
4 minute read
PH Clinical Sessions
Endothelial and Smooth Muscle Cell Interaction Via FoxM1 Mediates Vascular Remodeling and Pulmonary Arterial Hypertension
Dai Z, Zhu MM, Peng Y, Jin H, Machireddy N, Qian Z, Zhang X, Zhao YY Northwestern University, Chicago, IL, USA
Advertisement
Background: SMC-specific FoxM1 or Cxcr4 knockout mice, EC-specific FoxM1 or Egln1 knockout mice, as well as EC-specific Egln1/Cxcl12 double knockout mice were used to assess the role of FoxM1 on SMC proliferation and pulmonary hypertension (PH). Lung tissues and cells from PAH patients were employed to validate clinical relevance. FoxM1 inhibitor Thiostrepton was used in Sugen 5416/ hypoxia- and monocrotaline-challenged rats.
Methods: SMC-specific Foxm1 or Cxcr4 knockout mice, EC-specific Foxm1 or Egln1 knockout mice, as well as EC-specific Egln1/Cxcl12 double knockout mice were used to assess the role of FoxM1 on SMC proliferation and PH. Lung tissues and cells from PAH patients were employed to validate clinical relevance. FoxM1 inhibitor Thiostrepton was used in Sugen 5416/hypoxia- and monocrotalinechallenged rats. Results: FoxM1 expression was markedly upregulated in lungs and pulmonary arterial SMCs of idiopathic PAH (IPAH) patients and 4 discrete PH rodent models. Mice with SMC- (but not EC-) specific deletion of FoxM1 were protected from hypoxia- or Sugen 5416/hypoxia-induced PH. The upregulation of FoxM1 in SMCs induced by multiple EC-derived factors (PDGF-B, Cxcl12, ET-1 and MIF) mediated SMC proliferation. Genetic deletion of endothelial Cxcl12 in Egln1Tie2Cre mice or loss of its cognate receptor Cxcr4 in SMCs in hypoxia-treated mice inhibited FoxM1 expression, SMC proliferation and PH. Accordingly, pharmacological inhibition of FoxM1 inhibited severe PH in both Sugen 5416/hypoxia and monocrotaline-challenged rats.
Conclusions: Angiocrine factors derived from dysfunctional ECs induced expression of FoxM1 in SMCs and activated FoxM1-dependent SMC proliferation which contributes to pulmonary vascular remodeling and PH. Thus, targeting FoxM1 signaling represents a novel strategy for treatment of PAH.
Figure 1: FoxM1 disruption in SMCs inhibits SuHx-induced PH in mice. Figure 2: Foxm1 disruption in SMCs inhibits SuHx-induced PH in mice
Figure 3: Endothelial and smooth muscle cell interaction mediate FoxM1 upregulation, SMC proliferation and PH
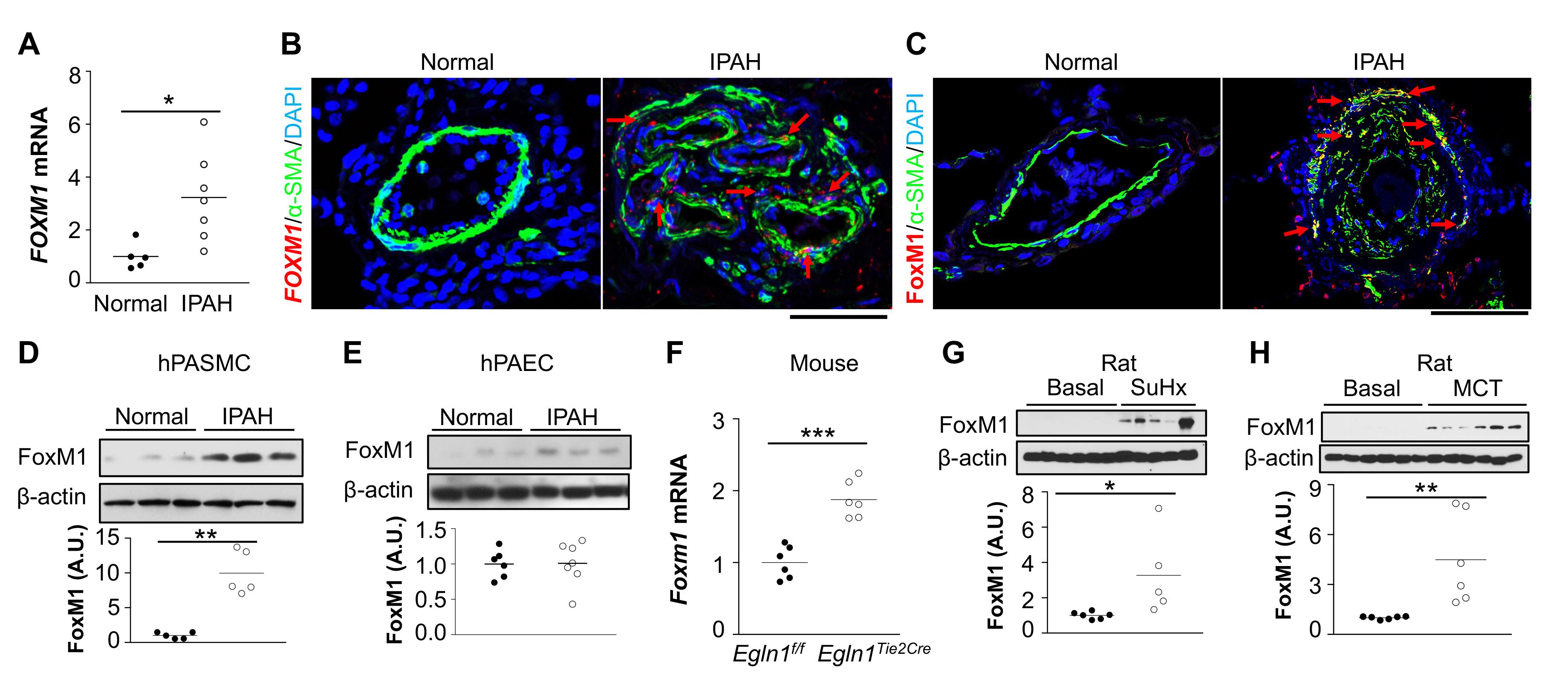


Figure 4: Pharmacological inhibition of FoxM1 inhibits both SuHx and MCT-induced PH in rats
Gender Difference on the Cellular Level: Distinct Stress Responses in Male and Female Endothelial Cells Isolated From Mouse Lungs
Rafikova O, Zemskova M, Kurdyukov S, Rafikov R University of Arizona, Tucson, AZ, USA
Background: Pulmonary arterial hypertension (PAH) is a disease with a well-established sexual dimorphism. While females generally being associated with higher susceptibility to PAH, males are showing lower survival rate and predisposition to develop RV failure. Sex hormones mediated gender difference is well described for many vascular diseases, including PAH. In this study, we investigated whether the contribution of gender goes beyond the effects of sex hormones by comparing the profile of pulmonary endothelial cells isolated from both genders.
Methods: Mouse lung endothelial cells (MLEC) were obtained from male and female 8 wk old mice using positive selection with anti-PECAM-1 antibody conjugated to Dynabeads and cultured for 3 passages. After 3th passage, MLEC were additionally purified by a second positive selection using ICAM2 antibody and validated by FACS analysis with antiCD31-FITS antibody, which yields 98% pure population of MLEC. Gender difference in the MLEC omitted from effects of sex hormones was analyzed by evaluation of cell morphology, proliferation rate, mitochondrial function, and capability to tolerate the stress.
Results: Male MLEC were found to be smaller in size and possessed about 2 times higher rate of proliferation comparing to female. The level of mitochondrial polarization was also higher in male cells, suggesting an increased cellular energy and metabolic output associated with male gender that could be also responsible for the higher proliferative state. Exposure of cells to 2% hypoxia for 24 hours induced a strong apoptotic response in female but not male MLEC. In contrast, treatment with mitochondrial respiratory Complex III inhibitor Antimycin A (AA) mediated a severe necrosis specifically in male MLEC, while female cells responded again primarily by apoptosis. Taken together these results suggest that male cells appeared to be protected against the mild stress conditions, possibly due to an increased mitochondrial biogenesis. In contrast, female cells are protected against more damaging stimuli, such as mitochondrial dysfunction. Indeed, the apoptotic response to AA in females was similar to hypoxia. Besides, apoptosis as a controlled and immune silent type of cell death represents a more physiological response to stress compared to necrosis.
Conclusions: This study revealed that isolated and cultured pulmonary endothelial cells retain a gender difference even in the absence of sex hormone stimulation, suggesting the importance of genetic mechanisms in gender dimorphism. The discovered difference in the ability to tolerate the stress is of a great importance for the PAH known to be closely associated with different types of stress conditions, including hypoxia and mitochondrial dysfunction.


