other infections
Do not stop or adjust steroid doses without discussing it with your health care provider


Steroid Side Effects
• Irritability, mood swings, depression
• Difficulty sleeping (insomnia), fatigue
• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Increase in blood pressure, water retention

• Blurred vision, cataracts
• Flushing/sweating
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increase in blood sugar levels, diabetes

Rajkumar SV, et al. Lancet Oncol. 2010;11(1):29-37. King T, Faiman B. Clin J Oncol Nurs. 2017;21(2):240-249.
& 60 Physical Psychological
PAIN PREVENTION AND MANAGEMENT
Pain can significantly compromise quality of life
Sources of pain include bone disease, neuropathy and medical procedures
Management
Prevent pain when possible
Bone strengtheners to decrease fracture risk; anti viral to prevent shingles; sedation before procedures
Interventions depends on source of pain
May include medications, activity, surgical intervention, radiation therapy, etc
Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)

Tell your health care provider about any new bone pain or chronic pain that is not adequately controlled


Physical
Faiman B, et al. CJON. 2017;21(5)suppl:19-36.
61
PERIPHERAL NEUROPATHY MANAGEMENT

Peripheral neuropathy happens when there is damage to nerves in the extremities (hands, feet, limbs).
Damage can be the result of myeloma, treatment or unrelated conditions (i.e. diabetes).
Symptoms:
•Numbness
•Tingling
•Prickling sensations
•Sensitivity to touch
•Burning and/or cold sensation
•Muscle weakness
Prevention / management:
•Bortezomib once-weekly and/or subcutaneous administration
•Massage area with cocoa butter regularly
•Neuroprotective Supplements:
• B-complex vitamins (B1, B6, B12)
• Green tea
•Safe environment: rugs, furnishings, shoes
Report symptoms of peripheral neuropathy early to your healthcare provider; nerve damage from neuropathy can be permanent if unaddressed
If neuropathy worsens, your provider may:
•Adjust your treatment plan
•Prescribe oral or topical pain medication
•Suggest physical therapy

B, et al. CJON. 2017;21(5)suppl:19-36. Tariman, et al. CJON.2008;12(3)suppl:29-36. Zhao T, et al. Molecules. 2022;27(12):3909.
Faiman
Physical
62
•Risk Factors
UNDERSTANDING CHANGES TO KIDNEY FUNCTION
• Active multiple myeloma (light chains, high calcium)
• Other medical issues (ex: Diabetes, dehydration, infection)
• Medications (MM treatment, antibiotics, contrast dye)
• Poor Nutrition
•Prevention
• Stay hydrated – drink water
• Avoid certain medications when possible (eg, NSAIDs), dose adjust as needed
•Treatment
• Treatment for myeloma
• Hydration
• Dialysis



Many myeloma patients will experience kidney issues at some point; protecting your kidney function early and over time is important

et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76.
Brigle K,
Physical
63
ADDITIONAL SUPPORTIVE CARE OPTIONS

Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:6076. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.

DVT/PE Prevention Bone Health Fatigue Anxiety
Medications Blood thinners Ex: Aspirin, DOACs Bone Strengthening Agents Calcium Vitamin D Stimulant medications Red Cell Transfusion (anemia) Anti-depressants Anti-anxiety Nonmedication Therapies Compression stockings Radiation Surgery Immobilization Physical therapy Relaxation Meditation Therapy Relaxation Meditation Lifestyle Options Activity Stop smoking Weight loss Activity Activity Improved sleep Activity Improved sleep
Supportive
Physical
64
Rest and Relaxation contribute to good health
�� Adequate rest and sleep are essential to a healthful lifestyle
Short and disturbed sleep increase risk of
• Heart related death
• Increase anxiety
• Weaken immune system
• Worsened pain
• Falls and personal injury
�� Things that can interfere with sleep
• Medications : steroids, stimulants, herbal supplements
• Psychologic: fear, anxiety, stress


�� Sleep hygiene is necessary for quality nighttime sleep, daytime alertness
• Engage in exercise but not too near bedtime
• Increase daytime natural light exposure
• Physiologic: sleep apnea, heart issues, pain
• Avoid Daytime napping
• Establish a bedtime routine - warm bath, cup of warm milk or tea
Associate your bed ONLY with sleep
Sleep aid may be needed
• Avoid before bedtime:
Caffeine, nicotine , alcohol and sugar
Large meals and especially spicy, greasy foods
Computer screen time
Rod NH et al 2014. PloS one. 9(4):e91965; Coleman et al. 2011. Cancer Nurs. 34(3):219227.
Mustian et al. Journal of clinical Oncology. Sep 10 2013;31(26):3233-3241.
Stan DL, et al. Clin J Oncol Nurs. Apr 2012;16(2):131-141.

Zeng Y et al., Complementary therapies in medicine. Feb 2014;22(1):173-
65 Physical Psychological

FINANCIAL BURDEN
Financial burden comes from
•Medical costs
• Premiums
• Co-payments
• Travel expenses
• Medical supplies
•Prescription costs
•Loss of income
• Time off work or loss of employment
• Caregiver time off work
Contact the Social Services department at your hospital or clinic to talk to a social worker for assistance.
Funding and assistance may be available
•Federal programs
•Pharmaceutical support
•Non-profit organizations
•Websites:
• Medicare.gov
• SSA.gov
• LLS.org
• Rxassist.org
• NeedyMeds.com

• HealthWellFoundation.org
• Company-specific website

Financial
66

FRAMING YOUR CARE
Know your care team, Telehealth & Meeting Prep, & Shared Decision Making

CARE TEAM COLLAGE
You are central to the care team
Be empowered
Ask questions, learn more
Participate in decisions
Communicate with your team
Understand the roles of each team member and who to contact for your needs
Participate in support network















You and Your Caregiver(s) Support Network



Allied Health Staff
Myeloma Specialist General P h a r m a c i s t
Subspecialists
68
PREPARE FOR VISITS & CONSIDER TELEMEDICINE
Come prepared:
Bring a list of current medications, prescribed and over the counter
Write down your questions and concerns. Prioritize them including financial issues
Have there been any medical or life changes since your last visit?
Current symptoms - how have they changed (improved, worsened, stable)? Keep a symptom diary. Bring it along
Communicate effectively: your health care team can’t help if they don’t know
Know the “next steps”, future appointments, medication changes, refills, etc
Check with your healthcare team –Is telemedicine an option?


Similar planning for “in-person” appointment PLUS:
• What is the process and what technology is needed?
• Plan your labs: are they needed in advance? Do you need an order?
• Plan your location: quiet, well-lit location with strong wi-fi is best
• Plan yourself: consider if you may need to show a body part and wear accessible clothing
• Collect recent vital signs (blood pressure, temp, heart rate) self-serve blood pressure cuff is available at many pharmacies and for purchase

IMF Telemedicine Tip Sheet. In development.
69
SHARED DECISION MAKING
Ask for time to consider options (if needed/appropriate)
Understand options; consider priorities
Use reliable sources of information
Use caution when hearing stories of other peoples’ experiences
Consider your goals/values/preferences

Express your goals/values/preferences; create a dialogue
My top priority is [goal/value]; additional [preferences] are also important.
I think [treatment] may be a good choice given my priorities… What do you think?
Arrive at a treatment decision together




70

LIVE LIFE IN COLOR

71
CARE PARTNERS ARE VITAL FOR SUCCESS
If you want to go fast, go alone, if you want to go far, go together
Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions
Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)



African Proverb
Caring for the Care Partner
Recognize that caregiving is difficult/stressful

Encourage care partners to maintain their health, interests, and friendships The IMF has information and resources to help care partners



IMF Care Giver Tip Card
72
HEALTHY BEHAVIORS FOR PATIENTS & CARE PARTNERS
• Stress reduction, management
• Rest, relaxation, sleep hygiene
• Maintain a healthy weight, eat nutritiously
• Activity / exercise / prevent falls, injury
• Stop smoking
• Mental health / social engagement
• Sexual health / intimacy
• Complementary or alternative therapy


• Have a PCP for general check ups, preventative care, health screenings, vaccinations
• Have specialists for dental care, eye exams/screening, skin cancer screening





73
73
DEVELOP A CARE NETWORK
•Multiple studies demonstrate that strong social ties are associated with
• Increased longevity including people with cancer
• Improved adherence to medical treatment leading to improved health outcomes
• Lower risk of cardiovascular diseases
• Increased sense of purpose & life satisfaction
• Improved mood and happiness
• Reduced stress and anxiety
• Enhanced resilience

•Strategies for enhancing social connection
• Deepen existing relationships with family, friends, and loved ones
• Build new relationships by participating in a support group, joining clubs or organizations, or volunteering
Martino J, et al. Am J of Lifestyle Med. 2015;11(6):466-475. Yang YC, et al. Proc Natl Acad Sci U S A. 2016;113(3):578-583. Pinquart M and Duberstein PR. Crit Rev Oncol Hematol. 2010;75(2):122–137.



Tip: Start with small steps outside your comfort zone. Call a loved one you haven’t spoken to in a while. Invite a person you’d like to know better for lunch, coffee, or a walk.
Hetherington C. Healthnews. https://healthnews.com/longevity/healthspan/soci al-connection-and-longevity/#:~:text=Research %20consistently%20demonstrates%20that %20people,of%20fulfillment%20in%20your%20life. Accessed Feb 1 2024.

74



























IMF OFFERS MANY RESOURCES You are not alone! eNewsletter: Myeloma Minute Website: http://myeloma.org IMF Videos Download or order at myeloma.org IMF InfoLine 1-800-452-CURE 9am to 4pm PST 75
YOU ARE NOT ALONE


Questions? 76

COFFEE BREAK
Thank you to our sponsors!









Frontline & Maintenance Therapy
Doug Sborov, MD, MS
Huntsman Cancer Institute/University of Utah
Salt Lake City, UT


What we know and where we are going with 1st line therapy in multiple myeloma
Douglas W. Sborov, MD MS
Associate Professor
Director – HCI Hematology Disease Center and Plasma Cell Dyscrasias Program
Huntsman Cancer Institute at the University of Utah


Sborov Disclosures
•Consultant/Advisory: GSK, Janssen, Sanofi, AbbVie, BMS, Pfizer, Bioline, Arcellx, AstraZeneca
•Research: Pfizer
•Steering Committee: Janssen (AURIGA)
•Data Safety and Monitoring: Karyopharm
•Independent Review Committee: Parexel


Physicians
Aman Godara
Brian McClune
Manni Mohyuddin
Amyloidosis
Jo Abraham
Kelsey Barrell
Spencer Carter
Joseph Stehlik
Jill Waldron
APCs
Lindsay Maxwell
Eliza Parkin
Sam Shewan
Mary Steinbach
Meg Vigil

BMT/CAR
Thank you to our patients and the PCD team
New
Maddie
ADAPT
Sharmilee Nuli
Montserrat
Biobanking
Shannon Buckley
Tony Pomicter
Justin Williams
RNs Kaitlyn Bushman Nicole Felkel Melanie Murphy Bri Peterson
Pulsipher Amanda ReitzHamner Lynnette Shimmin Matt
Social Work TBN Pharmacy Baylee Bryan Maren Campbell Michael Filtz Jessica Hudson Kelley Julian Charlotte Wagner
Kaitlyn
Whooley
Coordinators
Bellerive
Bleak Tiffany DeHammer Emily Doxey Ben Johnson Ryan Lombardi Support Staff
Carrie
Steve
Camille Breiholz
Case Rhys
Johnson Emma Kirby
Trials Office Collind Boyington Shaylee Cannon Jimmy Chapman Brody Coleman Catherine Cromar Kyle Morgan Nicole Schmidt Max Schultz Frankie Tibbits
Enloe Rashelle
Livia Koehler Clinical
Patient Coordinators
Lewis
Nelson
Support Zac
Call
Kylee
Sarah
Administrative
Francom Kaleb
Justo
Garrido Bailee Daniels
Talking points
•Introduction to multiple myeloma
•Treatment options for patients not intended for early autologous transplant
•Treatment options for patients intended for early transplant
•Maintenance strategies
•Hole in the arsenal - High-risk myeloma and plasma cell leukemia



Introduction to multiple myeloma
Multiple Myeloma (MM)
•Incurable blood cancer characterized by the malignant transformation of plasma cells – a white blood cell that normally resides in the bone marrow and functions to make antibodies
•Associated symptoms include:
• Bone pain resulting from fractures
• Fatigue due to anemia
• Elevated levels of calcium
• Kidney dysfunction
• Infections


Incidence and mortality

Incidence rates, 2014 - 2018
Death rates, 2014 - 2018


Myeloma is twice as common in black patients
Rate of new cases per 100,000 persons by race/ethnicity and sex


8.8 8.2 16.3 4.8 6.8 8.2 8.9 5.7 5.0 12.1 3.1 4.9 6.0 5.7 All Races White Black Asian/Pacific Islander American Indian/Alaska Native Hispanic Non-Hispanic MALE FEMALE SEER 21 20144-2018, Age-Adjusted Death rate per 100,000 persons by race/ethnicity
sex 4.0 3.8 7.2 1.9 3.9 3.3 4.1 2.5 2.3 4.9 1.3 2.5 2.2 2.5 All Races White Black Asian/Pacific Islander American Indian/Alaska Native Hispanic Non-Hispanic MALE FEMALE U.S. 2015-2019, Age-Adjusted SEER Cancer Stat Facts: Myeloma. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/mulmy.html
and
Diagnostic evaluation of plasma cell dyscrasias

Test
Potential MM findings
cbc with differential ↓hgb, ↓wbc, ↓plt
Complete metabolic panel + uric acid + crp
Serum and urine (24-hour) protein electrophoresis (SPEP/UPEP) +/- IgD and IgE (if no m-spike)
Serum and urine immunofixation (IFE)
Serum free light chain assay
↑Cr, ↑Ca, ↑uric acid, ↓albumin
↑monoclonal protein, ↓normal antibodies
Identifies type of monoclonal protein
↑involved light chain, ↓uninvolved
ß2-microglobulin (B2M), lactate dehydrogenase (LDH) ↑B2M, ↑LDH
proBNP, BNP
↑may be elevated in patients with cardiac amyloidosis
Imaging: PET +/- full body MRI (non-contrast) Evaluation for bone lytic lesions + EMD
Bone marrow biopsy with CD138-selected FISH > 10% monoclonal PCs


Multiple myeloma diagnostic criteria
MGUS
• M-protein < 3 g/dL
• Clonal marrow PCs < 10%
• No SLiM-CRAB
Smoldering Myeloma
• M-protein > 3 g/dL (serum) or > 500 mg/d (urine)
• Clonal marrow PCs 10 – 59%
• No SLiM-CRAB
S Clonal plasma cells in BM ≥ 60%
Li Serum FLC ratio ≥ 100
M >1 focal lesion ≥ 5 mm on MRI
Multiple Myeloma
• Clonal marrow PCs > 10% or > 1 biopsy-proven plasmacytoma
• SLiM-CRAB
Any of these criteria is associated with ~80% risk of progression to development of CRAB criteria in 2 years
C Calcium elevation (> 11 mg/dL or > 1 mg/dL higher than ULN)
R Renal insufficiency (CrCl < 40 mL/min or serum creatinine > 2 mg/dL)
A Anemia (Hb < 10 g/dL or 2 g/dL < normal)
B Bone disease (≥ 1 lytic lesions on skeletal radiography, CT, or PET/CT)


Rajkumar, Lancet Onc, SLiM CRAB
Myeloma is a heterogeneous disease

Understanding that each patient’s disease is different helps us define the best possible individualized approach to care


International Myeloma
Staging tells us how to approach each patient



R2-ISS includes 1q21 gain/amplification + accounts for double and triple hit disease


Palumbo, JCO, 2015; D’Agostino, JCO, 2022 Risk Feature Points ISS 2 1 ISS 3 1.5 Del(17p) 1 LDH > ULN 1 t(4;14) 1 1q+ 0.5 Group Total Score mOS, yr Low (I) 0 NR Low-intermediate (II) 0.5 – 1 7.4 Intermediate-high (III) 1.5 – 2.5 4.7 High (IV) 3 – 5 2.8
R-ISS R2-ISS
Risk is more than just disease biology
Disease Factors
• Cytogenetics/molecular (R-ISS)
• CRAB criteria (renal injury)
• PCL & EMD
• Coexisting amyloidosis


Organ function




Patient Factors
Performance status
Frailty index
Comorbid conditions
Prior DVT
Neuropathy
Caregiver support
Financial toxicity (copays)
Any of these issues can impact access to newer therapies only available in clinical trials


L
Myeloma treatment paradigm
Tumor burden
Induction
Induction followed by continuous therapy “Consolidation” Maintenance
Disease control and reversal of symptoms and signs
Maximize disease control to provide durable disease control while optimizing QoL


H
C
E l i g
b l e H S C T I n e l i g i b l e D i a g n o s i s a n d r i s k s t r a t i f i c a t i o n
S
T
i
GOA
The expanding toolbox is improving outcomes

. . . but we are not yet curing our patients


DARATUMUMAB (IV)
ELOTUZUMAB + Rd
DARATUMUMAB (IV) + Rd
DARATUMUMAB (IV) + Vd
DARATUMUMAB (IV) + Pd
ELOTUZUMAB
DARATUMUMAB + VTd
DARATUMUMAB + Rd
SELINEXOR + Dex
SELINEXOR +
24 FDA approvals since 2015

at least 3 prior lines including IMiD and PI
1 - 3 prior lines
at least 1 prior line
Transplant eligible NDMM
Transplant ineligible NDMM

combination Approval Year Indication
Drug +/-
2015 RRMM
2015 RRMM
2016 RRMM
2016 RRMM
at least 1 prior line
2017 RRMM
PI
at least 2 prior lines including
and lenalidomide
2018 RRMM at least 2 prior lines including PI
lenalidomide
+ Pd
and
2019
2019
2019 RRMM at least
prior lines including IMiD
PI
4
(2),
(2), and aCD38 mAb
2020 RRMM at least 1 prior line
Kd 2020 RRMM 1–
prior lines
(SC) 2020 RRMM at least 3 prior lines including IMiD
PI
double-refractory
2020 RRMM
(SC)
Vd 2020 RRMM at least 1 prior line BELANTAMAB MAFODOTIN 2020 RRMM at least 4 prior lines including IMiD, PI, and aCD38 mAb ISATUXIMAB
Pd 2020 RRMM at least 2 prior lines including PI and lenalidomide ISATUXIMAB + Kd 2021 RRMM 1 – 3 prior lines IDECAPTAGENE VICLEUCEL 2021 RRMM at least 4 prior lines including IMiD, PI, and aCD38 mAb DARATUMUMAB (SC) + Pd 2021 RRMM at least 1 prior line including IMiD and PI DARATUMUMAB (SC) + Kd 2021 RRMM at least 1 prior line including PI and lenalidomide CILTACABTAGENE AUTOLEUCEL 2022 RRMM at least 4 prior lines including IMiD, PI, and aCD38 mAb TECLISTAMAB 2023 RRMM at least 4 prior lines including IMiD, PI, and aCD38 mAb TALQUETAMAB 2023 RRMM at least 4 prior lines including IMiD, PI, and aCD38 mAb ELRANATAMAB 2023 RRMM at least 4 prior lines including IMiD, PI, and aCD38 mAb
Vd
DARATUMUMAB (IV) +
3
DARATUMUMAB
and
or
DARATUMUMAB (SC) + Rd
at least 1 prior line DARATUMUMAB
+
+
Steinbach, TAH,

Treatment options for NDMM
General Principles of Initial Therapy
•Most patients will be given a combination of drugs to control the disease quickly
•We don’t “save the best for later” because early use of highly effective drugs have a long-term effect on survival
•We seek a DEEP and DURABLE response
•We decide early whether someone should be considered for transplant
•We use a combination of the following for 1st line therapy:
• Immunomodulatory drug (IMiD) = Lenalidomide (revlimid)
• Proteosome inhibitor (PI) = bortezomib (velcade) or carfilzomib (kyprolis)
• CD38-directed monoclonal antibody = daratumumab (darzalex) or isatuximab (sarclisa)



Newly diagnosed multiple myeloma (NDMM): Transplant ineligible
Initial therapy regimens for multiple myeloma
S0777, IFM2009
Triplet Regimens Quadruplet Regimens
Velcade-Len-Dex (VRd) Dara-RVd
Carfilzomib-Len-Dex (KRd) Dara-KRd
Velcade-Thal-Dex (VTd)
Velcade-MEL-Dex (VMP)
Daratumumab-Len-Dex (Dara-Rd)
GMMG-HD7, IMROZ IsKia, CONCEPT
Quad-induction is now considered SOC for transplant eligible patients, though triplet may be adequate for less fit patients


GRIFFIN,
MASTER
MAIA
PERSEUS FORTE, ENDURANCE
CASSIOPEIA ALCYONE
Dara-VTd
Dara-VMP
Isa-RVd
Isa-KRd
CyBorD
Four primary trials in non-transplant NDMM
07771















MAIA2






ALYCONE4









Adapted from Facon, IMW, 2021; 1. Durie, Lancet, 2017; 2. Facon, NEJM, 2019; 3. O’Donnell, BJH, 2018; 4. Mateos, NEJM, NDMM intent for immediate autoHSCT
525] Rd (n = 261) [6 × 28-day cycles] VRd (n = 264) [8 × 21-day cycles] Rd Len 25 mg d1–21, Dex 40 qwk [Until PD, toxicity, or withdraw] MAINTENANCE Stratified by ISS & intent to transplant INDUCTION Primary endpoint: PFS SWOG
VRd Rd Median age 63 63 Age ≥75 (%) 38 48 High-risk cytogenetics % -VRd lite
NDMM ≥65 years [P2: N = 50] VRd lite [9 × 35-day cycles] VR [6 × 28-day cycles] INDUCTION Primary endpoint: ORR ITT Median age (range) 73 (65–91) Age ≥75 (%)High-risk cytogenetics % 12 Non-transplant eligible NDMM [rP3: N = 737] Rd (n = 369) [Until PD, toxicity, withdraw] DRd (n = 368) [Until PD, toxicity, withdraw] INDUCTION Primary endpoint: PFS
[rP3: N =
3
DRd Rd Median age (range) 73 (50–90) 74 (45–89) Age ≥75 (%) 44 44 High-risk cytogenetics % 15 14 Non-transplant eligible NDMM [rP3: N = 706] RVMP
356) [9
D-VMP
350) [9
INDUCTION Primary endpoint: PFS
(n =
× 6-week cycles]
(n =
× 6-week cycles]
D-VMP VMP Median age (range) 71 (40–93) 71 (50–91) Age ≥75 (%) 30 30 High-risk cytogenetics % 17 15 Daratumumab
[Until PD, toxicity,
qmonth
withdraw]
SWOG S0777: Triplet beats doublet

Patients younger than 65 had better outcomes with triplet RVd. Older patients might do better.


Durie, ASH, 2018; Durie, BCJ, 2020
KRd without transplant is not better than VRd

PRIMARY ENDPOINTS
PFS (induction)
OS (maintenance)









ENDURANCE [rP3 (N = 1087)] VRd Vel 1.3 mg/m2 d1,4,8,11 Len 25 mg d1–14, Dex 40 qwk KRd CFZ 36 mg/m2 d1,2,8,9,15,16 Len 25 mg d1–21, Dex 40 qwk 2 years
INDUCTION (9 cycles) R2 Indefinite Kumar, Lancet Onc, R1 Outcome VRd [N(%)] KRd [N(%)] P Value Safety (G3/4 AE) Neuropathy AKI Cardiac 44 (8) 3 (1) 9 (2) 4 (1) 13 (3) 30 (7) Response ORR ≥ VGPR ≥CR MRD–84% 65% 15% 7% 87% 74% 18% 10% Survival mPFS 34.4 m 34.6 m P = .74
LEN MAINTENANCE
. . . in patients with R-ISS stage 1 or 2 NDMM without intent for immediate autoHSCT
(Patients required to be R-ISS 1 or 2)
MAIA: Established SOC for transplant ineligible NDMM
Primary Endpoint: Progression Free Survival
28-day cycles until disease progression or intolerability
Daratumumab in


autoHSCT
the frontline setting improves outcomes in patients unfit for
MAIA
R A N D O M I Z A T I O N Dara-Rd Dara
C1-2,
q4w C7+ Len
Rd Len
Mateos,
NEJM, 2018; Facon, NEJM, 2019
Dara-Rd vs Rd [P3 (N = 737)]
16 mg/kg qwk
q2w C3-6,
25 mg/d d1-21, Dex 40 mg qwk*
25 mg/d d1-21, Dex 40 mg qwk* *Reduced to 20 mg/wk if > 75 yrs of age or BMI < 18.5
MAIA: Overall response rate and MRD (10-5)
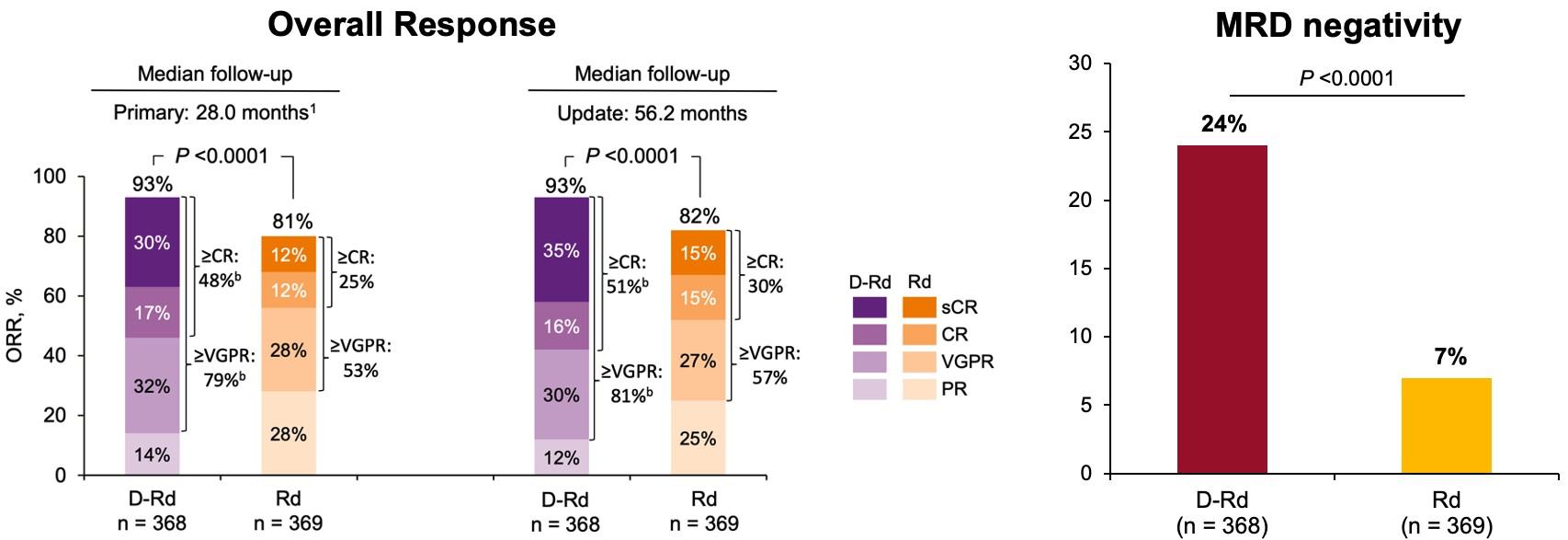
Overall Response Minimal Residual Disease (10-5)
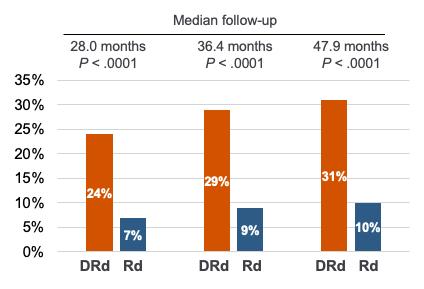
Addition of daratumumab improved response rates and depth of response

 Facon, NEJM, 2019; Facon, Lancet Onc, 2021
Facon, NEJM, 2019; Facon, Lancet Onc, 2021
MAIA: PFS and OS (median follow-up: 56 months)
Progression-Free Survival Overall Survival
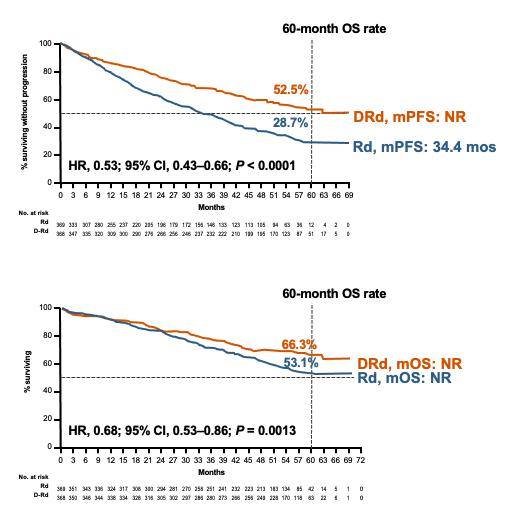

At 5-year follow-up, mPFS not reached in the DRd group vs 34.4 months in Rd group; HR, 0.53; P <.0001
Median OS not reached in either group; HR, 0.68; P = .0013)
In tiNDMM, DRd is associated with unprecedented depth of response and PFS. DRd is considered the SOC for this patient population


Facon, NEJM, 2019; Facon, Lancet Onc, 2021
All patients > 75 years of age or older start at Dex 20 mg Transplant ineligible NDMM – HCI algorithm
Consider isatuximab and daratumumab interchangeable


frail
DRd
D-RVd DRd +/- V lite Dd
If
Clinical trial or …
or

Newly diagnosed multiple myeloma (NDMM): Transplant eligible
DETERMINATION: AutoHSCT + maintenance
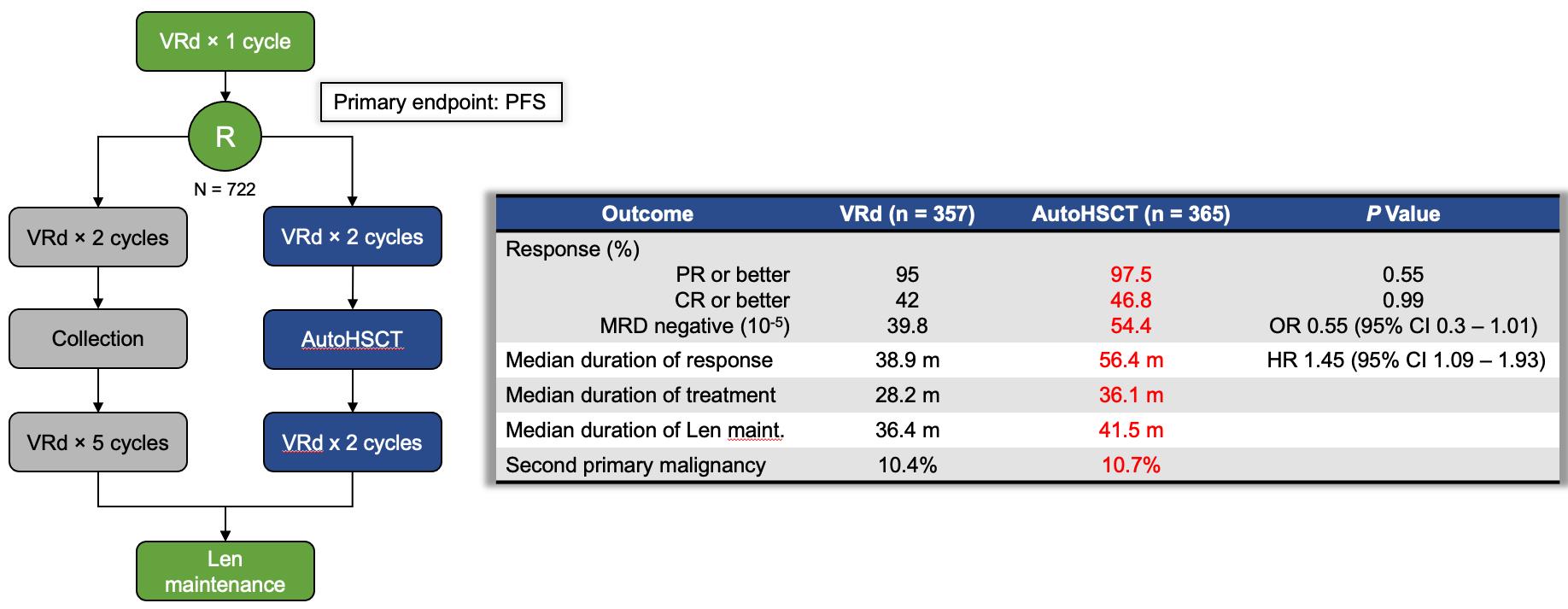
Study design initially paralleled IFM 2009, but following CALGB 100104, maintenance was changed to indefinite Len or until intolerance or progression


Richardson, NEJM, 2022
DETERMINATION: AutoHSCT improves mPFS
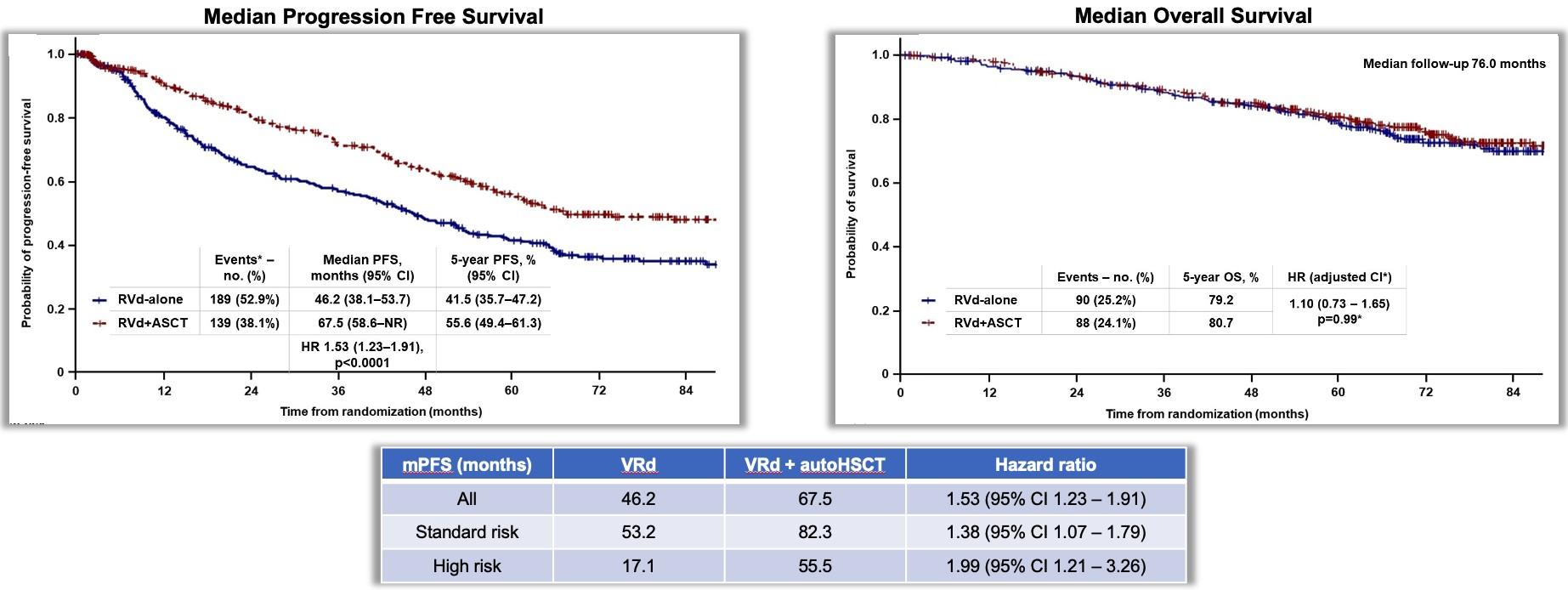
Authors attribute lack of OS difference to availability of


Richardson, NEJM, 2022
novel therapeutics
GRIFFIN: Addition of daratumumab to RVd
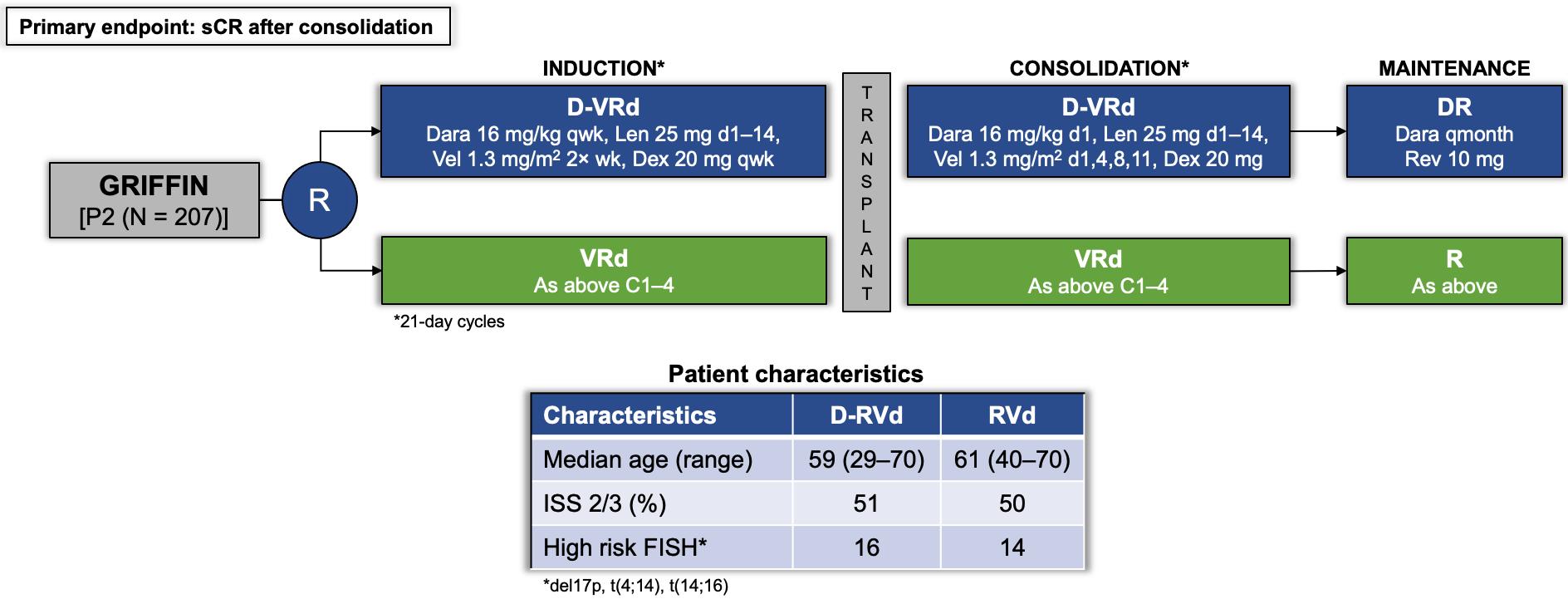
GRIFFIN was the first quad-based trial design in the US and the first to investigate the addition of daratumumab to a lenalidomide-based triplet

 Voorhees, Sborov, Lancet Heme,
Voorhees, Sborov, Lancet Heme,
GRIFFIN: Responses deepened over time
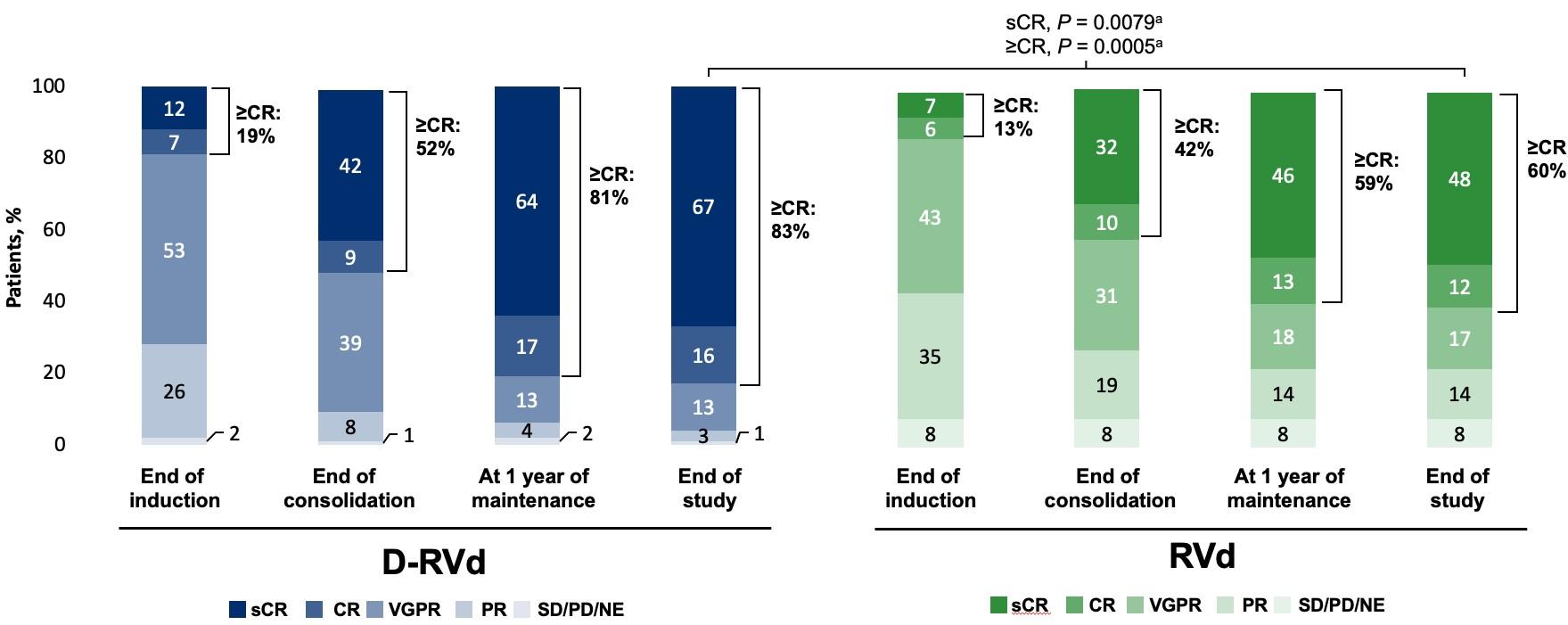
At all time points, response rates were higher for D-RVd; rates of ≥CR improved over time and were deepest at the end of study maintenance


Sborov, IMS,
At a follow-up of 49.6 months, mPFS was NR
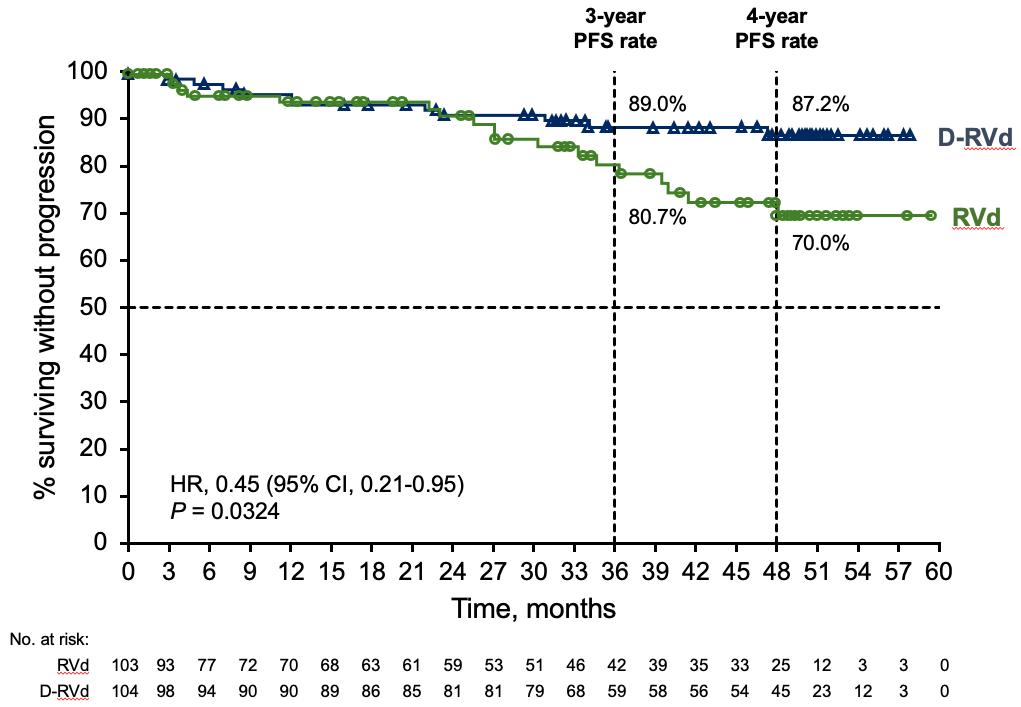
D-RVd/DR was associated with a clinically meaningful 55% reduction in risk of progression or death. The PFS curves separated beyond 1 year of maintenance

 Voorhees, Sborov, Lancet Heme,
Voorhees, Sborov, Lancet Heme,
PERSEUS – The rP3 follow-up of GRIFFIN

*Defined as del17p, t(4;14), t(14;16)
*After 24 months of DR maintenance, discontinue D for patients with > CR and MRD negativity for at least 12 months –Restart dara if loss of CR or MRD- without PD
PERSEUS was a multi-center trial based in Europe that investigated the D-RVd quad in a randomized phase 3 trial powered for PFS


Sonneveld, NEJM, PERSEUS D-RVd vs RVd [P3 (N = 709)] R A N D O M I Z A T I O N Dara-RVd Dara SQ1800 mg qw C1-2, q2w C3-4 +V Len 25 mg/d d1-21, Dex 40 mg d1-4, 912 RVd Bortezomib 1.3 mg/m2 d1,4,8,11 Len 25 mg d1-21, Dex 40 mg d1-4, 9-12 Primary
PFS INDUCTION 4 – 28-day cycles T R A N S P L A N T Dara-RVd Dara SQ 1800 mg q2wk + V Len 25 mg/d d1-21, Dex 40 mg d1-4, 912 RVd Bortezomib 1.3 mg/m2 d1,4,8,11 Len 25 mg d1-21, Dex 40 mg d1-4, 9-12 CONSOLIDATION
R Lenalidomide 10 mg Continuous
MAINTENANCE Until progression DR Dara q4w 24 months DR R* MRD negative MRD positive
Endpoint:
2 – 28-day cycles
d1-28
Characteristic D-RVd RVd Median age (range) 61 (32-70) 59 (31-70) ISS 2/3 47.6% 49.6% hrFISH* 21.4% 22.0% Patient characteristics
PERSEUS – Progression free survival
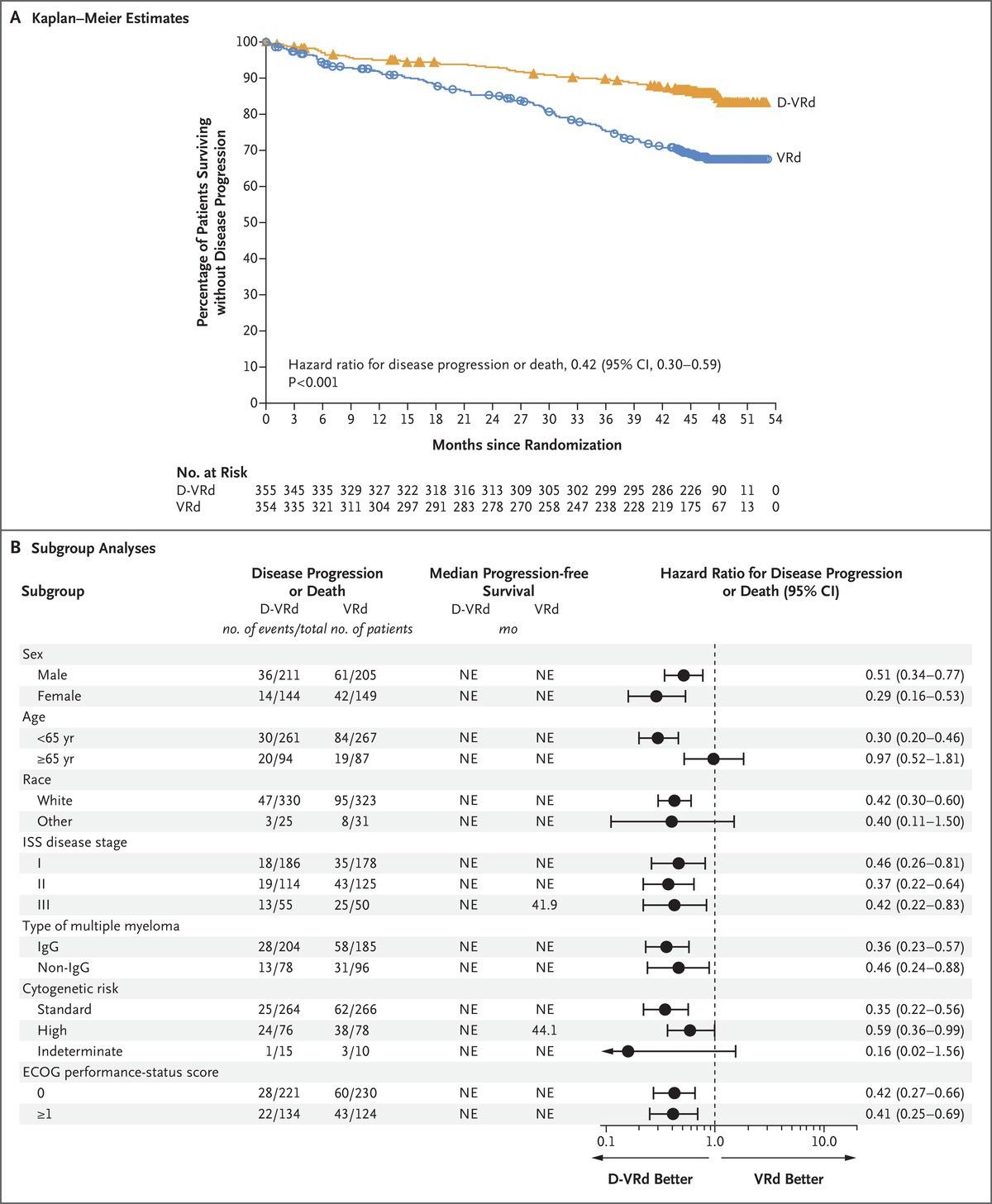
48-month PFS
At a median follow-up of 47.5 months, D-RVd was associated with a 58% reduction in the risk of


NEJM,
Sonneveld,
D-RVd 84.3% RVd 67.7%
GMMG-HD7 – Trial design
Primary endpoint 1: MRD rate post-induction


 Goldschmidt, Lancet Heme,
Goldschmidt, Lancet Heme,
GRIFFIN vs. GMMG-HD7

Endpoints sCR post-consolidation
Drug exposure during induction
Randomization 1: Rate of MRD negativity post-induction
Randomization 2: PFS after 2nd randomization
Mobilization G-CSF +/- plerixafor CAD (Cyclophosphamide, doxorubicin, dexamethasone)
Tandem allowed?
Patients treated with tandem No
for patients not in > CR Not reported
Response after consolidation
GRIFFIN GMMG-HD7
Phase 2 3
Monoclonal antibody Bortezomib Lenalidomide 12 doses 16 doses 56 doses 11 doses 24 doses (+8 doses) 84 doses (+28 doses)
MRD testing 10-5 (NGS) 10-5 (NGF)
ORR > CR > VGPR MRD negativity 98.0% vs 92.0% 7.1% vs 6.2% 72.0% vs 57.0% 22.0% vs 8.0% (Delta 14%) 90.0% vs 83.6% (p=0.02) 24.2% vs 21.6% (p=0.46) 77.3% vs 60.5% (p<0.001) 50.1% vs 35.6% (Delta 14%)
Tandem
Response after induction
ORR > VGPR > sCR MRD negativity 99.0% vs 92% 90.0% vs 73% 42.0% vs 32% 50.0% vs 20% NA Dropout rate pre-autoHSCT 5% (D-RVd), 20% (RVd) 5.4% (Isa-RVd), 10.6% (RVd) High risk cytogenetics 16% (D-RVd), 14% (RVd) 17.5% (Isa-RVd), 20.1% (RVd)
D-KRd may be the next quad king


Costa, JCO,
IsKia – rP3 investigating KRd +/- isatuximab
Primary Endpoint: Rate of MRD- post-consolidation INDUCTION
4 – 28-day cycles
Patient characteristics

*Defined as del17p, t(4;14), t(14;16) per IMWG, + 1q21 per R2-ISS
Cytoxan mobilization
CONSOLIDATION
4 – 28-day cycles


Gay, ASH, IsKia Isa-KRd
R A N D O M I Z A T I O N Isa-KRd
KRd
Len 25 mg
40 mg qweek
vs KRd [P3 (N = 302)]
Isa 10 mg/kg qwk C1
q2w thereafter KRd as below
Carfilzomib 20/56 d1,8,15
d1-21, Dex
T R A N S P L A N T Isa-KRd
KRd
Isa 10 mg/kg q2w KRD as below
Carfilzomib 20/56 d1,8,15 Len 25 mg d1-21, Dex 40 mg qweek
Characteristic D-RVd RVd Median age (range) 61 (55-66) 60 (54-63) R-ISS 2/3 65.0% 66.0% hrFISH per IMWG 18.0% 19.0% 2+ hrFISH per R2-ISS 9.0% 11.0%
IsKia results in context of other relevant quads

Quadruplet induction + transplant +/- consolidation is SOC for most 3- vs 4-drug question is answered and it is time to investigate more novel approaches


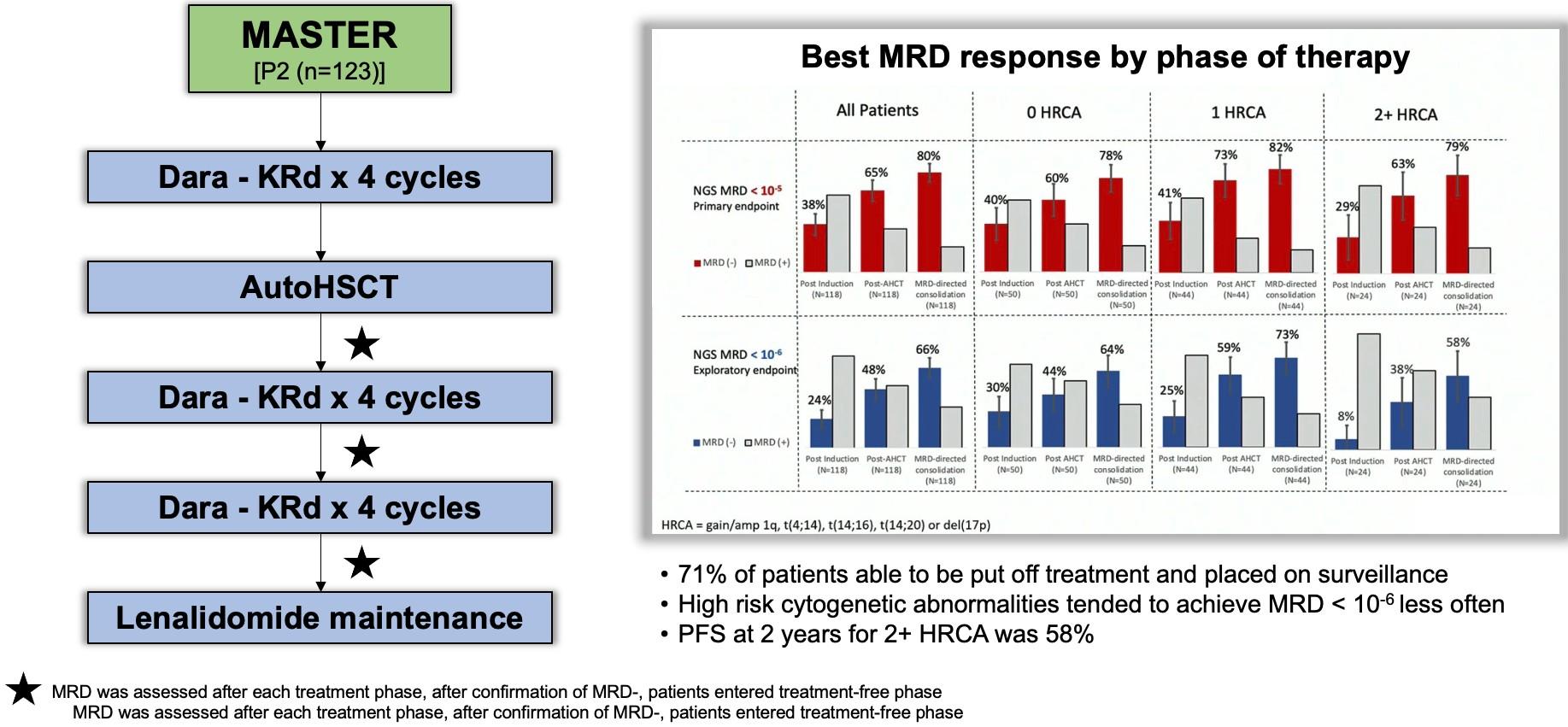
1Voorhees, Sborov, Lancet Heme, 2023; 2Sonneveld, NEJM, 2024; 3Goldschmidt, Lancet Heme, 2022; 4Costa, JCO, 2022; 5Gay, ASH, Trial Population Phase N PI CD38 mAb % MRD- (10-5) Post-induction % MRD- (10-5) Post-Consolidation Overall MRD(10-5) GRIFFIN1 teNDMM 2 207 V Dara 22% 50% 63% PERSEUS2 teNDMM 3 709 V Dara NR 58% 75% GMMG-HD73 teNDMM 3 660 V Isa 50% NA NA MASTER4 teNDMM 2 123 K Dara 38% 80% 80% IsKia5 teNDMM 3 302 K Isa 45% 77% 77%

Maintenance Therapy
Let’s keep the terms clear
•Induction: Intense and short-term therapy with goal to achieve rapid disease control
•Consolidation: Intense and shorter-term therapy with the goal of deep remission
•Maintenance: Less intense and longer-term therapy with goal of prolonged disease control (PFS) and survival (OS)
•Ideal maintenance regimen will obtain:
• Deep, prolonged remission
• Easy drug administration
• Minimal toxicity



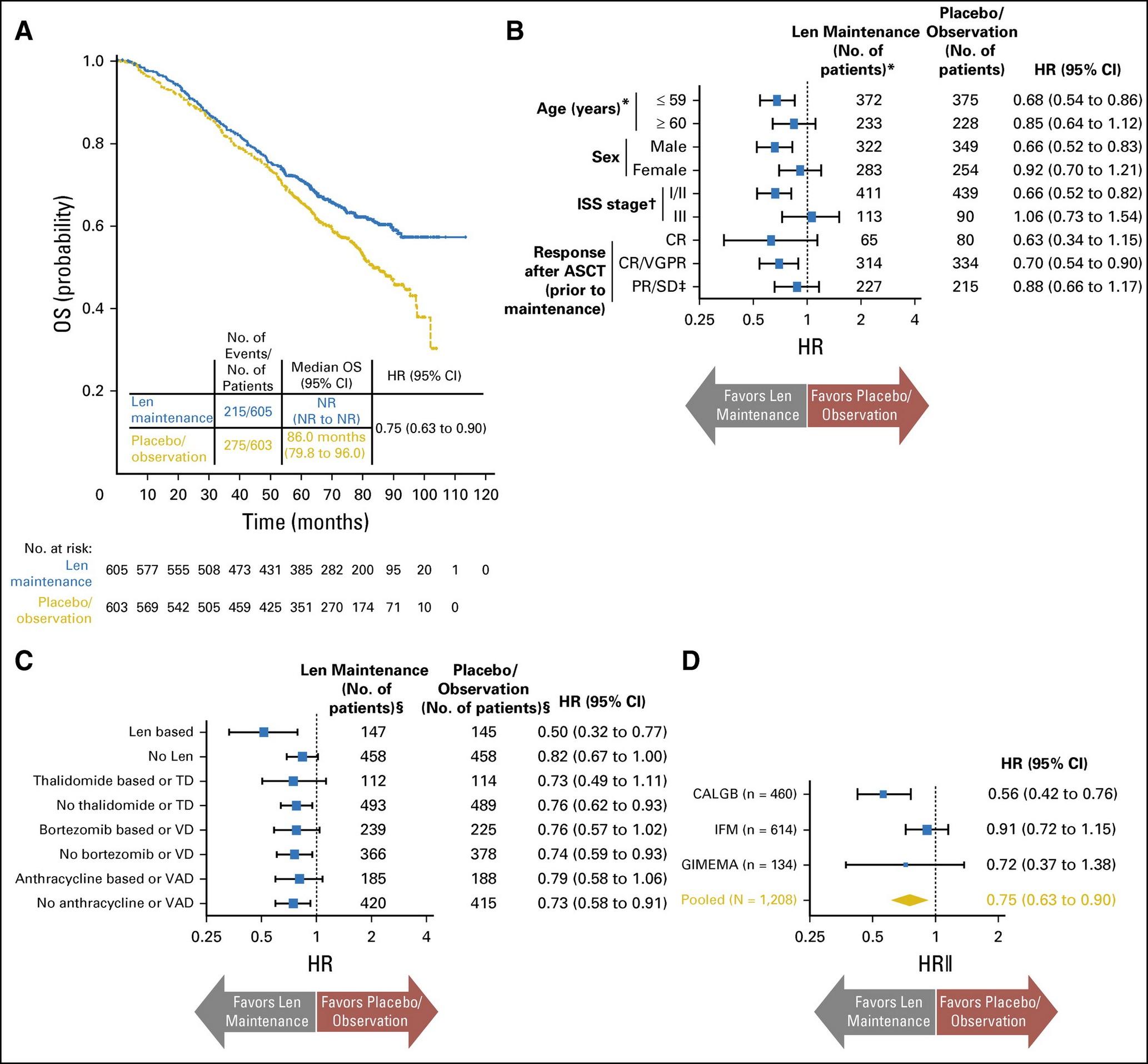
Indefinite lenalidomide maintenance is considered standard of care in all ”standard risk” patients

McCarthy, JCO,
Key take home points from this study
•Average follow-up = 6.6 years
•PFS: 52.8 months (lenalidomide) vs 23.5 months (placebo)
•PFS2: 73.3 months (lenalidomide) vs 56.7 months (placebo)
•Average overall survival (OS): NR vs 86 months
•All responding patients benefited from maintenance therapy
•29% patients discontinued lenalidomide maintenance
•Second primary malignancy (SPM) occurred more frequently (6.1%) with lenalidomide maintenance than placebo (2.8%)


What’s the optimum duration of maintenance
Myeloma XI trial



Pawly
Key take home points from this study
•Average duration of lenalidomide therapy = 28 cycles (range 1-96)
•There is an ongoing PFS benefit associated with continuing lenalidomide maintenance > 4-5 years in the overall patient population
•Even in those patients that are MRD negative, there is evidence of benefit for continuing maintenance for at least 3 years
•In MRD+ patients, continue lenalidomide maintenance until progression
Ultimate question: Which patients need to continue indefinite maintenance vs stopping at some earlier time point like 2 or 3 years


<65 years, NDMM [rP2 (N = 396)]
Combination maintenance therapy

CCd (n = 138)
CFZ 20/36 mg/m2
Cytoxan 300 mg/m2
(d1,8,15)
Dex 40 mg qwk

INDUCTION (4 cycles) R 1


KRd (n = 132)
CFZ 20/36
(d1,2,8,9,15,16)
Len 25 mg d1–d21
Dex 40 qwk

KRd (n = 126)
CFZ 20/36 mg/m2
(d1,2,8,9,15,16)
Len 25 mg d1–d21
Dex 40 mg qweek



CONSOLIDATION × 4

CCd
CFZ 20/36 mg/m2
Cytoxan 300 mg/m2 (d1,8,15)
Dex 40 mg qweek KRd
CFZ 20/36 mg/m2
(d1,2,8,9,15,16)
Len 25 mg d1–d21
Dex 40 mg qweek


KRd
CFZ 20/36 mg/m2
(d1,2,8,9,15,16)
Len 25 mg d1–d21
Dex 40 mg qweek


Len 10 mg d1–d21 Until progression

CFZ 20/36 mg/m2
(d1, 2, 15, 16)*
Len 10 mg d1–d21 Until progression
*Up to 2 years
Primary endpoint: Rate of ≥ VGPR
Secondary endpoints: Rate of sCR and MRD–, incidence of G3/4 AE, survival** **Three induction/consolidation and 2 maintenance arms

Gay, ASCO,
KR
R
M O B
L I Z A T I O N
I
KRd 4 cycles AutoHSCT MAINTENANCE R
FORTE
2
FORTE: Progression-Free Survival




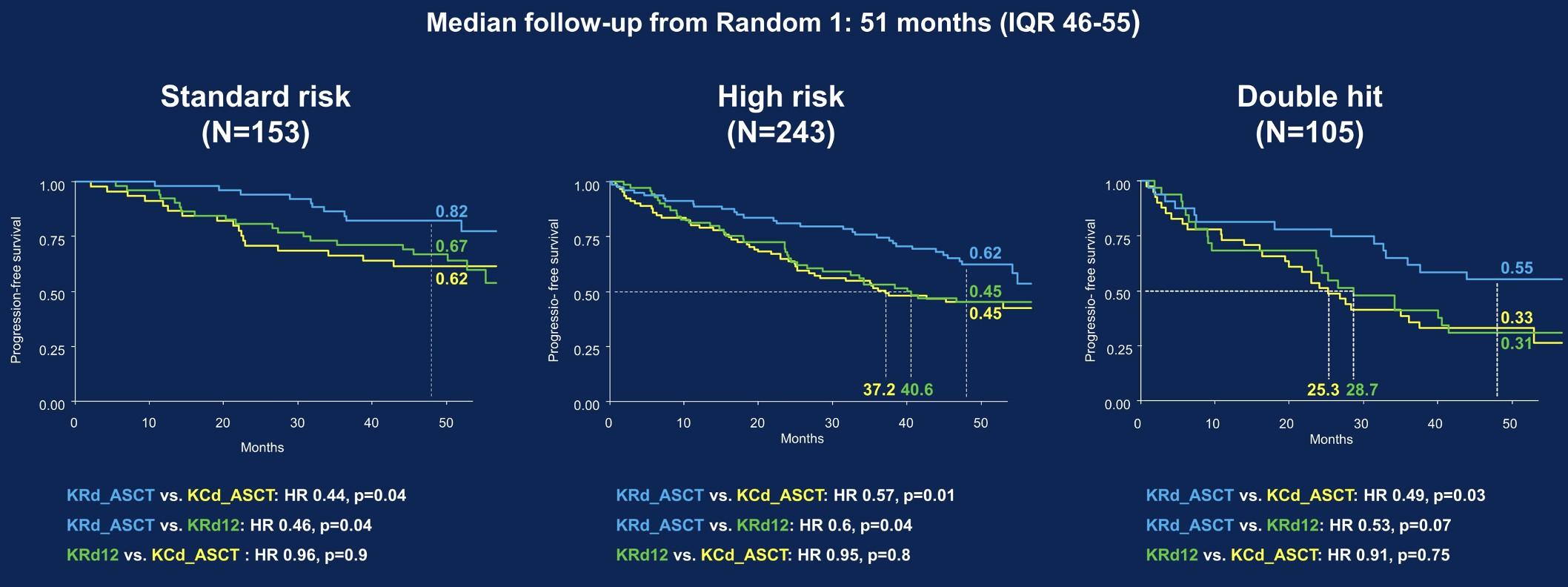
KRd + Auto significantly prolonged PFS vs KRd12 in standard- and high-risk patients.
KR significantly prolonged PFS from the start of maintenance vs R in all patients

Gay, ASCO,
KRd + Auto vs KRd12 vs CCd + Auto KR vs R
FORTE: PFS by Risk in Randomization 1


Gay, ASCO,
KRd + Auto vs KRd12 vs CCd + Auto
FORTE: PFS by Risk in Randomization 2

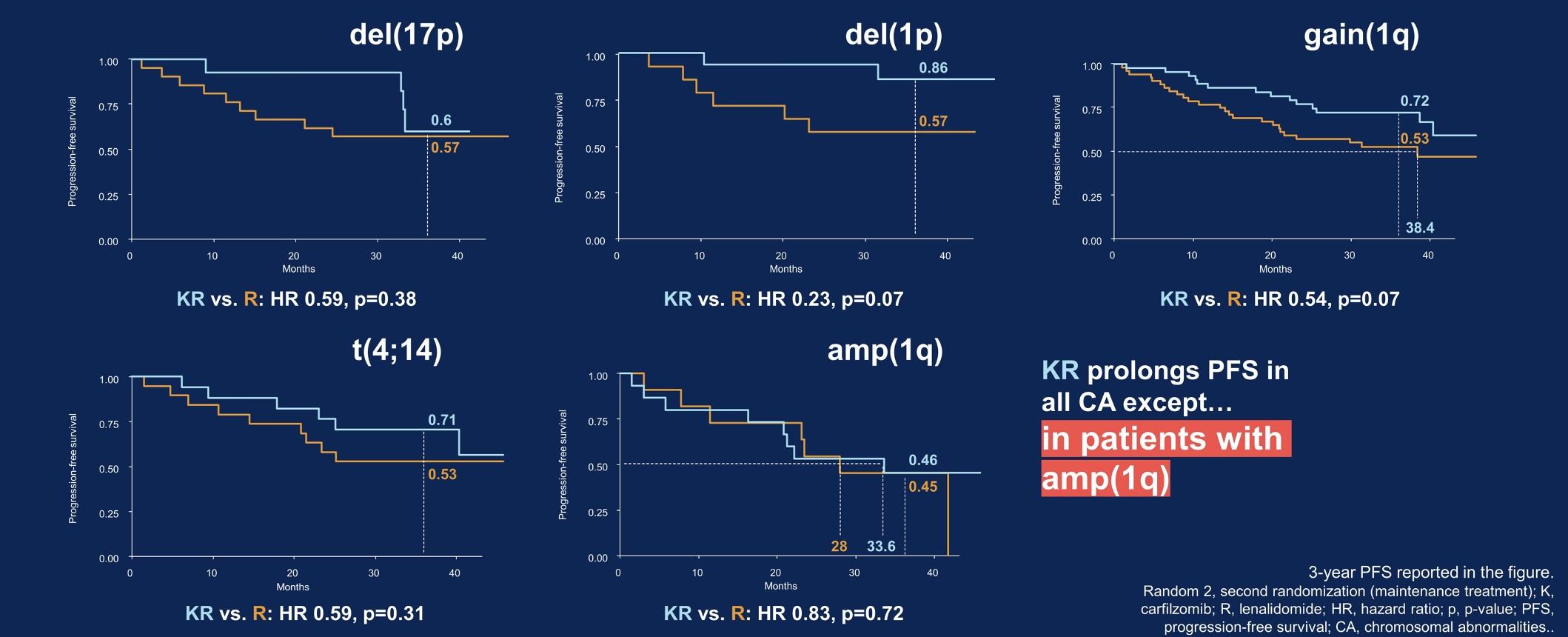
PFS was seen in all risk subgroups except those patients with >3 copies of 1q

Gay, ASCO,
KR vs R
Some patients (high risk features) benefit from 2drug therapy, but which 2 drugs?
•Lenalidomide + proteosome inhibitors
• Emory dataset, FORTE
•Daratumumab
• CASSIOPEIA – Single agent daratumumab > placebo (PFS)
•Lenalidomide + daratumumab
• GRIFFIN, PERSEUS, AURIGA, S1803 (DRAMMATIC)
• Addition of daratumumab deepens response (CR and MRD negativity
• Still so much to learn and we need more and longer term data


Improving response & survival by adding dara
Inclusion
aCD38 mAb naïve
Induction/autoHSCT
< 12 months of transplant
MRD+ at screening


Primary endpoint - MRD conversion rate after 12 month



DRAMMATI

Inclusion
Induction/autoHSCT
<








AURIG A rP3 [n = 214]
Dara SQ + Lenalidomide 10 mg qday Lenalidomide 10 mg qday Risk stratification – High risk vs standard/unknown 36 cycles Primary endpoint
OS
-
C rP3
1100]
[n =
6 months of transplant Dara SQ + Len 10 mg qday Len 10 mg qday Dara SQ + Len 10 mg STOP Dara SQ + Len 10 mg qday 24 months MRD + MR DR R STOP Len 10 mg qday MR DLen 10 mg qday R MRD + R Now that AURIGA is closed and DRAMMATIC is near the end of enrollment, we are opening RAMP-UP – investigating isatuximab + Revlimid in patients that are MRD+
Maintenance therapy summary remarks
•The choice of maintenance therapy should be risk adapted
• Patients with standard-risk disease should be treated with single-agent, dosereduced lenalidomide
• Patients with high-risk features including (but not limited to) EMD and high-risk cytogenetics should be considered for lenalidomide-based doublet maintenance
• Choice of doublet maintenance partner will be patient specific
•Data support that maintenance should be given indefinitely; however, we are likely overtreating some patients
•SWOG S1803 (DRAMMATIC) and others will help define this space in the future



Plasma Cell Leukemia
Primary plasma cell leukemia is an untamed beast
OS of primary PCL based on t(11;14)
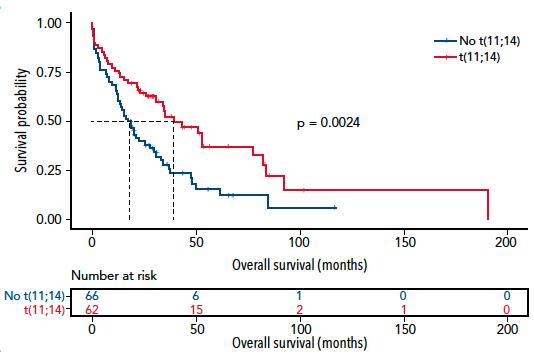

Estimated 3-year OS is 55% in t(11;14) patients and 25% in non-t(11;14) patients


Blood, 2022
Cazaubiel,
PCL: Novel therapy vs conventional chemotherapy
Retrospective analysis of pPCL (n = 110)
VRd vs. dara-quad vs. velcade-based regimens vs. conventional chemotherapy
In all-comers: ORR 83%
At a median follow-up of 51 months, VRd/dara-quad had:
Higher CR (41% vs. 17%; p=0008)
Improved mPFS (25 m vs. 13 m; p=0.03)
Improved mOS (NR vs. 20 m; p<0.001)
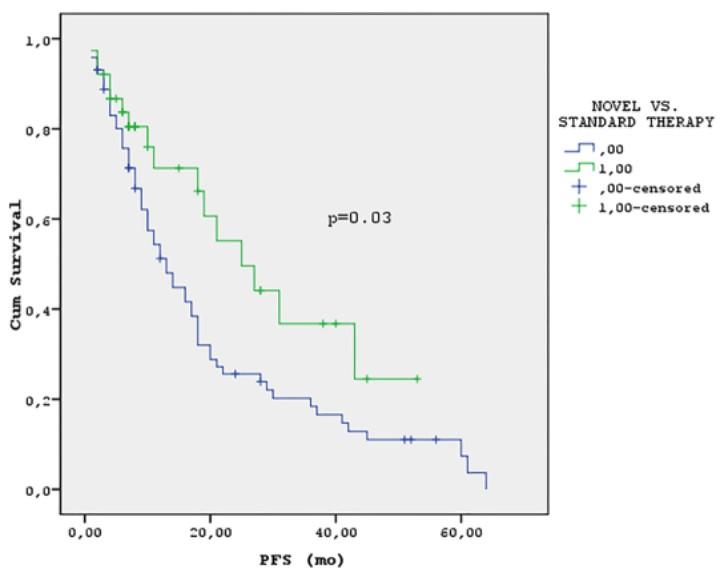



Moreau, NEJM, 2022; van de Donk, ASCO, 2023
A phase 2 trial investigating quadruplet induction followed by novel consolidation in patients with primary PCL
Stratified on receipt of preprotocol therapy and presence of high risk CGs
Tec+Tal X4 cycles
Re g t(11;14)?
SOC = Regimen received during induction therapy
MRD- rate comparison for t(11;14) pts
SOC X4 cycles
AutoHSCT MEL 140 or 200 mg/m2
Tec+Tal X4 cycles
AutoHSCT MEL 140 or 200 mg/m2
Stratified on receipt of preprotocol therapy, t(11;14) status and induction rx: t(11;14) with D-KRd vs. t(11;14) with Ven-DKd vs. non-t(11;14) with D-KRd
SOC X4 cycles
OS comparison pooled across both t(11;14) and non-t(11;14) pts
MRD- rate as co-primary endpoint
The trial has preliminary approval from Alliance, SWOG, BMTCTN, ECOG, CTEP, Janssen, and Abbvie, protocol writing is underway (National PI: Sborov), and basis of my R50 submission


N o Ye s DKRd R VenDKd DKRd
R
K-R maintenance K-R maintenance
LUNCH BREAK



Regional Community Workshop
April 13th, 2024 –
IMF
after lunch
1:00 PM Local Patient & Care Partner Panel, Sandy
Lisa Mahoney
1:10 PM Q&A 1:10 – 1:30 PM Maintenance Therapy, Nisha Joseph, MD
– 1:40 PM Q&A
– 2:25 PM Relapsed Therapies & Clinical Trials, Jonathan Kaufman, MD 2:25 – 2:35 PM Q&A 2:35 – 2:45 PM Closing Remarks 2:45 – 3:00 PM Coffee / Network
Agenda
12:40 –
& Joe Brown, Jim &
1:00 –
1:30
1:40

May 18th, 2024
IMF
Regional Community Workshop
Agenda after lunch 12:40 – 12:50 PM Engaging & Partnering with the IMF, Sylvia Dsouza 12:50 – 1:10 PM Local Patient & Care Partner Panel, John and Ann Bailey 1:10 – 1:20 PM Q&A 1:20 – 1:40 PM Maintenance Therapy, Doug Sborov, MD, MS 1:40 – 1:50 PM Q&A 1:50 – 2:30 PM Relapsed Therapies & Clinical Trials, Peter Forsberg, MD 2:30 – 2:45 PM Q&A 2:45 – 3:00 PM Closing Remarks/Coffee & Networking
–
Thank you to our sponsors!







Partnering with the IMF
By Sylvia Dsouza, Vice President, Development


WHO AM I WHAT DO I DO?

Vice President of Development for the IMF
Securing support and resources for the IMF through diverse mechanisms

Oversee a team of passionate and determined fundraising professionals who are committed to advancing the mission of the IMF
Have the incredible honor of working with dedicated volunteers from the US and across the globe.
144
Become a Partner, Be a Change Agent
Peer-to-Peer Fundraising
• Peer-to-Peer Fundraisers are created from YOUR ideas. Starting a Fundraiser is easy and fun. They also make a world of difference in the myeloma community.
• Engage your family, friends, co-workers, your network who honor your journey with myeloma and want to support you. Let them show you that you are not alone.
Join the HOPE Society (Recurring Monthly & Annual Giving Program)
• Help us cultivate the future by joining the International Myeloma Foundation's Hope Society.
• Monthly gifts starting at $10 support IMF core programs, including educational events, publications, the toll-free InfoLine, and more.
• Turn your monthly contribution into a yearly commitment.
Transformative Gifts (Major Giving and Principal Giving)



• Gifts can be designated toward a specific program, project or initiative .
Unrestricted, Direct and Endowment

145

What will your legacy be?
Planned Giving
• Join the Brian D. Novis Legacy Society and make a planned gift!
• Gain immediate tax benefits
• Potentially increase your income during your lifetime.
• Continue to fund our core programs and four pillars.
• Make a bequest (a gift from your estate)
• Include a provision in your will or living trust.
• Designated us as a beneficiary of a life insurance policy, or retirement plan (IRA, 401(k), or 403(b).

• Leave us in your will is one of the most profound ways to support the people and causes important to you.
Corporate and Foundation Gifts
• Your organization can contribute a corporate gift or foundation grant
• Provide seed funding that is necessary to accelerate the path to a cure.


146

3 Ways to Engage
Philanthropy
• Make a donation to support research, patient programs, and advocacy efforts.
• Sponsor or participate in fundraising events such as walks, runs, or galas.
• Create a fundraising campaign online to raise awareness and funds.
Community/network
• Join your local support group/become a Support Group Leader
• Volunteer your time through mentorship or support programs.
• Engage on social media to connect with others affected by myeloma.
Intellectual capacity
• Attend conferences/webinars to stay updated on the latest news.
• Offer your expertise as a speaker or panelist at events.



147
Start the Conversation




We welcome you to continue to learn more about our programs, projects, and initiatives at the IMF and find alignment with your own myeloma journey as well as ways to deepen and strengthen your engagement with us.
Reach out to the IMF Development Team to start a conversation on how you can make a difference in the lives of the people impacted by myeloma.
•Sylvia Dsouza - IMF Vice President, Development sdsouza@myeloma.org | 818.487.7455 ext. 268
148


Join us - Miles for Myeloma on Saturday, May 4

149
QUESTIONS?



Local Patient & Care Partner
John Bailey, Patient
Ann Bailey, Care Partner

Caregiver’s Guide to Sailing Through Multiple Myeloma!

1.Be Prepared

Get your ship in shape!

Huntsman’s Daily Dozen for Caregivers

Anchors
•Educate yourself to make decisions
• Meds, terms, procedures, side winder problems
•Find a specialist
• Meds, terms, procedures, side winder problems
•Know Patient’s Meds
• and manage If needed

•Patient Binder so you know what’s happening
•Financial Affairs in order
•Manage appointments

•Myeloma Bag with healthy snacks, water, tissues, mints, etc for patient or you if needed!
2. Take Charge-Advocate!
…..control your sails, chart the course!
•Know options
•Assess your resources
•Compile questions before see Dr. - Don’t be intimidated!
•Find support groups
•Be alert to patient’s needs
•Sense when to take over and when to back off
•Minimize stress
•Don’t borrow trouble from other patient’s journeys

•Designate person to be PR person when going through transplants, etcso it is off your plate
• Well-meaning friends who have a cure for cancer! Or want to know all the details--Trust your judgement-
3. Give Thanks
….for the journey!
•Angels surround you

•Team effort-Bright medical professionals-Drs.,Nurses,
• Social workers, Insurance specialists, therapists, etc
•Family, friends, neighbors, other patients/caregivers
•Think higher whether it is a faith or philosophy-hopeful!
•“Because I Knew You, I Have Been Changed for Good”-Wicked
• You can be the calm in someone else’s storm. Lift other patients!
Cancer does not have to:
…cripple love
…shatter hope
…corrode faith
… destroy peace
…kill friendship
…suppress memories
…silence courage
…invade the soul
…steal eternal life
…conquer the spirit


Our Well-trained Crew!!!



• The pessimist complains about the storm,
the optimist hopes it will turn and
the realist adjusts the sails.
•
•
Relapsed Therapies & Clinical Trials
 Peter Forsberg, MD
Peter Forsberg, MD
Colorado Blood Institute
Denver, CO
Relapsed/Refractory Myeloma and Clinical Trials
IMF Salt Lake City Regional Community Workshop
Peter Forsberg MD Co-Director of Plasma Cell Disorders
Colorado Blood Cancer Institute


Disclosures
•Consultant - BMS, GSK, Karyopharm, Sanofi
•Research Support - Karyopharm, Genentech, BMS, Janssen


Lets start with some definitions
•Relapsed myeloma-
• Myeloma that becomes active after prior therapy
•Refractory myeloma
• Myeloma that fails to respond to therapy or becomes active during therapy after initial control
• You can be refractory to certain therapies while still having multiple viable treatment options (even in the same drug class)
•You can receive a treatment but if it’s stopped before myeloma progresses we wouldn’t consider you refractory to it (we call this exposed)


How about a few more definitions
•We categorize certain groups of drugs that act in similar ways to target MM as “classes”
• These include IMiDs (eg Revlimid, Pomalyst), proteasome inhibitors (eg Velcade, Kyprolis), monoclonal antibodies (eg Darzylex, Sarclisa)
• If you have received at least one IMiD, one PI and one antibody you’d be considered ”triple class exposed”
• If your myeloma has progressed while receiving at least one IMiD, one PI and one antibody you’d be considered “triple class refractory”
•A “line of therapy” is a treatment/combination or planned sequence of treatments to target myeloma


How do we decide on therapy in RRMM?
•It’s complicated!
•There are more considerations to factor in than ever before
•As always in MM it starts with the patient
•Our tool box continued to get broader and more diverse in terms of therapies
•Need to have an open discussion around pros/cons of different approaches in each individual situation


Early lines treatment are important!
1st Relapse 2nd 3rd 4th 5th and beyond
Fewer patient are eligible for therapy in each subsequent line of therapy (LOT)

Figure adapted from: Yong, K et al. Br J Haematol 2016;175(2):252-264

Multiple Myeloma is Not One Disease!
RVD+ASCT+Lenalidomide Maintenance
Relapse 2

Relapse 1
Relapse 3
Relapse 2
Relapse 1
Relapse 1
Time: Years!

D i s e a s e A c ti v i t y
Slide stolen from Joe Mikhael
Notable Phase 3 Combination Trials in RRMM


Study Name Experimental Arm Control Arm Experiment al PFS Control PFS Hazard Ratio Endeavor Car/Dex Vel/Dex 18.7 9.4 0.53; p<0.0001 Aspire Car/Rev/Dex Rev/Dex 26.3 17.6 0.69; P=0.0001 Candor Car/Dara/Dex Car/Dex 28.6 15.8 0.63; P=0.0014 Ikema Car/Isa/Dex Car/Dex 35.7 19.15 0.53; p=0.0007 Castor Dara/Vel/Dex Vel/Dex 16.7 7.1 0.31; p<0.0001 Pollux Dara/Rev/Dex Rev/Dex 44.5 17.5 0.44; p<0.0001 Apollo Dara/Pom/ Dex Pom/Dex 12.4 6.9 0.63; p=0.0018 Eloquent 3 Elo/Pom/Dex Pom/Dex 10.3 4.7 0.54; p=0.008
What to use at first relapse (if revlimid refractory)?
Lancet Oncol 2021)

Lancet Oncol 2022)
Lancet 2019)
2022, Moreau Lancet 2021)

Dara/Pom/Dex Dara/Carfil/Dex Isa/Pom/Dex Isa/Carfil/Dex Carfil/Pom/Dex # visits per month 4->2->1 7->6 (in practice 4->3) 4->2 7->6 (in practice 4->3) 6 (in practice 3) mPFS (total) 12.4 months 28.6 11.5 35.7 12 mPFS Len Refractory 9.9 months 28.1 Not available Not available Not available Median Prior Lines of Therapy 2 2 3 2 4 % Len Refractory 79% 32% 92% 34% N/A Source APOLLO
CANDOR Update
ICARIA (Attal
IKEMA/Update (ESMO
Jakubowiak CLML
Anti-CD38 mAb Pomalidomide Dexamethasone Anti-CD38mAb Carfilzomib Dexamethasone Carfilzomib Pomalidomide Dexamethasone
(Dimeopoulos
(Usmani
2019, pooled analysis
Until a month ago how did you decide on for 2nd or 3rd line therapy?
If Revlimid
Refractory Dara or Isa/Carfil/Dex (Likely best PFS) Dara or Isa /Pom/Dex (Most Convenient dosing) Dara/Vel/ Dex (if preexisting CHF)
If Rev+CD38 mAb Refractory Vel/Pom/Dex (If PI naïve) Carfil/ Pom/Dex (If vel exposed) Carfil/Cytoxan/ Dex (If Pom exposed)


If IMiD+PI+CD 38 mAb Refractory Clinical Trial CAR-T Bispecific Selinexor based combo
Why did you say until a month ago?
Immunotherapies have crashed the party!
Antibody–Drug Conjugates
Cytotoxic Payload Released Into Cell

CAR T-Cells Bispecific
Antibodies or T-Cell Engagers

BCMA MM Cell Death
BCMA MM Cell Death scFv Viral Vector Signaling Domain Cytotoxic Cytokines CD3 BCMA MM Cell Death
Yu. J Hematol Oncol. 2020;13:1. Bispecific T-cell Engager Bispecific Antibody
Cytotoxic Cytokines
The CAR-T Era in MM is firmly upon us
•T-cells engineered to target something on the surface of cancer cells
•Abecma: First CAR-T approved for use in relapsed MM in 2021
•Carvykti: Second approval in 2022
•Both initially approved for use in patients with 4 prior lines of therapy
•Mar 2024: Abecma approved in patients with patients with 2 prior lines of therapy
•April 2024: Carvykti approved in patients with 1 prior line of therapy
Ide-Cel/Abecma Cilta-Cel/Carvykti
BCMA-specific extracellular scFv-targeting domain with 4-1BB co-stim and CD3ζ signaling domains

Contains 2 BCMA-targeting single-chain antibody designed to confer avidity

scFv scFv CD3 ζ 41BB
CD8α hinge VHH VHH Binding Domains CD3 z 41BB
How does CAR-T work?
Leukapheresis Manufacturing Infusion
Collect patient’s white blood cells Isolate and activate Tcells Engineer Tcells with CAR gene

Targeting element (eg, CD19, BCMA)
Expand CAR T-cells Infuse same patient with CAR T-cells
Spacer



Transmembrane domain
Costimulatory domain (eg, CD28 or 4-1BB)
CD3 (essential �� signaling domain)
Median manufacturing time: 17-28 days

















Patients undergo lymphodepleting (and possibly salvage/bridging) therapy

Majors. EHA 2018. Abstr PS1156. Lim. Cell. 2017;168:724. Sadelain. Nat Rev Cancer. 2003;3:35. Brentjens. Nat Med. 2003;9:279. Park. ASH 2015. Abstr 682. Axicabtagene


ciloleucel PI.
PI. eg, CD19, BCMA
Activity
vector with CAR DNA CARengineere d T-cell
Tisagenlecleucel
Tumor cell
Viral
Slide credit: clinicaloptions.com
CAR T-Cell Therapy Patient Journey







3 Lymphodepletion (chemotherapy) 4 Infusion (Hospitalization or daily outpatient monitoring) 1 Apheresis 2 (Manufacturing) Patients return home Immune cells from the patient are collected
used to create “immunologic
to CAR T cells to expand Standard of care therapy is permitted until CAR T cells are ready for infusion 1 day 4–6 weeks 3 days 2 weeks Within 2 weeks 5 Close Follow up Required period close to CAR-T center Caretaker support needed
Fludarabine and Cytoxan are
space”
CAR T: Expected Toxicities

Cytokine release syndrome (CRS) Neurotoxicity (ICANS)

Cytopenias

Infections

CRS ICANS
Onset 19 days after CAR T-cell infusion 29 days after CAR T-cell infusion
Duration 5 11 days 3 17 days
Symptoms
• Fever
• Difficulty breathing
• Dizziness
• Nausea
• Headache
• Rapid heartbeat
• Low blood pressure
• Headache
• Confusion
• Language disturbance
• Seizures
• Delirium
• Cerebral edema
Management
• Actemra (tocilizumab)
• Corticosteroids
• Supportive care
• Antiseizure medications
• Corticosteroids
*Based on the ASTCT consensus; †Based on vasopressor; ‡For adults and children >12 years;
§For children ≤12 years; Only when concurrent with CRS
Xiao X et al. J Exp Clin Cancer Res. 2021;40(1):367. Lee DW et al. Biol Blood Marrow Transplant. 2019;25:625; Shah N et al. J Immunother Cancer. 2020;8:e000734.

Late Line Abecma: KarMMa-2 Trial



Munshi NC et al. N Engl J Med 2021;384:705-716
All
84%,
Neurotoxicity All
Cytokine Release Syndrome
Gr
Gr 3+ 5%
Gr 18%, Gr 3+ 3%
Earlier Line Abecma: KarMMa-3 Trial
•Abecma vs investigator selected standard of care regimen in patients with 2-4 prior lines of therapy including prior CD38 monoclonal, proteasome inhibitor and IMiD
•Camparison arm options: DPd, DVd, Ird, Kd, EPd
•65% triple refractory P Rodriguez-Otero et al. N Engl J Med 2023;388:1002-1014.

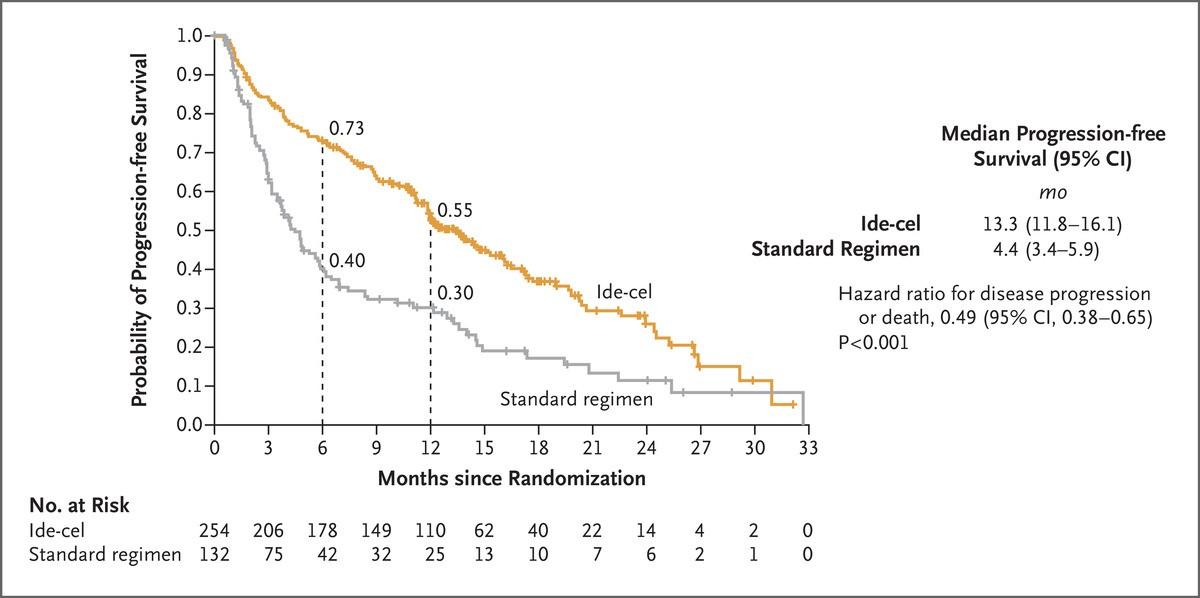

Late Line Carvykti: CARTITUDE-1 Trial


3.1% ORR by IRC: 97.9% P a ti e n t s ( % ) 100 80 60 40 20 0 sCR: 82.5% ≥ VGPR: 94.9% 82.5% Best response = sCR VGPR PR 12.4% Martin et al. Journal Clin Oncol 2022; 41 (6): 1265-1274 Cytokine Release Syndrome All Gr 95%, Gr 3+ 4% Neurotoxicity All Gr 22%, Gr 3+11% Most recent update median PFS 34.9 months
Earlier Line Cavykti: CARTITUDE-4 Trial
•Carvykti vs standard of care in MM with 1-3 prior lines of therapy
•Len refractory
•15% triple refractory
•Comparison options: PVd, DPd
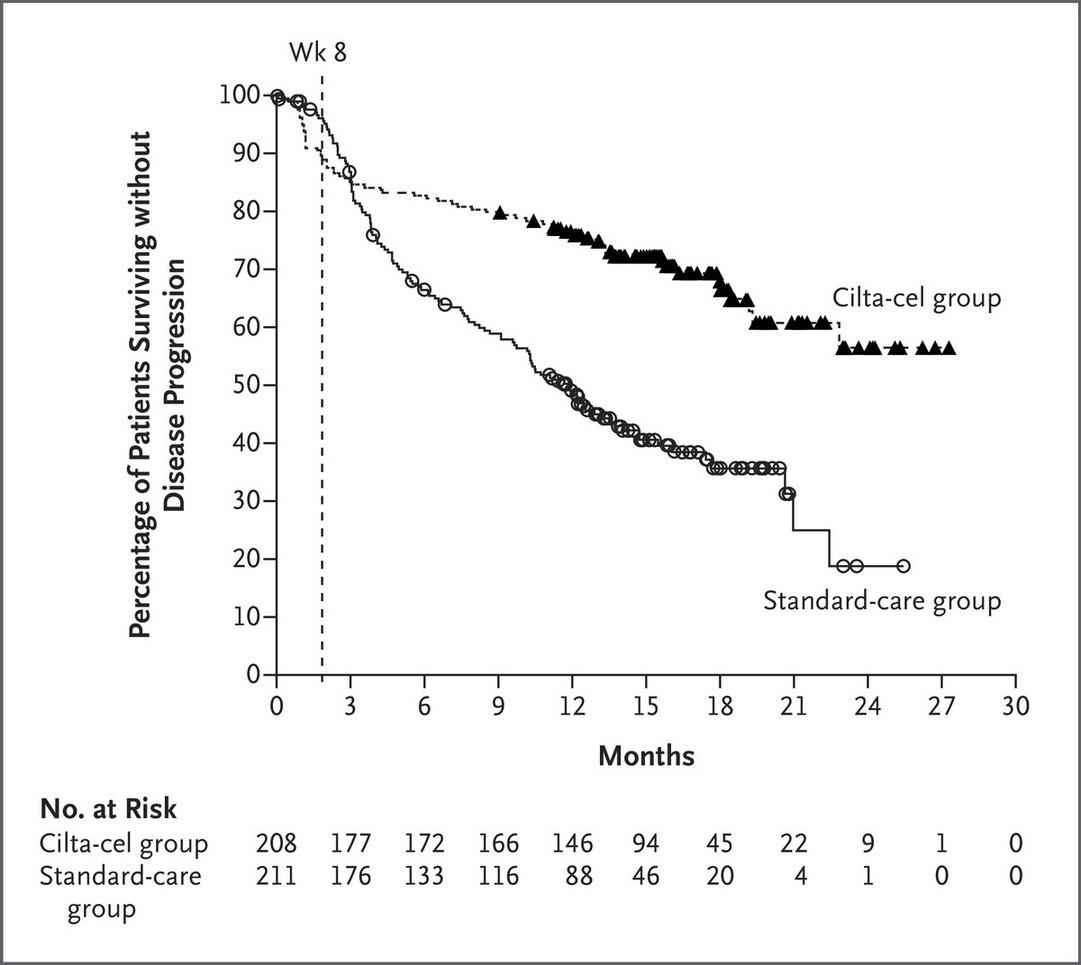


J San-Miguel et al. N Engl J Med 2023;389:335-347.
CARTITUDE-4 Safety
- Similar rates of infection, grade 3+ infection - 66% IVIg use in CAR arm - One primary concern with Carvykti is atypical neurological toxicity - Almost 5% on CARTITUDE-1 with late-onset movement or neurocognitive issues
- <1% in CARTITUDE-4 - 9% cranial nerve palsy
- Similar rate of secondary malignancy in both arms- So far



J San-Miguel et al. N Engl J Med
2023;389:335-347.
What are bi-specifics?
•Antibodies that bind to T-Cells and cancer cells and bring them close together-> T-cell killing
•Teclistamab (Tecvayli): Approved Dec 2022
•Elranatamab (Elrexfio) and Talquetamab (Talvey) approved Aug 2023
•Teclistamab and Elranatamab target
•Talquetamab targets GPRC5D
•Currently approved for late line use

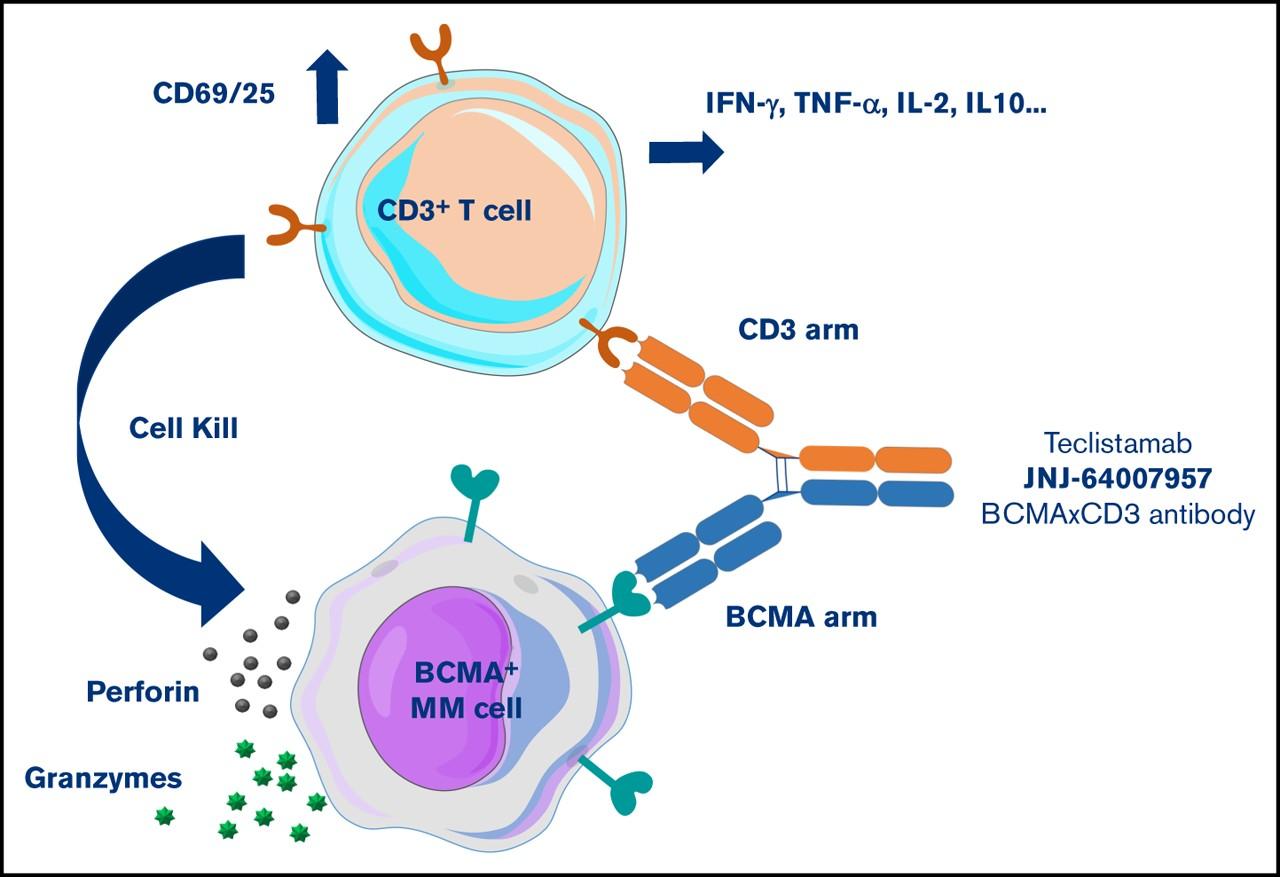

BCMA
Moreau P
al. N Engl J Med 2022;387:495-505 Pillarisetti
al. Blood Adv 2020;4(18):4538-49
et
K et
Teclistamab: MajesTEC-1 Trial
Cytokine Release Syndrome All Grade 72%
Infection All Grade 76%, Gr 3/4 45%


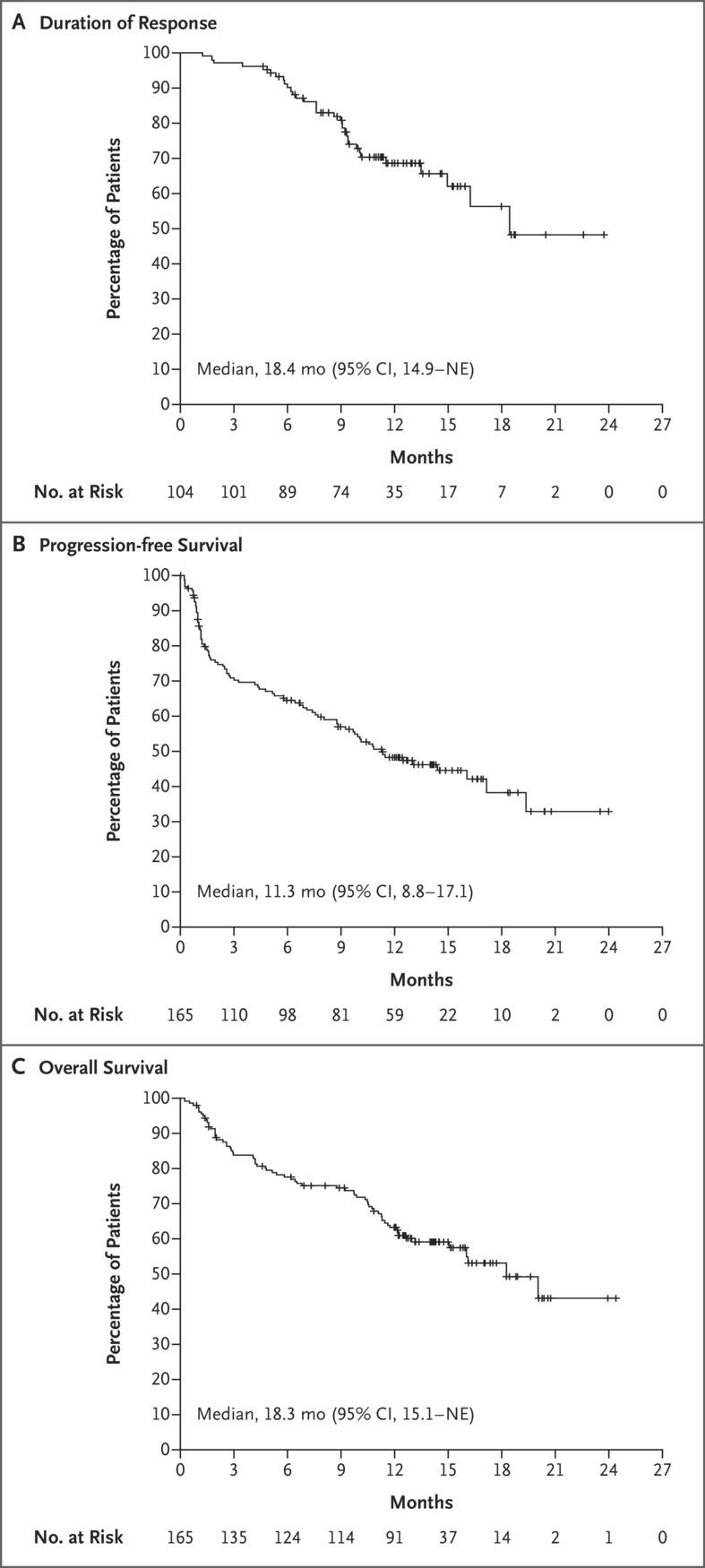

Moreau P et al. N Engl J Med2022;387:495-505
Elranatamab
•MagnetisMM-3 trial
•Median PFS around 15 months
•CRS 56%, no grade 3 or higher
•Infection 70%, grade 3+ 40%
•ICANS 3.4%
•Update from MagnetisMM-1 trial: analysis of 13 patients with prior BCMA therapy ORR 54%2
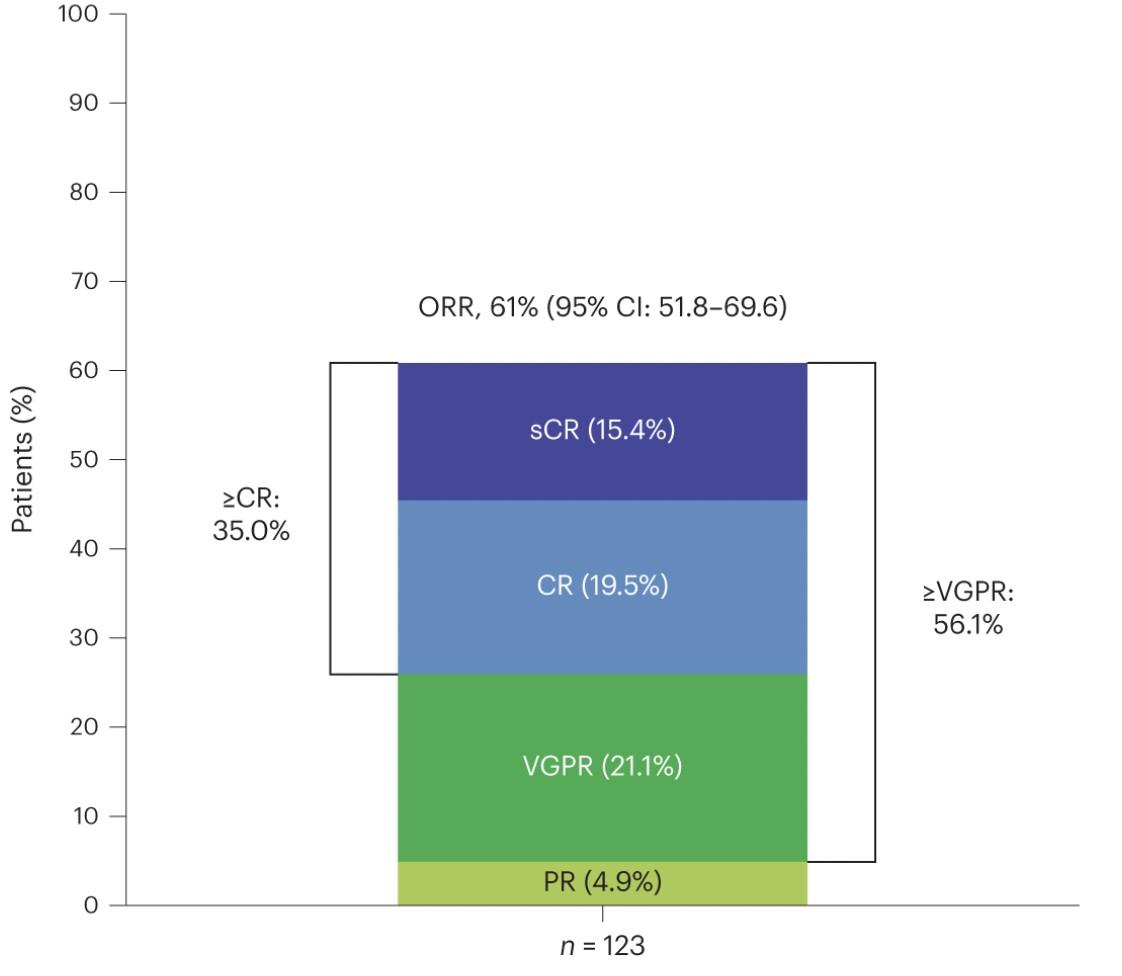

 1. Lesokhin et al, Nature Medicine 2023 29; 2259-2267
2. Raje. ASH 2022. Abstr 158
1. Lesokhin et al, Nature Medicine 2023 29; 2259-2267
2. Raje. ASH 2022. Abstr 158
Talquetamab
•Novel bispecific targeting GPRC5D
Phase 1 dose escalation study with 232 patients
• 130 with subcutaneous dosing
•Common adverse events included:
• CRS in 78% (only 1 grade 3/4)
• Skin-related events 69%
• Altered taste 59% (dry mouth very common
• Nail related events 39%

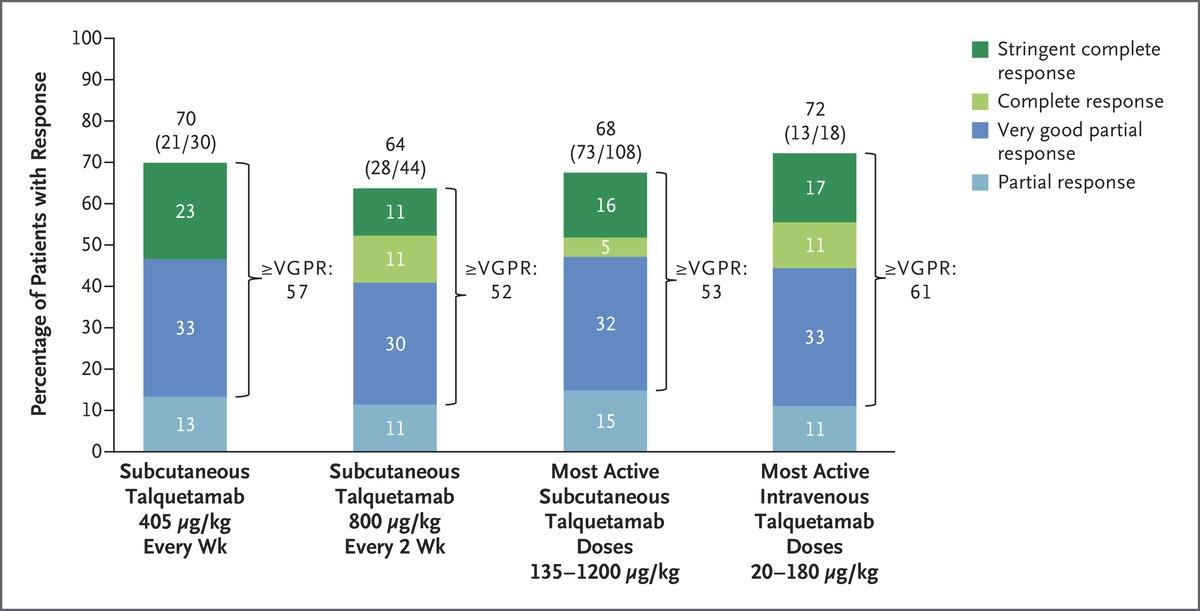

A Chari et al. N Engl J Med 2022. DOI: 10.1056/NEJMoa2204591
How do I choose between a CAR-T and a Bispecific?
CAR T-Cells Bispecific Antibody
Personalized
Targeted immuno-cytotoxicity
Single infusion (“one and done”)
Potentially persistent
FACT-accredited center required (hospitalization likely required)
CRS and neurotoxicity; requires ICU and neurology services
Dependent on T-cell health (manufacturing failures)
Requires significant social support; caregiver required

Off the shelf
Targeted immuno-cytotoxicity
No lymphodepletion Minimal steroids
Initial hospitalization required
CRS and neurotoxicity possible
Dependent on T-cell health (T-cell exhaustion)
Requires repeated administration

D i s a d v a n t a g e s A d v a n t a g e s $$$$ $$$ Adapted from Shah, ASH 2021
So in this new landscape what’s the right fit for me?
•Treatment options are quite different
•In 1st/2nd relapse the diversity of options give us more opportunity to find the right treatment fit for each patient without sacrificing effectiveness
•Increased need for open conversation, sharing your goals/preferences with providers and engaging with a myeloma specialty center
• Discussing availability and openness regarding second cancers/neurologic events are key
•Always ask about trial options!


Clinical Trials - Overview
Remember some of the important principles of clinical trials:
•The drive of research has brought us to where we are
•No one is expected to be a “guinea pig” with no potential benefit to them, there are strong checks/balances to prevent this
•Research is under very tight supervision and standards
•Open, clear communication between the physician and the patient is fundamental
•Some trials don’t evaluate new treatment but seek to collect patient data or samples to help answer other questions about myeloma


Clinical Trials – Why Me??
•Every patient is unique and must be viewed that way
•Benefits of trials are numerous and include:
• Early access to “new” therapy
• Delay use of standard therapy
• Contribution to myeloma world – present and future
• Financial access to certain agents
•Must be balanced with potential risks
• “toxicity” of side effects
• Possibility of lack of efficacy


Overview
of New Drug Development
Identify a target for therapy in the laboratory
Confirm the anticancer activity in laboratory and animal studies
Clinical trials (human studies) to determine safety, dosing and effectiveness
The whole process costs millions of dollars and years of effort!


Clinical Trials - Phases


Phase I Phase II Phase III
Tests safety Tests how well treatment works Compares new treatment to standard treatment
Phase 1 Clinical Trials
•All patients receive the experimental therapy
•Phase 1 trials find the optimal dose of a new drug or drug combination
•Patients get higher doses as the study continues
•Determine side effects of new drugs or combinations
•Explore how the drug is metabolized by the body
•Important for all stages of myeloma


Phase 2 Clinical Trials
•Determine if a new drug or combination is effective against the cancer
•May be added to a phase 1 study once the ideal dose is found
•Patients usually receive the experimental therapy
•In some cases, the study may include two “arms” comparing either two different doses or a different treatment (another combination of drugs)


Phase 3 Clinical Trials
•Highest form of clinical evidence. Typically a large number of patients are required…usually required for full FDA approval
•Patients receive either an experimental therapy (one or more drugs) or the current standard treatment
• The patient is randomly assigned to a treatment—a process called randomization
• Neither the physician or the patient can determine which treatment is given
•May be placebo controlled, if no standard treatments are available
•Very closely monitored for effectiveness and side effects


Differences between early (phase 1) and late (phase 3) trials
•Potential risks and benefits may be pretty different
• In early phase trials:
• Effectiveness and potential toxicities from a treatment may not be well understood
• May be a higher chance that treatment is not effective or that it is being used below effective dose
• These studies are more often conducted in settings where standard of care options are limited (the alternative is ineffective, not well tolerated or there is no real alternative)
• In later phase trials:
• Treatment has shown promising effectiveness and reasonable safety in earlier phase studies
• Needs to be compared to standard options to see how it stacks up
• Key to bringing new therapies into earlier lines or treatment


Clinical trial study design or protocol
•Each cancer clinical trial has a written detailed study design called a protocol that includes:
• Why the clinical trial is needed
• Purpose of the clinical trial
• What drug or drug(s) are being tested, with a treatment and follow-up schedule
• Safety measures throughout the clinical trial program
• How outcomes will be measured
• Who is eligible for the clinical trial
• How the clinical trial will be organized, one site or multiple sites
• If the clinical trial is a multi-site trial, all participating physicians must follow the same protocol


Diversity in Clinical Trials
•There has been a lack of diverse representation in clinical trials in myeloma. In the U.S., approximately 20% of all myeloma patients are of African descent, but only 5%–8% of patients in myeloma clinical trials are of African descent.
• This is significant for the following reasons:
• All patients of all races and ethnicities should be able to benefit from clinical trials.
• Diverse patient representation in clinical trials is required to ensure that the outcomes are applicable to all patients.
•Reasons for underrepresentation in clinical trials are complex and include accessibility of clinical trials, sensitivity to diversity by medical professionals, misconduct in medicine in the past, the lack of trust in the system, and more


Commonly Asked Questions
How does the study work? How often will I need to see my doctor or visit the cancer center?
Will I need to undergo additional tests?
What is currently known about the new drug or combination?
What benefits can I expect?
What side effects should I expect? Who should I notify if I have side effects?
Can I take my vitamins or other medications?
Can I get the treatment with my local doctor?
Will my insurance pay for my participation in the clinical trial?










Considering Entering a Clinical Trial?
•Discuss whether or not you are eligible for a clinical trial with your physician
•Work with your physician to determine the best trial for you
•Meet with the clinical research nurse or trials coordinator to discuss the trial
•Carefully review the provided “Informed Consent”
• Describes
the study and any potential safety concerns related to the experimental medication




Ongoing Trials in Myeloma
SO many areas being studied right now including…
•Innovating with CAR T Cell therapy (used earlier in myeloma, new targets, faster manufacturing, even allo CAR T!)
•Expanding on bispecific therapies (new targets, use in earlier lines, without hospitalization, as part of combinations with other bispecifics or other approved therapies) and even trispecifics
•New molecules – CelMods, …
And MANY more!



Questions?

Thank you to our sponsors!








Upcoming IMF Events

Patient and Family Seminars

Los Angeles – August 16th & 17th, 2024 – Hilton Los Angeles/Universal City; please check the website for updates!

Thank you for attending today’s program!
May 18th, 2024, International Myeloma Foundation’s
Regional Community Workshop – Salt
Lake City

























































































































































































































































































































