
20 minute read
How Acute Care Surgery Team Managed the COVID-19 Surge
By VICTORIA STERN
When COVID-19 began to surge in the United States in March 2020, hospitals had to act quickly. The acute care surgery (ACS) team at Tufts Medical Center, in Boston, was no exception.
“We felt a sense of urgency to prepare our hospital for an influx of critically ill patients,” said Horacio M. Hojman, MD, the chief of the Division of Trauma and Acute Care Surgery and surgical director of the Surgical Intensive Care Unit at Tufts University School of Medicine. “As trauma surgeons, we see emergencies every day and are trained to come up with a plan fast.”
Despite limited information about the coronavirus, the four-surgeon team quickly pivoted their normal operations to meet the moment. First, the surgeons devised a system to separate COVID-19‒positive and uninfected patients into two surgical ICUs, and to provide around-the-clock coverage of trauma, emergency general surgery and surgical critical care patients, leaving a handful of surgeons in reserve in case anyone got sick.
“Many of those systems were created on the spot by extrapolating from our disaster preparedness plan deployed during the Boston Marathon bombing, military training, and the experiences our colleagues in Italy and China had with COVID19 even earlier in the pandemic,” said Nikolay Bugaev, MD, the executive director of research in the Department of Surgery and an assistant professor of surgery at Tufts University School of Medicine. “We saw our colleagues across the country and the world facing a similar challenge, and decided to document our experience to understand how well our disaster planning worked.”
In late November, Drs. Hojman and Bugaev, and their colleagues published a review comparing patient demographics, needs and outcomes two months before and after the March 2020 COVID-19 surge to assess their system and provide a guide for others (Am Surg 2020;86[12]:1629-1635). The authors found that during the initial surge months, trauma and emergency general surgery volumes at Tufts dropped by about 51% overall, while critically ill patient volumes increased by 64%.
Between March and May, the needs of patients admitted to the ICU also shifted. Overall, the majority of people admitted to the surgical ICU were not surgical patients. The surgeons tracked the types of procedures performed and reported that, in the months before COVID-19 hit, emergent laparotomies were the most common procedure encountered by the ACS team (35%), but during the surge that shifted to tracheostomies and percutaneous endoscopic gastrostomies (36%).
To protect clinicians from aerosolized particles during tracheostomies, the surgeons created a localized negative-pressure environment by overlaying the top of the bed with a plastic, nonsterile sheet and connecting an air filter (Am Surg 2020 Dec 7).
Of note, the surgeons found that hospital mortality did not differ significantly between the pre‒COVID-19 and surge groups (9.6% vs. 13.4%, respectively; P=0.4) or between critically ill COVID-19 patients treated by ACS and non-ACS ICUs during the surge (13.4% vs. 13.5%, respectively).
“I was pleased at our great results,” Dr. Bugaev said. “The ACS service was able to apply general emergency concepts and escalate them rapidly in order to continue providing trauma and emergent surgical services during the surge, while also delivering intensive medical care to nonsurgical patients.”
Six weeks into the new system, the influx of COVID-19‒positive patients began to plateau and the ACS service gradually deescalated its disaster plan. With this experience, Drs. Hojman and Bugaev now feel prepared if there is a next time.
“After creating and implementing this self-sustaining emergency plan, I think we’re better equipped to handle an emergency of this magnitude,” Dr. Hojman said. “We were lucky that our expertise managing disaster scenarios could be put to good use during the pandemic.” ■
DO YOUR PATIENTS HAVE MESH HESITANCY?
With all the recent publicity surrounding synthetic mesh, is it any wonder that more and more hernia patients are in search of alternatives? OviTex® Reinforced Tissue Matrix can help address these concerns by providing your patients a More Natural Hernia Repair™. To learn more about OviTex’s proven clinical performance and how it can help address mesh hesitancy, visit our website at v.TELABIO.com/GoNatural, or scan the QR code below.
Natural is the new normal.
www.telabio.com
THE SCIENCE BEHIND POSITIVE PATIENT OUTCOMES
Tri-Staple™ 2.0 Reinforced Reloads: Preloaded Buttressing for Bariatric Procedures
Alfonso Torquati, MD, MSCI
Bariatric Surgeon Rush University Medical Group Chicago, Illinois
Advances in Staple Line Reinforcement
Staple line buttressing is increasingly recognized as a standard of care for an array of thoracic, abdominal, and gastrointestinal resections.1-3 In fact, the majority of bariatric surgeons were using buttressing for sleeve gastrectomies by 2013,3 and multiple bariatric surgery studies associate staple line buttressing with a reduction in complications, particularly bleeding.4,5 Over the past several years, device manufacturers have advanced the materials and device design to optimize strength and seal, and improve procedural efficiency.6 The incorporation of 3 staple lines and preloaded buttressing material are among the most significant of these advances.7
Alfonso Torquati, MD, MSCI, a bariatric surgeon at Rush University Medical Group, in Chicago, Illinois, reinforces the staple line in gastric bypass, sleeve gastrectomy, and revision procedures. Dr Torquati has witnessed the evolution of the technology throughout his career, and although he has extensive experience with numerous stapling devices, he has used the Tri-Staple™ 2.0 reinforced reload (Figure 1) almost exclusively from the time of its introduction and for every case. “When I joined the practice at Rush, we were buttressing with both of the staplers we had available, but it wasn’t long before all the bariatric surgeons switched to the Tri-Staple™ device,” Dr Torquati said.
Efficiency
Tri-Staple™ reinforced reloads are the only reloads that come with prefixed buttressing, which speeds operating time (Figure 2). In fact, a survey of 250 surgeons and nurses found that using a preloaded buttress saved 7 to 10 minutes compared to a separately loaded buttress.8,a According to Dr Torquati, several devices incorporate methods to facilitate loading of the buttressing material, but additional steps can introduce chances for error. “Our OR scrub technicians are much happier with the Tri-Staple™ reinforced reloads. They no longer spend time pulling strings or taking the other required steps to ensure proper alignment,” he said. “It means more efficiency during the procedure.”8,a
The Technology
Reload Size
Tri-Staple™ 2.0 reinforced reloads provide the benefit of individualized color-coded staple loads. The black cartridge is appropriate for extra thick tissue, and has staple heights ranging from 4.0 mm in the innermost row to 4.5 and 5.0 mm in the middle and outer rows, respectively. The purple cartridge is designed for medium/thick tissue and provides staple heights of 3.0, 3.5, and 4.0 mm. Both black and purple cartridges are available in 45 and 60 mm lengths. Dr Torquati says he starts with the black loads in most patients and, as appropriate, moves to purple loads for thinner tissue. Occasionally in thick tissue, he will fire 2 staple lines from black cartridges.
Dr Torquati thinks the ability to adjust to tissue thickness represents an important advance in initial bariatric surgeries as well as revisions, which represent a significant proportion of his cases. He typically uses the black reload when tissue is thickened by inflammation to ensure adequate reinforcement. “Different loads allow me to adjust the closure to the patient’s anatomy, while the variable height adds strength without impairing the vascularization needed for healing,” Dr Torquati said.9,10,b-d

Figure 1. Tri-Staple™ 2.0 reinforced reloads.
The Material
Relative to composite materials, the polyglycolic acid buttressing material used in the Tri-Staple™ 2.0 reinforced reload device was developed with small uniformly packed fibers, resulting in less material mass at implantation with greater strength, 9,b,c and according to Dr Torquati, this represents an important advance. “This current material is flexible. It is stable but resorbs readily,” he said. “In my experience, it is very well tolerated.” Dr Torquati estimates that extra reinforcement, such as oversewing, is required in fewer than 1 in 20 of his cases. “An example might be very thick tissue close to the duodenum,” he said. “We might also consider a clip for larger vessels when we see oozing, but these are uncommon situations.” He added: “The combination of the buttressing material and the 3 rows of staples means it is rarely necessary to use additional reinforcement.”
Reduced Bleeding11,e
The strength of the closure with Tri-Staple™ 2.0 reinforced reloads is reflected in a “solid” quality at the staple line, according to Dr Torquati. “When attempting to move tissue following closure, there was always a risk of bleeding in the absence of reinforcement. With Tri-Staple™ closures, there is less concern about pulling the tissue in order to move it into view,” he said. “The reduction in intraoperative bleeding provides better visualization of the surgical field.11,f Less bleeding means less time clearing the field. Better visualization as a result of less bleeding allows me to
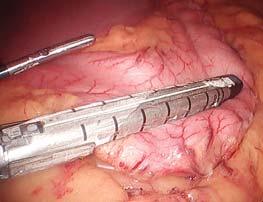
Figure 3. Tri-Staple™ 2.0 reinforced reloads with the smart Signia™ stapler.
ascertain during the procedure whether I am achieving a good staple formation.”
Research indicates that buttressing (vs not buttressing) the staple line in bariatric surgery is associated with a 30% reduction in postoperative bleeding, 12 and Dr Torquati has found that his bleeding rate is at least as low as what has been reported in the literature since adopting the Tri-Staple™ 2.0 reinforced reloads. His leak rate is even lower.
“Leaks can be a more serious complication than bleeding, but they are rare,” Dr Torquati said. In fact, he has had none in his series and attributes this absence of leaks to his reliance on staple line reinforcement. “We perform an endoscopy after every procedure to confirm the quality of the closure within the lumen of the stomach. We have consistently seen good hemostasis. I have not yet needed to perform additional steps on the basis of the endoscopic view,” he said.
Smart Technology
The Tri-Staple™ 2.0 reinforced reload has a stepped cartridge face that provides gradual compression across tissue types. Many bariatric surgeons use the reload with the Signia™ stapler, which automatically adjusts speed to improve staple formation when forces increase in challenging applications (Figure 3). 13,14,g Dr Torquati prefers to staple without smart technology when demonstrating the fundamentals of closure to his fellows, but he is quick to recognize the advantages of using the technologies together. The Signia™ stapler is fully powered, and he likes the audible feedback and variable speed functions it offers. “With thicker tissue, it is helpful to close at a slower pace,” he said. Manufacturer studies have found that adjusting the speed of firing in different tissue thicknesses can reduce the percentage of malformed staples.13-15,g Dr Torquati is also enthusiastic about the advantages of smart stapler technology for less experienced surgeons. “It’s important to select the appropriate staple size. Staplers that help surgeons assess tissue thickness will be a big help for those developing skill in bariatric surgery,” he said.16,c
Conclusion
The progress in stapling technology for bariatric surgery has substantially reduced patient complications and improved the surgeon’s experience.6-8,12,a Dr Torquati believes the Tri-Staple™ 2.0 reinforced reload features create procedural efficiencies, a provide adequate strength, g,h and permit the blood supply necessary for healing.8,10,17,d For him, strength at the closure line means less intraoperative bleeding and fewer postoperative complications.11,18,f Dr Torquati thus relies on the Tri-Staple™ 2.0 reinforced reload for weight loss surgery.
a Compared with an aftermarket buttress. Results based on a sample of 125 OR nurses and 125 surgeons. b In vitro tensile strength compared with Seamguard™ at days 7 and 14.
c Bench test results may not necessarily be indicative of clinical performance. d Compared with flat-faced cartridges with single-height staples.
e Compared with nonbuttressed reloads. f Based on international consensus of an expert panel of bariatric surgeons.
g Preclinical results may not correlate with clinical performance in humans. h Based on a benchtop comparison at 0 and 7 days in vivo (2 sample t-test: P=0.000 and 0.0007).
References
1. Deguchi H, Tomoyasu M, Shigeeda W, et al.
Reduction of air leakage using linear staple device with bioabsorbable polyglycolic acid felt for pulmonary lobectomy. Gen Thorac Cardiovasc Surg. 2019;68(3):266-272.
2. Shigeeda W, Deguchi H, Tomoyasu M, et al. The utility of the stapler with PGA sheet for pulmonary wedge resection: a propensity score-matched analysis.
J Thorac Dis. 2019;11(4):1546-1553.
3. Gagner M, Deitel M, Erickson AL, et al. Survey on laparoscopic sleeve gastrectomy (LSG) at the
Fourth International Consensus Summit on Sleeve
Gastrectomy. Obes Surg. 2013;23(12):2013-2017. 4. Gill RS, Switzer N, Driedger M, et al. Laparoscopic sleeve gastrectomy with staple line buttress reinforcement in 116 consecutive morbidly obese patients. Obes Surg. 2012;22(4):560-564.
5. Shikora SA, Mahoney CB. Clinical benefit of staple line reinforcement in gastrointestinal surgery: a meta-analysis. Obes Surg. 2015;25(7):1133-1141. 6. Chekan E, Whelan RL. Surgical stapling device—tissue interactions: what surgeons need to know to improve patient outcomes. Med Devices (Auckl). 2014;7:305-318. 7. Moussaoui IE, Limbga A, Mehdi A. Staple line reinforcement during sleeve gastrectomy with a new type of reinforced stapler. Minerva Chir. 2018;73(2):127-132.
8. Data on file. Medtronic. ORC International. Survey of tissue reinforcement users to determine waste and time loss attributed to separately loaded buttress materials in the OR. Online U.S. national sample of 125 surgeons and 125 OR nurses. November 9, 2011. 9. Data on file. Medtronic. Internal report #RE00240873.
Mass, thickness, area measurements, pH measurements, and glycolic acid released for polyglycolic acid buttress (PGA) and Seamguard™ buttresses. [Reinforcement buttress material tested using 2-sample t-tests. Data calculated with 95% confidence using Minitab™ (P<0.0001)]. December 2, 2019.
10. Data on file. Medtronic. Internal test report #2128-002-2. Final analysis of staple-line vascularity using MicroCT. July 2015. 11. Rosenthal RJ, Diaz AA, Arvidsson D, et al. International
Sleeve Gastrectomy Expert Panel consensus statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8(1):8-19. 12. Zafar SN, Felton J, Miller K, et al. Staple line treatment and bleeding after laparoscopic sleeve gastrectomy.
JSLS. 2018;22(4):e2018.00056. doi: 10.4293/
JSLS.2018.00056
13. Data on file. Medtronic. Internal test report #R2146-173-0. ASA verification testing with slow speed force limit evaluation. 2015.
14. Data on file. Medtronic. Internal test report #R2146-151-0. Powered stapling firing speed DOE analysis and ASA parameters. 2015. 15. Data on file. Medtronic. Internal test report #RE00218740. Signia™ stapling adaptive firing technology data calculations and references.
August 7, 2019. 16. Data on file. Medtronic. Internal test report #RE00055515. Surgeon evaluation testing Signia™ stapling system sensing technology and real-time feedback. August 4, 2016. 17. Data on file. Medtronic. Internal test report #RE00088418. In-vitro tensile strength comparison.
April 11, 2017. 18. Nagahisa Y, Morikawa A, Kato T, et al. Feasibility of Endo GIA™ reinforced reload with Tri-Staple™
Technology for delta-shaped anastomosis.
Asian J Surg. 2018;41(5):448-453.
Disclosure: Dr Torquati reported receiving honoraria from Medtronic.
Disclaimer: This is one physician’s experience, results, and recommendations, so results may vary. Please see the package insert for the complete list of indications, warnings, precautions, and other important medical information.
On Learning to Land

continued from page 1


I fly a Cessna 172, a single-engine plane that can seat four adults uncomfortably, with my flight instructor, Brian, who is in his mid-30s, tall and bald by choice. He used to be a high school football coach but is built more like a basketball player. He’s unassuming, mildmannered and not shy. In a recent lesson, he decided it was time for me to practice landings. We flew to a small airfield outside of Nashville, Tenn., where traffic is sparse, and the radio communication requirements are minimal. We arrived with a plan to practice “touch and go’s” all day: entering the traffic pattern, landing on the field, then quickly taking off again, and repeating. The weather was nice, the winds were smooth, and the airspace was empty. But despite these near-perfect conditions, I struggled to land safely. While Brian didn’t say anything, I could feel he was adjusting the flight controls as I made my approaches so we would land safely. After my sixth attempt with the same bumpy result, I was frustrated and surprised that Brian hadn’t said anything up to that point. So, I finally just asked him: What am I doing wrong here? All he said was, “I think you want the plane on the ground too badly.”
One of the more interesting ethical questions that surgical training presents stems from the perception of tension between patient safety and effective surgical training. If developing surgical skills requires the novice to develop those skills while caring for a patient, how do we protect the patient from the risk inherent from a novice’s skills?
When I was a resident, I felt the effects of this tension much differently from what I do now as a surgeon recently accused of being mid-career. This makes sense, too: As a trainee, my goal was to evolve from a novice to an expert. I wanted my future patient to be cared for by the kind of surgeon every patient has a right to see: a competent surgeon. I think it’s clear, at least in my experience, that the trainee bears a great deal of the moral weight that arises from thinking about the future patients they will expose to the knife. But does the training surgeon bear some of this weight, too? I think I’ve always considered my duty as surgical educator as a duty to the trainee—the surgical resident. But where does that duty really get its moral power? If not from the future patient, then where? And if that’s the case, are the ethics that shape my clinical practice as a surgeon formed not just by the obvious obligation I have to the patient on the operating room table today, but also that future patient who will be on this trainee’s table someday, too?
The funny thing about landing a plane, in my experience, is that it takes a completely different mindset from any other aspect of flying. It’s a balance of controlling the orientation of the aircraft while making fine adjustments in the throttle so you start falling. It feels, at least to me, like you’re trying to convince the airplane it’s still flying while making subtle adjustments that bring it closer to the earth without making you feel like you’re falling from the sky. You have to get this thing that is designed to fly—it wants to go up—and get it to go down. It’s not an obvious skill to develop. Part of what makes it less than obvious is because the best way to land is as much about what you don’t do as it is about what you do. As challenging as it is to learn, my instructor’s challenge is as acute a dilemma as I can imagine. He has to let me learn enough that I can eventually do this on my own, which means he has to let me do some of it on my own. In essence, he has to allow me to make as many errors as necessary for me to learn, while keeping me from killing both of us. There is a famous thought experiment that ethicists, like me, love to invoke





L S
I





whenever possible. It’s called the “Trolley Problem.” Originally described by the philosopher Judith Jarvis in 1967, the trolley problem essentially goes like this: Imagine a train track that splits into two directions. In one direction, there is a single person tied to the tracks, and in the other direction, there are five people tied to the tracks. Imagine also that there is a trolley that is barreling down the main track and is heading directly toward the five people. Now imagine you are standing next to a lever, that if pulled will redirect the trolley away from the five people on the track and toward the track with the one person tied there. There is no way to communicate with the trolley to stop. What would you do?
The trolley problem is a perfect analogy to surgical training as long as we consider patient safety as antagonistic to effective surgical training. As we shelter patients from surgical trainees in the name of patient safety, we put at risk all the future patients they will treat as a surgeon in the future. We’ve seen the effects of this over the course of years. Ever since Mike Wallace’s “60 Minutes” program exposed the realities of surgical training, the resulting oversight and subsequent constraints on surgical trainees have been significant. But so has the effect on producing competent general surgeons. In one study of recent graduates, 38% could not perform a laparoscopic cholecystectomy by themselves. What do you call a general surgeon who can’t do a lap chole? I don’t know, either.
But what if we’re thinking about this incorrectly? When I’m flying with Brian, the safest way to land the plane is to let Brian do it. But then how do I reach the part where it’s safe to send me up there on my own? At some point, every flight instructor must make a call about whether their student can solo: the process of stepping out of the airplane and letting their student take off and land by themselves. Usually, the instructor gets out of the cockpit and waits on the tarmac with a handheld radio and watches the whole thing take place. Instructors take this part of their job with incredible earnestness. A failure of effective training or judgment is a failure of the highest order. And the ownership and moral responsibility for that failure is clear and immediate in-flight instruction. Is surgical training any different?
For whatever reason, when Brian told me, “I think you want the plane on the ground too badly,” something clicked. I knew what I needed to do to get the plane safely on the ground, and as I entered the final leg of the traffic pattern, I lined up on the center line, I adjusted the power to let the plane slowly lose altitude, and I adjusted the nose of the airplane to bring us down near the numbers. Then we got to a few feet off the ground, I brought the throttle back to idle, and as we floated down the runway, I resisted the urge to push the nose down, and instead settled in and just let the plane come down on its own. It was as smooth a landing as I had ever experienced in a small plane. Brian had kept his hands and feet off the controls completely. All he said was, “You can fly on your own if you fly like that.”
The truth is the surgeon’s obligation is to the patient on the table in front of them today and that obligation has a weight of almost religious proportions, established in tradition and culture and history. I would argue, though, that we haven’t considered enough the ways in which that obligation extends to the future patients of our current surgical residents. Sometimes we conflate a responsibility to our trainees when what we really mean is a moral obligation to their future patients. Thinking about my ethical duties in this way has changed my perception of the moral duty of this role. It’s not enough to become the best surgeon; the moral duty isn’t honored until we consider the ways in which we are caring for the future patient, too.









