EUROPE AN EDITION
MEDICAL PLASTICS news NEW ASSEMBLY METHODS FOR FILTER CARTRIDGES TIPS FOR DESIGNING DEVICES WITH POROUS PLASTICS NEW CHALLENGES FOR MANUFACTURERS SUBMITTING IN THE EU
CAPITALISING ON LOCAL MANUFACTURING
OPPORTUNITIES
Integrated moulding systems deliver major benefits ISSUE 63
November - December 2021
WWW.MEDICALPLASTICSNEWS.COM
You expect precision. We deliver. contract manufacturing injection moulding medical devices Our manufacturing includes cleanroom environments, automation, and assembly services, delivering total value solutions that reduce investment and improve cost.
www.carclo-ctp.co.uk +44 208 685 0500 sales@carclo-usa.com United States
• United Kingdom
• Czech Republic • India
• China
CONTENTS Nov/Dec 2021, Issue 63
Regulars 5 Comment Corrine Lawrence refl ects on 2021. 6 Digital spy 12 Cover story As new opportunities emerge, medical injection moulding can turn to local production and integrated systems says Thomas Bontempi of Husky. 30 11:2021
Features 9 Tubing, catheters & stents: Moving beyond convention Mike Winterling of Junkosha takes us through the latest developments in the company’s PHST portfolio.
14 Biocompatibility: ISO 10993-18:2020’s new challenges for manufacturers submitting in the EU WuXi AppTec advises how medical device manufacturers can best navigate the recent updates of ISO 10993-18:2020. 18 Designing medical devices: The extended wear challenge Miguel Solivan of Avery Dennison Medical discusses wearable medical device growth and design considerations to enable longer wear times. 23 3D printing: Making the impossible possible Arburg’s Lukas Pawelczyk explains how the APF process with the freeformer is particularly suitable for AM in medical technology. 26 Components & assembly: New assembly methods for PP dialyser filter cartridges Didier Perret of Branson Welding and Assembly at Emerson evaluates new assembly methods that best suit alternative materials.
WWW.MEDICALPLASTICSNEWS.COM
3
It’s nice when new life can rely on CYROLITE®: our BPA- and DEHP-free high-
BPA- and DEHP-free: CYROLITE® acrylics
have just what it takes to save lives.
performance acrylics are safe to use in medical devices, especially in prenatal and neonatal applications. Additionally, CYROLITE® can be reliably sterilized and is resistant to medical fluids and disinfectants. This has impressed newborns and healthcare professionals alike: CYROLITE® easily meets the requirements of USP Class VI, ISO 10993-1, and REACH. We give you all the reasons you need at www.cyrolite.com.
editorial content producer | corrine lawrence corrine.lawrence@rapidnews.com advertising | caroline jackson caroline.jackson@rapidnews.com
Editor’s Comment
vp sales & sales talent | julie balmforth julie.balmforth@rapidnews.com
C O R R I N E L AW R E N C E
head of studio & production | sam hamlyn graphic designer | matt clarke publisher | duncan wood Medical Plastics News Europe Print Subscription – Qualifying Criteria UK & Europe – Free US/Canada – £249 ROW – £249 Medical Plastics News NA Print Subscription – Qualifying Criteria US/Canada – Free UK & Europe – £249 ROW – £249 FREE on iOS and Android devices Subscription enquiries to subscriptions@rapidnews.com Medical Plastics News is published by: Rapid Life Sciences Ltd, Carlton House, Sandpiper Way, Chester Business Park, Chester, CH4 9QE T: +44(0)1244 680222 F: +44(0)1244 671074 © 2021 Rapid Life Sciences Ltd While every attempt has been made to ensure that the information contained within this publication is accurate the publisher accepts no liability for information published in error, or for views expressed. All rights for Medical Plastics News are reserved. Reproduction in whole or in part without prior written permission from the publisher is strictly prohibited.
BPA Worldwide Membership ISSN No: 2047 - 4741 (Print) 2047 - 475X (Digital)
2
A SHOT IN THE ARM
020 was a year many of us would care to forget for obvious reasons. It was a year during which many businesses found themselves digging deep to come up with workable solutions to satisfy financial and supply chain challenges ... because there was no official road map to recovery. The medical industry however, along with countless others, became adept to working differently. Quite simply, it had to. But it was far from ideal. Face-toface interactions, or specifically the lack of them, was a particularly hard blow — not only did this gaping hole affect organisations’ daily dealings with customers and prospects, it also hammered trade fairs — a sector we rely on to showcase our technologies, innovations, and expertise, as well as the opportunities they afford to reconnect with established acquaintances or forge new connections. ‘Virtual’ has its place, and we’ve been thankful for its ability to bail us out of a few tight spots, but it can never fully substitute being physically in front of people: how many business deals have been secured not just because of impressive technologies or competitive pricing, but rather because of rapport and intuition gained only through physical interaction? As we ushered in 2021, the weak light flickering at the end of the tunnel took on a new, brighter glow as vaccination programmes began to roll out across the land. As the days passed, optimism grew. Businesses that learnt new ‘survival’ tricks in 2020 became more efficient, which in turn has attracted more customers in
WWW.MEDICALPLASTICSNEWS.COM
2021. Others have now established a ‘medical’ arm to their business after realising they can adapt their capabilities and know-how to help redress the industry’s high demand/ low supply issue. There’s nothing quite like a crisis to highlight weak spots … and the pandemic certainly achieved that. The international just-in-time supply chains relied on by many proved to be organisations’ Achilles’ heel. But where there’s a cloud, there’s (usually) a silver lining, and the silver lining here is that many businesses are now choosing to work with local partners — a move that keeps industrial cogs turning while benefitting local economies. To keep pace with this trend, companies have, therefore, chosen to invest in additional capacity either through expanding current facilities or through acquisitions. And yet, despite all of this — or, perhaps because of all of this — the industry has continued to innovate, particularly in the sustainability arena: another major theme of 2021. Many medical plastics companies have already signed up to net zero initiatives or are developing environmentally friendly alternatives to their main product portfolios. Regardless of what comes out of COP26, companies have a duty of care to assume responsibility for their own facilities, processes and products. We’re all keen to return to normal, but pollution? Well, that’s one ‘normal’ we don’t want back.
5
DIGITAL
spy
POLYMERS UPDATE
EXTRUSION UPDATE
www.teknorapex.com
New co-extrusion line greatly enhances Teknor Apex’s technical capabilities
T
eknor Apex has added a complete new Arvitec co-extrusion line in its application development laboratory in Rothenburg ob der Tauber (Germany), enhancing the capabilities of its European Centre of Excellence for plastics R&D. This extrusion line has been fully commissioned and trialled and is ready to support clients’ development projects. Along with running trials and prototypes for customers, the company can use the line to speed up its own internal material developments “Arvitec machinery is popular amongst our customers within Europe, so this allows us to do development and problem solving on the very same
equipment on which they run their product,” said Thomas Aschenbrenner, application development laboratory manager. The custom-built line includes dual Genesis extruders, a calibration table, haul-off, and a rotary blade providing a clean and burr-free cut. It provides the latest in touchscreen controls, with an intuitive, easy-tooperate design. These advanced controls offer a complete automated performance with control reports and recording of setups for ease of repeatability. Arvitec can also provide remote support with the ability to fully monitor and control the system in real-time from their location in Spain.
www.sabic.com/en
S
SABIC reveals performance of aromatic polyol
ABIC has announced its recently launched NORYL AP2001G aromatic polyol can improve the performance of hot cast polyurethanes (PUs) based on methylene diphenyl diisocyanate (MDI) and polytetramethylene ether glycol (PTMG). By boosting the hardness, toughness, and stiffness properties of cast PU by double digits, NORYL AP2001G polyol can enable MDI formulations cured with 1,4-butanediol (BDO) to deliver equivalent or better performance compared to toluene diisocyanate (TDI) and PTMG PU formulations cured with MOCA (4,4’-methylene bis(orthochloroaniline). Due to increasing scrutiny in the use of MOCA as a substance of very high concern (SVHC) in the European Union, many PU manufacturers have adopted alternative formulations, including MDI-BDO. Existing MDI-BDO systems pose performance challenges including lower mechanical and chemical
properties vs. TDI-MOCA systems. SABIC’s NORYL AP2001G polyol for MDI-BDO systems addresses both the SVHC issue and performance deficiencies of existing materials, providing formulators with an alternative solution to TDI formulations and an opportunity to reduce or eliminate MOCA. Antonello Cerullo, senior business development manager, SABIC, said: “As manufacturers began to transition away from SVHCs, SABIC understood the need to assist customers with this challenge. We collaborated with Troy Polymers to validate the notable performance benefits of our new NORYL polyol for MDI-BDO systems. Thanks to our innovative new modifier, customers may now achieve results comparable to – or even better than – what they experienced with TDI-MOCA and MDI-BDO formulations, while staying ahead of the tighter restrictions that are being placed on the MOCA curative.”
SUSTAINABILITY UPDATE
World’s first biobased, certified renewable high-performance amorphous polymer
S
ABIC has launched a new portfolio of biobased ULTEM resins that offer sustainability benefits while delivering the same high performance and processability as incumbent ULTEM materials. These polyetherimide (PEI) materials are the first certified renewable high-performance, amorphous polymers available in the industry. Using a mass balance approach, for
6
every 100 kg of ULTEM resin produced, SABIC replaces 25.5 kg of fossil-based feedstocks with biobased materials derived from waste or residue. This advanced offering is a drop-in material option for current ULTEM materials and can support customers’ sustainability goals for challenging applications where high temperature, dimensional
WWW.MEDIC ALPL ASTICSNEWS.COM
stability or demanding mechanical performance is required. The company says biobased ULTEM resins can reduce carbon footprint by up to 10% compared with fossil-based incumbent grades, giving the material the International Sustainability and Carbon Certification Plus (ISCC+) designation.
DIGITAL SPY
3D PRINTING UPDATE
www.nexa3d.com
N
Nexa3D strikes material development agreement with Henkel
exa3D, the maker of polymer 3D printers, has struck an exclusive material development agreement with functional polymers firm Henkel. This agreement builds upon the companies’ partnership and deepens their joint commitment to advancing the capabilities of additive manufacturing (AM) for volume production. As part of the expanding partnership, Nexa3D and Henkel are developing a new casting material which can be used to produce
complex geometries to reduce weight and consolidate parts, resulting in affordable lightweight parts at high production volumes. The new class of functional material is fully optimised for ultrafast 3D printing workflows. Use of advanced design for additive manufacturing tools will further optimise results possible with the material, enabling reductions in material and energy consumption as well as final part weights and costs. Kevin McAlea, COO of Nexa3D, said: “We have found that fewer than 5% of the more than 45,000 foundries globally currently use 3D printing, with adoption typically constrained by technology being either too slow or too expensive. Compared to traditional stereolithography printers, the combination of this new material and our ultrafast technology offers 20x productivity and produces far more robust parts. Foundries and patternmakers now have access to a complete digital workflow that enables them to speed up production and post-processing to develop patterns faster.”
EXTRUSION UPDATE
https://davis-standard.com
Gamut Capital to acquire Davis-Standard G amut Capital, a New York-based middle-market private equity firm, has signed a definitive agreement to acquire Davis-Standard from ONCAP, the middle-market private equity platform of Onex. Davis-Standard is a supplier of extrusion and converting systems and related aftermarket products and services for the rigid packaging, flexible packaging and infrastructure end-markets with an installed base of approximately $7.5 billion of equipment globally. Jim Murphy, CEO of DavisStandard, said: “We are excited to
partner with the Gamut team during this next phase of Davis-Standard’s long history as a provider of highly engineered solutions to an extensive base of industry-leading customers. The resources Gamut brings to this investment will enable us to not only accelerate growth within our markets but also transform Davis-Standard into a leading, value-added global process solutions business. We achieved great growth and performance under ONCAP’s successful ownership and look forward to leveraging the strong foundation we built as we move into our next phase of growth.”
talking
POINT
www.engel.com
SEBASTIAN PAYRLEITNER, HEAD OF PRODUCT MANAGEMENT SERVICE AND AFTER SALES, ENGEL AUSTRIA What is the e-connect.expert service and how does it work? It is a new web application which enables live videos to be used for online support and remote maintenance. Video telephony is supported on smartphones and tablets and using augmented reality (AR) glasses. The only pre-requisite is an internet connection. The ENGEL service technician sends the customer a link to the e-connect.expert platform to start the collaborative work immediately. Do customers need to be tech-savvy to use this technology? Not at all. Smartphones and tablets enable very easy access to the new service options. The full feature scope of e-connect.expert view is available independently of the hardware used. How does it benefit customers? As the number of support cases that can be resolved online has grown due to live videos, e-connect.expert view increases the availability of production cells, minimises downtime and reduces service costs. Some three quarters of all urgent service cases can be resolved remotely in this way. Troubleshooting times are reduced by 70%. Does e-connect.expert have longevity? Can it be developed further? The pandemic has accelerated the development of digital service solutions. Many plastic processing companies are now concerned with the question of how they can ensure their productivity and delivery capacity in the event of future crises. Online support and remote maintenance play a key role here, and the possibilities are gradually being expanded.
TUBING, CATHETERS & STENTS
MIKE WINTERLING, JUNKOSHA’S VICE PRESIDENT OF BUSINESS DEVELOPMENT FOR USA & EUROPE, TAKES US THROUGH THE LATEST DEVELOPMENTS IN THE COMPANY’S PHST PORTFOLIO AND INTRODUCES THE NEWLY LAUNCHED PTFE LINER CHALLENGE.
MOVING BEYOND
F
CONVENTION
or manufacturers and consumers in the medtech sector, everadvancing tubing and catheter applications have highlighted several unmet needs. To address these gaps,
Junkosha has designed peelable fluorinated ethylene propylene (FEP) heat shrink tubing (PHST) to give catheter manufacturers cost-effective, lower tolerance baseline materials to achieve reflow into a single smooth construct. The key advantage to PHST is its peelable design, which makes removing it significantly easier compared with the traditional method of skiving, thereby saving time and reducing risk of damage to the underlying construct. Junkosha’s PHST solutions are ideal for particularly small devices such as micro-catheters due to their simplicity in removal. Conventional skiving techniques do not work well on smaller reflowed catheters, frequently leading to scoring or kinking of the catheter shaft, rendering them useless. The move towards miniaturisation in medical tubing is picking up pace, with medical device manufacturers demanding catheter solutions that can readily penetrate harder to reach anatomy to treat a broader range of patients and conditions. Junkosha has developed a range of medical tubing innovations, including the first ultra-small PHST — a tubing solution suited for laminated jacket coating of tiny guide wires (down to 0.011” and 0.014”),
WWW.MEDICALPLASTICSNEWS.COM
9
TUBING, CATHETERS & STENTS
leveraging the fact that PHST has a recovered interior diameter (ID) down to 0.009”. These miniature guide wires are perfect for applications such as the navigation of vessels to hard-to-reach locations in the brain. Beyond ultra-small, the company’s 2.5:1 PHST solution equips catheter manufacturers with the highest shrink ratio currently possible in peelable FEP. Last year, the company also launched Cut-To-Length and Slit PHST which eliminates the need for customers to make the initial slit, further simplifying the manufacturing process. Junkosha also provides clear PHST, which exhibits the same product quality as its ‘natural’ PHST but is optically transparent (Figure 1). This visual clarity is a critical requirement for manufacturers seeking to bond, weld or tip catheters, as well as needing to see what is happening underneath along the underlying substrate for quality control.
durability has been welcomed by manufacturers of small format catheters worldwide. For example, in some situations customers had been considering the use of a tie layer, which is an additional thermoplastic coating on the outside of the PTFE liner, to minimise the appearance of small imperfections as well as increase the bond strength between the layers of the catheter.
Using the clear format of PHST enables manufacturers to visually inspect the catheter shaft before and after reflow, enabling identification of any bubbles in the tubing and any gaps or overlap within the joints of the catheter shaft. This is particularly useful for manufacturers using catheter constructs with joints in tight proximity, or multiple Pebax layers demanding close inspection to ensure extrusions do not shift.
In doing so, however, they had added unnecessary thickness and stiffness, therefore finding that the tie layer limited the amount of stretch they could use in their manufacturing processes. Overall, Junkosha has yet to come across a customer application where the performance of its liners required the addition of a tie layer to meet performance or yield targets for the device.
THE PTFE LINER CHALLENGE Thanks to proprietary processing techniques, the company’s etched polytetrafluoroethylene (PTFE) liners (EPL) provide considerable robustness, high tensile strength, have superior consistency in elongation, and are available with ultra-thin walls to deliver increased flexibility and smaller outer diameters for increased catheter real estate (Figure 2). In addition, they incorporate consistent etched surface treatment to provide confidence of adhesion during reflow even in the most demanding catheter designs.
LOOKING AHEAD As increasingly intricate procedures become viable, medical devices will become even more complicated. Device manufacturers are utilising combinations of braiding, coils, laser cut tubing, and multiple durometer jacketing materials to increase flexibility where it is needed, while maintaining the mechanical performance necessary for a smooth delivery. With a number of milestones already under our belt, we are looking forward to leveraging our technologies and enabling technology innovators well into the future.
Earlier this year, Junkosha launched its PTFE Liner Challenge by making a pledge to manufacturers — by partnering with Junkosha, they will not need a tie layer to improve yields for applications under 3.0 mm ID. Evidence of the robustness of Junkosha’s EPL products has emerged through customer feedback. In a field that demands high levels of consistency and reliability, the
10
Consistent positive feedback from customers regarding its EPL range has led the company to formulate a service package which makes it easy for customers to experience the product benefits for themselves. This includes free standard samples for testing, free application support including a full problem audit and solution identification, alongside shorter lead times than competitors.
Thanks to proprietary processing techniques, the company’s etched PTFE liners provide considerable robustness, high tensile strength, have superior consistency in elongation
W W W. M E D I C A L P L A S T I C S N E W S . C O M
Polymers for Healthcare Applications
Whether it’s medical devices, pharmaceutical packaging or diagnostic equipment – countless healthcare applications can be realized using polymers from our portfolio. Our team of experts will provide technical service, product safety, risk management and a deep understanding of market needs. Do you process polymers? Then we are your reliable partner.
ALBIS (UK) Ltd. albisuk@albis.com www.albis.com
Sales Manager (m/w/d) Become part of our international sales team in Germany for the medical technology sector
Join one of the leading Masterbatch producers in Europe
Apply now!
www.gabriel-chemie.com/karriere
COVER STORY
AS NEW OPPORTUNITIES EMERGE, MEDICAL INJECTION MOULDING CAN TURN TO LOCAL PRODUCTION AND INTEGRATED SYSTEMS SAYS THOMAS BONTEMPI HEAD OF MEDICAL BUSINESS DEVELOPMENT AT HUSKY INJECTION MOULDING SYSTEMS.
CAPITALISING ON LOCAL
MANUFACTURING OPPORTUNITIES
M
Integrated moulding systems deliver major benefits
edical injection moulding continues to enjoy strong growth as worldwide demand increases for medical devices, according to industry observers. New business opportunities are emerging globally as the industry expects medical device sectors to see compounded annual growth of greater than 10%. The rising prevalence of chronic disease and the ageing population are the key market drivers. In addition, emerging economies with higher GDPs are seeing increasing expenditures for healthcare. Another major influence has been the COVID19 pandemic, which has spurred the explosive growth of lab consumables for testing, vaccinations, and other diagnostics. Among the growing business opportunities are pipette tips and blood collection tubes, utilising integrated manufacturing approaches which assure the highest levels of certainty, scalability, and fastest time-to-market.
12
LOCALISED PRODUCTION IS A TOP PRIORITY Skyrocketing demand for medical devices during the pandemic resulted in major supply disruptions. One issue that resurfaced during the COVID-19 outbreak was the realisation that 85% of the world’s countries rely on imports of medical devices and components.1 The imbalanced supply situation existed previously but the pandemic accelerated demand and raised concerns about worldwide shortages of medical products — many essential in the fight against the virus. To counteract future uncertainty and ensure supply, many political and governmental initiatives have made the creation of local supply chains a top priority. According to McKinsey & Co. more than 90% of medtech multinational companies (MNCs) expect greater localisation in the industry after the outbreak.2 Meanwhile, about 60% of medtech MNCs believe that local manufacturing will be favourable for business success. The scenario presents lucrative opportunities for both existing players and new entrants. The looming question is whether manufacturers will be prepared to capture these new business opportunities. IDENTIFYING THE HIGH-GROWTH OPPORTUNITIES Clearly, processors and end users are poised to capitalise on potential growth applications. Selecting the right application means balancing the risk and opportunity. Making a risk/rewards evaluation is important in selecting business opportunities that present a reasonable risk and complexity level, combined with high volume for fast payback. Amidst the pandemic, the production of pipette tips, blood collection tubes, and prefilled syringes have emerged as high-growth tracts that are ripe for expanded penetration. Pipette tips — which dispense medical fluids and liquids — are a high-volume application which are predicted to see a 9.7% compounded annual growth rate (CAGR) through 2025.3 Growth opportunities are fuelled by COVID-19, nationalisation, and demand for quality parts. Pipette tip design has several critical quality criteria including dimensional tolerances, part filling (especially below 0.5-mm wall thickness), concentricity (run-off or run-out), inside and outside flash, and insert split lines positioning. Another promising application is blood collection tubes which have seen dramatic growth due to the need for pandemic-related products. They consist of a PET tube, an HDPE closure, and a rubber stopper. Tubes are delivered ready-to-use to the end user for specific sample analysis with features such as labels, vacuum sealed to draw a specific volume, and prefilled with reagent. Key design requirements include dimensional accuracy, no brittleness, transparency, oxygen permeability, vacuum maintenance, water vapour permeability, and gate quality.
W W W. M E D I C A L P L A S T I C S N E W S . C O M
COVER STORY
monitoring service with proactive troubleshooting capability which allows for early detection and traceability. It analyses the operating system and traces variation down to the subsystem and hardware components. This capability is enabled by the intimate understanding of all the building blocks of the integrated solution since they are all delivered from Husky as a single-source supplier.
A further growth area is pre-filled syringes, which have emerged as one of the fastest-growing choices for unit dose medication, driven by the increasing adoption of self-care devices and the high demand for improved safety in injectables. Increasingly, pre-filled syringes are manufactured using plastics as they are more break-resistant than glass, and can be manufactured for specific sizes, inner diameters, and finger flange designs while maintaining tight tolerances during production. INTEGRATED MOULDING SYSTEMS DELIVER MAJOR BENEFITS To meet the requirements of these high-volume applications, processors and OEMs are moving increasingly to integrated manufacturing systems that provide better quality, eliminate artificial barriers between suppliers, and provide faster time-to-market. Medical device manufacturing can be highly complex, and the process of self-aggregating different pieces of equipment can be time-consuming, costly, and inefficient. Integrated solutions offer maximum part performance and precision and repeatability in high-cavitation manufacturing processes, bringing validated medical parts to the market at unimaginable speeds and volumes. This is accomplished while minimising waste and increasing the bottom line. The goal is to bring together the subcomponents of the injection moulding system so they’re not simply integrated but designed and operating as a system. Husky has translated its extensive experience and expertise in turnkey PET systems for packaging to the medical segment. Along with its subsidiary Schöttli a leading medical mouldmaker, the company delivers integrated systems for high-volume medical applications like pipette tips, blood collection tubes, and pre-filled syringes. Husky brings injection machines, hot runners, controllers, and auxiliaries whereas Schöttli provides medical mould design and mouldmaking expertise. Together, they deliver a total system solution which has single-source responsibility and is specifically configured for an application. For pipette tips, a typical fully integrated work cell for a two- to 128-cavity application, incorporates Husky’s injection machinery and Schöttli’s mould components. It also includes automation equipment such as racking, vision control, filter assembly, packing, and sterilisation, from third-party suppliers. The system delivers reduced complexity, less risk, and faster time-to-market. The Schöttli mould solution features a compact design for high cavitation, built around the eight-cavity cluster concept. It also includes star nozzle hot runner technology, component accessibility from main parting lines, and highly efficient cooling for faster cycles. Concentricity and quality are achieved through adjustable cores and mold design concept. A cycle time of 4.9 seconds is estimated for a 200 μL pipette tip. For blood collection tubes, Husky’s Ichor integrated system is based on a proven PET platform, and provides high performance, low risk, and fast timeto-market. The work cell includes the injection machine, hot runner, auxiliaries, controller, and cold half mould. It delivers a 5.4-second cycle time and 97% overall system efficiency. Various levels of IMM integration are possible with downstream automation including takeout devices, inspection, labelling, and packing. Typical integrated systems run in ISO Class 8-9 Cleanrooms and incorporate downstream automation from third-party suppliers. Husky’s integrated systems also feature Advantage+Elite, a remote
SYRINGE START-UP SCALES UP QUICKLY IN RUSSIA A successful case study involving a Russian manufacturer illustrates how integrated production systems can deliver business success. Husky helped Moscow-based Pascal Medical, one of Russia’s leading medical device suppliers, to scale-up fast to produce a high-quality product, and quickly become an industry leader. Pascal Medical wanted to reverse import trends by setting up local production of disposable PET syringes to meet local and international standards in a short time, starting from scratch. Pascal Medical was looking for technology to enable them to produce the highest quality syringes in Russia. They lacked upfront capabilities to set up such an operation, so they enlisted Husky to develop a turnkey system. Pascal Medical relied on Husky to guide them towards the right approach for setting up their injection moulding capabilities. In this case, Husky supplied multiple integrated injection systems including moulds, injection machines, and hot runners to mould high-volume quantities of disposable syringes. The company is now the largest Russian supplier for these products, producing 450 million disposable syringes per year. As processors and end users look to capitalise on these future business opportunities, they are acutely aware of the challenges coming out of COVID-19. They see the vital need to create local manufacturing to avoid being hamstrung by a reliance on imports. Integrated or turnkey production is expected to play a key role as processors and OEMs aim to collaborate with partners that can deliver the necessary technical expertise and know-how to efficiently scale up operations and quickly bring end products to market. REFERENCES 1. McKinsey & Co., New York, NY 2. McKinsey & Co., New York, NY 3. GlobalData, London, UK
WWW.MEDICALPLASTICSNEWS.COM
13
BIOCOMPATABILITY
MARK CABONCE, PRINCIPAL TOXICOLOGIST, SANDI SCHAIBLE, SENIOR DIRECTOR OF ANALYTICAL CHEMISTRY AND REGULATORY TOXICOLOGY, AND DR SHERRY PARKER, SENIOR DIRECTOR OF REGULATORY TOXICOLOGY, WUXI APPTEC ADVISE HOW MEDICAL DEVICE MANUFACTURERS CAN BEST NAVIGATE THE RECENT UPDATES OF ISO 10993-18:2020.
ISO 10993-18:2020’s
NEW CHALLENGES FOR MANUFACTURERS SUBMITTING IN THE EU
T
he International Organization for Standardization (ISO) significantly updated the standard for chemical characterisation when it revised 10993-18:2020 — Chemical characterisation of medical device materials within a risk management process. The revision built a framework for identifying and quantifying medical device materials and constituents in concentrations that could potentially cause toxicological concern. Two primary components introduced in the standard are the analytical evaluation threshold (AET) and intensified extraction requirements. Both components have contributed to a massive increase in the amount of testing each medical device undergoes to support safety. Given the broad and fundamental changes inherent in ISO 1099318:2020, regulatory bodies in the US and the EU have taken a stepwise approach to recognise, adopt and implement the standard. The US Food and Drug Administration (FDA) partially recognised 10993-18:2020 in July 2020 but stopped short of full recognition.1 Still, FDA regulators expect manufacturers submitting products for use in the US to address the requirements of the standard.
what constitutes state-of-the-art analytical chemistry is not explicitly defined in the regulation. The MDR empowers notified bodies (organisations that help shepherd products through regulatory submission) to interpret the meaning of state of the art and work with manufacturers to ensure compliance with standards before submission. Although potentially confusing, EU regulators did not set out to create a challenging system. The European Council and Parliament implemented the MDR to alleviate subjectivity in the submission process and align Europe with evolving global standards. The byproduct of their efforts is a process that requires solid partnerships and precise time management to achieve regulatory success. ISO 10993-18:2020’S IMPACT ON THE EU Expectations for physical/chemical information introduced in ISO 10993-1:2018 — Evaluation and testing within a risk management process, and now ISO 10993-18:2020 have compelled EU and US regulators to prioritise chemistry. Exaggerated and exhaustive extractions and a low AET can yield hundreds, sometimes thousands, of compounds for toxicologists to assess. The number of chemicals to identify and quantify adds a significant amount of time to submission preparation. It also increases the likelihood of unfavourable or “equivocal” findings (that is, those for which toxicologists deem the level of risk unacceptable, given the available information), which could mean more time for risk mitigation.
In the EU, the process is more nuanced. In May 2021, the Medical Device Regulation (MDR) replaced the Medical Device Directive (MDD) as a new, more comprehensive regulatory framework for medical devices in the EU. The regulation framework changed how devices are classified in the EU and introduced stringent requirements that prioritise patient risk. The MDR accepts the revised ISO 10993-18:2020 as “state of the art”; that is, it is the most recent published version of an accepted standard — and holds manufacturers to the standard. But the EU has yet to harmonise the standard across member states. Yet, 14
W W W. M E D I C A L P L A S T I C S N E W S . C O M
BIOCOMPATABILITY
Rigorous chemistry is the new normal in the EU, so manufacturers should consider adjusting their programme timelines accordingly. The scarcity of regulatory assistance is also real. Manufacturers with risk management strategies and timelines responsive to the EU’s regulatory environment and ISO 10993-18’s chemistry requirements will be positioned well for success.
Before ISO 10993-18:2020, assuming two months for chemistry studies prior to regulatory submission would have likely been reasonable. Today, regulatory expectations are much higher, and timelines include preliminary chemistry testing, extractables/leachables (E/L) testing, toxicological risk assessment and risk mitigation (if necessary). Although timelines are never absolute, manufacturers are finding submissions today take far longer than before. Budgeting 8–12 months for chemical characterisation, biocompatibility and risk assessments is now a more realistic timeline. Regulatory backlogs in the EU also contribute to lengthier timelines. Harmonised standards (HAS) consultants assess to what extent European standards comply with relevant EU legislation. Manufacturers are responsible for ensuring their products meet EU safety, health and environmental protection requirements. To sell products in the EU, manufacturers must conduct a conformity assessment, create a technical file, draw up an EU declaration of conformity (DoC) and affix a ‘CE’ mark to their products. HAS consultants can help manufacturers verify the legal definition of their products and clarify the category into which they fall. All documentation must be contained in a technical dossier and provided during regulatory submission. The process can be lengthy, and there are a limited number of qualified HAS professionals to consult.
WORKING WITH NOTIFIED BODIES Notified bodies help verify a medical device’s design and quality. They assist with interpreting regulatory guidance and, as noted above, ensure manufacturers adhere to state-of-theart standards. Notified bodies also provide a window into expectations for their conformity assessment — they can even supply a checklist of factors to consider based on the product and classification. The questions they ask and the data they request will likely reflect the rigour inherent in MDR and ISO 10993-18:2020, making notified bodies valuable members of the manufacturer’s submission strategy team. When working with notified bodies, the bottom line is to secure one as soon as possible. It does not matter where a manufacturer’s product is in the submission process; the key is to get in the queue. To ensure compliance, notified bodies now recommend manufacturers file applications at least 18 months before a product’s MDD expiration date. Each notified body has a portfolio of clients, and once capacity is reached, manufacturers are out of luck until new notified bodies are accredited. The EU offers guidance documents, FAQs and transition plans on its website for manufacturers that have questions while waiting for access to a notified body.2 PREPARING FOR SUBMISSION POST-MDR Added transparency in the submission process is anticipated in the EU, but for now, notified bodies and manufacturers are navigating the new guidance together. As notified bodies receive more submissions, they will start to ask more detailed questions. And, as they gain exposure to MDR feedback, notified bodies can prepare their partners more thoroughly. And as manufacturers go through the process, they will gain valuable experience that will serve them well in the future. All these factors will help set better expectations and create a smoother process for future submissions.
WWW.MEDICALPLASTICSNEWS.COM
15
Vyon® for Porous Plastics Solutions Precisely engineered components designed and manufactured for medical and life science applications. Filtration
Media Support
Separation
Wicking
Diffusion
Absorption
Venting
It’s the little things. Vyon®, is the leading brand of porous plastic material found at the heart of innovative product solutions. As small as it can be, Vyon® can be precisely engineered to tight tolerances and has the versatility to be manufactured into a wide range of shapes and sizes to ensure you get the perfect fit for your product or application. Learn more about Vyon® at:
Drop our team a message at:
www.vyonporousplastics.com
enquiries@porvairsciences.com
Qosina offers a wide selection of stock and custom tubing solutions for medical and bioprocessing applications. Choose from over 100 tubing options available in a variety of brands, types and materials.
Brands
Stock Materials
Tygon ® C-Flex ® SaniPure ™ BDF ™ PharMed ® BPT PharmaFluor ® TuFlux ®
DEHP-free PVC TPE HDPE FEP Platinum- and peroxide-cured silicone Multi-layer
Qosina is a leading global supplier of thousands of stock components to the medical and pharmaceutical industries. Qosina Corp.: Qosina Europe:
2002-Q Orville Drive North, Ronkonkoma, NY 11779 USA
qosina.com
Viale Giacomo Matteotti, 26, 20095 - Cusano Milanino (MI) - Italy
+1 (631) 242-3000
+39 02 66401337
info@qosina.com info@qosinaeurope.com
BIOCOMPATABILITY
For now, using data that isn’t state-of-the-art could cause serious problems with a manufacturer’s submission. Regulators understand that standards are updated periodically and inconsistently, but manufacturers who submit a device with outdated data can expect a regulatory inquiry and a request for justification. In these instances, EU regulators can ask for a “hard pause”. A hard pause is not necessarily a request for retesting; it just means regulators want additional time to consider the data and supporting evidence for a device’s safety claims. Any manufacturer submitting a device with outdated data should proactively conduct a gap analysis to identify weak areas of its submission. Internal teams or laboratory partners can conduct gap analyses, but they should include representation from an organisation’s quality control, regulatory, R&D, finance, product management and procurement departments. ISO 10993-18:2020 presents some of the biggest challenges manufacturers face. Due to materials characterisation requirements, manufacturers need to disclose whether any carcinogens, mutagens, reproductive toxicants or endocrine disrupters (CMRs) are present in their devices in amounts ≥0.1% w/w. This applies to most devices, regardless of contact duration or risk class. For many manufacturers, materials characterisation compliant with ISO 10993-18 will be the best approach for addressing CMRs. STREAMLINING EU SUBMISSIONS Some manufacturers have found success by gaining FDA approval for their devices before submitting them for review in the EU. Regulatory bodies differ, and expectations can be subjective, but in some instances, receiving a green light from the FDA has effectively demonstrated device competency and data validity en route to regulatory approval. The primary recommendation for manufacturers submitting in the EU is to overcommunicate. Be prepared to communicate with notified bodies and laboratory partners consistently. Overcommunication ensures clarity among partners and protects programme timelines.
Manufacturers with risk management strategies and timelines responsive to the EU’s regulatory environment and ISO 10993-18’s chemistry requirements will be positioned well for success
It is also critical for manufacturers to take advantage of whatever opportunity they have with notified bodies. As mentioned, these consultants are in short supply, and there is little evidence to suggest that trend will change any time soon. A manufacturer’s relationship with a notified body could mean the difference between regulatory success or failure for its medical device. Finally, building a solid relationship with a laboratory partner supports safety. A suitable laboratory partner can help manufacturers gather reliable data and avoid potential pitfalls. More than that, a partner with experience navigating the nuanced regulatory environment in the EU can provide crucial guidance, help set expectations and avoid uncertainty. Without this relationship, manufacturers could be putting their products and patients at risk. THE FINAL WORD Submitting a medical device for regulatory approval in the EU is no easy feat. Regulatory delays and confusing submission pathways persist despite the extra year to prepare for the MDR rollout. Fortunately, manufacturers do not have to navigate the landscape alone. Experienced laboratory partners can help clarify MDR, guide manufacturers through the EU’s unique regulatory system and, ultimately, provide the safest products possible to EU patients. REFERENCES 1. https://www.accessdata.fda.gov/ scripts/cdrh/cfdocs/cfStandards/ detail.cfm?standard__identification_ no=41050 2. https://ec.europa.eu/health/md_ sector/new_regulations/guidance_ en
FACTORS TO CONSIDER DURING A GAP ANALYSIS PRIORITIES: EU regulators may prioritise devices that could potentially affect many patients or fall into a lower risk class. TIME: The length of time a product has been on the market and the time since last submitting documents can ease regulatory concern. Newer products that have undergone testing recently may have fewer gaps and may require less time for review.
HISTORY: A history of regulatory success appeals to regulators, but withholding information about past issues will certainly derail a submission. CHANGES: Major and minor changes to a device can affect data applicability. Be sure to document any changes to sterilisation methods, materials, packaging or vendors. Notified bodies will be looking for anything that could potentially interfere with device chemistry or functionality.
WWW.MEDICALPLASTICSNEWS.COM
SAFETY: Disclosing any patient safety or performance problems — including how they’ve been addressed — will demonstrate innovation and transparency.
17
DESIGNING MEDICAL DEVICES
MIGUEL SOLIVAN, A GLOBAL PRODUCT MANAGER FOR AVERY DENNISON MEDICAL, DISCUSSES WEARABLE MEDICAL DEVICE GROWTH AND DESIGN CONSIDERATIONS TO ENABLE LONGER WEAR TIMES.
E
THE EXTENDED WEAR CHALLENGE
xtended wear times are the new gold standard for many wearable medical devices. Where once three days or a week was the norm, now use cases call for 14, 21 and more than 30 days of continuous wear. The longer wearable devices are worn, the more uninterrupted data healthcare providers can collect and analyse. Furthermore, they enable patients to go about their daily activities without stopping to change their device or visit the doctor’s office for a new one.
Longer wear times strengthen the economics for wearables. Less frequent device replacement usually means less expense for the patient and healthcare system. Still, although extended-wear device benefits are easy to see, it’s not so easy to achieve long wear times, especially for stick-toskin devices. But with careful planning and device design, a wearable can perform for many days or even weeks. WEARABLE DEVICE GROWTH Global wearable medical device revenue should expand at a compounded annual growth rate of
18
26.8% to reach $111.9 billion in 2028, according to Grand View Research Inc. The research organisation points to drivers such as: • Home healthcare • Remote patient monitoring • Sedentary lifestyles and related chronic diseases requiring continuous monitoring • Demand for COVID-19 early symptom detection.1 Wearable applications include continuous glucose monitoring (CGM), drug delivery, pulse oximetry, vital signs sensing and sleep apnoea detection. Wearable devices have both diagnostic and therapeutic capabilities. On the diagnostic side, they help healthcare providers and patients monitor vital signs, activity levels, blood sugar and respiration, for example. On the therapeutic front, they can deliver medications and help patients and providers manage asthma, addiction recovery and post-operative rehabilitation. The pandemic has accelerated wearables growth. “COVID-19 pushed telehealth to the forefront of the healthcare market” as “caring for patients beyond the confines of the hospital became an urgent need,” said VitalConnect, maker of the VitalPatch RTM wearable monitoring device.2 By staying home, patients reduce their risk for hospital-acquired infections. Wearable medical devices come in multiple forms, from wrist bracelets and strap-on headbands to skin-worn patches. This article focuses on the latter. EXTENDED WEAR HURDLES For patients and healthcare providers, extended device wear time affords greater convenience and quality of life. One example is Abbott’s FreeStyle Libre 2 CGM system. The system’s self-applied sensor is designed to stay on
W W W. M E D I C A L P L A S T I C S N E W S . C O M
Trusted to protect… Life science products that provide care, improve health and save lives We are a global technology provider to the life sciences industry, providing innovative packaging solutions and complementary products and services. We drive our customers’ success by delivering the best total value by combining superior quality and customer support, and the most efficient technology. Custom Packaging Solutions Focused 100% on Healthcare Custom Medical Packaging solutions that provide superior quality and protection. Nelipak®-designed device packaging is based on market expertise from concept to the point-of-use. For more information, contact us: email: info@nelipak.com | phone: +31.478.529.000
Sealing Machines A range of custom-built medical tray and blister heat sealing machines
www.nelipak.com www.bhp-europe.com
Flexible Packaging Materials A wide range of flexibility in materials and functionalities
®
Nelipak Corporate Office Cranston, RI, USA
®
Nelipak Elsham, United Kingdom
®
Nelipak Phoenix, AZ, USA
®
Nelipak Venray, The Netherlands
Nelipak Galway, Ireland
®
Nelipak Heredia, Costa Rica
Nelipak Derry, Northern Ireland, UK
®
Nelipak Humacao, Puerto Rico
®
®
Nelipak Clara, Ireland
Medical Device and Pharmaceutical Packaging Full-service solutions that provide superior quality and protection
®
®
Nelipak Whitehall, PA, USA
DESIGNING MEDICAL DEVICES
the back of the upper arm for 14 days. Patients with diabetes can shower, bathe, exercise and go about other daily activities while wearing the sensor.3
Cover/Overlay Tape Device
Two other examples of extended-wear devices are from BioIntelliSense. The firm’s BioSticker is designed to be worn for 30 days, and its BioButton is designed for 60-day wear. Both devices provide continuous vital signs monitoring.4 To deliver long wear times in bodyworn applications, medical device manufacturers face the following challenges: • Moisture management — Human skin contains oils and perspires. Activities such as showering, swimming and exercise add to fluidhandling hurdles. Only specially engineered medical adhesives can adhere to this complex surface for long-term wear. • Secure hold — An adhesive material must remain secure amid skin twisting and stretching. Also, beyond moisture, human skin regularly releases dead cells. The exterior layer, the epidermis, sheds every 7–14 days. These cells can saturate the device material.5 • Atraumatic removal — A wearable device eventually must come off, preferably with minimal discomfort. It is a challenge for device makers to balance reliable adherence with relatively painless release when it’s time to remove the device. • Repositionability — Sticking a bodyworn device exactly where it’s supposed to go, on the first attempt, is another challenge. This especially can be the case for patients selfapplying their wearable devices. Device developers must plan for this contingency. SOLVING THE CHALLENGES Stick-to-skin patches typically contain layers of thin, plastic materials held together and to the body by biocompatible medical adhesives (Figure 1). Compared with other options such as metal and glass, plastic materials are lightweight, flexible and relatively easy to process. They enable device developers to achieve a sleek profile, which is important for discretion and comfort. Quite often, the chosen materials to achieve the desired look and function will include one or more low surface energy (LSE) plastic materials, which pose a predicament for many adhesive formulations. Following are
20
Tie Layer Skin Contact Layer
Compared with other options such as metal and glass, plastic materials are lightweight, flexible and relatively easy to process some considerations to keep in mind when designing wearable devices. • Skin-contact layer — This layer must manage moisture and keep the patient’s skin comfortable for the full wear-time duration. Breathable materials contain tiny pores that allow perspiration and other fluid to evaporate. Absorbent materials are another option for handling fluid. These materials absorb moisture and hold it away from the skin. The skin-contact layer also should be soft and stretchable. Polyester and polyurethane (PU) nonwovens are popular carrier materials. • Tie layer — The tie layer, also known as the construction layer, must be compatible with both the device and the skin-contact layer. If a device is made of an LSE material, then the tie layer adhesive must have a lower SE than the device material, or the device surface must be specially treated to allow the adhesive layer to wet out consistently across it.6 Differential two-sided acrylic adhesive tapes are ideal tie-layer materials and adhere well to films, foams, nonwovens and other LSE substrates. • Overlay/cover layer — This layer, which can be optional, offers device developers a way to provide additional securement as well as another layer of protection from moisture and bacteria. Breathability and transparency are desirable, as is die cuttability, so that manufacturers can design a hole in the overlay to expose part of the device, if desired. The wearable medical device market is growing rapidly, in tandem with telehealth’s surge. Longer device wear times will continue to play an important role in chronic disease management, medication selfadministration, remote patient monitoring and other at-home care. With the right materials and design, device developers can deliver wearable solutions with optimal comfort and performance. REFERENCE 1. https://www.grandviewresearch.com/industry-analysis/wearable-medicaldevices-market 2. https://vitalconnect.com/vitalconnect-achieves-multiple-milestonesaccelerates-growth-in-2021/ 3. https://www.freestyle.abbott/content/dam/adc/fds/us-en/documents/getstarted-guide.pdf 4. https://biointellisense.com/ 5. https://medical.averydennison.com/content/dam/medical/medicalproducts/ products/wearables/ADM-21006_8_5x11_White%20papers_031521_ Material.pdf 6. https://www.adhesives.org/adhesives-sealants/adhesives-sealantsoverview/structural-design/surface-energy-and-wetting
W W W. M E D I C A L P L A S T I C S N E W S . C O M
Medtech | Digital HealthTech | Medical Plastics | Manufacturing | Software | Inspection and Metrology Regulation | Design | Early-Stage | Innovation | Pharmaceutical | Manufacturing
Exhibit with us Generate Leads Position Your Brand Make Connections Accelerate Your Launch Demonstrate Live
Med-tech innovation expo
8-9
JUNE 2022
NEC | BIRMINGHAM | UK
Book your stand!
PLATINUM PARTNER
www.med-techexpo.com Co-located Shows
Medical Device Supply Chain Intelligence
UN3373 Compliant Sample Transport
Packaging Solutions
Ophthalmology Cardiosurgery Enteral Feeding Infusion Orthopedics Drug Delivery Pharma Fluid Handling
All the Packaging Components You Need for Your Kit Construction When developing diagnostic or clinical trials kits, consideration of compliant patient sample return is critical. Alpha Laboratories can help with qualified advice and essential supplies. ■ SpeciSafe® All-in-One Secondary
Packaging Solutions
■ ShuttlePouch™ Secondary Mailing Pouches
Market-oriented. Customized. RAUMEDIC. At RAUMEDIC, we develop and manufacture customized solutions for a wide range of therapeutic areas. Let us turn your product idea into reality – tailored to the market and your specific application. RAUMEDIC can support you throughout the product development process – from material selection to medical device certification.
Learn more now at: raumedic.com/application-areas
21-08-Imageanzeige-MPN-265x86-EN-RZ2.indd 1
■ Absorbent Sheets ■ ADR and IATA 95kPa
Secondary Pouches
■ Rigid Outer Boxes ■ Seals and UN3373 Labels ■ Complete Sample Packaging Kits
Contact our UN3373 experts for more information or to discuss your requirements and join our long list of satisfied customers. Web: www.UN3373.co.uk Phone: +44 (0)23 8048 3000 Email: marketing@alphalabs.co.uk
08.09.21 12:13 MPN_UN3373wShP_FINAL_Oct21.indd 1
08/10/2021 14:23:49
3D PRINTING
LUKAS PAWELCZYK, HEAD OF FREEFORMER SALES, ARBURG, EXPLAINS HOW THE APF PROCESS WITH THE FREEFORMER IS PARTICULARLY SUITABLE FOR AM IN MEDICAL TECHNOLOGY.
Making the impossible possible
T
he medical plastics market was one of the first to make extensive use of additive manufacturing (AM) technology, creating complex and often custom-designed components and devices in relatively small numbers. This trend has accelerated as the imagination of medical designers and new 3D printing equipment have made the previously impossible possible. Now, a different type of system is extending those boundaries even further by allowing the use of the same plastic granules used in injection moulding, including biocompatible, resorbable, sterilisable, and FDA-approved original materials. Developed and built by Arburg, the German manufacturer of precision injection-moulding machines, the freeformer machine, with its Arburg Plastics Freeforming (APF) process, facilitates sophisticated medical applications that cannot be achieved with any other process.
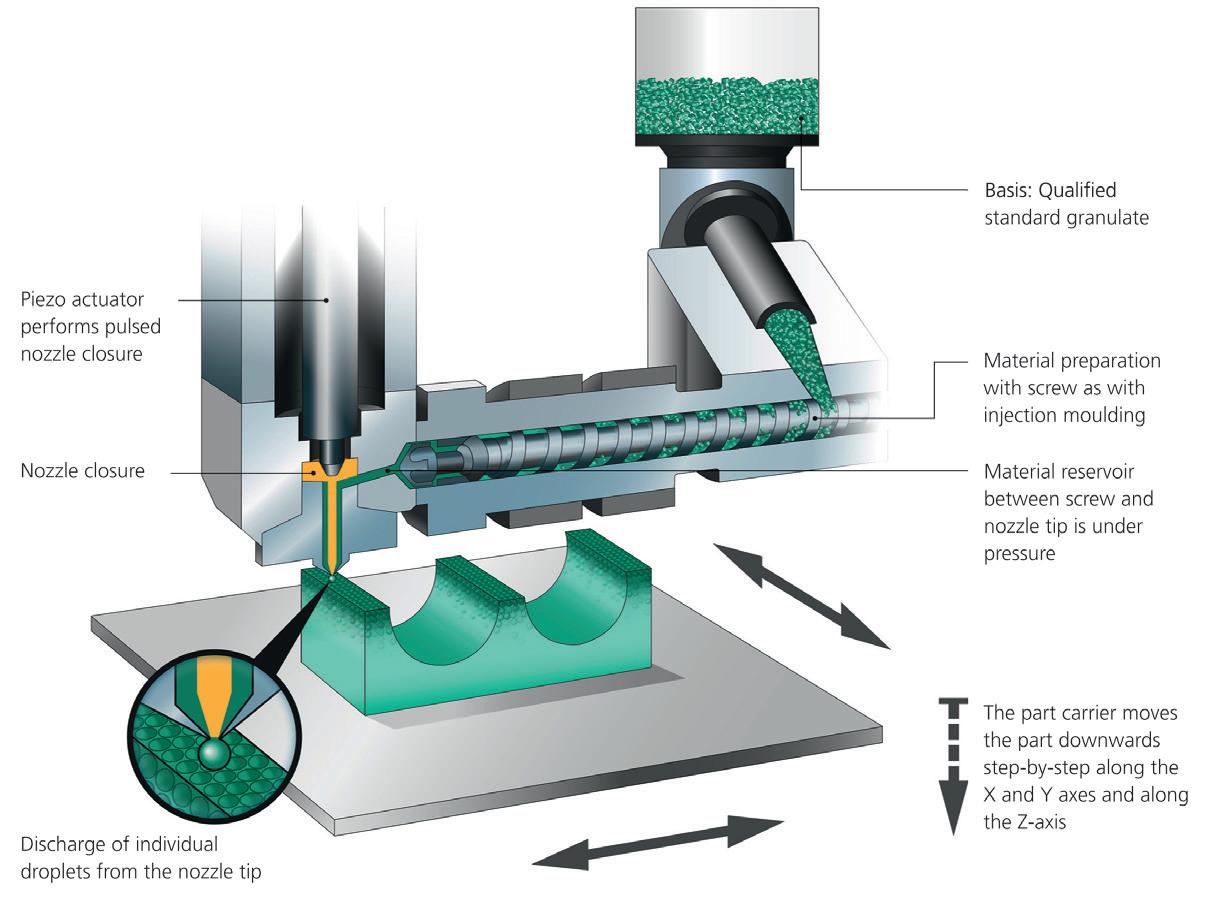
Figure 1: The APF process is based on plastic granules. The qualified original material is melted in an injection unit and discharged via a nozzle in the form of droplets.
‘OPEN SYSTEM’ IS THE KEY Similar to injection moulding, the freeformer operates by melting conventional plastic granules via a heated plasticising cylinder. A high-frequency pulsing rigid nozzle then discharges tiny droplets of the liquid plastic melt (Figure 1). The part carrier, which can be moved along three axes, enables each individual droplet to be set down precisely. The applied droplet bonds with the existing surrounding material so that, layer-bylayer, three-dimensional components with a high mechanical strength are produced. Part production starts from 3D CAD data in basic stereolithography STL format. Unlike conventional filamentfed AM systems, freeformers work using a wide variety of qualified standard plastics granulates. In addition, users can process their own custom-compounded materials with this ‘open system’ and optimise the droplet size and process themselves. Alternatively, they can access Arburg’s material database and select certified plastic granules, such as ABS (acrylonitrile butadiene styrene), amorphous PA (polyamide) and PC (polycarbonate), elastomeric TPU (thermoplastic polyurethane) and semi-crystalline PP (polypropylene), PLLA (poly-L-lactic acid) and other special and certified original materials including biocompatible, absorbable,
WWW.MEDICALPLASTICSNEWS.COM
23
3D PRINTING sterilisable, and FDA-approved original materials. RESORBABLE IMPLANTS An outstanding example of the use of the APF process in medical technology is the processing of Resomer LR 706 (composite of poly L-lactide-coD,L-lactide and ß-TCP) from Evonik to create implant plates that are inserted directly into the body in the case of bone fractures. The polymer composite, which is modelled on human bone, contains 30% ceramic additives, known as ß-TCP. This makes the component stronger and releases calcium to promote bone regeneration. After a given time, the implant dissolves completely. Resorbable cranial bones, cheekbones, and finger bones have also been made from medical PLLA (Purasorb PL18, Resomer LR 708). In addition, the plastic granules can be loaded with anti-inflammatory agents, for example, to minimise rejection.
To date, the freeformer is the only AM system that can process the FDA-approved TPE Medalist MD 12130H used in physiotherapy, emitting an acoustic signal as soon as an injured arm or operated knee is overstretched or understretched. FILLING LEVEL CAN BE SELECTIVELY CHANGED To date, the freeformer is the only AM system that can process the FDAapproved TPE Medalist MD 12130H (hardness 32 Shore A) and, without changing processing parameters, adjust the filling level of the part — how close together the droplets are — to fine-tune mechanical properties and achieve different hardnesses (Figure 2). For example, at a fill level 100% (that is, drops as dense as possible), maximum mechanical strength and stiffness is achieved. At a fill level of 20%, for example, there is a greater distance between the drops and the part is more flexible. It is even possible to create different material densities in different parts of the same a component. A current research project at the University of Belfast, Ireland, is looking at how vaginally inserted rings loaded with active ingredients can protect women from HIV infection.1 Using medical-grade TPU, rings with different filling levels (100, 50 and 10%) were investigated. The lower the filling level, the more porous the TPU ring and the greater the active ingredient release. Result of the study: at a filling level of 50%, about 60 of a total of 111 mg of active ingredient are released over a period of 30 days. This compares with only five out of a total of 190 mg for an injection-moulded ring. In addition, the APF process is also gentler than injection moulding, so there is less temperature degradation, less stress and the active ingredient remains more stable.
Permanent implants are also being produced using the APF process. For instance, spinal implants have been made using Bionate thermoplastic PCU (polycarbonate polyurethane), and a multimaterial meniscus (using different types of polyurethane) were developed within a few days eliminating the time-consuming (and more complicated) development of an overmoulded part produced by conventional overmoulding. MEDICAL AIDS The APF process is also used for medical devices and aids. The freeformer processes medically approved SEBS (styrene-ethylene/ butylene-styrene) (Cawiton PR13576) with a hardness of 28 Shore A, for instance. This very soft material is dense and tear-resistant and is suitable for producing items such as functional bellows. Another typical example is sawing templates made from PA, which are used as customised surgical aids. Flexible and electrically conductive strain gauges are one example of future developments. These consist of soft TPU material (Desmopan) with carbon components and an inserted LED. The two-component functional part produced with the freeformer is both flexible and electrically conductive. Depending on the strain and thus the electrical resistance, the LED lights up with different brightness. Strain gauges of this kind could be
FREEFORMERS ARE SUITABLE FOR CLEANROOMS With just a few minor modifications, all freeformers are suitable for use in cleanrooms. They operate with low emissions, are virtually dust-free, and their build chamber is generally made from stainless steel. An optional robotic interface allows the AM to be automated and the freeformer to be integrated into IT-networked production lines. Process quality can be reliably documented and the components individually traced if required. CONCLUSION The APF process with the freeformer is particularly suitable for AM in medical technology. Geometric freedom combined with material freedom opens up completely new plastic applications, including for use within the human body. REFERENCE 1. https://www.sciencedirect.com/science/article/abs/pii/S0378517319307707
Figure 2: Part density can be selectively influenced with the APF process. Honeycomb structures can be produced from TPE Medalist MD 12130H (hardness 32 Shore A). 24
W W W. M E D I C A L P L A S T I C S N E W S . C O M
Cofinanciado por:
COMPONENTS
AS MANUFACTURERS OF DIALYSER FILTER CARTRIDGES INCREASINGLY MOVE AWAY FROM USING PC, DIDIER PERRET, MEDICAL BUSINESS DEVELOPMENT MANAGER, BRANSON WELDING AND ASSEMBLY AT EMERSON EVALUATES NEW ASSEMBLY METHODS THAT BEST SUIT THE ALTERNATIVE MATERIALS.
NEW ASSEMBLY METHODS
FOR PP DIALYSER FILTER CARTRIDGES
P
atients suffering from endstage renal failure rely on haemodialysis (or simply dialysis) for survival. A machine known as a dialyser acts like an external, artificial kidney, removing blood from the body, filtering out harmful impurities and returning the blood to the body. All such machines include a cartridge containing extremely fine filtration media encased in a plastic housing (Figure 1). The industry, however, is undergoing a major shift in the type of polymer used to make these cartridges, which is affecting all players in the market, from contract manufacturers to brand owners. Historically, these tubular housings have been commonly made of polycarbonate (PC), an amorphous thermoplastic polymer with excellent dimensional stability, high strength, and good heat and cold resistance. Yield strength is high for this polymer (in the 58–70 MPa range), and its coefficient of friction is approximately 0.40. These properties are advantageous when mechanical assembly methods (screw-type fasteners) are used. Properly tightened screws will not loosen for up to 48 months. The surface energy of PC (46 dynes/cm) is also high among plastic materials, so gluing — using a range of affordable glue types — has also been a common bonding technique. Until recently, these assembly techniques and materials have been used by most of the major filter-cartridge manufacturers, but that situation is changing. Not only is PC a relatively expensive polymer, costing two to five times more than polypropylene (PP), it is also more difficult to process because it must be dried before it can be molded or extruded. Even more concerning to manufacturers and brand owners in the kidney dialysis market is that PC contains bisphenol A (BPA) — a chemical that has been linked to adverse health effects.1 BPA-containing plastics have not
26
Figure 1: Kidney dialysis machines (dialysers) incorporate a filtering cartridge such as this one in lower right. They consist of extremely fine filtration media encased in a plastic housing. Image courtesy of Emerson. been banned primarily because normal kidney function easily eliminates low levels of this compound. According to recent studies, however, serum BPA levels accumulate as renal function decreases, and are highest in individuals with chronic kidney disease who are on haemodialysis. The safety of BPA in the general population, therefore, cannot be extrapolated to the dialysis population. THE PP ALTERNATIVE Given the issues with PC, dialyser filter manufacturers are increasingly turning to alternative materials. In some cases, acrylonitrile butadiene styrene (ABS), polyphenylene sulphide (PPS), polysulfone (PSU), poly(phenylene sulfide− phenyleneamine) (PPSA) or polyethylene (PE) are used, but polypropylene (PP), in most cases, is the preferred choice. PP is nontoxic, tasteless, low density, unaffected by humidity, and relatively inexpensive and easy to process. Unfortunately, this thermoplastic polymer becomes brittle at low temperature, has a low coefficient of friction and low yield strength (approximately 12–40 MPa), all of which makes it unsuitable for screw-type fasteners. Even under compression, a correctly tightened assembly will loosen within a relatively short time. Likewise, PP is a poor candidate for gluing, as its surface energy is so low (30 dynes/cm). Making the gluing process effective requires plasma or other expensive and time-consuming surface treatment. The best solution for PP assembly, therefore, is welding, for which there are at least three good options: ultrasonic welding, laser welding and spin welding. Each method has
W W W. M E D I C A L P L A S T I C S N E W S . C O M
COMPONENTS
ABOVE | Figure 2: Typical joint configurations are shown here. Graphics courtesy of Emerson. BELOW | Figure 3: This schematic drawing shows various elements of a typical dialyser and indicates which joining technique(s) can be used for specific components. Courtesy of Emerson.
90° typical PC ABS…
110° typical PP PE…
Alternative for PP PE…
advantages and disadvantages. By considering the full picture and the best investment/benefit ratio, manufacturers can receive the best technology to satisfy their needs. ULTRASONIC, LASER AND SPIN WELDING Ultrasonic welding has been used for more than 70 years to join thermoplastic parts that would be too complex or prohibitively expensive to mould in one piece. The process is fast, flexible and economic, making it ideal for large series production, and it is easily controlled and automated. The latest systems set a new industry standard for outstanding weld quality, process reliability, intelligent process controls and data gathering. Ultrasonic welding does require a one-time, upfront investment in the necessary equipment and application-specific tooling to precisely hold the various plastic components in place during the welding process, which usually takes one second or less. Other than power, there are no incremental consumable or assembly costs, regardless of production volume, so return on investment is normally predictable and rapid. Ultrasonic welding can also positively influence a product’s sustainability rating due to the lack of solvents and improved energy efficiency. Spin welding can be used to bond both ends of a filter cartridge. It can provide a strong bond with simple joint designs (Figure 2) but this can create some undesirable particulates. The newest trend in dialyser filter assembly is laser welding, which requires a higher capital investment for equipment compared with ultrasonic welding.
Given the issues with PC, dialyser filter manufacturers are increasingly turning to alternative materials
Components are pre-assembled before welding, and no vibration or movement is required to produce clean, particulate-free welds. In operation, multiple laser beams apply energy along the full length of weld surface. One surface freely transmits the laser energy (without itself being affected) through to the second (laserabsorbing) surface where laser energy is converted to heat that is conducted across the interface, creating the weld. Usually, the transmissive surface is more or less clear and the absorptive layer is darker, but that does not always have to be the case. Using an overmoulding method or various coatings and additives, an otherwise laser-transparent material can be made to absorb laser radiation, making it possible to create clear-on-clear assemblies. OTHER APPLICATIONS IN KIDNEY DIALYSIS SYSTEMS Filtration cartridges are just one component in a complex system of pumps, flow meters, valves, a ‘bubble trap’ (which prevents any air bubbles going back to the patient’s body), as well as controls and proportioning systems for dialysate solution, which pulls toxins from the patient’s blood. These devices are made of polymeric material and are assembled effectively using the aforementioned welding processes. Figure 3 shows these devices and indicates which process(es) are commonly used to make them. Similar technologies may be used to produce the fibre filtration elements housed in the filtration cartridge. The fibres may be cut to length using ultrasonics, and the cut ends may be cauterised using an Emerson IR emitter — a metal-foil heater that emits infrared wavelengths matched to the absorption characteristics of the polymer fibres. CONCLUSION Most dialyser filters are produced by contract manufacturers and then assembled into finished products by a machine builder who supplies them to the market authorisation holder or brand owner/marketer. As the industry turns from traditional materials such as PC to cheaper, BPAfree PP, manufacturers can simplify their products, reduce costs and improve performance by shifting from adhesives and fasteners to plastic welding methods. REFERENCE 1. https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC4602822/
WWW.MEDICALPLASTICSNEWS.COM
27
ADVERTISEMENT FEATURE Figure 1. Representative illustration and scanning electron microscope image (inset) of sintered porous plastics.
TOP TIPS FOR DESIGNING MEDICAL DEVICES WITH SINTERED POROUS PLASTICS HILARY BISHOP, PRODUCT MANAGER – VYON® POROUS PLASTICS AND RICHARD MORGAN, PROCESS MANAGER — VYON® POROUS PLASTICS EXPLAIN HOW SINTERED POROUS PLASTIC MATERIALS CAN PLAY AN INTEGRAL ROLE IN THE PERFORMANCE OF MEDICAL DEVICES. New product development (NPD) for medical devices is a lengthy, multistep process often layered with regulatory requirements. In a rapidly evolving market, designers and engineers are under pressure to deliver new and innovative medical devices to meet changing market demands. The global response to the Covid-19 pandemic crisis increased pressure for supply of not only final products, but their components and raw materials. Sintered porous plastics may not always be the first material or even the most familiar material to which engineers turn when designing a medical device. Yet, the versatility and ability to adapt to diverse
28
applications have made sintered porous plastics a key component found at the heart of world leading medical devices. WHAT IS A SINTERED POROUS PLASTIC? Sintered porous plastics are manufactured from thermoplastic polymers to meet different market demands. Through a process of heat and pressure, plastic powders are bonded together to create a porous network composed of tortuous interconnected pathways (Figure 1), a structure that ultimately defines the key characteristics and behaviour of the final material.
KEY PROPERTIES The top two properties of porous plastics are the size and distribution of pores (pore size). They are key determinants of the material’s permeability, filtration efficiency, porosity and strength. Permeability is a material’s capacity to allow the flow of liquids and gases through the porous structure, whereas filtration efficiency is a measure of the material’s ability to stop particles passing through. Finally, porosity is the measure of pore or void space within the material. APPLICATIONS Filtration: The pore size and tortuous path of sintered porous plastics offers
W W W. M E D I C A L P L A S T I C S N E W S . C O M
excellent depth filtration for liquids and gases and are, therefore, effective at filtering out a large variety of particulates. In addition, they can act as efficient bacterial filters by preventing microbial ingress — characteristics often desirable for point-of-care and surgical devices. Venting: From simple to complex medical devices, venting is typically required to relieve pressure from a closed system or prevent substances from escaping into the external environment. Permeability and pore size play a key role in the material’s ability to efficiently vent a system.
ADVERTISEMENT FEATURE
… the versatility and ability to adapt to diverse applications have made sintered porous plastics a key component found at the heart of world leading medical devices Absorption: Sintered porous plastics’ uniform porosity enables the controlled wicking of substances such as chemicals, fragrances and drugs. This makes them ideal materials for use in lateral flow tests, which require good capillary flow through the device. Lateral flow devices have always played a significant part in medical testing such as pregnancy tests but are now even more prevalent as they are used for many Covid-19 tests. Application: The selfsupporting and rigid structure of a sintered porous plastic together with its controlled pore size and porosity enables it to be ideal for use in topical (device to body) applicators, such as wart removal and wound closure devices. This is particularly useful in both drug delivery and wound closure applications. Media support: It is the pore size and self-supporting structure of sintered porous plastics that will retain the required media without any breakthrough. Media support is a key attribute in medical devices such as dialysis cartridges. TOP TIPS TO START STRONG Collaborate, collaborate, collaborate Working openly with your partner or supplier
is the number one tip for any engineer or product developer. Although this sounds like a nobrainer, often delays and miscommunication can arise during the early stages of the development process. Open collaboration allows innovative and practical design ideas to come to life; most importantly, it ensures you find the best solution. Whether you are replacing a known porous plastic component or exploring porous plastics for the first time as part of a new design, working early in product development process with experienced, knowledgeable, and collaborative partners is key. Function As part of your medical device design, you’ll have a plethora of constraints to consider but it is key to know i) exactly what you want the porous plastic to do ii) or whether you want it to fulfil more than one function. Porous plastics designed for filtration can look very different to those used in wicking and absorption applications. Sharing these required functions early will enable you to evaluate which sintered porous plastics are most closely aligned with your application. Design This is where working closely with your supplier(s)
can pay dividends. Although porous plastics can be formed into a wide range of geometries from large, machined sheets to small discs and complex 3D shapes, you don’t want to complete your design before learning whether it can be effectively manufactured. Conducting manufacturing feasibility from the outset will save you time and cost in your design process. Identifying the key parameters of your product, including critical dimensions and tolerances, will enable them to be incorporated into any test or measurement procedures during the manufacturing process. Approvals and regulations Medical devices and regulations work hand-inhand. Many sintered porous plastics available meet common FDA, USP and EP regulatory approvals. Knowing what you need will ensure that the chosen material which meets this is used to develop your porous component. This also applies to the chemical compatibility. Some sintered porous plastic materials react with different fluids better than others. Assembly How is the porous plastic component going to be assembled into your final medical device? Sintered porous plastics can be incorporated into devices using a variety of different methods including over moulding, welding and press-fitting. In press-fitting, the degree of interference fit is important to ensure a good seal. Sterilisation or post processing Does your assembled device need to go through a sterilisation cycle? Many porous plastics can be sterilised using, for example, gamma irradiation,
WWW.MEDICALPLASTICSNEWS.COM
ethylene oxide or electron beam. In addition, porous plastics can be treated in several ways including hydrophilic plasma treatment and ultraclean treatments to ensure there are low levels of contaminating leachables and extractables. In addition, the material can be treated to bestow chemical and biological functionality for applications such as the attachment of biomolecules. Timelines and qualification Familiarise yourself with the timelines and milestone you need to hit throughout the development process. Consider whether your final assembled product including the porous plastic component needs external and internal approvals. With many sintered porous plastics being manufactured to bespoke designs, it is essential to share this information with your supplier early in the process. This will ensure the collaborative team will deliver the sintered porous plastic solution at the right time. SUMMARY Sintered porous plastic materials can play an integral role in the performance of medical devices and so require careful thought and understanding throughout the development process. Collaboration is key and technical accuracy is paramount; harmonising the two ensures greater success in both the manufacturing of sintered porous plastics and design of medical devices.
enquiries@porvairsciences.com www.porvairsciences.com
29
On show at Compamed & Medica
1
Coveris has presented recyclable solutions at Compamed following guidelines on better packaging solutions.
2
Mackwell Health has showcased a combination of wearables and support toolkits at this year’s Medica 2021.
3
Cathetrix has presented its new catheter stabiliser for prevention of UTIs and accidental Foley catheter extractions at Medica 2021.
4
Benvic exhibited at Compamed 2021 this year, for the first time represented together by Modenplast and Luc & Bel.
11:2021 COVESTRO LAUNCHES NEW DURABLE MATERIAL
C
ovestro has launched new durable materials for medical devices housings and hardware. The new Makrolon M6011 FR, a medical polycarbonate, and Makroblend M5005 FR, a medical polycarbonate/polyester blend, offer a differentiated composition of properties that improve chemical resistance while using next-generation flame retardants. Covestro has more than 50 years of polycarbonate manufacturing experience in the medical devices industry. As Hospital Acquired Infections (HAIs) rise and the COVID-19 pandemic persists, these two new materials provide improved disinfectant resistance in response to today’s challenging market requirements for housings of medical devices. The new polycarbonate
materials now comprise the most stringent UL rating – UL 94 V-0 – and nextgeneration flame retardancy compared to previous inmarket products. In addition, these medical-grade products offer enhanced impact strength, increased rigidity, as well as UV resistance and improved flowability. Irving Paz Chagoya, Covestro’s healthcare segment manager in EMEA, said: “The value proposition for these new durable materials is two-fold. First, these products use nextgeneration phosphorous based flame retardants. Second, these new polycarbonate materials comprise a variety of attributes— improved chemical resistance, impact strength, heat resistance, and so on—in a single solution.”
Compounding Solutions Europe becomes Evonik Ireland distributor
C
ompounding Solutions Europe, a custom medical polymer compounding specialist, has been named the distributor of Evonik’s portfolio of VESTAMID Care ML polyamide 12, VESTAMID Care ME polyether block amide (PEBA), TROGAMID Care transparent polyamide, and VESTAKEEP Care PEEK medical grade polymers for the Ireland medical device market. Evonik’s medical grade high-performance polymers are used throughout the medical device industry in a variety of applications including housings, imaging devices, surgical tools, and precision components. Compounding Solutions will offer Evonik medical grade polymers in quantities of 25kg to support the entire medical device development process,
from early stage prototyping through full-scale production. Scott Neal, general manager of Compounding Solutions, said: “I am excited about this opportunity for both Compounding Solutions and Evonik. Having the Evonik medical product range available locally in Ireland, backed by Compounding Solutions market leading customer support and material expertise will be mutually beneficial to both companies and the industry as a whole.” Marc Knebel, head of the medical systems business at Evonik, added: “Evonik and Compounding Solutions already have a successful partnership in the U.S. market which will now be strengthened in the long term by cooperation in Europe.”
Owen Mumford announces emissions reduction targets
O
wen Mumford has announced its emissions reduction targets through the Science Based Targets initiative (SBTi). The company has set a target to achieve net zero by 2045 (to mirror the UK NHS target) in alignment with the recommendations of the 2015
30
Paris Agreement. Using science-based targets, companies can determine how much and how quickly they need to reduce their greenhouse gas (GHG) emissions to prevent the worst effects of climate change. The Science Based Targets initiative
(SBTi) sets science-based emissions reduction targets in line with climate science. As part of its sustainability action plan, Owen Mumford has joined the Business Ambition for 1.5°c commitment and is one of the first 1,000 global businesses to sign up. Launched in 2019,
W W W. M E D I C A L P L A S T I C S N E W S . C O M
the initiative asks members to pledge to achieve net zero emissions by the latest 2050 following the United Nations Intergovernmental Panel on Climate Change (IPCC) warning that an increase of 1.5°c in global temperature will have catastrophic effects.
From design to manufacturing, we partner in your device strategy
NEMERA: YOUR COMBINATION PRODUCT PARTNER
Patients first. Always.
Partnering in drug delivery devices. Holistically.
Going the extra-mile. Together.