Schrödinger &
MCPHS Biopharmaceutical Industry Fellowship Program Fe Pr
(2025 – 2027) Industry



(2025 – 2027) Industry


Our mission at Schrödinger is to improve human health and quality of life by transforming the way therapeutics and materials are discovered.
At Schrödinger, we are united by a common goal — to change the world through scientific advancement.

• With over 800 employees across more than a dozen offices, Schrödinger’s footprint spans the globe. Our team brings diverse perspectives, experiences, and backgrounds, united by a shared purpose.
• We are driven to be the world leader in transforming drug discovery and materials design by relentlessly pursuing scientific and technology breakthroughs.
• We are committed to achieving the best possible outcomes for our customers, partners, patients, and other stakeholders.
• We deeply value our dedicated employees and invest in their growth, development, and well-being. We help and support each other, generously and with compassion.
• We pursue a diverse, equitable, and inclusive workplace where teamwork and collaboration are valued, and open, respectful debate is welcome and encouraged.
• We strive to do the right thing, applying the highest ethical standards to our work and always considering how our actions impact individuals and communities who depend on us.


For more than 30 years, we have invested in advancing the science underlying our computational platform. As our technology advanced, our company evolved along with it.
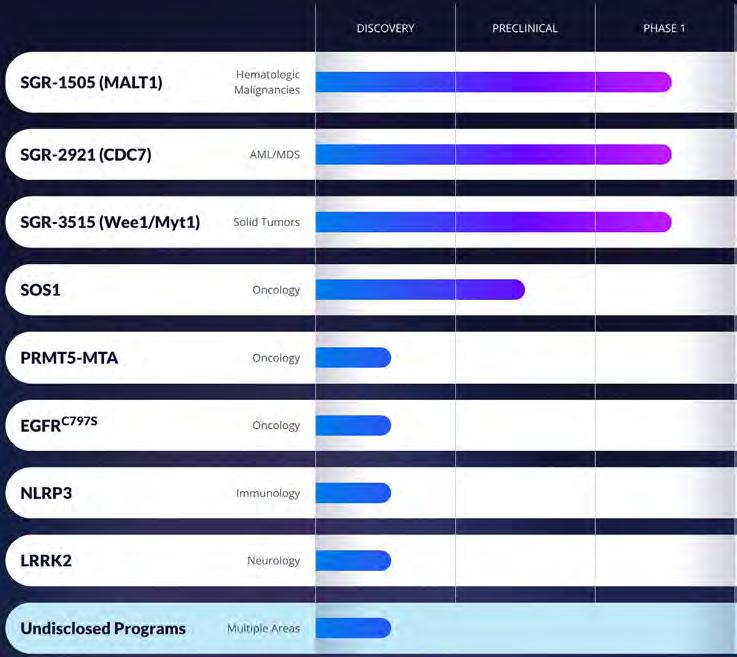
Recognizing the opportunity to leverage the full power of our platform to drive internal discovery of molecules and clinical candidates for unmet medical needs, we formed Schrödinger’s therapeutics group.

In collaboration with MCPHS, we are excited to introduce a two-year PharmD post-doctoral fellowship program specializing in the clinical development of First-In-Human Phase I investigational agents. This unique program offers you extensive, hands-on experience across various functional areas in Phase I drug development, with a focus on clinical science and operations. You’ll also gain insights into the study of adult hematologic and solid tumors, clinical pharmacology, translational science, toxicology, drug manufacturing, and regulatory affairs.
In Clinical Development, we are committed to advancing best-in-class compounds through clinical proof of concept. Our close-knit, cross-functional team thrives in a biotech setting, promoting agile collaboration across departments. As part of Schrödinger’s therapeutics group (STG), we play a crucial role in a multidisciplinary drug discovery ecosystem. By leveraging our proprietary physics-based drug design platform, we accelerate the transition of assets from discovery to the clinic, providing a dynamic, fast-paced, and strategically engaged environment perfect for fellows who are seeking diverse and impactful experiences in drug development.
With over 30 years of commitment to research & development, we are well-equipped to support educational collaborations, including our new partnership with MCPHS.
Year 1: Foundation Building
• Industry Overview: Develop a broad and comprehensive understanding of the pharmaceutical industry’s key functional areas. Develop knowledge of Good Clinical Practice (GCP) and International Council on Harmonization (ICH) guidelines.
• Clinical Knowledge Expansion: Engage and hands-on experience and learnings of clinical trial management. Leverage clinical and scientific expertise to strategically support the design and execution of early-phase clinical studies in selected adult hematology and solid tumor indications.
• Cross-Functional Collaboration: Work with cross-functional teams to gain insights into various industry sectors.
Year 2: Specialization and Leadership
• Specialized Training: Focus on a particular functional area of interest within a therapeutic area of interest, such as clinical science, clinical operations, clinical pharmacology, regulatory affairs, etc.
• Project Leadership: Take on leadership roles in special projects to develop management and decision-making skills. Training on strengthening presentation skills.

• Career Readiness: Build a robust professional network of various functional and clinical colleagues and build a solid skill set of knowledge and competencies for future employment opportunities.

Margaret Dugan, MD Chief Medical Officer
Margaret Dugan is the Chief Medical Officer at Schrödinger where she oversees clinical development and regulatory strategy for the company’s expanding pipeline of programs. A board-certified medical oncologist and hematologist with over 30 years of experience, Margaret Dugan served as Chief Medical Officer at Dracen Pharmaceuticals and held senior leadership roles at Novartis, where she led global oncology drug development, securing multiple regulatory approvals during her 20-year tenure. Her commitment to nurturing future clinical leaders is evident in her role as a preceptor, where she guided numerous classes of fellows.

Allison Upalawanna, PharmD
Vice President of Global Clinical Operations & Strategy
Allison Upalawanna is the Vice President of Global Clinical Operations & Strategy at Schrödinger, where she plays a pivotal role in advancing the company’s therapeutic assets. She brings extensive experience from leading oncology clinical trials across both big pharma and biotech, including first-in-human to registration studies. Before joining Schrödinger, Allison served as Vice President at Innovent Biologics, managing clinical operations, data management, and monitoring. At Novartis, she led strategic transformations in Oncology Global Development, managed several oncology clinical programs and studies, and precepted post-doctoral fellows in Clinical Development, Strategy & Operations. Allison holds a Doctor of Pharmacy from UNC, a Bachelor of Science in Biochemistry from NC State, and completed her Post-Doctoral Fellowship at Rutgers University.


Francois Lafleur, MPH
Senior Director of Clinical Operations
Francois Lafleur is the Senior Director of Clinical Operations at Schrödinger, bringing a distinguished background and experience in medical affairs, clinical research and operations from 9 pharmaceutical companies. While serving as Head of US Medical Affairs at Pfizer, his team was able to initiate and secure a two-year award for the PharmD Fellowship program sponsored by Rutgers University. This program, alternating annually between hematology and oncology, successfully exposed the fellows to the various functional areas involved in medical affairs, and eventually resulted in placement of fellows in desired roles at leading pharmaceutical companies.
Michele Corrado MS, RPh
Director of Clinical Operations
Michele is the Director of Clinical Operations within the Clinical Development team at Schrödinger, supporting clinical trials in leukemia and lymphoma. She has worked in the pharmaceutical industry for more than 20 years, with past roles supporting late-phase registration trials across numerous therapeutic areas, including hematology, neurology, ophthalmology, and transplantation, as well as clinical trial supplies management. She has also served as a preceptor to undergraduate interns and pharmacy residents. Prior to switching careers to the pharmaceutical industry, Michele was a clinical pharmacist at a large teaching hospital in New York City.
The Massachusetts College of Pharmacy and Health Sciences (MCPHS) provides an academic environment to guide and support Fellows toward a successful career in the biopharmaceutical industry. As a private institution with a history of specialization in health sciences, MCPHS offers programs that embody scholarship, professional service, and community outreach. Through MCPHS, the Fellow will have the opportunity to gain teaching and research experience in an academic setting. MCPHS faculty and company program leaders mentor fellows according to scholarly and professional interests throughout the two-year program.
As an adjunct instructor at MCPHS, the fellow may have the opportunity to:
• Develop, coordinate, and teach courses
• Co-precept pharmacy students on advanced experiential rotations
• Create and publish scholarly research and/or review articles
• Present research at scientific and clinical meetings
• Participate in professional development seminars

Amee Mistry, PharmD, RPh
Director of the Postdoctoral Biopharmaceutical Industry Fellowship Program
Dr. Amee Mistry is Professor of Pharmacy Practice and has been with MCPHS since 2006. Dr. Mistry earned her PharmD at the Albany College of Pharmacy and completed a PGY1 Community Practice Residency with Walgreens and MCPHS. In 2015, Dr. Mistry took over as Director of the MCPHS Biopharmaceutical Industry Fellowship program. She works directly with leaders in the area to continue to foster growth and development of the post-graduate program, and to assist the fellows in attaining positions within the pharmaceutical industry.
In addition, she is advisor for the student IPhO chapter at MCPHS, co-advisor for APhA-ASP, a national trainer for the APhA Pharmacy-Based Immunization training program and is actively involved with the Massachusetts Pharmacists Association.
Other essential members of the MCPHS team include Samantha Nganju and Tara Miskell, who provide crucial support to Amee in the effective execution and coordination of the fellowship program.

Samantha Nganju, MBA Fellowship Program Manager

Tara Miskell Fellowship Program Coordinator
The MCPHS Biopharmaceutical Industry fellows will be selected on a nationally competitive basis. Applicants must have a Doctor of Pharmacy degree from an ACPEaccredited college of pharmacy at the commencement of the program.
• Candidates must have strong written and verbal communication skills and a strong interest in pursuing a career within the biopharmaceutical industry.
• All candidates must have authorization to work in the United States throughout the duration of the oneor two-year fellowship. No visa sponsorship will be provided (i.e., TN, H-1B, STEM OPT, etc.).
The MCPHS application portal (SMApply) will open on Monday October 7, 2024. Applicants must upload the following application materials to the online portal, (mcphs.smapply.io) by Monday November 4, 2024:
• Letter of intent
• Curriculum vitae
• Unofficial college transcript
• Contact information for three references. References will receive an electronic recommendation form to complete separately
Three recommendation evaluation forms must be submitted no later than November 20, 2024 via the online portal. This is NOT a letter of recommendation but an online form that the recommender will receive for completion from SMApply.
Following a review of submitted applications, prescreens and preliminary interviews will begin in October. Additional interviews, including final rounds, will take place in December during ASHP. Candidates will be notified if selected for an interview. The process is rigorous and competitive; therefore candidates should submit their applications well in advance of posted deadlines as priority will be given to those who apply early.

The fellowship program will be conducting in-person interviews at the ASHP Midyear Clinical Meeting in New Orleans, LA. Applicants are strongly encouraged to attend, but it is not required. Candidates attending in-person will not be able to interview without registering for both ASHP and PPS. Please refer to the ASHP & PPS website for registration details.
Top candidates may be invited for interviews at the sponsoring company’s location.
The choice of a Postdoctoral Fellowship is an important decision. MCPHS, in conjunction with the Alliance of Industry Fellowship Associates (AIFA), has aligned to extend offers for Fellowships no earlier than December 16th, 2024. We believe this is a positive reflection of the cultures our Programs offer and that culture is a critical consideration in choice of Fellowship.
We hope that other academic and non-academic Fellowship Programs will NOT pressure candidates to accept offers prior to this aligned offer date.
Final candidates will be required to go through additional screening / onboarding as required by MCPHS.
