
8 minute read
Feature
Weed Management Strategies in Nursery Crop Propagation
Plant propagation is a key component of the nursery industry. Some nurseries only propagate for their own use while other nurseries specialize in propagating liners for sale and growing on to a finished salable size. Many nursery crops are propagated by seed including many tree species and rootstock used for grafting. Other crops, including herbaceous perennials and woody shrubs, are produced from vegetative cuttings. Weeds are a major issue during propagation (Fig. 1), yet limited weed control options are available during propagation. As weeds become established, they compete with rooted cuttings for light and resources, and many weed species can produce seed within a few weeks, quickly infesting larger areas in liner production beds. Although actively growing weeds can be removed from containers and flats prior to shipping, the remaining weed seeds will germinate and be a problem during later stages of crop production. Manual weed removal (hand weeding) is the most common method of weed control in propagation, but these efforts are time consuming and costly, and the work requires extra labor demands from staff members who have, in recent years, become less available for hire. As a result, development of improved weed control methods is needed to reduce labor and cost inputs during propagation and to improve finished crop quality.

Sanitation Practices
Weeds that infest propagative material can be spread by seed coming from contaminated container substrates, used containers, floors within the propagation area and surrounding areas, stock plants, and by workers. To prevent weed seed infestation, container substrates, especially pine bark in bulk piles, should be stored in a protected area or indoors. Propagation containers that are re-used should be thoroughly cleaned with high pressure water sprays to remove weed seeds, especially seeds with a sticky outer coating such as bittercress (Cardamine spp.) and woodsorrel (Oxalis spp.). Surrounding production areas including nearby container production blocks, floors of the propagation spaces (Fig. 2), and stock plants used for cuttings should be maintained weed free to prevent spread of seeds from weeds in established crops and nonproduction areas, roadways, and nursery borders.

Post-emergent herbicides can be used to control actively growing weeds, but care must be taken to avoid contact with foliage of desirable crops. Several post-emergent herbicides are labeled for use in enclosed structures (such as greenhouses) and can be used to control weeds during propagation. These products include diquat (Reward®), glufosinate (Finale®), glyphosate (Roundup®), and pelargonic acid (Scythe®). Preemergent herbicides are used to prevent weed seed establishment in container-grown crops in production, on gravel production pads, and in non-crop areas such as gravel drives and walkways. Pre-emergent herbicides such as flumioxazin (Sureguard®) and indaziflam (Marengo®) can be used on greenhouse and shade house floors, but these products must be applied prior to moving in flats and containers with plants.
Herbicide Use in Propagation
Propagation by seed and cuttings involves the initiation, growth, and development of new roots that will be sensitive to certain herbicides. As a result, pre-emergent herbicides have not been widely used during propagation. Pre-emergent herbicides function by inhibiting germination or root/shoot development. Sensitivity to active ingredients in pre-emergent herbicides can vary by chemical class and among plant species. Pre-emergent herbicides in the dinitroaniline family act as root inhibitors (by inhibiting mitosis in root cells) and numerous research reports noted reduced rooting percentage and root development when used in cutting propagation. Root inhibiting herbicides such as oryzalin (Surflan®), pendimethalin (Pendulum®), prodiamine (Barricade®), and trifluralin (Treflan®) should not be used in cutting propagation.
Several other pre-emergent herbicides are considered non-rootinhibiting resulting from their different modes of action. For example, oxadiazon (Ronstar®) and oxyfluorfen (Goal®) are Group 14 herbicides that disrupt cell membrane synthesis while isoxaben (Gallery®) is a Group 21 herbicide that inhibits cell wall synthesis. Previous research reported oxadiazon was safe to apply prior to sticking cuttings of several crop species including Abelia spp., Buxus spp., Euonymus spp., Ilex spp., and Rhododendron spp. (Johnson and Meade, 1986; Thetford and Gilliam, 1991). In another study, oxyfluorfen was reportedly safe when applied prior to germination of several large-seeded tree species including Carya spp, Juglans spp., and Quercus spp. (South and Carey, 2005). In other studies, isoxaben could be safely applied over-the-top of small seedlings of several crop species and during cutting propagation of Loropetalum spp. (Cochran et al., 2008; Halcomb and Fare, 1997). Nevertheless, limited information is available for using pre-emergent herbicides in propagation.
Research At Tennessee State University
Most work that previously has evaluated pre-emergent herbicide uses in propagation was completed over twenty years ago. There are newer products and formulations that may be viable for weed control in propagation systems. Other types of products such as mulches may be alternatives for weed control in sensitive crops (like many herbaceous perennials, dogwood, and hydrangea) and inside greenhouses. In recent years, several studies have been completed at the Tennessee State University Otis L. Floyd Nursery Research Center in McMinnville, TN evaluating pre-emergent herbicides and mulches in seedling and cutting propagation.
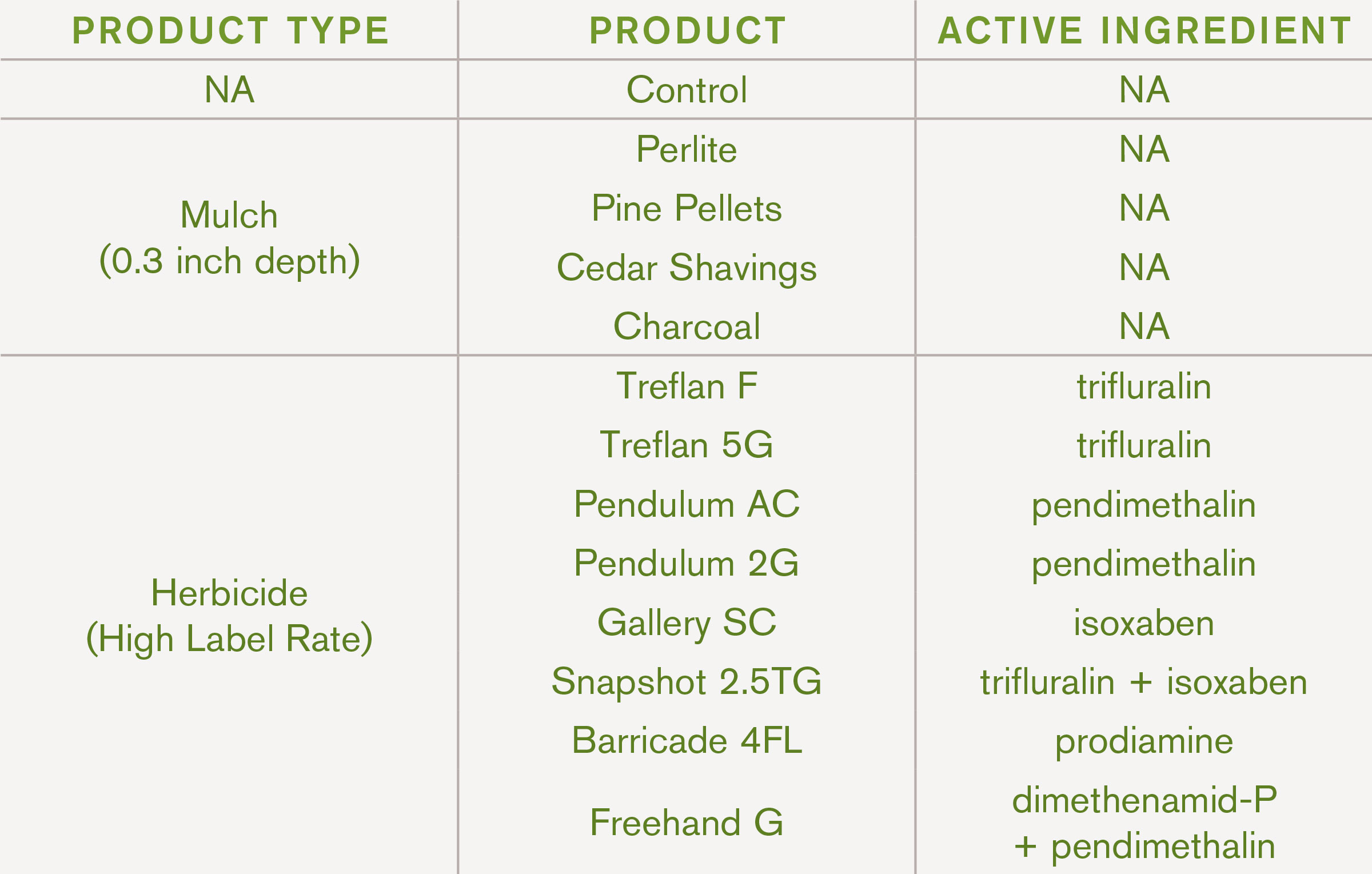

In a second study, three pre-emergent herbicides (isoxaben, isoxaben+dithiopyr, and oxadiazon+oxyfluorfen) were applied at three different timings (two weeks before sticking, at sticking, and two weeks after sticking cuttings). Cuttings of butterfly bush (Buddleja davidii ‘Nanho Blue’), holly (Ilex cornuta ‘Dwarf Burford’), and crape myrtle (Lagerstroemia indica ‘Catawba’) were tested. Compared to the non-treated control, herbicide type and application timing had no negative effects on root development for crape myrtle or holly; however, the herbicides containing isoxaben reduced rooting for butterfly bush. Neither of those products were labeled for use in butterfly bush. Oxadiazon+oxyfluorfen (Regal O-O®) also provided excellent control of four common weed species: bittercress (Cardamine hirsuta), crabgrass (Digitaria sanguinalis), creeping woodsorrel (Oxalis corniculata), and mulberryweed (Fatoua villosa)]. Applying pre-emergent herbicides to substrates prior to sticking cuttings would provide more uniform coverage to the container substrate surface and reduce labor costs.
In a third study, mulch type and depth were evaluated for rooting cuttings of three crop species: butterfly bush, crape myrtle, and hydrangea (Hydrangea paniculata ’Phantom’). Mulches included coarse vermiculite, paper pellets, pine pellets, and rice hulls applied at 0.5- or 1-inch depth prior to sticking cuttings (Fig. 4). No differences in rooting percentage were observed for any treatments. Crape myrtle root dry weight was lower for paper pellets (both depths), but no differences were observed for butterfly bush or hydrangea. Both depths of pine pellets and paper pellets provided effective weed control of bittercress, crabgrass, creeping woodsorrel, and mulberry weed seeds (Fig. 5). By contrast, rice hulls provided poor weed control, which is surprising due to their effectiveness when used in container production. The frequent mist required for cutting propagation likely negated the hydrophobic properties of the rice hulls. Economically, pine pellets are comparable in cost to rice hulls based on coverage area. Paper and pine pellet mulches can be alternatives to pre-emergent herbicides and used with sensitive crop species and within enclosed structures.


Currently, there are no pre-emergent herbicides labeled for use in propagation. Many products have restrictions that limit use in small diameter containers (less than four inches) and on seedlings and/or non-rooted cuttings. Additionally, there are no pre-emergent herbicides labeled for use within enclosed structures because they may become volatile once activated by irrigation. Nevertheless, some products lack restrictive language thus may be allowed for use in outdoor (shade house) propagation at the grower’s own risk. Mulches and some pre-emergent herbicides may have potential for use in seedling and cutting propagation, however further research is needed to screen pre-emergent herbicide safety to additional crop species. Growers should always read herbicide labels to identify any use restrictions (that may include site, sensitive crop species, container sizes, etc.). Growers should also conduct small trials with individual products and crop species prior to large scale adoption.
Additional Reading References:
Cochran, D.R., Gilliam, C.H., Eakes, D.J., Wehtje, G.R., and Knight, P.R. (2008). Herbicide use in propagation of Loropetalum chinense ‘Ruby’. J. Environ. Hortic. 26:139-143.
Halcomb, M.A., and Fare, D.C. (1997). Tolerance of seedlings to preemergence herbicides. Proc. South. Nurs. Res. Conf. 42:315-318.
Johnson, J. R., and Meade, J.A. (1986). Pre-emergent herbicide effect on the rooting of cuttings. Proc. Intl. Plant Prop. Soc. 36:567-570.
South, D.B., and Carey, W.A. (2005). Weed control in bareroot hardwood nurseries. USDA Forest Serv. Proc. 35:34-38.
Thetford, M., and Gilliam, C.H. (1991). Herbicide use in propagation: Effects on rooting and root growth of stem cuttings. J. Environ. Hort. 9:21-23.
For more information on weed control practices in nursery crop propagation and production, contact Dr. Anthony Witcher (awitcher@tnstate.edu).





