11 minute read
Up to the test
Founded in June, biotech firm Salient Bio has hit the ground running with what it believes to be the fastest-ever mass scale Covid-19 diagnostics platform, as HealthInvestor UK reports
This year’s global health crisis has had an immense impact on the world economy. Of course, many businesses have suffered, some have had to shut down completely, yet some have shown resilience and were able to adapt and grow, particularly tech companies. Despite this, you might not think of 2020 as the best year to launch a start-up. Nevertheless, Salient Bio did just that.
Founded in June by previous members of Imperial College London’s Biofoundry unit, biotech firm Salient Bio has hit the ground running with what it believes to be the ‘fastest- ever’ mass scale Covid-19 diagnostics platform, utilising top-of-the-line robotics to facilitate the development of what the co-founders have coined the ‘Lab of the 21st Century’. An unlikely birth For a year and half, experts in robotics and microbiology, Marta Ciechońska and Miles Priestman, worked at a specialist unit at Imperial College London called the London Biofoundry which focuses on the commercialisation of synthetic biology and experimental biological processes.
In January this year, the Biofoundry offered to help an affiliated Chinese university as the coronavirus epidemic began to peak in China. The Biofoundry team had the insight that one of the largest issues facing centralised, automated, mass-scale diagnostics was the resilience of the supply chain. The Chinese university asked the Biofoundry team to use its expertise to design a supply chain agnostic workflow, which

Automatable format robot
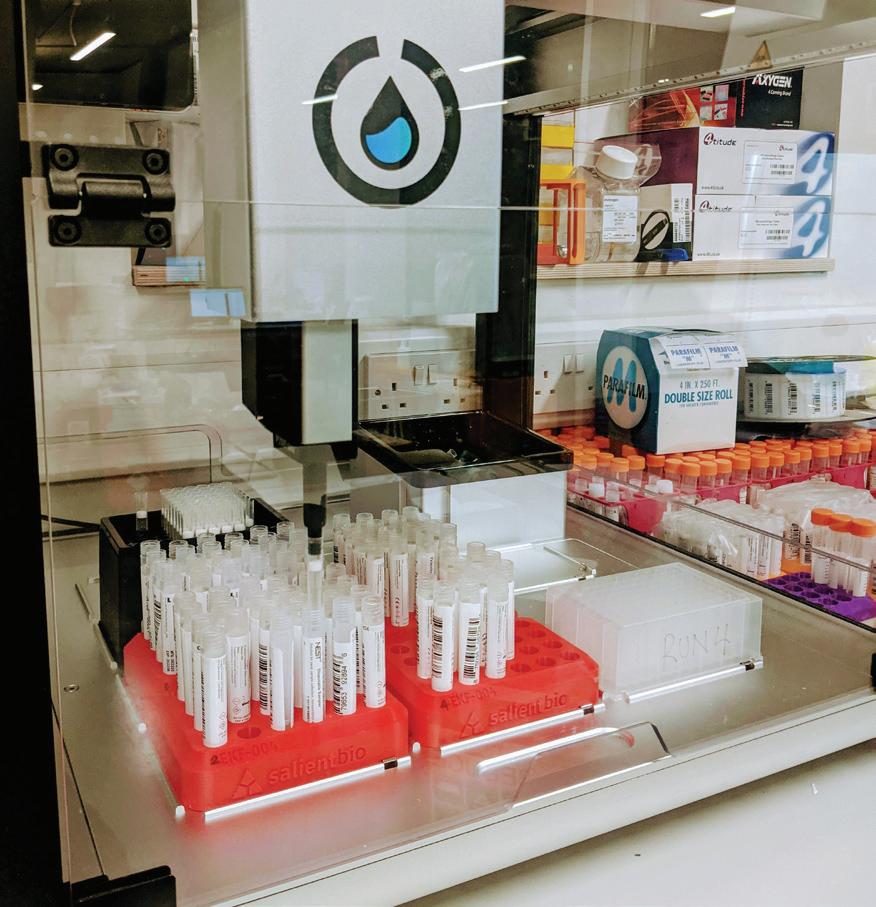
has the ability to facilitate population-scale Covid-19 testing.
To achieve this, the team took state-of-the-art, modifiable robots and designed a molecular diagnostic ‘experiment’, at the same time developing software to have the robots run that experiment. After working on this for some months, the pandemic had well and truly reached the UK.
Focusing efforts closer to home, the Biofoundry team’s completed platform had passed a rigorous NHS validation process with flying colours and had it fully enrolled in two hospitals that are part of the Imperial College NHS Trust. These hospitals started using this platform, diversifying their reliance on the mainstream diagnostic machines typically used in centralised diagnostic laboratories.
It was at this point that Marta Ciechońska and Miles Priestman came to Jack Priestman, the commercial brain behind Salient Bio’s operations, as they believed this approach to diagnostics could be a commercially viable proposition. Not only could this approach be used for mass Covid-19 testing, the team could see it having broader applications to population-scale testing of a far wider array of medical conditions. Saying goodbye to the Biofoundry and taking the leap into independence, Marta, Miles and Jack formed the Salient Bio we see today and proceeded to pursue their mission of making diagnostics accessible to all.
The innovative platform After this colourful history, it’s worth peeking under the hood of Salient Bio’s diagnostic platform itself. The process is completed in four stages, with robots used to automate the analysis process. Stage One is sample prep, the most manual part of the process. This involves the laboratory workers unboxing the tests and removing the swabs from the tubes. At Stage Two, the samples are transferred in an automatable format using 3D printed hardware designed by Salient Bio. This standardises each sample for the benefit of the robots. Stage Three involves the highly precise, liquid handling robots extracting RNA from samples ready for the polymerase chain reaction testing (PCR). Finally, at Stage Four, a series of robots prepare the plates for testing before the thermocycling PCR analysis is run. This produces results which can be sent to the customer.
So, what does this all mean in essence? When the platform is operating at its ideal state, test kits are bought by users and sent out, Salient Bio receives these throughout the day, it runs those tests through its automated workflow which, if the kits are received before 1pm, produces the results to be sent back to the customer on the same day. The team says this sounds fairly straightforward only because their automated system “really makes it that simple.”
Moreover, because the platform is open and modular, as each step in the process completes, another machine starts the second step and so on. Meanwhile, the first machine begins work on a second batch. This means the system can process multiple batches at once. This modular approach creates meaningful productivity gains overall. Salient Bio also uses high-quality PCR machines that have high temperature modulation speeds reducing the time needed for temperature changes to occur, thus expediting the thermocycling process even further. Contrastingly, in the standard closed process, one batch monopolises all machinery until it is completely processed.
PCR prep robot

“Our innovative workflow means our current team of three has the same capacity as that of a 20-person team.” said Ciechońska
Funding and business model Salient Bio sought investment to get its vision off the ground. In its initial seed round of funding, it looked for £200,000 with a view to working without salaries for the foreseeable future until the platform was up and running. However, within just three weeks, the total raised had more than doubled the original goal and the company has ultimately raised far more than this to date.
“We raised more than five times our initial seed round goal of £200,000 in just three months,” said Jack Priestman,
The money received has bolstered Salient Bio’s offering. It now have a lab, which takes up one tenth of the floor plan of its traditional counterpart, that has thousands of times the applicability in terms of the number of diseases it can test for, with a much higher throughput, not only due to the automation of the platform, but also because its input supply chain agnostic approach allows it to use supply chains that are a fraction of the cost of the norm. Consequently, this enables processing of 2,000 tests a day, though it claims it has “lots of operational contingency” above that.
When asked how Salient Bio compares to the competition, Jack remarks that, “the truth of the matter is we aren’t doing anything novel on the testing side – we aren’t inventing a new form of test – instead, what we have designed is an industrial process.” Salient Bio believes this is of immense value on the business side as it doesn’t have to invest time, effort and money into something that ultimately might not work. Rather, it caters to the growing number of people who are interested in ditching the traditional lab that offers bad service and huge inefficiencies for a more modern alternative.
“We have landed on the blueprint of the lab of the 21st century – a fully automated and extremely versatile lab which can make diagnostics available to all,” said Ciechońska.
The initial injection of capital put Salient Bio in a place to think beyond Covid-19 and begin refining its long-term strategy. Indeed, it says its lab is far more productive, easier to run and, because of its smaller size, can be more localised than a traditional lab, adding this gives the consumer far more choice in what diagnostics they want to target at a much lower price.
In terms of future funding rounds, the team says there will likely be a period of lower revenue after the next six months if Covid-19 is brought under control. It will have finalised its long-term strategy at this stage and will look to a funding round in the next 12 to18 months to turbocharge that future plan.
The long-term strategy So, what’s the future plan? Generally, it is to apply its fast, affordable, easily accessible, highly accurate diagnostics to a wide range of medical conditions to facilitate early intervention and improve overall health outcomes.
Although this has not been fully refined, Salient Bio believes that a shift is occurring as the market moves from reactive healthcare, where, for example, you might find a lump and then test for malignancy, to a much more proactive and preventative state of affairs. The team foresees a situation where healthcare will become more about performing ‘community scale MOTs’ which are not searching for any particular medical condition but can track an individual’s health and catch any concerning deviations from the norm.
Once the pandemic is under control, the excess centralised
capacity of standardised Covid PCR testing will require Salient Bio to carve out a niche for itself. To Salient Bio, the coronavirus pandemic is not the cash-cow many biotech firms have viewed it as. Rather, “it has been an opportunity to realworld test and refine our innovative workflow under some of the most stress it could ever be subjected to,” the team told HealthInvestor UK, “this has informed us of the capability of our platform to tackle much wider medical issues at scale.”
Salient Bio’s next direction will probably seek to develop its platform to facilitate these community-scale ‘MOTs’ through Next Generation Sequencing (NGS), specifically microbiome testing. A working microbiome diagnostics service will operate similarly to blood test tracking, where blood composition is tracked over time and monitored for unusual deviations, but instead track gut health. The health of our gut is potentially more indicative of overall health than blood testing, which means this form of tracking could provide a more nuanced view of how one’s health is progressing than blood testing does. Furthermore, the team says that this more niche discipline commands a far greater profit margin too.
Beside the NGS route, the team has noticed one area not currently being executed on at all is bringing mass scale diagnostics to the developing world – and that this is an opportunity that has the potential to command vast market share in the space moving forward. Much of the developing world is locked out of conducting mass-scale diagnostics due to the cost of the equipment and reagents. A supply chain agnostic approach enables access to massively lower cost supply chains and, potentially more crucially, local supply chains, without compromising on quality of testing. Further, the cost of the equipment is a fraction of those currently available from the large incumbents.
So, Salient Bio has identified the niches it sees as viable for it to explore. However, Jack commented that the versatility of its platform places it in an especially beneficial position where it can fulfil the customer’s requests rather than have customers mould their needs to fit Salient Bio’s platform.
“We will uniquely have the opportunity to ask the customer what they want, instead of offering them a commoditised set of services to choose from,” Jack says.

Incoming challenges While Salient Bio remains optimistic, it is aware of the challenges it faces, both immediately and long term. As previously stated, it’s unlikely that Covid-19 testing will be feasible as the sole operation of the business in the second half of next year as it is presently. As it is not venture capital funded like most start-ups, it must be more cautious about how it spends its money.
To remedy this, a future funding round within the next year will be an important step. Building up a watershed of capital will be vital in keeping the business ticking over during the inevitable post-Covid slump. The team told HealthInvestor UK that once reaching this stage, Salient Bio will most likely operate much like a normal biotech start-up, spending most of its money on salaries and R&D.
Where will Salient Bio be in five years? When start-ups are asked the ‘five-year’ question, the answer is usually more interesting than that of larger, more established businesses, purely because ambition often outweighs capability at the early stages of a company’s life – especially in the tech sector. However, the team’s answer, while still ambitious, seems pragmatic and achievable.
The direction of the firm in the long term is heading towards the provision of tech-led, fully automated labs, and Salient Bio want to be a leader in this space. Jack explains the logic behind how it aims to achieve this.
He believes the value chain speaks volumes. Indeed, at present, this chain consists of the patient; the intermediary (that is, hospital, clinic or pharmacy) which makes 20% margins; the lab, which the intermediary requests services from, which makes 30% margins; and then the equipment and consumables, which the lab uses to run the tests, the providers of which make 50% margins.
Following the pound through, Salient Bio is already capturing 80% of this margin, designing its own diagnostic workflows and being its own lab.
While it is looking for ways in which to remove the intermediaries in the very long term, it is clear that investing in being the lab is attractive. Of course, the challenge in achieving this goal while maintaining growth is the scalability of this plan. Namely, opening automated labs more widely in other countries. However, for now, the team feels this is “quite far off to be too concerned with at present”.
In closing, Salient Bio appears to be a healthy, ambitious start-up with a service filling a gap in the market and commanding a majority share of the value chain. Of course, it will certainly face challenges as it adapts its business model to a post-pandemic world and must navigate a scalability challenge in the longer term.
But, with clients across healthcare, logistics, sports and education, and A-list recording artists already utilising its affordable and time-efficient offering, this company’s journey in the new year will be one to watch. n