15 minute read
Research and Funding
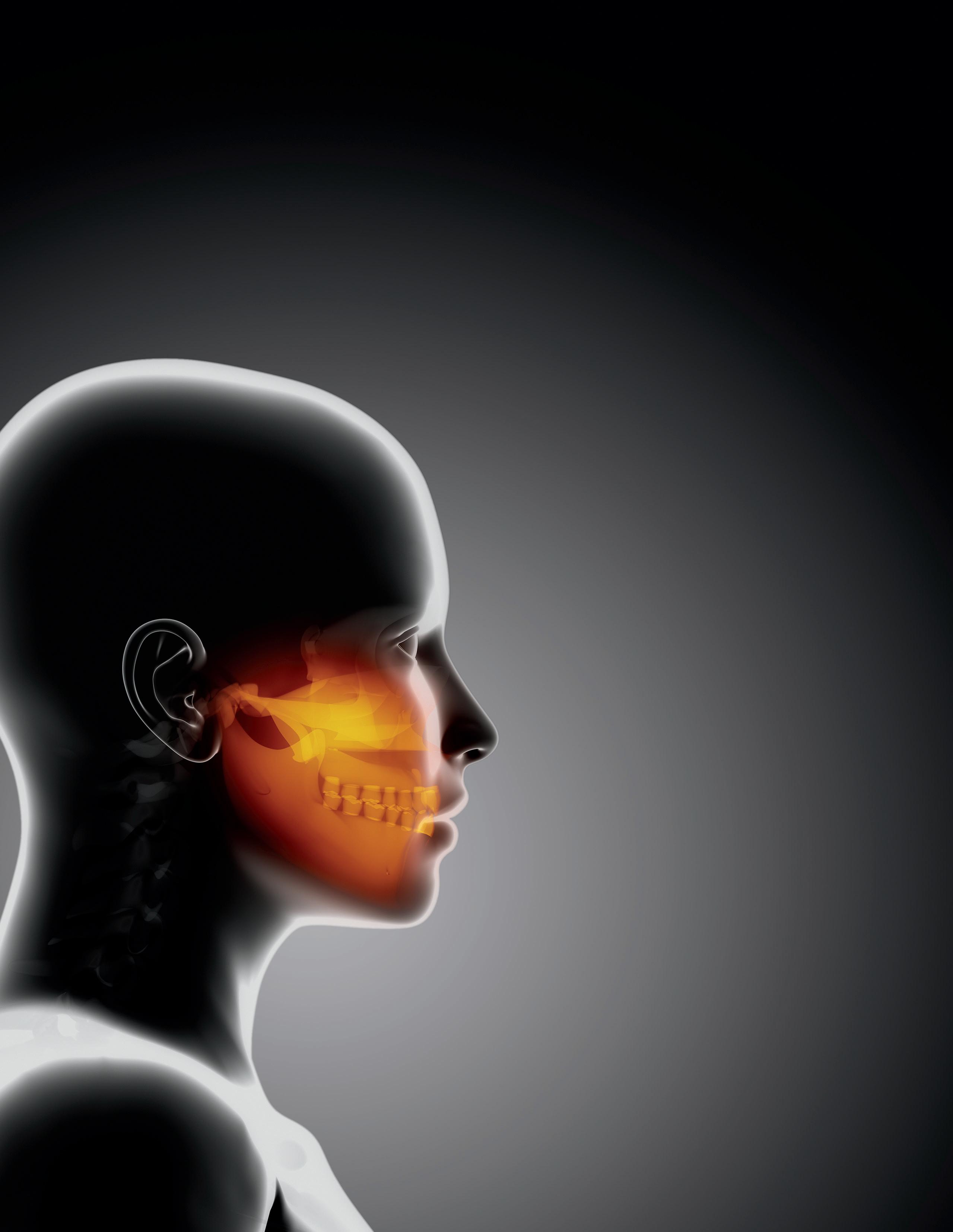
UCI ENGINEERS AIM TO PIONEER TISSUEENGINEERING APPROACH TO TMJ DISORDERS
One in four people are impacted by defects of the temporomandibular – or jaw – joint. Despite the pervasiveness of this affliction, treatments are lacking, and many sufferers resort to palliative measures to cope with the pain and debilitation it causes.
Advertisement
“The TMJ is central to chewing, talking and so many other daily activities, so when this crucial joint is impaired, there are significant negative effects on quality of life,” said Kyriacos A. Athanasiou, UCI Distinguished Professor of biomedical engineering.
“The problem may start with slight pain and clicking and get progressively worse to the point where it’s not just impacting the jaw but the entire body.”
Athanasiou is senior author on a paper published recently in the Cell Press journal Trends in Molecular Medicine that examines the causes of temporomandibular disorders, past failures in treating them and new approaches based on tissue-engineering innovations developed in his
laboratory. Co-authors are Ryan Donahue, UCI graduate student researcher in biomedical engineering, and Jerry Hu, UCI principal development engineer in biomedical engineering.
Temporomandibular disorders can be the result of sudden injuries or wear and tear over time. The cartilage disc between the mandible and the temporal bone is subject to thinning or perforation. The condition usually affects
patients between the ages of 20 and 50. Most strikingly, premenopausal
women are eight times more likely to experience jaw joint problems than men – which Athanasiou calls the TMJ gender paradox.
Typical treatments include physical therapy, splints and adjustments, corticosteroid injections and pain medications. Only about 5% of sufferers are candidates for surgical interventions. The TMJ is a joint like many others in the body, but surgeries to repair it are rare because of its location.
“It has to do with the proximity of the TMJ to the brain,” Athanasiou said. “Back in the 1980s, many patients – primarily women – came forward with issues they had with the TMJ. The solution at the time was to insert a spacer between the two bones articulated in the jaw.” The spacer was made of Teflon, a material approved by the U.S. Food and Drug Administration.
“It turns out that Teflon was an absolute catastrophe for all of those women,” Athanasiou said. “Because of the large mechanical forces generated in the jaw, the Teflon broke up into pieces, and because of the proximity of the TMJ to the brain, those pieces somehow found their way into the brain.”
This fiasco set back therapies for temporomandibular disorders for decades, but now Athanasiou and his colleagues in UCI’s Department of Biomedical Engineering are working on new approaches that eschew synthetic materials entirely. They’re developing biological TMJ discs in the laboratory that will be suitable for implantation in humans.
“The end product that we aspire to use for treating afflictions of TMJ discs is a tissue-engineered product that’s fully alive, biological and mechanically comparable to the real thing,” Donahue said. “So even if it breaks down, it will be like any other biological component, without having pieces of foreign material entering the brain.”
NIH GRANT TO FUND NEW ELCACT INSTRUMENT

This fall, a new state-of-the-art, highly sensitive microscope will be installed at UCI’s Edwards Lifesciences Center for Advanced Cardiovascular Technology (ELCACT), giving researchers the capability to expand and accelerate their research. The instrument is funded in part by a highly competitive S10 equipment grant from the National Institutes of Health’s Office of Research Infrastructure Programs. UCI’s Office of the Vice Chancellor for Research provided matching funds.
The $594,000 grant will fund an Olympus Fluoview FV3000 with MPM System, a multispectral, multiphoton laser scanning microscope. The tool will open the door to new cardiovascular and tissue engineering possibilities at ELCACT, a center that seeks to better understand cardiovascular disease and tissue vascularization. “This will allow us to conduct research and science related to topics such as heart cell structure-function-force generation relationships, macrophage mechanobiology, and the mechanics of growing vessels into implanted medical devices,” said principal investigator Elliot Botvinick, ELCACT associate director and professor in the departments of biomedical engineering and surgery. Securing the grant was a five-year team effort led by Botvinick, Associate Professor Anya Grosberg and ELCACT Assistant Director Ann Fain. The effort included many biomedical engineering faculty who will use the equipment, including Tim Downing, Christopher Hughes, Abe Lee, Zhongping Chen, Arash Kheradvar, Kerry Athanasiou and Wendy Liu. The new microscope will have confocal and two-photon imaging, and onstage incubation for longitudinal studies. Some of these studies utilize environmentally sensitive tissues that cannot be transported out of the building without compromising the research. The new instrument will be housed adjacent to the center’s Core Tissue Culture Facility, opening avenues for research involving mechanically sensitive samples.

DIGMAN RECOGNIZED WITH NSF EARLY CAREER AWARD
Michelle Digman, associate professor of biomedical engineering, earned a National Science Foundation Faculty Early Career Development award. Digman will receive $500,000 for her project to develop an imaging platform with a fast-orbital tracking technique to follow mitochondria with nanometer precision and, at the same time, noninvasively measure metabolic changes at the local environment in cancer cells. “My lab is excited to continue to push the envelope in developing imaging technologies to noninvasively track mitochondrial recruitment and how environmental cues influence mitochondrial function within specific regions inside cancer cells, which may lead to aggressive cancer invasion.”
Among the NSF’s most prestigious, the CAREER award supports early career faculty who have the potential to serve as academic role models in research and education. Begun in 1995, the program provides recipients the opportunity to pursue outstanding research, excellence in teaching, and the integration of education and research.
BIOMEDICAL ENGINEERS DESIGN RAPID STD DETECTION DEVICE

Samueli School biomedical engineers have developed a novel microfluidic platform capable of rapidly detecting multiple sexually transmitted viral infections. The technology uses blood or saliva samples and can diagnose HIV, HPV and HSV simultaneously in less than 20 minutes.
The researchers used a five-step protein-array assay for the multiplexed detection of these viruses’ antibodies on an integrated microfluidic system. Investigators say the technology can be adapted with different protein microarrays to detect a variety of other infections, such as dengue or chikungunya virus. The device could provide a promising approach for identification, analysis and monitoring of infectious disease, particularly in lowresource settings.
“This technology would allow clinicians to extend their ability to diagnose and start treatment in the field or at the bedside, providing point-ofcare services for viral infections,” said Neha Garg, lead investigator and a graduate student researcher in the lab of Abe Lee, professor of biomedical engineering and co-investigator.
NANOTECHNOLOGY TREATMENT SHOWS PROMISE AGAINST MULTIPLE SCLEROSIS
A nanotechnology treatment derived from bone marrow stem cells has reversed multiple sclerosis symptoms in mice and could eventually be used to help humans, according to a new study led by UCI researchers. “Until now, stem cell therapies for autoimmune and neurodegenerative diseases have produced mixed results in clinical trials, partly because we don’t know how the treatments work,” said corresponding author Weian Zhao, associate professor of pharmaceutical sciences and biomedical engineering, who is affiliated with the Sue & Bill Gross Stem Cell Research Center. “This study helps unravel that mystery and paves the way for testing with human patients.” In past experiments, intravenously injected stem cells – taken from bone marrow and activated with interferon gamma, an immune system protein – often got trapped in filter organs before reaching their target. For this study, published in the journal ACS Nano, researchers avoided that problem by extracting nanosized particles called exosomes from the stem cells and injecting them into rodents with MS.
Loaded with anti-inflammatory and neuroprotective RNA and protein molecules, the exosomes were able to slip through the bloodspinal cord barrier. In addition to rejuvenating lost motor skills and decreasing nerve damage caused by MS, they normalized the subjects’ immune systems, something conventional drugs can’t do, said study colead author Reza Mohammadi, a UCI doctoral candidate in materials science and engineering.
More experiments are in the pipeline. “This novel treatment will be tested on humans in early 2020, initially on people with Type 1 diabetes,” said co-lead author Milad Riazifar, who worked on the study as a pharmacological sciences doctoral student in Zhao’s lab and is currently helping prepare it for a City of Hope clinical trial. “If successful, it could pave the way for treating other autoimmune diseases.”
NSF GRANT SUPPORTS CENTER TO DEVELOP MICROFLUIDICS-BASED SOLUTIONS
UC Irvine received phase 2 funding of $750,000 from the National Science Foundation to support the Center for Advanced Design and Manufacturing of Integrated Microfluidics (CADMIM). The center, which launched five years ago, has two sites – one at UCI and another at the University of Illinois at Chicago. CADMIM is an NSF Industry-University Cooperative Research Center, which fosters long-term partnerships among academia, industry and government in various technology sectors. Total phase 2 funding for the two-site center is $1.25 million over five years.
CADMIM focuses on developing miniature devices that can perform biochemical analytical functions quickly and cheaply. These chips have the potential to rapidly detect dangerous toxins in the blood, quickly screen hundreds of potential drugs, isolate cells for cancer diagnostics and treatment, or provide information on plant health that can improve crop outputs. The UCI site has expertise in microfluidic sample preparation (cell and molecular sorting/separation, tissue dissociation, etc.), droplet-based microfluidics, autonomous microscale fluidic handling and various noninvasive detection methods.
“It is gratifying to know that the National Science Foundation is recognizing and rewarding the many accomplishments of CADMIM in its first five years, in research, technology transfer and most importantly, in building a community of students, faculty and industrial members that bridges advanced research with real-world applications,” said Abe Lee, CADMIM director and professor of biomedical engineering at the Samueli School.
CADMIM has worked with several industry leaders over the last five years, including Beckman Coulter, Corteva Agriscience, KWS, Monsanto, QIAGEN, ThermoFisher Scientific, Canon U.S. Life Sciences, Procter & Gamble, GSK, Genomics Institute of the Novartis Research Foundation, Douglas Scientific, Amgen Inc., Genentech Inc., Corning Inc., Los Alamos National Laboratories and Air Force Research Labs.
CADMIM industry partners provide funding for university researchers to develop solutions for specific needs or problems. UCI and UIC CADMIM researchers working with GSK, for example, are developing a human liver culture platform using induced pluripotent stem cell technology. These tools can screen thousands of compounds in early drug discovery using a sustainable and genetically diverse source of patient-specific cells. “Cell micropatterning is also being employed to organize the liver cultures precisely at the cellular scale, allowing for optimization of liver function and rapid identification and assessment of each cultured cell type by automated microscopy,” added investigator Elliot Hui, assistant professor of biomedical engineering at the Samueli School. Jered Haun, Michelle Khine and Michelle Digman from UCI, and Salman Khetani, David Eddington and Jie Xu from UIC, are other key researchers at CADMIM.

NEW STUDY EXAMINES BLOOD FLOW LEAKAGE AFTER TRANSCATHETER HEART VALVE REPLACEMENT For people with heart disease, particularly aortic valve stenosis, valve replacement is the primary treatment option. A narrowing of the valve opening, aortic valve stenosis restricts blood flow from the heart to the rest of the body. Since 2011, when the FDA first approved it, transcatheter aortic valve replacement (TAVR) has been a less invasive choice than traditional open-heart surgery for patients at high surgical risk.
However, a tiny gap can occur between the patient’s natural heart valve and the implanted valve after TVAR. Called paravalvular leak, or PVL, this gap leads to blood flow leakage from the aorta back into the heart’s left ventricle (regurgitation).
Although mild PVL is usually not serious, moderate PVL may lead to symptoms of heart failure, including fatigue and difficulty in breathing, among other complications. Moderate to severe regurgitation after TAVR has a poor prognosis and is associated with higher mortality.
In a study published last fall in Nature’s Scientific Reports, UCI’s Arash Kheradvar and his team found that PVL leads to formation of an abnormal swirling pattern (vortex) in the heart’s left ventricle that clearly interferes with natural blood flow from the left atrium into the left ventricle. This negatively affects blood flow dynamics, including circulation, impulse and kinetic energy.
Using echocardiographic particle image velocimetry, an advanced method to measure the heart’s blood flow velocity based on echocardiography, the researchers were able to observe and study various fluid dynamic events that occur inside the heart relative to the location of the leak.
The study’s results emphasize the significance of the PVL location. “Our in vitro and in vivo studies show that posterior PVL is significantly more harmful than anterior PVL considering the blood flow dynamics inside the heart. According to those results, we predict that presence of posterior PVL may lead to worse patient survival and outcomes compared to anterior PVL and no PVL,” said Kheradvar, professor of biomedical engineering.
Accordingly, Kheradvar suggests more careful follow-up after TAVR, and if needed, early therapeutic interventions that may include PVL repair. Kheradvar also has published a new book, Principles of Heart Valve Engineering, available through Academic Press, a division of global publisher Elsevier.
Directed at biomedical engineers, cardiologists, cardiothoracic surgeons, academics and other scientists and engineers, the book focuses on the current state of the art in heart valve science and engineering, therapies, and pathways to developing safer and more durable heart valve devices in the future. It is an interdisciplinary, comprehensive resource for heart valve engineering.
According to Kheradvar, who is also a medical doctor, since the inception of heart valve engineering in 1960s, the field lacked an all-inclusive resource of up-to-date heart valve research and development efforts. He compiled a group of internationally known researchers in the field from more than 15 institutions, including bioengineers and cardiologists, to contribute. The result is a comprehensive textbook covering a wide range of topics, including heart valve anatomy, embryology, mechanobiology, epidemiology, surgery, tissue engineering, computer modeling and more.

BIOMEDICAL ENGINEERS DEVELOP WEARABLE RESPIRATION MONITOR WITH CHILDREN’S TOY
Samueli School biomedical engineers have developed a wearable, disposable respiration monitor that provides high-fidelity readings on a continuous basis. Designed to help children with asthma and cystic fibrosis and those with chronic pulmonary conditions, the inexpensively produced sensors were created using the popular children’s toy Shrinky Dinks, thin sheets of plastic that are painted or drawn on and then shrunk with heat.
Placed in two positions – one between the ninth and 10th ribs and another on the abdomen – the Band-Aid-like devices track the rate and volume of the wearer’s respiration by measuring the local strain on the application areas. The information gleaned could, in the case of asthma, help warn of an oncoming attack.
The devices are made by applying a very thin layer of metal to a sheet of the plastic toy and then heatshrinking it to cause corrugation. The film is then transferred to a soft, stretchy material – similar to a small bandage – that can be adhered to a patient. Signals from embedded sensors can be transmitted via Bluetooth to be displayed on a smartphone app.
The lab of Michelle Khine, where the devices were developed, is well-known for employing Shrinky Dinks as a platform for medical applications. About a decade ago, Khine innovated the use of the toy to produce microfluidic devices. “It’s amazing that this toy for kids has enabled us to create these robust sensors that may one day benefit children and others around the world,” she said.
The new technology has been tested on healthy subjects, and plans are underway for a pilot trial with a small number of asthma sufferers.
LIU CO-AUTHORS GUIDE FOR FEDERAL INVESTING IN SYNTHETIC BIOLOGY RESEARCH
Chang Liu is among a group of more than 80 scientists and engineers from 30 universities and a dozen companies who released a road map to guide and encourage government agencies to invest effectively in engineering and synthetic biology research endeavors.
Liu, assistant professor of biomedical engineering, is lead author of the document’s biomolecular engineering section. The Engineering Biology Research Consortium, partially funded by the National Science Foundation and headquartered at UC Berkeley, produced the treatise to not only improve public health, food crops and the environment, but also fuel the economy and maintain U.S. leadership in synthetic biology. Current efforts in the field include genetic modification of crops; microbe engineering to produce drugs, fragrances and biofuels; gene editing and human gene therapy. But the future will bring even more complex applications. The document’s authors outline synthetic biology’s challenges and opportunities to help decision-makers determine whether to make it a research priority for the U.S.
“The road map is backed by rich technical analysis and projections to focus our nation’s goals in engineering biology in a concrete manner,” Liu said. “I was impressed by the open and inclusive process that informed this project. We actively sought and incorporated the contributions, expertise and ideas of scores of researchers in the greater synthetic biology community, including postdocs and graduate student trainees who will define the future of our field.”
HAUN RECEIVES PILOT STUDIES FUNDING
Jered Haun received a 2019 Pilot Studies Award from the UCI Institute for Clinical and Translational Science. The $25,000 award supports Haun’s efforts to develop a microfluidic device platform for processing human fat for autologous therapies.
“Fat tissue contains a large number of stem cells that could readily be collected and used to heal wounds and treat diseases,” said Haun, assistant professor of biomedical engineering. “Funds from this award will help us develop and test new microfluidic device components that will make it possible to fully automate the processing of fat into a stem-cell-based therapeutic, paving the way to clinical applications.”
The ICTS Pilot Studies Award is designed specifically to support exceptionally innovative and/or unconventional research projects that have the potential to create or overturn fundamental paradigms.








