

2024 Boca Raton Patient and Family Seminar
March 15 & 16, 2024













The IMF Support Group Team is Here For You!
Shared Experiences Help to Better Understand the Myeloma Journey
• Support Groups Empower Patients & Care Partners with information, insight, & hope
• The IMF provides educational support to a network of over 150 myeloma specific groups

Support.myeloma.org



We are happy to help connect you with an existing support group or help form a new one! We assist with virtual, in-person, and hybrid options for meetings.
Reach out to us at
SGTeam@myeloma.org

Local
Support Groups: You Are Not Alone!
Miami Multiple Myeloma Support Group
Meets virtually on the 4th Wednesday of each month at 6:30PM
Melbourne Multiple Myeloma Support Group
Meets in-person on the 4th Monday of each month at 10:30AM
Palm Beach County Multiple Myeloma Support Group
Meets in a hybrid format on the 1st nonholiday Monday of each month at 6:30PM

Maitland Multiple Myeloma Support Group
Meets in-person on the 2nd Monday of each month at 6:30pm


Fort Myers Multiple Myeloma Support Group
Meets in-person on the 3rd Tuesday of each month at 6pm
Hollywood Multiple Myeloma Support Group
Meets virtually on the 1st Tuesday of each month at 6PM
Jacksonville Multiple Myeloma Support Group
Meets in a hybrid format on the 2nd Wednesday of each month at 6PM
Local Support Groups: You Are Not Alone!
Brooksville / Nature Coast Multiple Myeloma Support Group
Meets virtually on the 3rd Wednesday of each month at 6PM
Tampa CentralMultiple Reasons Support Group
Meets virtually on the 2nd Thursday of each month at 11AM


North Tampa Multiple Myeloma Support Group
Meets in-person on the 3rd Saturday of each month at 10:30AM

Naples Multiple Myeloma Support Group
Meets in-person on the 3rd Thursday of each month at 6pm
Tampa Bay/St Petersburg Multiple Myeloma Educational Group
Meets virtually on the 1st Saturday of each month at 10:30AM
Local Support Groups: You Are Not Alone!
Palm Coast Multiple Myeloma Support Group
Meets in-person on the 2nd
Thursday of each month at 3:30PM
Ocala Multiple Myeloma Support Group
Meets in-person on the 2nd
Saturday of each month at 11AM
Sarasota Multiple Myeloma Network & Education Group
Meets in-person on the 4th
Friday of each month at 11AM


The Villages Multiple Myeloma Support Group
Meets in-person on the 1st Tuesday of each month at 1PM

Panama City Multiple Myeloma Support Group
Meets in-person on the 2nd Saturday of each month at 10AM
Tallahassee Multiple Myeloma Support Group
Meets in-person on the 4th Monday of each month at 5:30PM
IMF – Special Interest Virtual Groups

Special interest groups are designed as a supplemental support for specific populations of patients, in addition to their local Support Groups
Las Voces de Mieloma
Designed for Spanish speaking patients only
Living Solo & Strong with Myeloma
Designed for patients without a care partner

Coming Soon!
Care Partners Only
Designed to address the needs of care partners only

Smolder Bolder
Created for people living with Smoldering Multiple Myeloma
MM Families

High Risk Multiple Myeloma
Designed to address the needs of the high-risk MM population
MGUS 4 Us
Created for people living with MGUS
For patients/care partners with young children

EVALUATION
Please be sure to complete your program evaluation today.
Questions 1 – 5 can be completed before the program begins.
Questions 7 & 8 can be worked on after each presentation.
Our team will collect all responses at the end of the day!
We greatly appreciate your time and feedback!


President & CEO Address
Yelak Biru
28-year Myeloma Survivor Patient
International Myeloma Foundation









The IMF is dedicated to improving the quality of life of myeloma patients while working toward prevention and a cure.




In November 2023, Susie stepped down from her leadership position to take time from what has been her 24/7 commitment to the IMF since •





In 2021 Poornima was Diagnosed with Multiple Myeloma



She had many questions!
Emotional

• This can't be happening to me.
• What will the future hold? How will this progress?
• Will treatment be painful? Will I be able to handle the side effects?
• How long do I have? What will my final days be like?
• Even after treatment, the fear of the cancer coming back can be very real.
• No one understands what I'm going through."
Medical
• What kind of doctor should I see?
• What is myeloma? What stage is it? What is my prognosis? Is it curable?
• What tests do I need to have done?
• What are my treatment options? What are the side effects of treatment?
• Are there clinical trials I can participate in?
• Will I be able to work and live a normal life?
Support
• What resources are available to help me cope with this disease?
• How will I pay for treatment?
• What kind of support groups are available?
• How do I talk to my family and friends about my diagnosis?



In 1995 Yelak was Diagnosed with Multiple Myeloma








A world where every myeloma patient can live life to the fullest, unburdened by the disease.



Purpose

Our calling is clear: to fight alongside you every step of the way.



The Why of the IMF: A Patient-Centric Approach

You have the right to live life to the fullest. This is why a cure alone isn't enough.
• Patient-centric approach
• Information and resources
• Support systems




















Is it Enough?

The IMF has made incredible strides in empowering patients. Imagine a newly diagnosed patient, overwhelmed and scared.
• Clear, concise information and Education
• Support groups
• We advocate on your behalf.
• We do and conduct research.

These are vital steps, but…
The answer is a resounding NO, we are not doing enough!


More Should Be Done, and Here’s How the IMF Plays a Role

• We envision a future where patients can thrive, not just survive.
• Myeloma shouldn't dictate your quality of life.
• You shouldn't have to choose between effective treatment and debilitating side effects.
• C U R E


Early Diagnosis

High-quality
Strategic collaboration
5
Research
that matters most to patients happens

Our Strategy Flywheel

4+ Generations
Millennials (22-41)
Gen-X (42-57)
Baby Boomers (58-75 Traditionalists (75-95)
1. Primary Destination
• Research, Support, Education, Advocacy
• Omnichannel way – Online, In Person, Telephone
2. Patients & Care Partners
• Shorten time from diagnosis to Hope –Time To Hope (TTH)
• Increase Time To next Treatment (TTT)
• Education
• Connection
• Overcome obstacles to access
• Address health equity
3. Healthcare Providers
• Timely Information + Education
• Treatment guidelines for newly diagnosed & initial relapse
• Expert 2nd opinion
4. Partner Ecosystem
• Credible partners in development, access, and policy
• Clinical trials – remove entry barrier + increase patient participation
• Research – Novel testing & therapy approaches
• Black Swan Research Initiative – BSRI
• Immunotherapy Data Marketplace
5. Sustainable Growth
• Strategic Plan
• Digital Innovation
• Investment in our People
• Bold Collaboration
6. Expand Capability
• Research
• Technology and Data







EVALUATION
Please take a moment to reflect and respond to the program evaluation.
Questions 7 & 8 can be worked on after each presentation.
We greatly appreciate your time and feedback!


What is the Future of Myeloma?
Saad Usmani, MDMemorial Sloan Kettering Cancer Center
New York, NY



The Future of Multiple Myeloma
Saad Z. Usmani, MD MBA FACP FRCP FASCO Chief of Myeloma ServiceProfessor, Weill Cornell Medical College, Cornell University



Disclosures
• Research funding: Abbvie, Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda.
• Consulting: Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Gracell Therapeutics, Janssen, Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio.

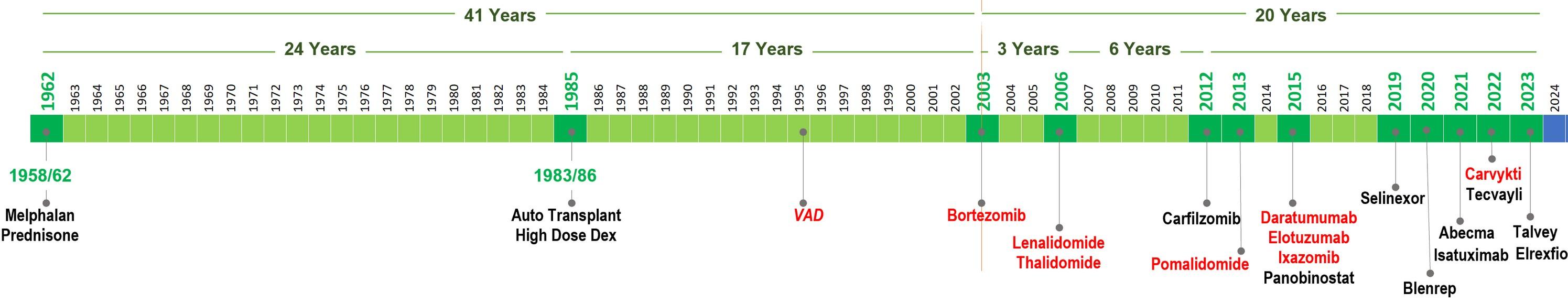
It Is Not a Matter of How, It Is a Matter of When We Will #CureMM!


Towards Curing Myeloma by 2034
• Comprehensively study the molecular and immunobiology of disease evolution and progression in MM.
• Recognize ‘real’ myeloma at the smoldering stage and intervene early for a defined duration of time.
• Pick different strategies for different disease biology and immune status.
• Incorporate frailty assessments in this algorithm.
• Optimize sequencing of existing therapies and incorporation of select novel MoAs based on disease biology.
• Accurately assess sustained minimal residual disease (MRD) negativity.
• Utilize novel imaging and novel peripheral blood assessments.
• Use MRD assessments guide treatment time and treatment strategy.
• Use Sustained MRD to stop treatment.
• Pay attention to supportive care.
• Address both short-term and long-term sequelae of treatments.
Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani
The Road to Victory….

CAR, chimeric antigen receptor; HDT, high-dose therapy; OS, overall survival; PD-1, programmed cell death 1; PD-L1, programmed cell death-ligand 1; PFS, progression-free survival; R/R, relapsed/refractory.
1. Laubach J, et al. Annu Rev Med. 2011;62:249-264. 2. Rajkumar SV. Am J Hematol. 2020;95(5):548-567. 3. Palumbo A, et al. N Engl J Med. 2014;371(10):895-905. 4. Zanwar S, et al. Blood Cancer J. 2020;10(8):84. doi: 10.1038/s41408-020-00350-x. 5. US Food and Drug Administration. FDA granted accelerated approval to belantamab mafodotin-blmf for multiple myeloma. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-granted-accelerated-approval-belantamab-mafodotin-blmf-multiple-myeloma. Updated August 6, 2020. Accessed May 6, 2021. 6. US Food and Drug Administration. FDA approves first cell-based gene therapy for adult patients with multiple myeloma. https://www.fda.gov/news-events/press-announcements/fda-approves-first-cell-basedgene-therapy-adult-patients-multiple-myeloma. Updated March 27, 2021. Accessed May 17, 2021.
Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani

NDMM: Principles of Therapy
• Picking the right strategy that gives the highest likelihood of the best depth of response in the first year of diagnosis is extremely important for survival outcomes.
– MRD 10-5 >> MRD 10-6 >> Sustained MRD 10-6
• Optimize induction, consolidation and maintenance based on:
– Disease biology (what kind?).
– Disease burden (how much?).
– Patient characteristics (PS, co-morbidities, frailty).
– Patient preference.
• Never under-treat, put your best foot forward!
– Especially true for high risk NDMM (HR-NDMM)
• Do not forget supportive care measures: bone health, infection prevention, pain management, physical therapy and rehabilitation, mental health.

Staging: Combining Nature with Size
Stage1 R-ISS1
Serum albumin ≥3.5 g/dL-1
I
Serum β2M <3.5 mg/L-1
No high-risk cytogenetics
Normal LDH level
II Not stage I or III
III Serum β2M >5.5 mg/L-1
High-risk cytogenetics: t(4;14), t(4;16), or del(17p) or elevated LDH
Risk2 Features
Trisomies
Standard
High
t(11;14) t(6;14)
t(4;14) t(14;16) t(14;20)
Del(17p)
p53 mutation
Gain/Amp 1q
High plasma cell S-phase
GEP high-risk signatures
Circulating Plasma Cells
Elevated LDH/EMD
Ultra-High Risk 2 or more features
Stage1 R2-ISS3
I 0 Points (Low Risk, 19% pts)
II 0.5-1 Points (Low-Intermediate Risk, 31% pts)
III 1.5-2.5 Points (Intermediate-High Risk, 41% pts)
IV 3-5 Points (High Risk, 9 % pts)
POINTS: ISS III= 1.5, ISS-II = 1, Del17p =1, elevated LDH =1, Chromosome 1q21+ = 0.5
High-Risk Consensus Definition for Trials4
• R-ISS III
• R-ISS II with 1q21+, Del17p, t(14;16), t(14;20)
• Circulating PCs >5%
• Extramedullary disease

Treatment Paradigm For Newly Diagnosed


SWOG S0777: RVd Versus Rd in Patients Without Immediate Intent for ASCT1

RVd
Presented

IFM 2009 Study: Early vs Late ASCT
3x R ASCT
3x
RVd 21-day Cycles
R: 25 mg d 1 – 14
V: 1.3 mg/m2 d 1, 4, 8, 11
2x
Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani
R Maintenance
R: 10-15 mg/d for 13 cycles
ORR, MRD, TTP, OS, safety

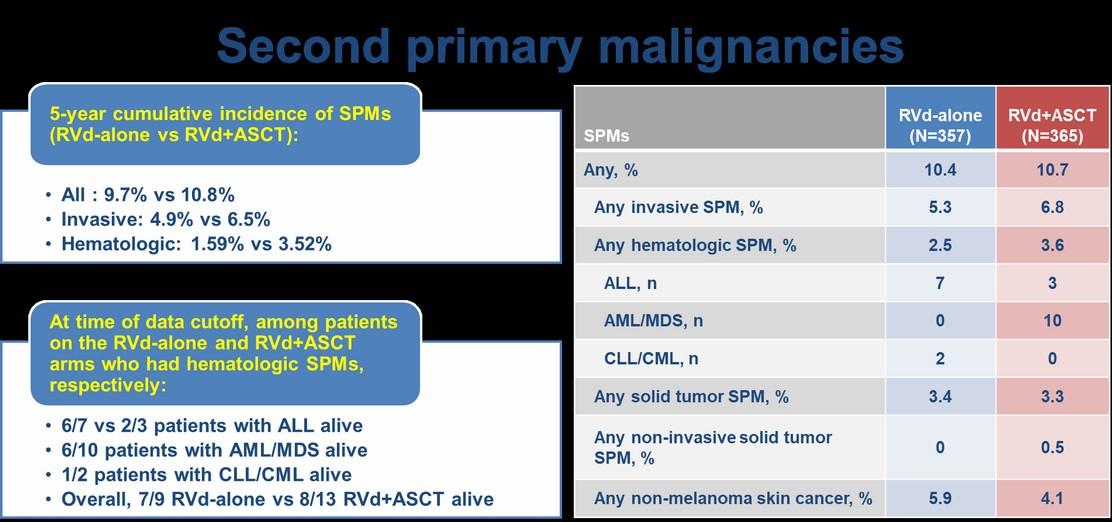
Superior PFS With ASCT vs RVd Alone

RVd + transplant was superior to RVd alone, even with undetectable MRD at 10-6
Presented by:
MRD, minimal residual disease.
Perrot A. Presented at: 62nd ASH Annual Meeting and Exposition; December 5-8, 2020; Abstract 143.

DETERMINATION: study design and patient disposition
DETERMINATION: Delayed vs Early Transplant with Revlimid Maintenance and Antimyeloma Triple Therapy
RVd cycle 1 (N=729)
Randomization (N=722)
Stratified by:
ISS disease stage
Cytogenetic risk

Arm A: RVd-alone (N=357)

Arm B: RVd+ASCT (N=365)
RVd cycles 2-3
Stem cell collection
RVd cycles 4-8
R maintenance (N=291)
RVd cycles 2-3

Each RVd cycle (21 days):
R 25 mg/day PO, days 1-14
V 1.3 mg/m2 IV/SC, days 1, 4, 8, 11
Dex 20/10 mg PO, days 1, 2, 4, 5, 8, 9, 11, 12
Stem cell collection
Melphalan 200 mg/m2 + ASCT (N=310)
RVd cycles 4-5

Induction ± ASCT + consolidation treatment duration = ~6 months
R maintenance (N=289)

Lenalidomide maintenance
Months 1-3: 10 mg/day
Month 4 onwards: 15 mg/day
• d/Dex, dexamethasone; DOR, duration of response; ISS, International Staging System; IV, intravenous; PO, orally; R, lenalidomide; SC, subcutaneous; TTP, time to progression; V, bortezomib
Primary endpoint: PFS
Secondary endpoints: response rates; DOR; TTP; OS; QoL; safety

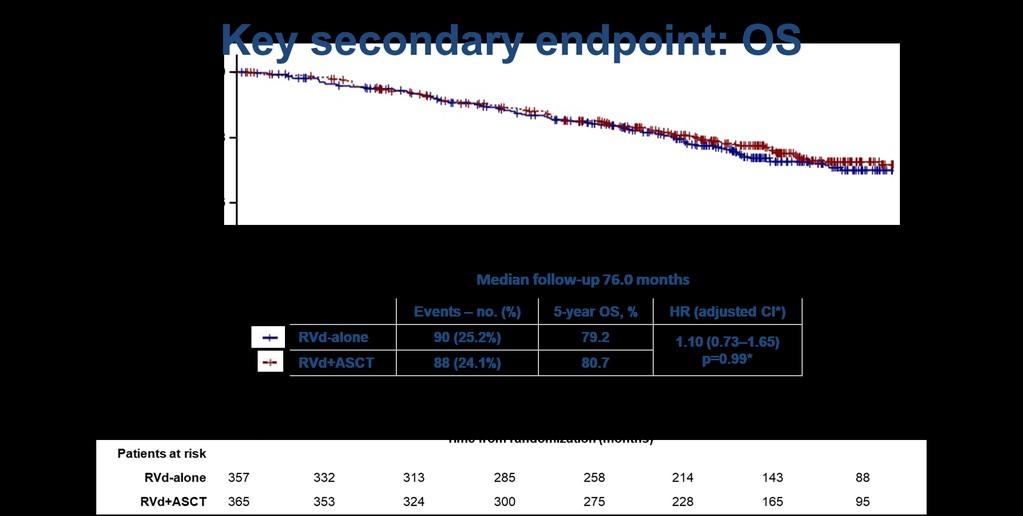
DETERMINATION: Endpoint Readouts
(Median follow-up 70 months)


Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani

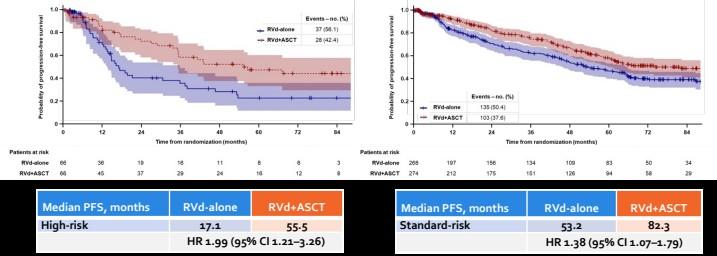
DETERMINATION Trial: PFS by Risk

GRIFFIN: Daratumumab Plus Lenalidomide, Bortezomib,
and Dexamethasone in Transplant-Eligible NDMM – 24 Months of Maintenance
Study design
Key eligibility criteria: TE NDMM; 18–70 years; ECOG PS 0–2; CrCl ≥30 mL/min2
D-R
N=207
Induction: C1–4 Consolidation: C5–6a Maintenance: C7–32b
D-RVd
D: 16 mg/kg iv D1, 8, 15
R: 25 mg po D1–14
V: 1.3 mg/m2 sc D1, 4, 8, 11
d: 20 mg po D1, 2, 8, 9, 15, 16
RVd
R: 25 mg po D1–14
V: 1.3 mg/m2 sc D1, 4, 8, 11
d: 20 mg po D1, 2, 8, 9, 15, 16
21-day cycles
D-RVd
D: 16 mg/kg iv D1
R: 25 mg po D1–14
V: 1.3 mg/m2 sc D1, 4, 8, 11
RVd
Patient disposition
n (%)
D: 16 mg/kg iv D1 q4w or q8wc
R: 10 mg po D1–21; C7–9
d: 20 mg po D1, 2, 8, 9, 15, 16 R
R: 25 mg po Days 1–14
V: 1.3 mg/m2 sc D1, 4, 8, 11
d: 20 mg po D1, 2, 8, 9, 15, 16
Stem cell mobilization with G-CSF ± plerixafor
• Primary endpoint: sCR by end of consolidation
D-RVd (n=104) RVd (n=103)
Treated with maintenance therapy 90 (87) 70 (68)
Completed maintenance therapy
15 mg po D1–21; C10+
R: 10 mg po D1–21; C79
15 mg po D1–21; C10+
21-day cycles 28-day cycles
67 (64) 44 (43)
Discontinued treatment during maintenance therapy 21 (20) 21 (20)
Adverse
• Secondary endpoints: MRD negativity (NGS 10-5), ORR, ≥VGPR, CR, PFS, OS
aConsolidation initiated 60–100 days post transplant; bPatients who complete maintenance cycles 7–32 may continue single-agent lenalidomide thereafter; cProtocol amendment allowed q4w dosing option. Phase 2 trial – patient enrollment between December 2016 and April 2018
Laubach JP, et al. ASH 2021, Virtual Meeting. Abstract 79
Presented

GRIFFIN: Daratumumab
Plus Lenalidomide, Bortezomib, and Dexamethasone in Transplant-Eligible NDMM – 24 Months of Maintenance
RVd ± Daratumumab x 6 cycles (4 pre- and 2 post ASCT) ASCT R ± Daratumumab maintenance x 2 years optional R maintenance
Voorhees PM et al. Lancet Haematology 2023.
Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani
MRD assessed in the ITT population

GRIFFIN: Longitudinal Outcomes



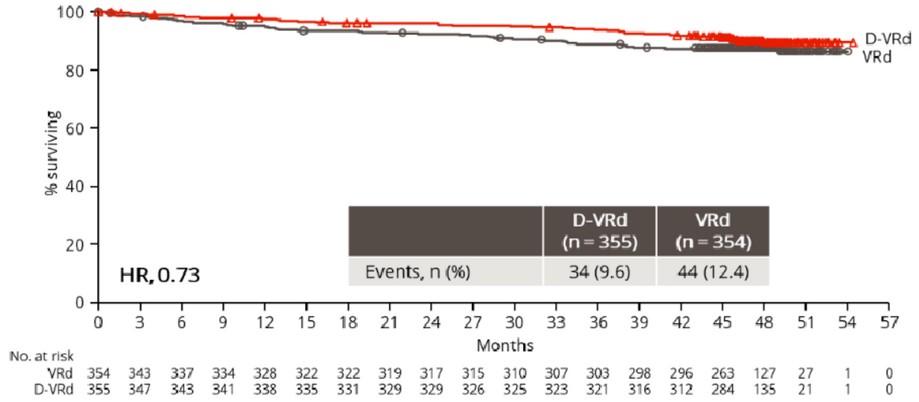
PERSEUS: DVRd vs VRd in Transplant-Eligible NDMM
Eligibility
• Transplant-eligible NDMM
• Age 18 – 70
• ECOG PS 0 – 2
4 cycles induction 2 cycles consolidation Transplant
28-day cycles
n = 355
R VRd
n = 354
28-day cycles
DVRd VRd
DVRd
Mel200 + ASCT
Induction/Consolidation Schedule
D: 1800 mg SC days 1, 8, 15, 22 in cycles 1-2, days 1, 15 in cycles 3-6
V: 1.3 mg/m2 days 1, 4, 8, 11
R: 25 mg PO days 1 – 21
d: 40 mg PO/IV days 1-4, 9-12
Primary endpoint: PFS
Key secondary endpoints: CR rate, MRD, OS
Maintenanc e until PD
DVRd VRd
Key Baseline Characteristics
n = 355 n = 354
Median age (range), y 61 (32 – 70)
High risk cytogenetics, n (%) 76 (21.4)
59 (31 – 70)
78 (22.0)
Extramedullary disease, n (%) 15 (4.2) 16 (4.5)
ISS stage, n (%) I II III
186 (52.4)
114 (32.1)
55 (15.5)
178 (50.4)
125 (35.4)
50 (14.2)
• ASCT, autologous stem cell transplant; CR, complete response; DVRd, daratumumab, bortezomib, lenalidomide, and dexamethasone; DR, daratumumab and lenalidomide; ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System; IV, intravenous; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; PFS, progression-free survival; PS, performance status; OS, overall survival; PO, by mouth; R, lenalidomide; SC, subcutaneous; VRd, bortezomib, lenalidomide, and dexamethasone.

PERSEUS: PFS and OS
Median follow-up 47.5 mo


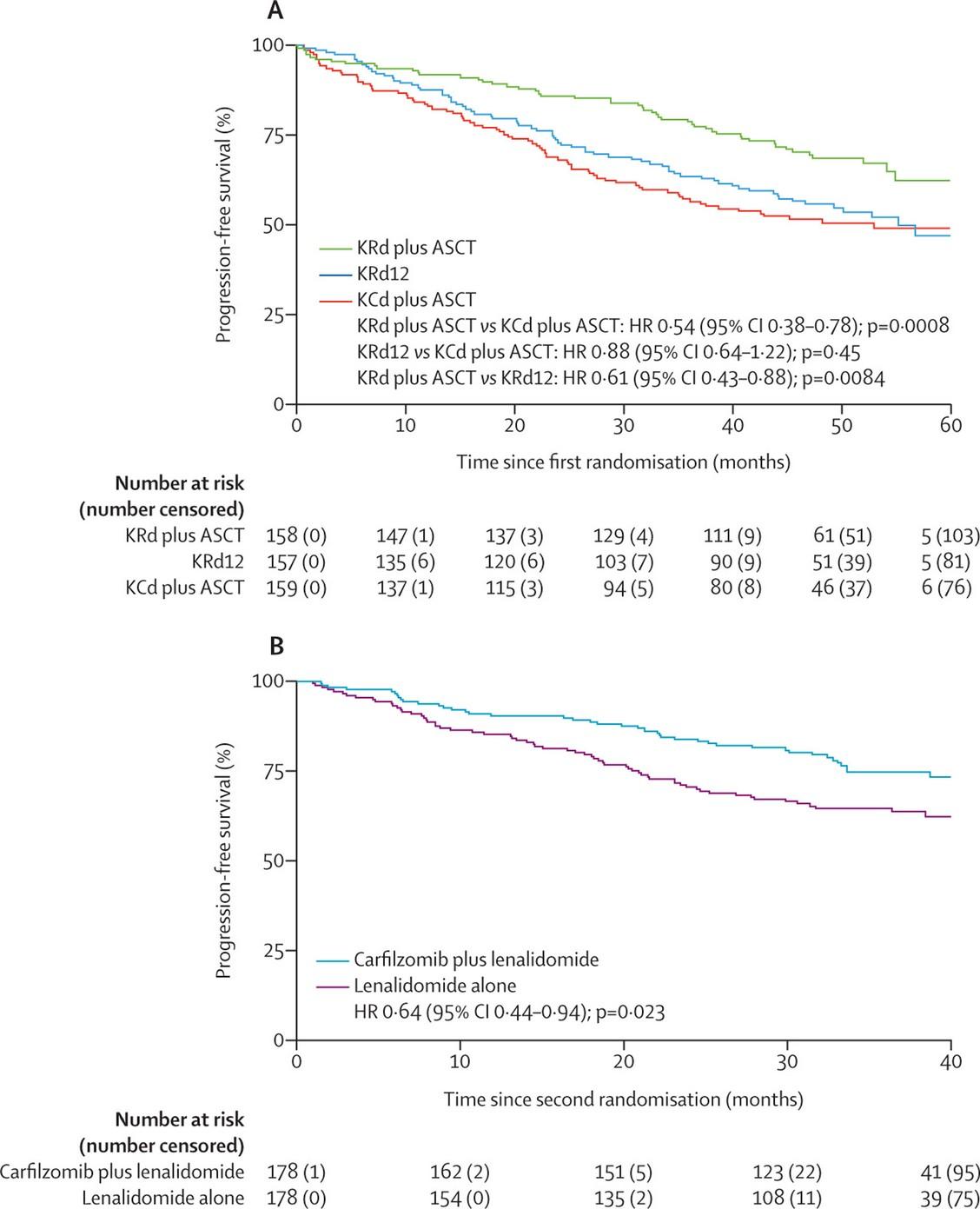
The FORTE Trial
Multicenter, Randomized (1:1), Open-Label, Phase 2 Study

Is MEL200 better than 16 additional weeks of KRD?
FORTE. Updated November 3, 2022. https://classic.clinicaltrials.gov/ct2/show/NCT02203643
Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani

FORTE: Depth of Response and PFS
Progression-Free Survival
Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani
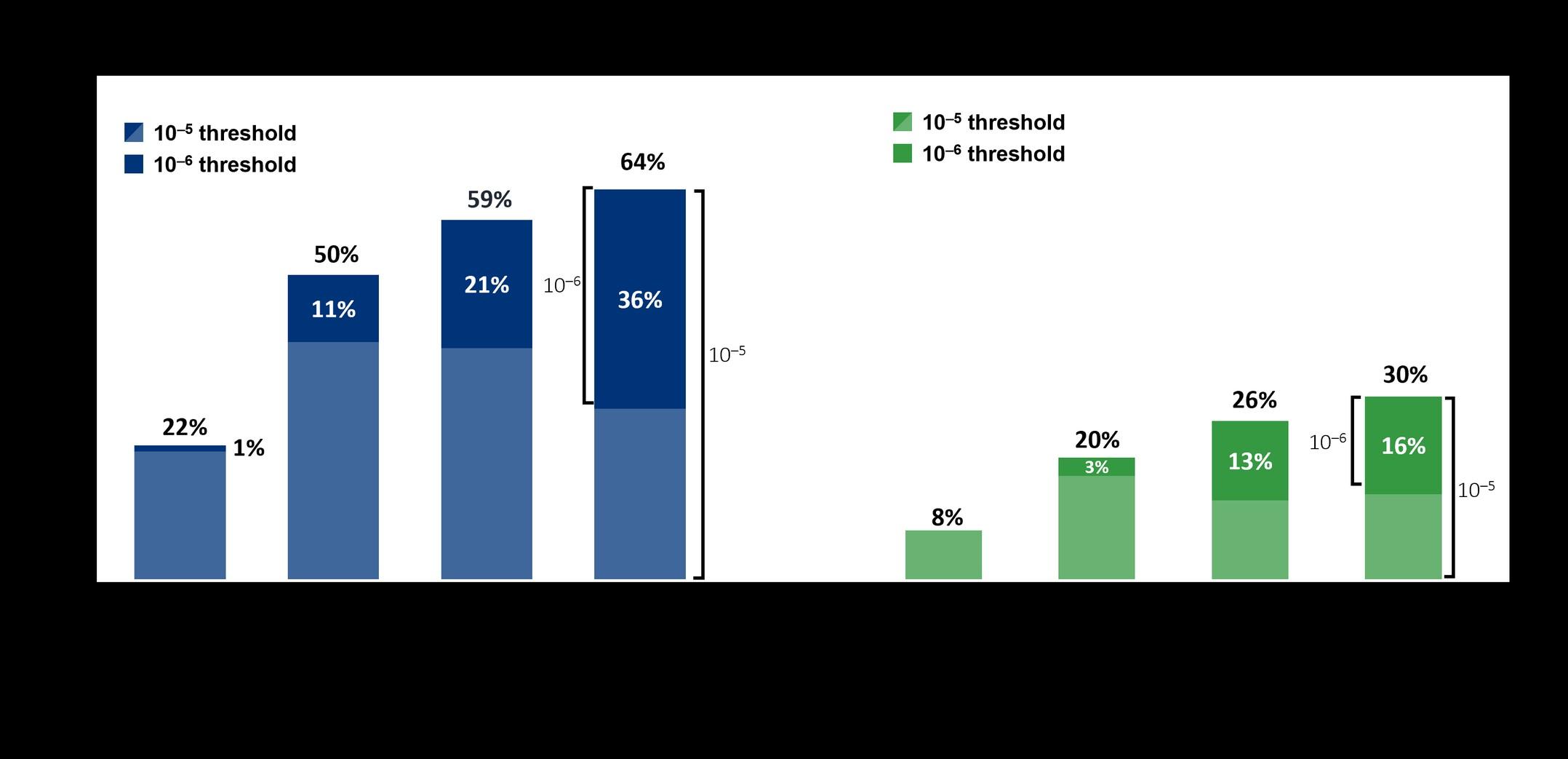
MRD Negativity
12 58% 35%
ASCT 43% 25%
† OR KRd ASCT vs KRd 12: 1.69 (95% CI 1.07 – 2.66, P = .024).
Progression-Free Survival With Sustained MRD Negativity

. 2021;22(12):1705-1720.


Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani

Daratumumab-KRd for NDMM
Study/Phase Patient Characteristics Responses PFS data Safety (Grade ¾)
Landgren O et al
JAMA Onc 2021
Phase II
8 cycles
N=41
High-risk = 49% (included gain 1q)
Median age: 60 years
Costa LJ et al
JCO 2022
Phase II
4 cycles
Bhutani M et al
ASH 2022
Phase II
8 cycles
ORR = 100% >CR rate = 95%
MRD-ve at 10-5 = 71%
N=123
High-risk = 57% (included gain 1q)
Median age: 60 years
N=23 (of 39)
High-risk = 43% (included gain 1q)
Median age:
ORR = 100%
>CR rate = 39%
MRD-ve at 10-5 = 80%
1-year PFS rate 100% Neutropenia 27%, Rash 9%
Lung infection 7%
Increased ALT 4% No TRM
2-year PFS rate 87% Lung infection 6% VTE 3% No TRM
ORR = 100%
>CR rate = 65%
MRD-ve at 10-5 = 70%
Not reported Hypophosphatemia 30%
Neutropenia 13%, HTN 13%
COVID19 7% No TRM

Rates of ≥CR (best response on study) by cytogenetic risk status* in MASTER and GRIFFIN trials

Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani

MRD negativity by cytogenetic risk status* among patients who received D-KRd in MASTER and D-RVd in GRIFFIN
MRD minimal residual disease, D-KRd daratumumab plus carfilzomib/lenalidomide/dexamethasone, D-RVd daratumumab plus lenalidomide/bortezomib/dexamethasone, HRCA high-risk cytogenetic abnormality, CR complete response, NA not available.
*HRCAs include any of the following genetic abnormalities: del(17p), t(4;14), t(14;16), t(14;20), and gain/amp(1q21) (≥3 copies of chromosome 1q21). Patients were grouped into categories: standard risk (0 HRCA), high risk (1 HRCA), or ultra-high risk (≥2 HRCAs).
†For MASTER, data are for all enrolled patients with available MRD data.
‡For GRIFFIN, the D-RVd group included patients from the randomized phase (n = 104) and the safety run-in phase (n = 16). Patients were grouped by HRCA: 0 HRCA (n = 67), 1 HRCA (n = 34), or ≥2 HRCAs (n = 13). 6 patients were not evaluable for cytogenetic abnormalities.

IsKia Isa-KRd vs KRd in Transplant-Eligible NDMM
Eligibility
• Transplant-eligible NDMM
• Age < 70y
4 cycles induction 4 cycles consolidation Transplant
28-day cycles

N = 302
28-day cycles
n = 151
R KRd
n = 151
Isa-KRd KRd
Isa-KRd
Mel200 + ASCT
Induction/Consolidation Schedule
Isa: 10 mg/kg IV days 1, 8, 15, 22 in cycle 1,
10 mg/kg IV days 1,15 in cycles 2 – 4
K: 20/56 IV mg/m2 days 1, 8, 15
R: 25 mg PO days 1 – 21
d: 40 mg PO days 1, 8, 15, 22
Primary endpoint: rate of post-consolidation MRDnegativity in ITT population
Key secondary endpoints: post-induction MRD-negativity, PFS
Key Baseline Characteristics
Isa-KRd KRd
n = 151 n = 151
Median age (range), y 61 (55 – 66) 60 (54 – 63)
High risk by IMWGa 25 (18) 26 (19)
# of HRCAb, n (%) 0 1 2 or more Missing
R-ISS stage, n (%) I II III
R2-ISS stage, n (%)
78 (56)
49 (35)
13 (9) 11
50 (35)
82 (58)
10 (7)
75 (54)
49 (35)
15 (11) 12
34 (24)
45 (32)
52 (37) 8 (6)
48 (34)
85 (59) 10 (7)
35 (25)
47 (34)
51 (37)
6 (4)
• a. del(17p), t(4;14), and/or t(14;16); b. del(17p), t(4;14), t(14;16), gain or amp(1q).
• Isa, isatuximab; KRd, carfilzomib, lenalidomide, and dexamethasone; R-ISS, Revised International Staging System; Mel200, melphalan 200 mg.

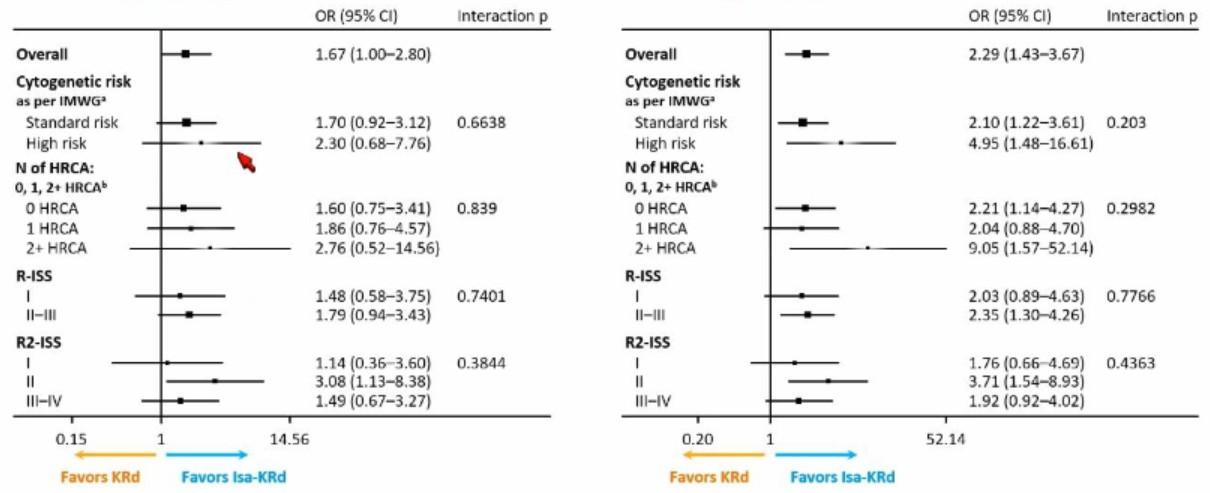
IsKia: Responses
Post-consolidation MRD-Negativity in ITT population
Primary endpoint
•
MRD advantage with Isa-KRd retained across all subgroups
Subgroup analysis

MSK Approach to Transplant Eligible NDMM
ASCT-Eligible Patients
Patients with good PS and adequate organ function
Standard risk
Dara-VRd × 4 cycles
High risk*
Consider VRd or KRd ×4 cycles
For Insurance reasons
DaraKRd × 4 cycles
Stem cell mobilization; adequate stem cell harvest (≥8×10 6 CD34 cells/kg) as per MSK ABMT SOP ASCT
Consider
with induction regimen for HR-NDMM patients who do not achieve CR or better
Consider VDPACE for Bulky EMD or PCL
Cytoreduction Consider planned Auto/Allo for Ultra-high risk
ASCT, autologous stem cell transplant; CR, complete response; DVRd, daratumumab, bortezomib, lenalidomide, and dexamethasone; IMiD, immunomodulatory drug; PI, proteasome inhibitor; PS, performance status; Tx, treatment.
*By R-ISS staging (R-ISS II/III) and/or cytogenetics (t[4;14], t[14;16], or del[17p]), elevated LDH, primary plasma cell leukemia 1. Attal. NEJM. 2017;376:1311. 2. Voorhees PM. Blood 2020. Gay. ASH 2020. Abstr 294. 4. McCarthy. J Clin Oncol. 2017;35:3279. 5. Nooka. Leukemia. 2014;28:690. 6. Dimopoulos. ASH 2018. Abstr 301. 7. Usmani. Lancet Haematol. 2021 Jan;8(1):e45-e54.

MSK Approach to Transplant Ineligible NDMM
ASCT-Ineligible Patients
Patients with poor PS not related to disease, ejection fraction <50%, pulmonary function test values <50%, concomitant multiorgan amyloidosis
RVd-Lite1×8-12 cycles
Lenalidomide maintenance until progression3
Consider DVd or VCd or Rd if VRd or DRd is not appropriate (eg, renal failure or other comorbidities)
DRd2
IMiD/PI maintenance until progression for high risk4
Continue treatment until progression
• DRd, daratumumab, lenalidomide, and dexamethasone; DVd, daratumumab, bortezomib, and dexamethasone; VRd-Lite, modified VRd regimen.
• Adjust dosing of lenalidomide based on renal function. Consider empiric age-adjusted dose reductions for all regimens, as needed.4
• 1. O’Donnell. Br J Haematol. 2018;182:222. 2. Facon. ASH 2018. Abstr LBA-2. 3. Larocca. ASH 2018. Abstr 305. 4.Usmani. Lancet Haematol. 2021 Jan;8(1):e45-e54.

RVd-Lite
• Regimen (N=53)
– Lenalidomide: 15 mg po days 1 to 21
– Bortezomib: 1.3 mg/m2 SC 1× weekly on days 1, 8, 15, 22
– Dexamethasone
• If ≤75 years, 20 mg 2× weekly
• If >75 years, 20 mg 1× weekly
• Results
– 86% ORR
– 66% ≥VGPR
– Median PFS: 35.1 months
– Median OS: NR
– Median follow-up: 30 months
– Median age: 73 years (range: 65-91)
– PN: 62%
– Only 1 patient had grade 3 symptoms
• PN, peripheral neuropathy. O’Donnell et al. Br J Haematol. 2018;182:222-230.

Phase 3 MAIA Study: Daratumumab Plus Rd in NDMM
• Stratified by ISS (I vs II vs III), region (North America vs other), and age (<75 vs ≥75 y)
• Primary endpoint: PFS
• Secondary endpoints: ≥ CR rate, ≥ VGPR rate, MRD negativity, ORR, OS, and safety
Patients with ASCTineligible NDMM, ECOG
PS 0-2, CrCl ≥30 mL/min (N = 737)
R
Daratumumab 16 mg/kg IV (every-wk cycles 1-2; every-2-wk cycles 3-6; every-4-wk cycles 7+) +
lenalidomide 25 mg/d PO on d 1-21 +
dexamethasone 40 mg/wka PO or IV (n = 368)
28-d cycles until progressio n
Lenalidomide 25 mg/d PO on d 1-21 +
dexamethasone 40 mg/wka PO or IV (n = 369)

Demographics and Baseline Characteristics (ITT)
D-Rd (n = 368)
Rd (n = 369)
73 (50-90)
4 (1)
74 (20)
130 (35)
160 (43)
Male,
74 (45-89)
4 (1)
73 (20)
131 (36)
161 (44)
Type of measurable disease, n (%)
IgG
IgA
Otherd Detected in urine only
Detected as serumfree light chain only
Cytogenetic profile,e n/total n (%)
Standard risk
High risk
Median time since initial diagnosis of MM (range), months
225 (61)
65 (18)
9 (2)
40 (11)
29 (8)
271/319 (85)
48/319 (15)
231 (63)
66 (18)
10 (3)
34 (9)
28 (8)
279/323 (86)
44/323 (14)
0.95 (0.1-13.3)
0.89 (0-14.5)

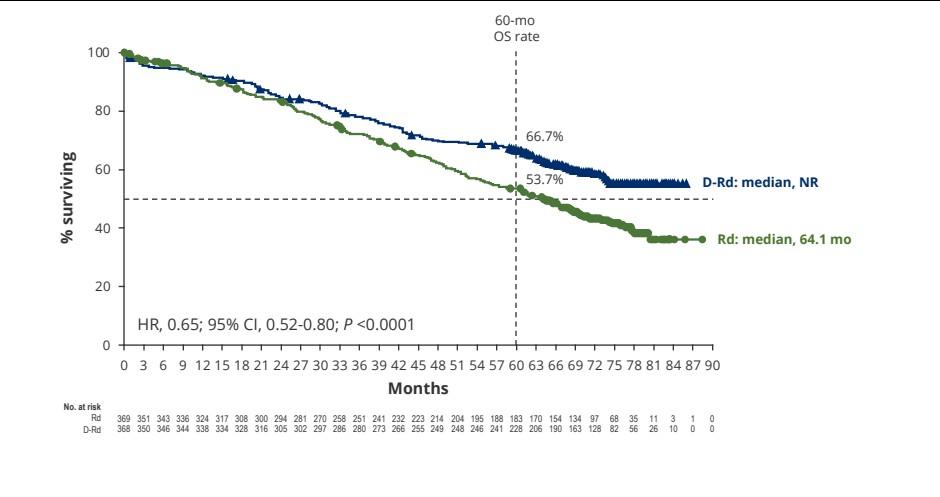
MAIA Phase III ORRa
Median follow-up
Primary: 28.0 months1
Median follow-up
56.2 months
• D-Rd induced deeper responses, with significantly higher rates of ≥CR and ≥VGPR, compared with Rd
• With >28 months of additional follow-up, responses deepened with continued daratumumab therapy
VGPR, very good partial response; PR, partial response; OR, odds ratio.
aITT population. bP <0.0001; P values were calculated from the Cochran-Mantel-Haenszel Chi-Squared test.
1. Facon T, et al. N Engl J Med. 2019;380(22):2104-2115.
Note: percentages may not add up to the total due to rounding.
Presented

MAIA Phase III Updated PFS/OS


CEPHEUS: Study Design
• Phase 3 study of DARA-VRd versus VRd in transplant-ineligible NDMM
Induction/Consolidation
V: 1.3 mg/m2 SC
VRd
Days 1, 4, 8, 11
Key eligibility criteria:
• Transplantineligible NDMM or deferred
• CrCl <40 mL/min
• ECOG PS ≤2
1 R a
R: 25 mg PO Days 1-14
d: 20 mg PO/IV Days 1,2,4,5,8,9,11,12
DARA SC-VRd
DARA: 1,800 mg SC
Cycles 1-2 QW
Cycles 3-8 Q3W
VRd: Same as control
Maintenance
8 Cycles of 21 days
Rd
R: 25 mg PO Days 1-21
d: 40 mg PO Days 1,8,15,22
Primary endpoint:
• Overall MRD negativity rate at 10-5
Secondary endpoints:
• PFS
• Durable MRD negativity at 1-yr
• Response
• PFS2
• OS
28 day cycles
Zweegman S, et al. Trials in Progress Poster presented at ASCO Annual meeting. May 31-June 4, 2019. Chicago, IL. Abstract TPS8066. ClinicalTrials.gov Identifier: NCT03652064. Accessed 24 February 2022

IMROZ: Study Design
• Open-label, multicenter, randomized phase III trial
Stratified by ISS (I or II vs III vs unknown), age (<70 vs ≥70 yr)
Induction (4 x 42 ± 3 d cycles) Continuous (cycle 5 onward 4-wk ± 3 d cycles)
Isatuximab
10 mg/kg on d 1, 8, 15, 22, 29 in cycle 1; every 2 wk after + RVd*
Transplant-ineligible patients with NDMM (N = 440) IsaRd
3: 2
Isatuximab
10 mg/kg q 2 wk until cycle 18; Q4W after + Rd† Rd†
*RVd: lenalidomide 25 mg PO d 1-14, d 22-35, bortezomib 1.3 mg/m2 SC d 1, 4, 8, 11, 22, 25, 29, 32;
• Primary endpoint: PFS (IMWG criteria)
dexamethasone 20 mg PO/IV d 1, 2, 4, 5, 8, 9, 11, 12, 15, 22, 23, 25, 26, 29, 30, 32, 33.
†Rd: lenalidomide 25 mg PO d 1-21, dexamethasone 40 mg PO/IV weekly.
• Secondary endpoints: ≥ VGPR, CR, OS, MRD negativity
Cross over to Isa-Rd if progressio n on Rd

MSK Approach to Transplant Ineligible NDMM
ASCT-Ineligible Patients
Patients with poor PS not related to disease, ejection fraction <50%, pulmonary function test values <50%, concomitant multiorgan amyloidosis
Dara-RVd-Lite OR Isa-RVd-Lite × 6-8 cycles
[Fit or Intermediate Fit]
Lenalidomide maintenance until progression
Consider DVd or VCd or Rd if VRd or DRd is not appropriate (eg, renal failure or other comorbidities)
DRd1 [Frail ]
IMiD/PI maintenance until progression for high risk
Continue treatment until progression with either Len or Dara maintenance based on tolerability
• DRd, daratumumab, lenalidomide, and dexamethasone; Dara-RVd-lite, daratumumab, lenalidomide, bortezomib, and dexamethasone; Isa-VRd-Lite, isatuximab, lenalidomide, bortezomib, and dexamethasone.
• Adjust dosing of lenalidomide based on renal function. Consider empiric age-adjusted dose reductions for all regimens, as needed.4
• 1. Facon. ASH 2018. Abstr LBA-2. 3.

The Promise of T-cell redirection

BCMAbispecific

CAR, chimeric antigen receptor; MM, multiple myeloma
CAR T-cell therapy is not yet FDA-approved for patients with MM.
Presented
bispecific T-cell engager
3


Adapted from Cho S-F et al. Front Immunol. 2018;9:1821.


Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani

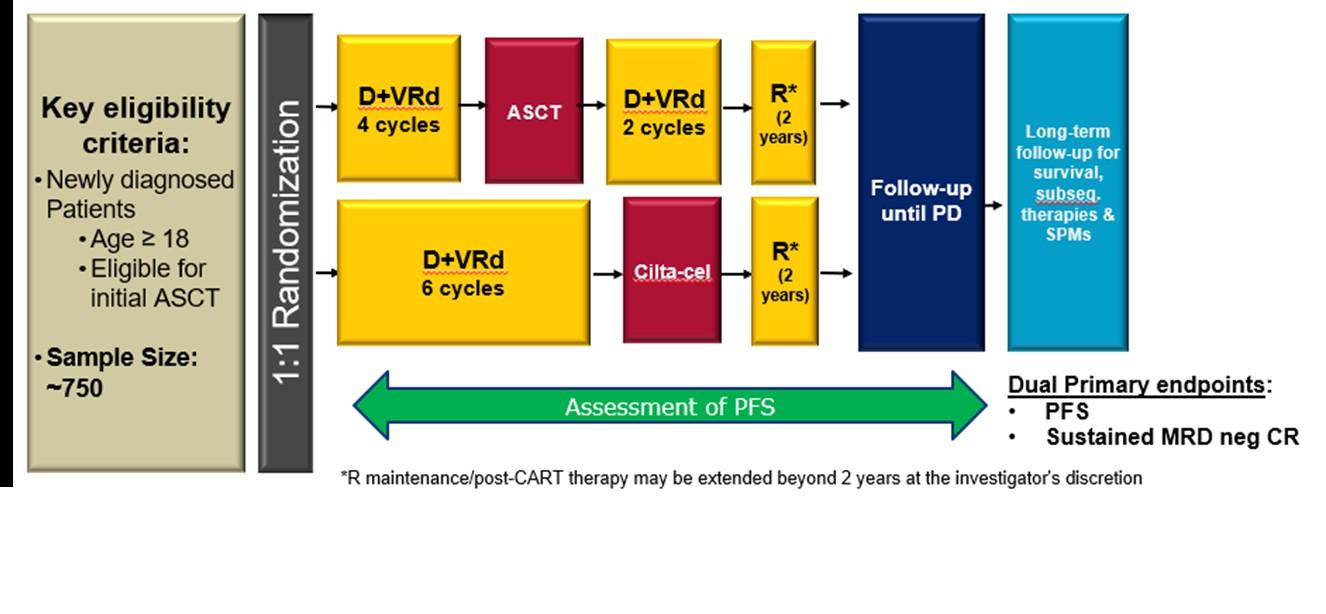
CARTITUDE-6: Randomized, phase 3 in NDMM, transplant eligible (NCT05257083)
Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani

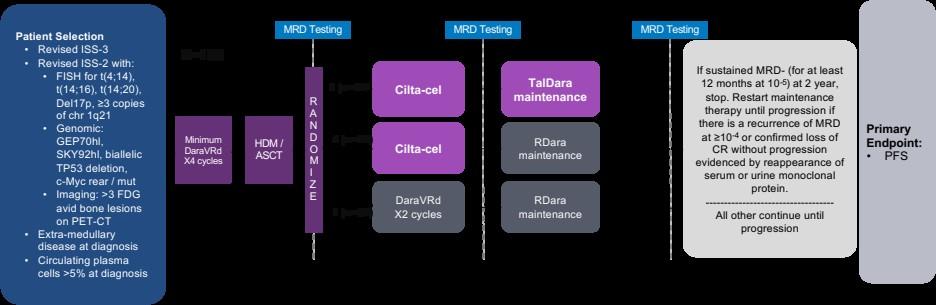
TRIUMpH Trial: Phase 3 BMT-CTN Study in HR-NDMM (In Development)
Presented by: Saad Z. Usmani, MD MBA FACP, @szusmani

A062104: Phase 2 trial investigating quadruplet induction followed by novel consolidation for Primary Plasma Cell Leukemia
Primary endpoint:
• Rate of MRD negativity (10-5) for t(11;14) patients post-induction
Key secondary endpoints:
• Rate of MRD negativity post-consolidation pooled across all patients
Registratio
INDUCTIO N 4 cycles
CONSOLIDATION 1 4 cycles
• Overall survival R
Teclistamab+
Talquetamab
CONSOLIDATION 2 4 cycles
MAINTENANC E 24 cycles
Teclistamab+
Talquetamab
KR
Ven-DKd or D-KRd*
Ven-DKd or D-KRd*
*Consolidation regimens will be the same as induction
Stratified on t(11;14) status and induction regimen t(11;14) with D-KRd vs. t(11;14) with Ven-DKd vs. non-t(11;14) with D-KRd
KRd, carfilzomib-lenalidomidedexamethasone





Newly diagnosed, standard risk concept
Induction Consolidation
Maintenance
DaraRVd x 4 cycles
1 : 1 R a n d o m i z a ti o n
ASCT
Tec+Talq (q2wk dosing as per RedirecTT-1) x 4 cycles
DaraLe n x 2 years
Len until PD
MRD assessment
Stem cell collection
MRD assessment
MRD assessment
• Co-primary endpoints: PFS (non-inferiority with planned superiority assessment) and cumulative incidence of severe infections
• Key secondary endpoints: OS, MRD-neg post consolidation, sustained MRD negativity after 2 yrs of maintenance
• QOL/PRO endpoint as correlative/exploratory endpoint: incorporate into design as an integrated biomarker with separate hypotheses, power statements and analysis plans
Presented by: Saad Z. Usmani, MD MBA FACP,




Conclusions
• Picking the right strategy that gives the highest likelihood of the best depth of response in the first year of diagnosis is extremely important for survival outcomes.
• Anti-CD38 monoclonal antibody-based quadruplets have helped optimize the ‘induction’ part of treatment by providing better depth of response, translating in to better PFS (GRIFFIN, CASSIOPEIA, PERSEUS, ?GMMG-HD7, ?IsKIA).
• Future strategies may incorporate:
– Biomarker-directed small molecules into induction.
– BsAb or CART as consolidation. – Fixed duration of maintenance. Presented by: Saad Z. Usmani, MD MBA

Building Translational Research Enterprise
Foci of Work
• Microbiome & Immune profiling.
• Computational Genomics
• Functional Imaging
• Blood-based MRD assessments
• Metabolomics
Collaborators
• Marcel van den Brink lab
• Sohrab Shah lab
• Vijay Joseph lab
• Omar Abdel-Wahab lab
• Jessica Chapman (Path/Clinical Proteomics)
• Kaz Murata (Path/Lab Medicine)
• Jason Lewis lab (Radiology)
• Gareth Morgan lab (NYU)
• Santosha Vardhana lab
• Samir Parekh (Mount Sinai)
• Madhav Dhodapkar (Emory)
Presented by: Saad Z. Usmani, MD MBA
Working Groups
• PCD Immune profiling/Microbiome
• PCD Genomics
• PCD Specimen Collection
• PCD Research Database
• PCD Imaging
















 Paula and Rodger Riney
Paula and Rodger Riney

MSKCC Myeloma Team – It Takes a Village!

Physicians:
• Parastoo Dahi (ABMT)
• Sergio Giralt (Deputy Chair, DHM)
• Alexander Lesokhin
• David Chung (ABMT)
• Hani Hassoun
• Malin Hultcrantz
• Neha Korde (Clinical Director)
• Heather Landau (ABMT)
• Kylee Maclachlan
• Sham Mailankody (Research Director)
• Dhwani Patel
• Sridevi Rajeeve
• Michael Scordo (ABMT)
• Gunjan Shah (ABMT)
• Urvi Shah
• Carlyn Tan
•
APPs:
• Isabel Concepcion
• Katie Jones
• Justina Kiernan (BER)
• Lori Lang (WES)
• Katelyn Kelly-Johnson (CMK)
• Jennifer Rielly
• Ashley Steinberger
• Jenna Wenzel
CTNs:
• Marcela Algave, RN
• Kelly Barnett, RN
• Jenna Blaslov, RN
• Julia Caple, RN
• Tara Sood, RN
• Ling Tran, RN
OPNs:
• Kelly Aliaga
• Grismer Canales
• Carolanne Carini (BER)
• Kathleen Considine (WES)
• Alexa Cracolici (MON)
• Kellie Donovan
• Mackenzie Galvin
• Anna Howard
• Kyla Lafond
• Michelle O’Hare (CMK)
• Pattie Scherer (BER)
PharmDs:
• Alice Wang
• Issam Hamadeh
Clinical Research Team:
• Miranda Burge
• Leah Gilbert
• Bianca Gonzalez
• Laura Guttentag (CRM, Myeloma)
• Selena Hamid
• Roger Huang
• Meredith Hyland
• Mosammed Kabir
• Emily Lei
• Guljar Nahar
• Alexis Nwakwo
• Garrett Preusz
• Anna Przemielewska
• Raisa Rahman
• Colin Rueda
• Jeannen Santos
• Tala Shekarkhand
• Felicia Slaton
• Clare Sullivan
• Kristina Vinzon-Baltazar
OCs:
• Fariha Ali
• Xavier Ayala
• Elhaji Ba
• Ruth Bien-aime
• Odali Espinal
• Eric Frazer
• Daniel Maldonado
• Krystal Soto
Service Manager/Admins:
• Kristen Hakuta
• Nicole Santiate
• Shaneeza Imran
• Gladys Acosta
EVALUATION
Please take a moment to reflect and respond to the program evaluation.
Questions 7 & 8 can be worked on after each presentation.
We greatly appreciate your time and feedback!


Myeloma Action Month
International Myeloma Foundation

 Jason London
Manager, Marketing & Communications
Jason London
Manager, Marketing & Communications
BREAK


Breakout Sessions #1: Treatment
Approaches in Myeloma
A Newly Diagnosed: An Approach to Frontline Therapy
Peter Voorhees, MD
Royal Palms Ballroom
An Approach to Relapsed Myeloma
Gurbakhash Kaur, MD
Grand Oasis Ballroom






Novel Therapies for Relapsed Myeloma
By Gurbakhash Kaur, MD MACo-Director of Amyloidosis Program
Assistant Professor of Internal Medicine
UT Southwestern Medical Center
Active Drugs in Multiple Myeloma
Anti-SLAMF7 moAb
Anti-BCMA CAR-T
Alkylators
Steroids
Anthracyclines
IMiDs
Thalidomide
Lenalidomide
Pomalidomide
Elotuzumab
Cilta-cel
Ide-cel
ddBCMA
Anti-CD38 moAbs
Daratumumab
Isatuximab
Felzartamab (MOR202)
TAK 079
Proteasome
Inhibitors
Bortezomib
Carfilzomib
Ixazomib
CELMoDs
Iberdomide
Mezigdomide
SAR 442085
Anti-BCMA antibody drug conjugate
Belantamab
Selinexor (XPO1 inhibitor)
Venetoclax (BCL-2 inhibitor)
Lumicar Anti-BCMA bispecifics
Teclistamab
REGN-5458
Alnuctamab
Elranatamab
TNB 383B
AMG 701
Novel bispecifics
Talquetamab (GPRC5D/CD3)
Cevostamab (FcRH5/CD3)

Modified from Rajkumar SV © 2022

Current MM Treatment Paradigm
Newly Diagnosed MYELOMA
Plateau remission
EARLY RELAPSE
CAR and BsAb
REFRACTORY RELAPSE 3+
Symptomatic MM
Front-line therapy
Induction
--- QUAD/Triplet Consolidation --- +/- SCT Maintenance
years
Frontline
Triplet/QUAD: Auto SCT Maintenance
2nd-line therapy
Early Relapse (1-3 Prior Lines)
Triplet
Delayed Transplant
OR CLINICAL TRIAL
3rd+ line therapy
Expected OS
<12 months
Late Relapse
BCMA - CAR, bispecifics
GPRC5D-Bispecific
Belantamab
Selinexor
Clinical Trial: BsAb, GPRC5D, etc.

Treatment Attrition in Multiple Myeloma
Patients reaching line of therapy, %
Attrition by line of therapy, %
Br J Haematol. 2016;175:252.
In every new line of therapy, 15%-35% of patients are lost
BMC Cancer. 2020;20:1087

Myeloma Tx At Glance
DP D
KR D
KP D

Selinexor Vd
PV D

Treatment Decision Rests On Interplay between
Disease Characteristics
Patient Characteristics
Treatment Characteristics
Clinical Nature of Relapsed Myeloma- All that Relapses is NOT the SAME
Biochemical relapse
Clinical Relapse
• Hypercalcemia
• Bone lesions
• EMD
• Renal Failure
Plasma cell Leukemia

Patient characteristics
Age Performance Status Comorbidities
Side effects and Toxicity to prior Therapy Support System
Distance to the Treatment Center

Disease Characteristics
High Risk Disease Cytogenetics
Depth and Duration of Response to Prior therapy
Timing of relapse
Aggressiveness of Relapse
• Extramedullary Disease
• Plasma Cell Leukemia
• Doubling time of the Myeloma Markers



Mechanism of Action (i.e Drug class, exposure vs refractory to a similar drug in the same class)
Frequency -> twice weekly, weekly, monthly TIME TOXICITY
Side effects: (Peripheral Neuropathy, Cardiovascular/Renal Dysfunction, Blood counts, immune recovery)
Route of Administration (Intravenous, Subcutaneous, Oral)
Concurrent Supportive Care & Monitoring
$$$$$$$ Financial Burden

**Triplets are preferred
**Preferred Options,
Lenalidomide
Sensitive
KRD
DRD
ERD IRD
DVd
DKd
Isa Kd
SVd Kd, Rd,
Lenalidomide
Refractory
DPD
Isa PD
DKD
KPD
PCD
CyBorD

Results of Recent Phase III Randomized Studies in Relapsed Multiple Myeloma
Rajkumar SV. Am J Hematol 2022;97(8):1086. Martin T et al. Haematologica. 2022;107:2485. Dimoupolus. Blood (2022) 140
Richardson ASH 2022 Abstract . ICARIA Update. Richardson. International Myeloma Society. 2022.

Results of Phase III Randomized Studies in Relapsed Multiple Myeloma
1.
5.

1st line: VRD-auto-len maintenance until PD
2nd line : CD38-Kd until PD
3rd line: Len + K + CD38 refractory after 2 lines
Regimen should include2 drugs patient has not seen yet
4th: ___?????___________
Clinical Trials, Venetoclax in high BCL2/t(11;14), Bendamustine,
5th line and beyond – Immunotherapy, Clinical Trials
The majority of patients are becoming triple-class (PI + IMiD + CD38) refractory after 2-3 lines of therapy

Where does Immunotherapy fit into this algorithm?

Amazing Success in Immunotherapy for MM



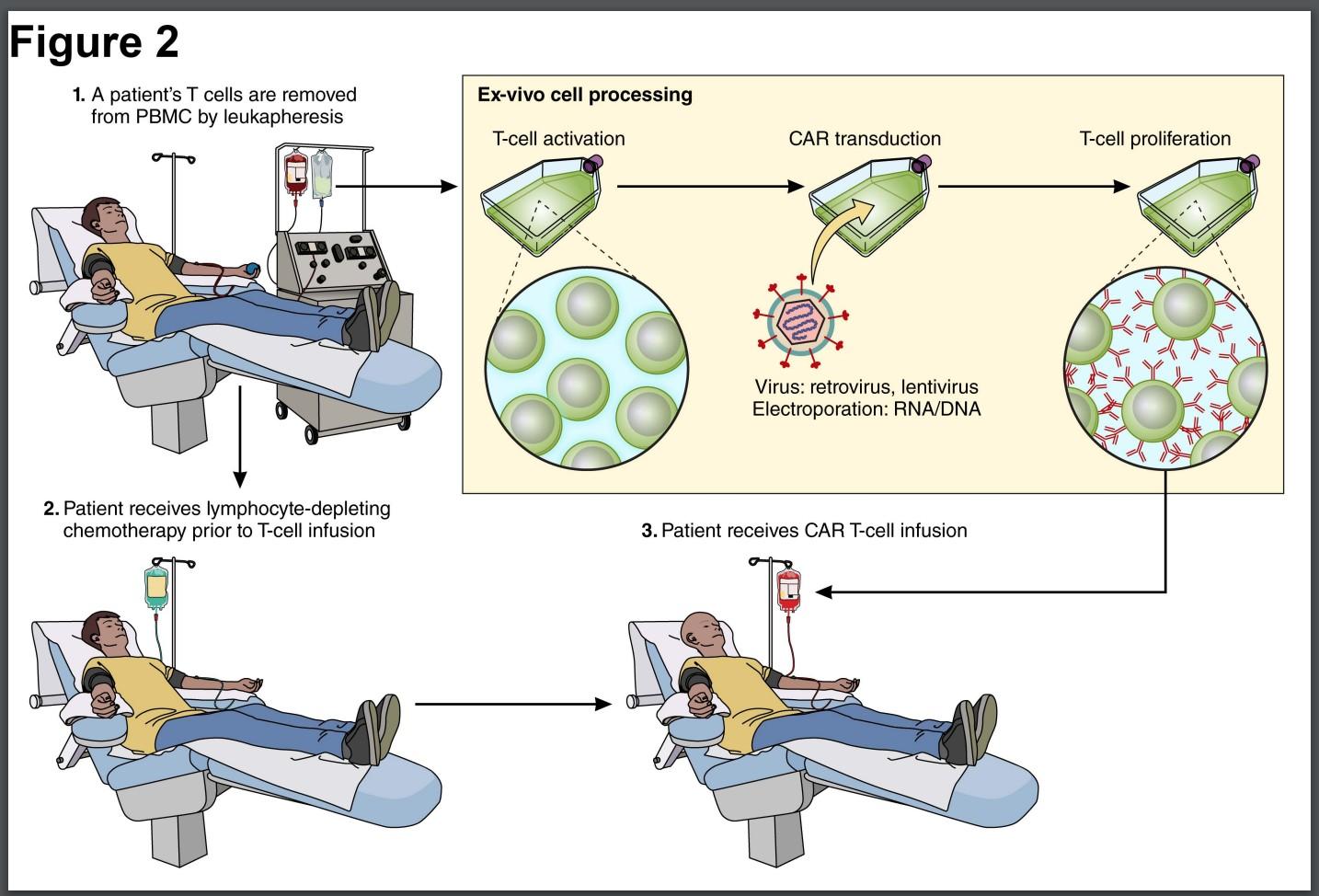

BCMA as a Target
BCMA: B Cell Maturation Antigen
Expressed on late memory
B-cells committed to PC differentiation and PCs
BCMA plays a role in survival of long-lived PCs
Cell lineage specific so avoids off target toxicity

CAR T-Cells
MM Cell Death
Cytotoxic Payload
Released Into Cell
T-Cell
Bispecific T-Cell Engagers BCM A
CD3
Bispecific Antibodies
T-Cell
CD3
Antibody–Drug
Conjugates
NKCells, Monocytes
Cho. Front Immunol. 2018;9:1821. Su. J Hematol Oncol. 2021;14:115. Tai. Expert Opin Biol Ther. 2019;19:1143. Slides adopted from


Idecabtagene
Vicleucel (ide-cel, Abecma)
6 median prior LOT
35% HR Cytogenetics
39% EMD
84% Triple Refractory
ORR: 81%
CR/sCR: 39%
PFS: 12.2 mo
3/26/2021
4+ lines, including IMID, prior PI and anti-CD38 mAB
300-460 x 106
CAR-positive viable T-cells IV
Ciltacabtagene
Autoleucel (cilta-cel, Carvykti)
6 median prior LOT
24% HR Cytogenetics
13% EMD
88% Triple Class
Refractory
ORR: 98%
CR/sCR: 39%
PFS: 34.9 mo
2/28/2022
4+ lines, including prior IMID, PI and anti-CD38 mAB
0.5-1.0 x 106
CAR-positive viable T-cells/kg IV

Cell
Idecabtagene
Vicleucel (ide-cel, Abecma)
BCMA
Ciltacabtagene
Autoleucel (cilta-cel, Carvykti)
BCMA
6 median prior LOT
35% HR Cytogenetics
39% EMD
84% Triple Refractory
ORR: 81%
CR/sCR: 39%
PFS: 12.2 mo
CRS:
6 median prior LOT
24% HR Cytogenetics
13% EMD
88% Triple Class Refractory
ORR: 98%
CR/sCR: 39%
PFS: 34.9 mo
CRS: ICANS:
3/26/2021
4+ lines, including IMID, prior PI and anti-CD38 mAB
300-460 x 106
CAR-positive viable T-cells IV
2/28/2022
4+ lines, including prior IMID, PI and anti-CD38 mAB
0.5-1.0 x 106
CAR-positive viable T-cells/kg IV

What is it?
CRS and ICANS in a nutshell
CRS (Cytokine Release Syndrome)
Common symptoms
When does it happen?
How is it treated?
Will it get better?
A reaction from a strong immune response after treatment.
Fever, nausea, headache, fast heartbeat, low blood pressure.
Usually within the first week after treatment.
Car-t Construct specific
Supportive care, steroids, and a specific medication (tocilizumab).
Usually improves with treatment, severe cases may need intensive care.
ICANS (Immune Effector CellAssociated Neurotoxicity Syndrome)
A condition affecting the brain and nervous system after treatment.
Confusion, agitation, seizures, trouble speaking.
Often after CRS, usually within the first few weeks after treatment.
Supportive care, steroids, and sometimes other medications.
Often gets better with treatment, but some effects can last longer.

Adverse Events Associated With CAR-T
Adverse Event Description
Cytokine Release Syndrome (CRS)
A condition where the immune system reacts strongly, causing symptoms like fever, nausea, and in severe cases, problems with breathing and blood pressure.
Occurs 70-95% - all grade; <5% grade 3 or higher
Neurotoxicity
ICANS and Parkinsonian Type
This can include changes in behavior, confusion, seizures, and in rare cases, severe brain swelling
Rare cases of delayed Parkinsonian movement disorder, motor neuropathy, and CN palsies, may be irreversible
Any grade CRS: 20-25%, Grade 3 or higher in <5%
Low Blood Cell Counts
Infections
Hemophagocytic Lymphohistiocytosis (HLH)
This includes a decrease in the number of red blood cells (anemia), white blood cells (neutropenia), and platelets (thrombocytopenia), increasing the risk of infections and bleeding.
Due to a weakened immune system, there is a higher risk of catching infections.
**Requires supportive care like IVIG, prophylactic antibiotics**
A rare but serious condition where the body's immune system becomes overactive, attacking its own tissues and organs, leading to severe inflammation.

Bispecific Therapy
BCM A
CD3 T-Cell Myeloma Cell
Antibodies with multiple binding domains
‒ Target different tumor antigens including BCMA, GPRC5D,FcRH5
‒ Also binds to immune cell targets including CD3 (T-cell)
Bispecific Therapy Approval Dosing
Teclistamab (Tecvayli™)
CD3 x BCMA 10/25/202 2
Step up Dosing: 0.06 mg/kg on Day 1, increase to 0.3 mg/kg on Day 4, and 1.5 mg/kg on Day 7; Day 14 and weekly thereafter: 1.5 mg/kg once ]
Recently, new FDA insight. Change to Q2 weekly after 6 months
Elranatama b (Elrexfio™)
CD3 x BCMA 8/14/2023
Step-up dosing: 12 mg on Day 1, 32 mg on Day 4, followed by 76 mg on Day 8
Day 8: 76 mg SQ followed by Day 15
76 mg once weekly SQ through Wk 24
Wk 25 onward: 76 mg every 2 wk
Talquetama b (TALVEY™) 8/9/2023
Image Credit: Modified from CCO Options
Step Up Dosing: Weekly option: Day 14 0.4 mg/kg SQ followed by 0.4 mg/kg SQ every wk

Immunotherapy What, When and How
What
• ADC
• Bispecifics
• CAR-T
WHEN Timing
HOW: Sequencing
• Early vs Late
• Impact of T cell exhaustion move treatment earlier in order to have more robust T cells
• Bispecifics CAR-T ADC
• CAR-T Bispecifics ADC
CombinationAlone or Together
• Combine mABs + Bispecifics
• Combine IMIDS


Mechanism of Action
Administration
Engages both the tumor antigen on myeloma cells and CD3 on T cells to induce T-cellmediated cytotoxicity.
Genetically modifies patient's T cells to express a chimeric antigen receptor (CAR) targeting a tumor antigen.
Manufacturing
Duration of
Response
Toxicity
Accessibility and Cost
Off-the-shelf, ready-to-use intravenous or subcutaneous injections.
Mass-produced, standardized product.
Continuous or repeated dosing to maintain response
Commonly associated with cytokine release syndrome (CRS) and neurotoxicity, usually milder compared to CAR-T.
Autologous therapy requiring cell collection, modification, and reinfusion.
Personalized, patient-specific production.
Potentially long-lasting responses with a single treatment.
Higher risk of severe CRS and neurotoxicity.
Patient Eligibility
Generally more accessible and less expensive than CAR-T therapy.
Broader eligibility, including patients with comorbidities or those ineligible for autologous stem cell transplant.
Requires specialized centers for administration, with higher costs.
Limited to patients who can undergo apheresis and tolerate the intensive treatment process.

Timing
•Can CAR-T Therapy be moved up in earlier lines of therapy
• KarMMA-3
• CARTITUDE -4
•Bispecifics
• Many trials are looking at this strategy right now


KarMMa-3: Phase III Trial of Ide-Cel vs SoC in R/R MM
International, open-label, randomized phase III trial
Lymphodepletion
Patients with R/R MM after 2-4 prior lines of therapy; including a PI, an IMiD, and daratumumab; disease progression in ≤60 days after last therapy
ECOG PS ≤1 (N = 386)
Stratified by age (<65 vs ≥65), prior therapies (2 lines vs 3-4 lines), and high-risk cytogenetics (present or absent)
Primary endpoint: PFS
Key secondary endpoints: ORR, OS, DoR, MRD detection, safety
Rodriguez-Otero. NEJM. 2023;388:1002.
2:1
Cyclophosphamide 300 mg/m2 + Fludarabine 30 mg/m2
CAR T-Cell Infusion (Day 0)
Idecabtagene Vicleucel
150-450 x 106 CAR T-cells (n = 254)
Investigators’ Choice SoC* (n = 132)
SOC : DPD(n= 43)
DVd (n = 7)
IRD (n= 22)
Kd (n – 30)
EPD (n= 30)
PD or unacceptable toxicity


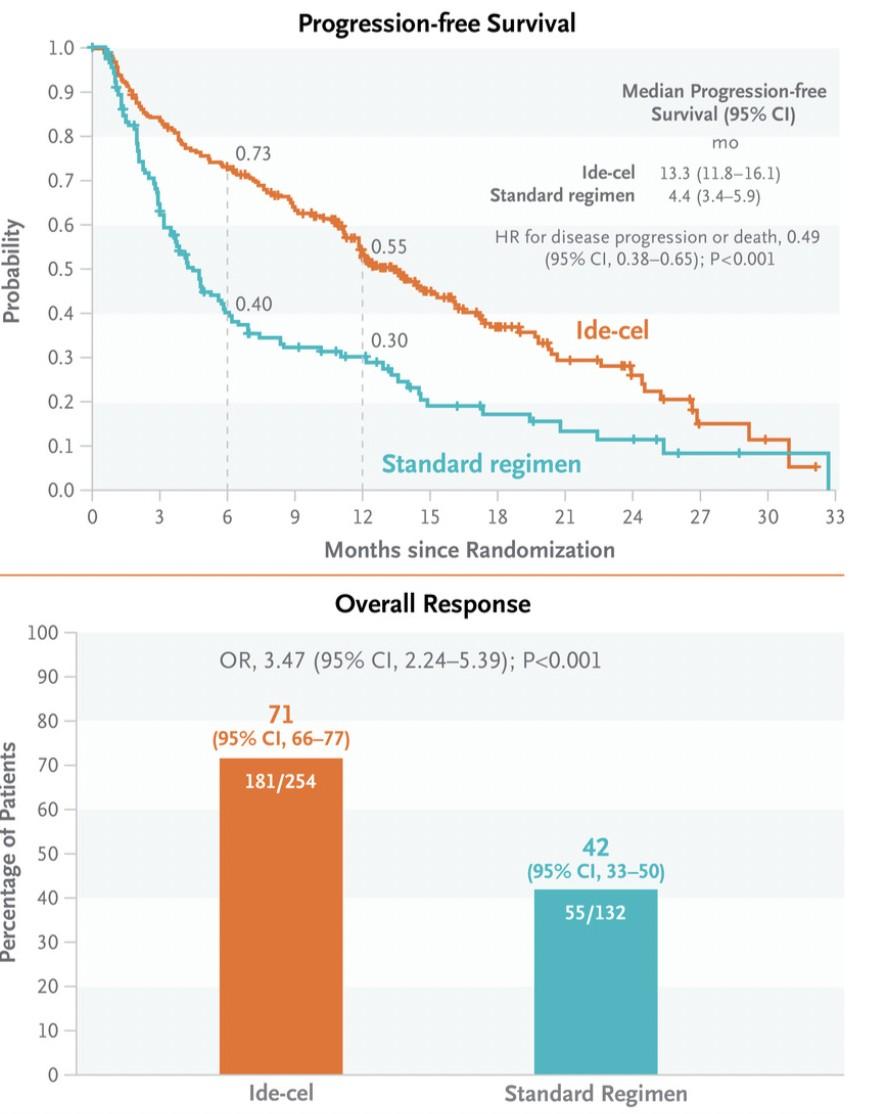


*Physician’s choice of PVd or DPd. †As-treated population (n = 176): 32 patients did not receive cilta-cel as part of study due to PD (n = 30) or death (n = 2) during bridging therapy/lymphodepletion.

CARTITUDE-4 (1-3 prior lines of therapy, Cilta-cel vs SOC (DPd or PVd)



Is Early CAR-T A Reality?
ODAC MEETING 3/15/2024
CARVIKTY
• 11-0 in favor of moving up CARVIKTY CAR-T to earlier line of therapy
• There is a safety signal early on (6 patients died due to disease progression, infections i.e COVID19) with CARVIKTY in CARTITUDE
ABECMA –
• 8:3 in favor of moving up ABECMA
• Cross over built in trial design so maybe OS benefit not so seen clearly

What would I do if CAR-T became available earlier?
Be in touch with reality
High risk patients (either with HR cytogenetics/functional high risk) will get early access to CAR-T with first relapse
There has to be a benefit with $$$, Efficacy and Toxicity
The current state where CAR-T and Bispecific therapy is available as 5th line of therapy is not practical as more patients become PI, IMID, mAB refractory at the end of 2-3 LOT
Access will remain an issue

Sequence BCMA-Targeted Therapies
100%
5% 8% sCR CR VGPR PR MR SD PD
Prior BsAb (n = 7) 5% 14% 5% 14% 25% 23% 29% 25% 31% 14%
8. 2
Cohen. Blood. 2023;141:219. Hansen. JCO. 2023;41:2087.
Ide-cel retrospective study: 75% of patients met KarMMa exclusion criteria
ORR: 87% ORR: 73% CR or sCR : 44 %
CR or sCR : 33 %
CR or sCR, MRD-
CR or sCR, MRD+
CR or sCR, MRD unknown
VGPR PR (n = 33) (n = 126)
Best ORR (n=159)



Prior BCMA
Targeted Therapy is an independent predictor of inferior outcomes for response, PFS, and OS


Treatment Landscape in Relapsed MM will change
BCMoreAntige n
CAR-T with non BCMA target in clinical trial
Bispecifics –
CT103
GPRC5D CART
CEVOSTOMA B -FcRH5
CelMODs Iberdomide
Targeted therapy
Belantamab Mafadotin
ddBCMA
DUAL CAR-T
Mezigodomid e
Future of Venetoclax in the t(11;14)

CAR-T cells in RRMM
We NEED more access
Approved CAR-T cells Academi c
Ide-cel KarMMa1 (n = 128)
US. US. China
Cilta-cel CARTITUDE-1 (n = 97) 2,3
CT103A7 (n= 79)
ARI0002h4 (n = 60)
Alternative manufacturin g
P-BCMA-101 PRIME5,6 (n = 53)
Novel Syntheti c AlloCAR GPRC5 D
ddBCMA7 (n= 40)
ALLO-715 UNIVERSAL8 (n = 43)
OriCAR -01790 (n= 13) Phase II Ib/II
CC-952669 (n= 70 )
scFv Chimeric mouse Chimeric Llama Human Humanized Chimeric mouse Synthetic Human Human Humaniz ed Biepitopic
*There, are no head-to-head comparisons of these data and naïve comparison should be conducted with caution
BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; EMD, extramedullary disease; HR cytog, high-risk cytogenetics; NA, not available; NR, not reached/not reported; ScFv, single-chain variable fragment; TCR, T-cell receptor; triple-R, triple-class refractory
1. Anderson L et al. ASCO 2021;abstract;8016 (poster presentation); 2. Berdeja J et al. Lancet 2021;398;314-24; 3. Lin Y et al. EHA 2022;abstract P961 (poster presentation); 4. Fernández de Larrea C, et al. EHA 2022;abstract S103 (oral presentation); 5. Costello C, et al. ASH 2020;abstract 134; 6. Mohyuddin GR et al. Blood Adv 2021;5(4):10971101; 7. Li C et al. EHA 2022;abstract S187 (oral presentation); 7. Li C, et al. ASH 2021;abstract 143; 8. Mailankody S, et al. ASH 2021;abstract 651; 9. Mailankody S, et al NEJM 2022. 10. Zhang et al Lancet Hematology 2023
AO, et al. ASH 2023 Frigault, M, et al. ASH2023. Du J, et al ASH 2023: Abstract 1022: FasT Bal S et al.

Summary:
•Multiple Options available at first relapse
•Nature of relapse, patient and treatment characteristics help decide the appropriate therapy
•In the era of quadruplets, many patients are triple class refractory after 2 nd line of therapy
•Huge gap in terms of effective options after triple class refractory until patient qualify for 5th line approved immunotherapies
•Immune therapy changed MM treatment landscape and eventually, will move from later lines to earlier lines of therapy
•Balance between side effects, $$$, efficacy
•Substantial progress by introducing newer therapies (19 FDA approved drugs in last 20 years, extending lives of patients and we have a long way to in introducing treatment regimens with low toxicity and improved QOL

Thank

EVALUATION
Please take a moment to reflect and respond to the program evaluation.
Questions 7 & 8 can be worked on after each presentation.
We greatly appreciate your time and feedback!


Welcome Back


Symptom Management & Living Well with Myeloma
Teresa Miceli, RN, BSN, OCN Mayo Clinic – Rochester, MN
International Myeloma Foundation
Nurse Leadership Board Member




Wintery Mix of Treatment Options Spring into Managing Side Effects Enjoy Life’s Bounty Summer of Success



Wintery Mix of Treatment Options
Diverse and Complex Treatment Combinations








Myeloma Treatment
Velcade® (bortezomib)
Lenalidomide
Common Combinations
DVRd, VRd, Vd
DVRd, VRd, Rd
Kyprolis® (carfilzomib) KRd, Kd, DKd, Isa-Kd
Pomalyst® (pomalidomide) Pd, DPd, EPd, PCd, Isa-Pd
Darzalex® (daratumumab)
DVRd, DRd, DVd, DPd, DVMP, DKd
Ninlaro®(ixazomib) IRd
Empliciti® (elotuzumab) ERd, EPd
Xpovio® (Selinexor)
Sarclisa® (Isatuximab)
XVd, XPd, XKd
Isa-Kd, Isa-Pd
Blenrep® (Belantamab mafodotin) Bela-d
Abecma® (Idecabtagene Vicleucel) --
Carvykti™ (ciltacabtagene autoleucel) --
Elrexfio™ (elranatamab) --

Prescribing information. NCCN Guidelines. Multiple Myeloma. V2.2024. Accessed February 5, 2024.
Tecvayli® (teclistamab)
Talvey™ (talquetamab)
Venclexta® (venetoclax)
Vd + ven
New agents or regimens in clinical trials are possible options
Stem Cell Transplant
ELIGIBILITY
Measuring Treatment Response
Determining Transplant Eligibility
Insurance Authorization
Collecting Stem Cells
Duration:
Approximately 2 weeks
Location:
Transplant Center


2
TRANSPLANT
High Dose Chemotherapy Stem Cell Infusion Supportive Care Engraftment
Duration: Approximately 3-4 weeks
Location:
Transplant Center


H A S E 3
POSTTRANSPLANT
Restrengthening
Appetite recovery
“Day 100” assessment
Begin maintenance therapy
Duration:
Approximately 10-12 weeks
Location: HOME


Upfront stem cell transplant remains the standard of care for eligible patients

CAR T: Another Treatment Approach
Ask for a referral to CAR Tcell center as soon as it is possible as next treatment option (ie, before relapse)

No driving for 8 weeks
“One & Done” with continued monitoring
T-Cell Collection
Manufacturing takes ≈ 4 to 6 weeks
Bridging therapy may be needed
• Away from home
• Often some hospital stay
• Care Partner needed
• Side effect management
• CRS, ICANS
• Low blood counts
• Fatigue and fever
• Some patients need ongoing transfusion support
Bispecific Antibodies
•Different bispecific antibodies have differences in efficacy, side effects
– Available after 4 prior lines of therapy (or clinical trial)
– About 7 in 10 patients respond
– Off-the-shelf treatment; no waiting for engineering cells
– CRS and neurotoxicity
– Risk of infection
•BCMA target: greater potential for infection
– Tecvayli® (teclistamab)
– Elrexfio™ (elranatamab)
T cell
BISPECIFIC ANTIBODIES
Cytotoxic cytokines

•GPRC5D target: potential for skin and nail side effects, GI issues of taste change, anorexia and weight loss
– Talvey™ (talquetamab)
MM cell
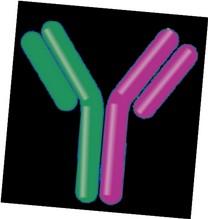

Target CD3
Bispecific antibody

MM cell death




Spring into Managing Side Effects
The Early Bird Gets the Worm:
Communicate Proactively with Your Healthcare Team
Your team may be able to help, but only if they know how you feel.
Unmanaged Myeloma can cause:
• Calcium elevation
• Renal dysfunction
• Low blood counts
• Infection Risk
• Blood clots
• Bone pain
• Neuropathy
• Fatigue





How You Feel

Side Effects of Treatment can cause:
• GI symptoms
• Renal dysfunction
• Low blood counts
• Infection Risk
• Blood clots






• Neuropathy
• Fatigue











Tip: Proactively discuss common side effects with your nurse and what to do if they occur

Tip: Keep a Symptom Diary and bring it to appointments







Infection Can Be Serious for People With Myeloma
[P]reventing infections is paramount
Infection remains the leading cause of death in patients with multiple myeloma. Several factors account for this infection risk, including the overall state of immunosuppression from multiple myeloma, treatment, age, and comorbidities (e.g., renal failure and frailty).
IMWG Consensus guidelines and recommendations for infection prevention in multiple myeloma; Lancet Haematol.2022;9(2):143–161.
Infection Prevention Tips
Good personal hygiene (skin, oral)
Environmental control (avoid crowds and sick people; use a high-quality mask when close contact is unavoidable)
IMWG = International Myeloma Working Group; HCP = healthcare provider. RAJE NS, et al. Lancet Haematol.2022;9(2):143–161. IMF Nurse Leadership Board


Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
As recommended by your healthcare team:
Immunizations: Flu, COVID, RSV & and pneumococcal vaccinations; avoid live vaccines
Preventative and/or supportive medications (next slide)
Medications Can Reduce Infection Risk
Type of Infection Risk
Medication Recommendation(s) for Healthcare Team Consideration
Viral: Herpes Simplex (HSV/VZV); CMV Acyclovir prophylaxis
Bacterial: blood, pneumonia, and urinary tract infection
PJP (P. jirovecii pneumonia)
Fungal infections
COVID-19 and Influenza
IgG < 400 mg/dL (general infection risk)
ANC < 1000 cells/μL (general infection risk)

.2022;9(2):143–161.
Consider prophylaxis with levofloxacin
Consider prophylaxis with trimethoprim-sulfamethoxazole
Consider prophylaxis with fluconazole
Antiviral therapy if exposed or positive for covid per institution recommendations
Consider IVIg
Consider GCSF 2 or 3 times/wk (or as frequently as needed) to maintain ANC > 1000 cells/μL and maintain treatment dose intensity
Some people receiving BCMA-targeting therapies have experienced infections that are less common like CMV, PJP and fungal infections

Feel Like a Spring Chicken: Prevent and Manage Pain
Pain can significantly compromise quality of life
Sources of pain include bone disease, neuropathy and medical procedures
•Management
– Prevent pain when possible
• Bone strengtheners to decrease fracture risk
• Antiviral to prevent shingles
• Sedation before procedures
– Interventions depend on source of pain
• May include medications, activity, surgical intervention, radiation therapy, etc
• Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)
• Scrambler therapy for neuropathy


Tell your healthcare provider about any new bone or chronic pain that is not adequately controlled

Peripheral Neuropathy Management
Peripheral neuropathy happens when there is damage to nerves in the extremities (hands, feet, limbs). Damage can be the result of myeloma, treatment or unrelated conditions (i.e. diabetes).
Symptoms:
•Numbness
•Tingling
•Prickling sensations
•Sensitivity to touch
•Burning and/or cold sensation
•Muscle weakness
Prevention / management:
•Bortezomib once-weekly and/or subcutaneous administration
•Massage area with cocoa butter regularly
•Neuroprotective Supplements:
– B-complex vitamins (B1, B6, B12)
– Green tea
•Safe environment: rugs, furnishings, shoes
Report symptoms of peripheral neuropathy early to your healthcare provider; nerve damage from neuropathy can be permanent if unaddressed
If neuropathy worsens, your provider may:
•Adjust your treatment plan
•Prescribe oral or topical pain medication
•Suggest physical therapy
Understanding Changes to Kidney Function
•Risk Factors
– Active multiple myeloma (light chains, high calcium)
– Other medical issues (ex: Diabetes, dehydration, infection)
– Medications (MM treatment, antibiotics, contrast dye)
– Poor Nutrition
•Prevention
– Stay hydrated – drink water
– Avoid certain medications when possible (eg, NSAIDs), dose adjust as needed
•Treatment
– Treatment for myeloma
– Hydration
– Dialysis



Many myeloma patients will experience kidney issues at some point; protecting your kidney function early and over time is important
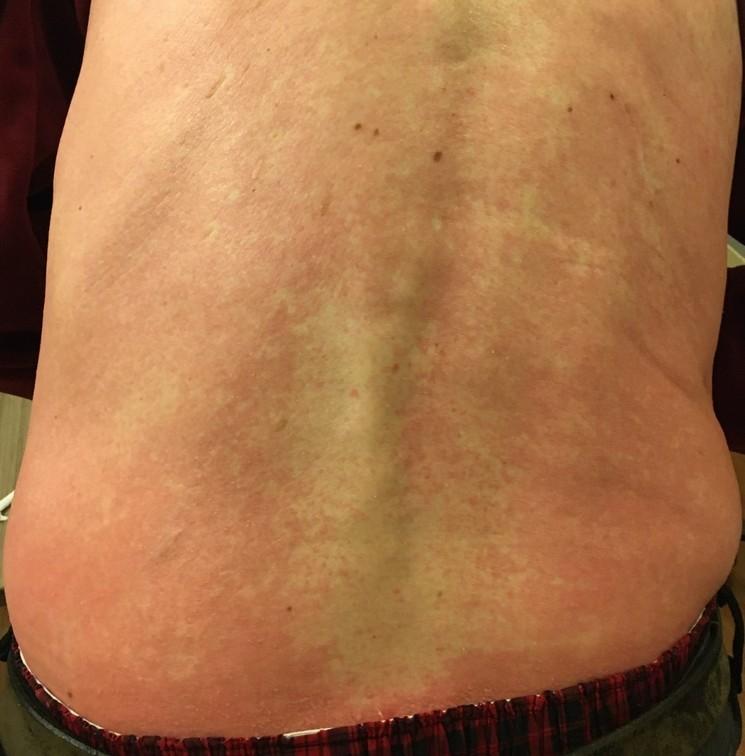
Skin Changes: Talvey™ (talquetamab)




Talvey™ (talquetamab): Common But Generally Mild and Painless Skin and Nail Side Effects
Body Rash:
Prevent dry skin; apply lotion
• Ammonium lactate 12% lotion
Steroids:
• Topical for grades 1-2,
• Systemic and topical for Grade 3 and dose hold
• Antihistamines, as needed
.
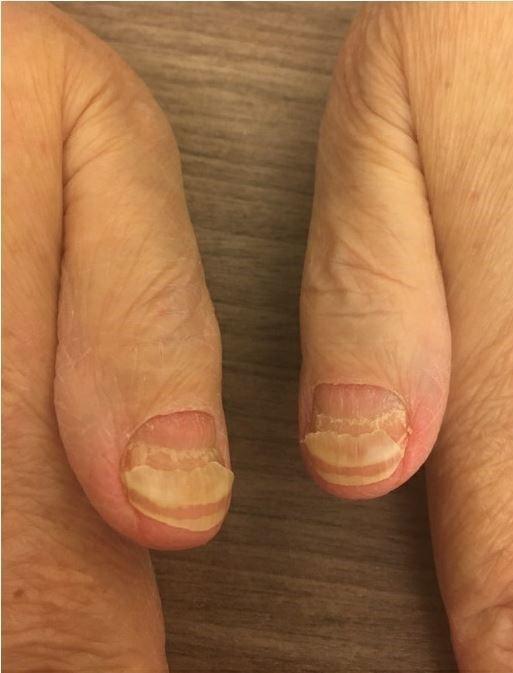
Nail Changes:
•Keep your nails short and clean. Watch for “catching and tearing”
Photos: Mount Sinai Hospital, NY, NY Nurse Leadership Board


•Apply a heavy moisturizer like Vaseline or salve. Wear cotton hand coverings to bed
•A nail hardener may help with thinning
•Tell the team if you have signs of a fungal infection, like thickened or discolored nails

Management of Talvey™ (talquetamab):
Oral Toxicities
Taste Changes
Dexamethasone oral solutions
“swish and spit” have been tried but with no proven benefit yet. Sour citrus or candies before meals are also recommended.
Glossitis and thrush
EARLY initiation of nystatin or Mycelex is key to manage symptoms.
Dry Mouth
OTC dry mouth rinse, gel, spray are recommended. Advise patients to avoid hot beverages.
Dietary modifications with small bites, eating upright, and sips with food can help manage symptoms.
• Weight loss and anorexia are associated with taste changes. Nutritionist involvement and dietary modifications are recommended to support patients. Appetite stimulant with Marinol, if indicated, can also be utilized.
• Education and emotional support are key strategies to manage oral toxicities.
GI Symptoms: Prevention & Management
Fluid intake can help with both diarrhea and constipation and helps kidney function
Diarrhea may be caused by medications and supplements
•Laxatives, antacids with magnesium
•Antibiotics, antidepressants, other (check with provider, pharmacist)
•Supplements: milk thistle, aloe, cayenne, saw palmetto, ginseng
Avoid caffeinated, carbonated, or heavily sugared beverages
Take anti-diarrheal medication if recommended
Discuss GI issues with healthcare providers to identify causes and make adjustments to medications and supplements

Constipation may be caused by medications and supplements
•Opioid pain relievers, antidepressants, heart or blood pressure medications (check with provider, pharmacist)
•Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency
Increase fiber
•Fruits, vegetables, high fiber whole grain foods
•Fiber binding agents – Metamucil®, Citrucel®, Benefiber®
Weight Management


Anorexia (difficulty eating) Weight loss; Steroids Weight gain
– Monitor weight for significant loss or gain
– Adjust diet (reduce calories or add supplements )
Side Effects
Confusion
Weakness
Fever
Diarrhea
Cytokine Release Syndrome
Fatigue
Headache
Nausea / vomiting
Shortness of Breath




CRS is a common but often mild & manageable side effect







Unique Side Effects


Neurotoxicity is a rare but serious side effect










Headache
Encephalopathy
Seizures
Confusion
NEUROTOXI
CITY
Tremors
Facial nerve palsy
Apraxia
Altered wakefulness
Ataxia
Hallucinations
Are Steroids Messing With Your Sunny Disposition?


Steroids enhance the effectiveness of other myeloma therapies
Your provider may adjust your dose. Do not stop or alter your dose of steroids without discussing it with your provider

• Irritability, mood swings, depression
Steroid Side Effects
• Difficulty sleeping (insomnia), fatigue
Managing Steroid Side Effects
•Consistent schedule (AM vs. PM)
•Take with food
•Stomach discomfort: Over-the-counter or prescription medications
•Medications to prevent shingles, thrush, or other infections

• Blurred vision, cataracts
• Flushing/sweating
• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increased blood pressure, water retention
• Increased blood sugar levels, diabetes
Don’t Hibernate Through Spring


98.8%
Fatigue

Fatigue is the most commonly reported symptom.
Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression
Anxiety
>35% of patients
Depression
≈25% of patients
Often, people do not share these symptoms with their providers. Talk to your provider about symptoms that are not well controlled or if you have thoughts of self-harm. Help is available.
Additional Supportive Care
Supportive Medications
DVT/PE
Prevention
Blood thinners
Ex: Aspirin, DOACs
Bone Health
Bone Strengthening Agents
Calcium
Vitamin D
Fatigue
Stimulant medications
Red Cell Transfusion (anemia)
Non-medication
Therapies
Compression stockings
Radiation Surgery
Immobilization
Physical therapy
Relaxation
Meditation
Activity
Lifestyle Options
Stop smoking
Weight loss
Anxiety
Anti-depressants
Anti-anxiety
Therapy
Relaxation
Meditation
Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al.
Financial Burden
Financial burden comes from
•Medical costs
– Premiums
– Co-payments
– Travel expenses
– Medical supplies
•Prescription costs
•Loss of income
– Time off work or loss of employment
– Caregiver time off work


Funding and assistance may be available
Federal programs, IRA & Medicare “Extra Help”
Pharmaceutical support
Non-profit organizations
– Websites:
• Medicare.gov


• SSA.gov
• LLS.org
• Rxassist.org
• NeedyMeds.com
• HealthWellFoundation.org
• Company-specific website
Contact the Social Services department at your hospital or clinic to talk to a social worker for assistance.

Summer of Success
Care Partners Are Vital for Success
If you want to go fast, go alone, if you want to go far, go together
•Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions
•Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)



•Caring for the Care Partner
– Recognize that caregiving is difficult/stressful
– Encourage care partners to maintain their health, interests, and friendships
– The IMF has information and resources to help care partners


Cultivate Strong Social Ties & Belonging
Multiple studies demonstrate that strong social ties are associated with
Increased longevity including people with cancer
Improved adherence to medical treatment leading to improved health outcomes
Lower risk of developing cardiovascular diseases
Increased sense of purpose and life satisfaction
Reduced stress and anxiety
Improved mood and happiness
Enhanced resilience

Martino J, et al. Am J of Lifestyle Med. 2015;11(6):466-475.
Yang YC, et al. Proc Natl Acad Sci U S A. 2016;113(3):578-583.
Pinquart M and Duberstein PR. Crit Rev Oncol Hematol. 2010; 75(2):122–137.
Strategies
for enhancing social connection
Deepen existing relationships with family, friends, and loved ones
Build new relationships by participating in a support group, joining clubs or organizations, or volunteering

Tip: Start with small steps outside your comfort zone. Call a loved one you haven’t spoken to in a while. Invite a person you’d like to know better for lunch, coffee, or a walk.


Hetherington C. Healthnews.
https://healthnews.com/longevity/healthspan/social-connection-andlongevity/#:~:text=Research%20consistently%20demonstrates%20that %20people,of%20fulfillment%20in%20your%20life. Accessed
Defining Your Success


Discuss your goals and priorities with your healthcare team
Myeloma Therapies
Rapid and effective disease control
Durable disease control
Improved overall survival
Minimize side effects
Promote good quality of life
Supportive Treatment
Prevent disease- and treatmentrelated side effects
Optimize symptom management
Promote quality of life

Enjoy Life’s Bounty
Harvest Good Health
Have a Primary Care Doctor
Have Recommended Health Screenings
• Blood pressure
• Cholesterol
• Cardiovascular disease
• Diabetes
• Colonoscopy
• Vision
• Hearing
• Dental checkups & cleaning
• Women specific: mammography, pap smear
• Men specific: prostate
Maintain a healthy weight
• Good nutrition
• Activity or exercise
• Sufficient Sleep (next slide)
An ounce of prevention is worth a pound of cure.Benjamin Franklin
Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56.
Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.

Plentiful Sleep: Important for Good Health
Adequate rest and sleep are essential to a healthful lifestyle
Shortened and disturbed sleep cause
– Increased heart-related death
– Increased anxiety
– Weakened immune system
– Worsened pain
– Increased falls and personal injury
Things that can interfere with sleep
– Medications: steroids, stimulants, herbal supplements
– Psychologic: fear, anxiety, stress
– Physiologic: sleep apnea, heart issues, pain
Sleep hygiene is necessary for quality nighttime sleep and daytime alertness
– Engage in exercise but not too near bedtime
– Increase daytime natural light exposure
– Avoid daytime napping
– Establish a bedtime routine - warm bath, cup of warm milk or tea
Associate your bed ONLY with sleep
– Avoid before bedtime:
• Caffeine, nicotine, alcohol and sugar
• Large meals and especially spicy, greasy foods
• Computer screen time
Sleep aid may be needed
Rod NH et al 2014. PloS one. 9(4):e91965; Coleman et al. 2011. Cancer Nurs. 34(3):219-227.National Sleep Foundation. At: http://sleepfoundation.org/ask-the-expert/sleep-hygiene Mustian et al. Journal of clinical Oncology. Sep 10 2013;31(26):3233-3241; Stan DL, et al. Clin J Oncol Nurs. Apr 2012;16(2):131-141; Zeng Y et al., Complementary therapies in medicine. Feb 2014;22(1):173-186.


























EVALUATION
Please take a moment to reflect and respond to the program evaluation.
Questions 7 & 8 can be worked on after each presentation.
We greatly appreciate your time and feedback!


Health Disparities in Myeloma
Patient and Family Seminars
Joseph Mikhael MD, MEd, FRCPCChief Medical Officer, International Myeloma Foundation
Professor, Translational Genomics Research Institute, City of Hope Cancer Center

What are Health Disparities?
•Health disparities are preventable differences in the burden of disease, injury, violence, or opportunities to achieve optimal health that are experienced by socially disadvantaged populations - Centers for Disease Control (CDC)
•Health equity generally refers to individuals achieving their highest level of health through the elimination of disparities in health and health care

What are the DRIVERS of Disparities in MM?
1. Systemic racism
2. The Healthcare system
3. Social Determinants of Health
4. Biology of the disease and concomitant comorbidities
5. Delayed Diagnosis
6. Access to Care – Triplets, Transplants, Trials and Car T
7. Lack of diversity, cultural sensitivity and optimal communication in healthcare professionals

A Call to Action Facts About African Americans and Myeloma
1.There is a longer time from symptoms to diagnosis among African Americans
2.African Americans and Latino Americans are younger by about 5-6 years on average at diagnosis
3.MM and MGUS are more than 2x as common in African Americans
4.African Americans and Latino Americansare less likely to receive the FOUR T’s: Transplant, Triplets, Trials and CAR Ts
5.African Americans have biologic differences with more t(11;14) and less high-risk cytogenetics with deletion 17p
6.Survival outcomes in African Americans are HALF of what is seen in White Americans
7.African Americans can achieve equal or better outcomes when they receive therapy

What are the DRIVERS of Disparities in MM?
1. Systemic racism
2. The Healthcare system
3. Social Determinants of Health
4. Biology of the disease and concomitant comorbidities
5. Delayed Diagnosis
6. Access to Care – Triplets, Transplants, Trials and CART
7. Lack of diversity, cultural sensitivity and optimal communication in healthcare professionals

M-Power = Myeloma Power
The core vision of this initiative is to improve the short- and longterm outcomes of African American patients with myeloma. We want to empower patients and communities to change the course of myeloma…
ENHANCE
ENGAGE
M-Power
Engage the community to increase awareness and provide support
Enhance access to optimal care by educating myeloma providers about the disparity and how to reduce it
EDUCATE
Shorten the time to diagnosis by educating primary care providers to recognize the disease and order the right tests

M-Power Is Both a National and Local Movement
Local Efforts
National Efforts


2023 M-Power Community Workshops

April 1, 2023
50+ attended
81% African American
• 100% rated v good or excellent
• 100% learned something new!

June 17, 2023
50+ attended
68% African American
• 80% at the Grace Baptist Church learned of the Workshop through their congregation
• 63% at the Riverside Church location learned of the Workshop from IMF

September 24, 2023
35+ attended
41% African American
• 96% of attendees rated the program as excellent
• 92% of the attendees reported learning something new

November 4, 2023
75+ attended
61% African American
• 100% plan to share something they learned
• 76% had not previously attended an IMF program
Facebook Live




Education of Primary Care Providers
Our goal is to reduce DELAYS in diagnosis among African Americans by educating the primary care community with a focus on:
• Recognizing the signs and symptoms of myeloma
• Discriminating myeloma from other diagnoses such as diabetes
• Capturing an accurate diagnosis through proper use of testing
• Providing referral guidelines for Hematology and Oncology

• Grand Rounds

• Postcards mailed to 6,000+ PCPs in target cities
• Free PCP CME course “Don’t Miss Myeloma”
• Cobb Institute talk
• Talk at NMA Annual Meeting
• Articles and pending publications

8,000 Learner s









Annual Meeting of the National Medical Association
Jane Cooke Wright Symposium on Health
Disparities
• Hosted by Dr. Edith Mitchell
• Keynote speaker - Dr. Monica Bertagnolli, the director of the National Cancer Institute (NCI)
• Dr. Mikhael spoke about health disparities in myeloma

Poster Walk
Student research was presented to Yelak Biru, Dr. Mikhael, Dr. Mitchell, Dr. Morgan (CEO of the Cobb Institute) and Dr. Bertagnolli.




M-Power Website:
•Web Stats: Over 40k Page views across main, city sites & myeloma.org
•Google PPC targeted web traffic
Email Stats:
•Total Sent: 18 emails
•Total Audience: 38k*
•Open Rate Avg: 31%*
*Note: We have continued to refine lists, contributing to a more engaged audience as evidenced in the Open Rates (The industry standard high-mark is 21%).
M-Minute Promotion
Stats:
•Total Sent: 19 emails
•Total Audience: 323k
•Open Rate Avg: 38.91%
M-Power Related Video
Stats:
• Total Views: Over 50k

Future Direction
Engage
• Planning for 2024 workshops in New York (Juneteenth) and other cities
• Expand online and social media strategy
sEducate
• Primary care program in Charlotte
• Lab based education
• Electronic Medical Record Initiative
Enhance
• Rolling out the Clinical Trial Mentor (CTM) as part of the Diversity in Clinical Trials initiative
• Nurse equity decision tool

Conclusions
•Health disparities are sadly prevalent across all diseases, but particularly in multiple myeloma
•There are MANY other types of inequity in myeloma, including geography, age, gender, orientation…
•Being aware of these disparities is critical to overcoming them
•The IMF’s M-Power is designed to reduce the inequity with specific emphasis on delayed diagnosis, access to therapy and diversity sensitive clinical care

What Can I Do??
•Be more conscious of the topics of health equity
•Evaluate the opportunities in your experience to reduce disparities
•Support the M-Power movement!

mpower.myeloma.org
EVALUATION
Please take a moment to reflect and respond to the program evaluation.
Questions 7 & 8 can be worked on after each presentation.
We greatly appreciate your time and feedback!













LUNCH


Breakout Sessions #2: Patients and Care Partners
Patients Only – Lessons Learned
Yelak Biru
Grand Oasis Ballroom
Care Partners Only
Nancy Bruno & Teresa Miceli
Royal Palms


Patients Only – Lessons Learned
Yelak Biru
Patient, President, CEO
International Myeloma Foundation


Bone Health in Multiple Myeloma
Matthew Drake, MD, PhDMayo Clinic
Rochester, MN


BONE HEALTH IN MULTIPLE MYELOMA
Matthew T. Drake, MD, Ph.D
Mayo Clinic College of Medicine
Boca Raton, Florida
March 16, 2024
Learning Objectives
Describe the physiologic/molecular bases for myeloma bone disease
Define how treatment with a bisphosphonate differs from treatment with denosumab and the potential for subsequent fracture risk with therapy discontinuation
Recognize the skeletal significance of MGUS
SKELETAL LESIONS IN MYELOMA
80-90% of patients with myeloma have bone involvement
• Spine, ribs, pelvis, skull, femur, humerus
Severe Features
• Intractable pain
• Increased fracture risk
• Hypercalcemia (high blood calcium)
• Risk for nerve compression
Also generalized bone loss (osteoporosis)

SITE-SPECIFIC FRACTURE RISK IN MYELOMA
SIGNIFICANT FACTORS FOR INCREASED RISK OF DEATH IN MULTIPLE MYELOMA
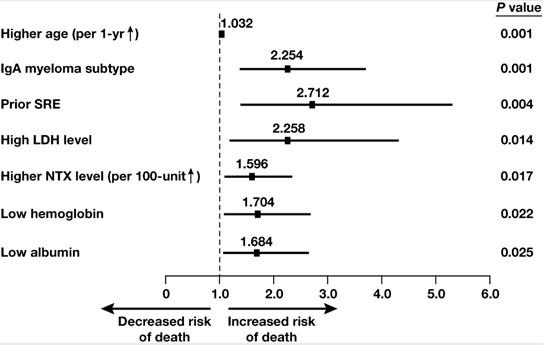
MYELOMA BONE DISEASE
Myeloma cells
Tumor-derived osteoclast activating factors
• Macrophage inflammatory protein-1 alpha
• Interleukin-3



Stromal cells

Osteoclast
Tumor-derived osteoblast inhibitory factors
• Dickkopf1 (DKK1)
• IL-3
• sFRP2
• IL-7 Bone
Osteoblasts
MYELOMA CELLS DISRUPT BONE REMODELING
Bone Resorption
Bone Formation
CLINICAL CONSEQUENCES OF MYELOMA BONE
DISEASE
•Pathological fractures
• Non-vertebral
• Vertebral compression
•Spinal cord compression/collapse
•Radiation therapy
•Surgery to bone
•Hypercalcemia
•Bone pain
•Use of analgesics
•Quality-of-life
*SREs
*SREs- skeletal-related events
PHARMACOLOGIC MANAGEMENT OF MYELOMA BONE DISEASE
•Current recommendations
- Pamidronate (Aredia) 90 mg/monthly (or less often)
- Zoledronate (Zometa) 4 mg/monthly (or less often)
- Denosumab (Xgeva) 120 mg/monthly
•Anti-resorptives improve skeletal outcomes
- Bone pain - Hypercalcemia - Fractures
BISPHOSPHONATES TARGET MATURE OSTEOCLASTS
Normal Osteoclast Bisphosphonatetreated
Apoptotic osteoclast

SKELETAL RELATED EVENT RISK –
ZOLEDRONATE VS PAMIDRONATE
DENOSUMAB (XGEVA) INHIBITS OSTEOCLAST FORMATION
Osteoclas t
Precursor
Activated Osteoclast
RANK Ligand RAN K
Denosuma
PRIMARY ENDPOINT: NON-INFERIORITY FOR TIME TO FIRST ON-STUDY SKELETAL RELATED EVENT

HR (95% CI) = 0.98 (0.85, 1.14); P=0.01 (Noninferiority)



DENOSUMAB (XGEVA) THERAPY CONSIDERATIONS
Denosumab does not cause osteoclast apoptosis like bisphosphonates; rather it prevents pre-osteoclasts from becoming active osteoclasts.
Therefore, any treatment with denosumab must be followed by a bisphosphonate (such as zoledronate) to limit rebound bone resorption.
The timing of bisphosphonate treatment relative to the last dose of denosumab is not clear.
RANKL is not specific to osteoclasts but has a role in the regulation of other immune cells. This may explain the slightly increased rates of infection in denosumab-treated patients.
Denosumab may be a good option in patients with kidney dysfunction
Multiple Myeloma without bone lesions
Limited data exists on the routine use of bisphosphonates in myeloma patients without osteolytic disease
Data from the MRC IX Trial showed that myeloma patients without bone lesions at baseline who received zoledronate had fewer skeletal related events compared to patients treated with clodronate
Guidelines from the European Myeloma Network suggest that patients with symptomatic patients but without lytic lesions can be treated with zoledronate based on this data
Morgan
BONE LOSS ALSO OCCURS IN MGUS
•MGUS is a common pre-malignant condition with an ~ 1% annual risk of progression to MM
•MGUS prevalence increases with age and affects ~ 3.4 million Americans
• 3.2% adults aged > 50 years
• 7.5% adults aged > 85 years
Kyle et al. (2006) NEJM 354:1363; Melton et al. (2004) J Bone Miner Res. 19:25.
FRACTURE RISK IS INCREASED IN MGUS
F o l d i n c r e a s e d f r a c t u r e r i s k
Femu r Any Axial Vertebra l
MGUS Patients have Decreased Volumetric Bone Mineral Density and Microstructure


Bisphosphonate Use in MGUS
Alendronate (70 mg/weekly) ↑ bone density at the spine and hip in osteoporotic MGUS patients
Zoledronic acid (4 mg/every 6 months) ↑ bone density at the spine and hip in MGUS patients with osteopenia/osteoporosis
Neither study was large enough to evaluate for fractures, although fractures generally follow bone density results
Treatment of the MGUS Patient
Patients with MGUS are at increased fracture risk
A pro-active approach is warranted
– May include DXA, counseling on fall risks, lifting recommendations, ensuring adequate calcium and vitamin D intake
In patients with documented osteoporosis (by DXA, history of a fragility fracture, height loss, or kyphosis), medical intervention with anti-resorptive therapy (such as a bisphosphonate) is warranted
In patients with osteopenia, medical therapy to limit bone loss and fracture risk may be appropriate and must be considered carefully
UPDATED IMAGING GUIDELINES IN MONOCLONAL PLASMA CELL DISORDERS
•Optimal imaging supports clinical care decisions
•Bone disease supports the immediate start of systemic therapy
•Imaging can identify painful bone lesions or skeletal sites at increased risk for pathologic fractures or neurologic complications (spinal cord compression)


QUESTIONS
EVALUATION
Please take a moment to reflect and respond to the program evaluation.
Questions 7 & 8 can be worked on after each presentation.
We greatly appreciate your time and feedback!


Ask – the – Experts Panel
Led by Dr. Joseph Mikhael
Guest Faculty:
Peter Voorhees, MD
Gurbakhash Kaur, MD
Teresa Miceli, RN, BSN, OCN
Matthew Drake, MD, PhD


EVALUATION
Thank you for joining us today! Please take a moment to complete your program evaluation form. Our team members will collect these from you on your way out.
We greatly appreciate your time and feedback!













