Review of Verticillium Wilt of Row Crops



PUBLISHER: Jason Scott
Email: jason@jcsmarketinginc.com
EDITOR: Marni Katz
ASSOCIATE EDITOR: Cecilia Parsons
Email: article@jcsmarketinginc.com
PRODUCTION: design@jcsmarketinginc.com
Phone: 559.352.4456
Outsmarting Your Fertilizer “Competition” to Improve Uptake Efficiency
Insights into Scouting for Pierce’s
Alejandro Hernandez
Graduate Student, Department of Plant Pathology, UC Davis
Steven Koike Director/Plant Pathologist, TriCal Diagnostics
Patricia Lazicki Ph.D., Postdoctoral Researcher, University of Tennessee, Knoxville
Mark C. Siemens Associate Specialist, Biosystems Engineering, University of Arizona
Mark S. Sisterson USDA-ARS, San Joaquin Valley Agricultural Sciences Center
Krishna Subbarao Plant Pathologist, UC ANR
Florent Trouillas
Associate Professor of Cooperative Extension, Department of Plant Pathology, UC Davis
Dr. Karl A. Wyant Director of Agronomy at Nutrien, Western Region Certified Crop Advisor Board Chair

Surendra Dara
Director, North Willamette Research and Extension Center
Kevin Day
UCCE Pomology Farm Advi sor, Tulare and Kings Counties
Elizabeth Fichtner UCCE Farm Advisor, Kings and Tulare Counties
Katherine Jarvis-Shean UCCE Orchard Systems Advisor, Sacramento, Solano and Yolo Counties
Steven Koike Tri-Cal Diagnostics
Jhalendra Rijal UCCE Integrated Pest Management Advisor, Stanislaus County
Mohammad Yaghmour
UCCE Area Orchard Systems Advisor, Kern County
The articles, research, industry updates, company profiles,

this
Crop
writers
does not assume
 By STEVEN KOIKE | Director/Plant Pathologist, TriCal Diagnostics and KRISHNA SUBBARAO | Distinguished Professor of Extension, UC Davis
By STEVEN KOIKE | Director/Plant Pathologist, TriCal Diagnostics and KRISHNA SUBBARAO | Distinguished Professor of Extension, UC Davis
Of the many plant pathogens that affect crops, few can rival the fungus Verticillium in its ability to infect a broad range of plants, persist in the soil for many years and cause crip pling economic crop losses season after season. While in this article we focus on Verticillium wilt of row crops caused by Verticillium dahliae, this disease also affects many ornamental crops, forest and landscape species, perennial tree and vine fruit crops, field and forage species, and even a number of weeds. While V. dahliae is known as a pathogen on the dicot group of flowering plants, it can also reproduce on monocots; colo
nization on monocots can augment soil inoculum levels and create problems for dicot hosts. Diseases caused by Verticil lium species further impact the agricul tural world because of the resources ex pended on studying the pathogens and fashioning management strategies. Huge financial commitments and the work of many dedicated research pathologists and plant breeders have been devot ed to dealing with Verticillium. Our understanding of Verticillium wilt has significantly increased over the years, though the disease remains a threat to many agricultural commodities.
As a soilborne pathogen, this fungus produces large numbers of microscopic, thick-walled, black pigmented masses of cells called microsclerotia. These microsclerotia can remain inert in the soil for many years until the root of a susceptible plant grows in the vicinity, at which time the microsclerotia germi nate and infect the root. Estimates vary, but researchers believe that dormant microsclerotia can remain viable in soil for as long as 8 to 10 years without a plant host being present. This long persistence in soil helps explain why V.
dahliae is such a long-term concern for growers.
In addition to being soilborne, iae can be seedborne in hosts such as lettuce and spinach. As plants develop flowers and later seed, move systemically in the plant xylem from infected root, through the stems and into these reproductive structures. The mature, harvested seed therefore can harbor the pathogen, which can later infect the developing seedling.

Many Verticillium ated with plants. The most important and widespread species is which is reported to cause disease on hundreds of hosts throughout the world, including a large number of row crops. Other Verticillium narrower niches; example, primarily infects plants in the brassica plant family. found in Europe and North America, primarily on potato. is thus far reported only from Japan, and the only row crop it infects is let tuce. Table 1 (see page 4) lium species that are associated with plants and provides examples of their respective row crop host ranges.
Researchers find that not all isolates of V. dahliae are the same. V. dahliae iso lates can be divided into groups called “races.” In plant pathology, races belong to the same species of fungus but have distinct genetic profiles and have the ability to infect crop cultivars that carry different resistance genes. Presently, V. dahliae isolates can be placed into one of three known groups: race 1, race 2 and race 3. These three races differ from each other in their molecular signatures and in the host cultivars they can infect. This race phenomenon can be very important when it comes to row crop cultivars. Plant breeders have developed cultivars that are resistant to V. dahliae race 1 in several row crops. However, when a new V. dahliae race appears, often the previously resistant cultivars become susceptible and significant crop loss can occur.

One label for multiple uses; industry-leading stability Short contact time & no additional rinse
Tank-mix friendly: boosts fungicide performance
Broad-spectrum, multiple modes of action
Stimulates plant immunity & prevents disease
Enhances tree health & overall stress tolerance
Active molecules prevent pathogen infections
Boosts plants ability to defend itself
Living spores provide protective shield from disease
Most Verticillium species are categorized as vascular plant pathogens because these organisms have the ability to colo nize and grow in the xylem tissues in plants. As V. dahliae becomes established in a host plant, it disrupts the flow of water in the xylem, resulting in foliar symptoms such as wilting, yellowing and necrosis (death of leaf tissue). Infected plants may remain small, stunted and non-productive. In advanced cases, the plant will collapse and die completely. Verticillium wilt disease does not cause rotting of the roots. Therefore, susceptible row crop hosts may be affected by Ver ticillium wilt but still have intact root systems.
Infection first occurs when roots grow near the dormant microsclerotia that reside in soil. The microsclerotia germi nate, grow into the root and colonize the water conducting tissue that makes up the xylem. From the root, the fungus grows up into the plant crown and then into the branches or petioles of the host. This systemic colonization blocks the xylem, the plant does not receive the water it needs and the wilting/yellowing/browning of the leaves will follow. Symptom expression is accentuated when the plant is under stress and needs additional water. Therefore, Verticillium wilt symptom severity tends to be worse during times of high water require ment, such as afternoons during the day and warm summer months during the year. Physiological stress factors can also trigger severe Verticillium wilt symptoms. Plants being grown
Vegetable crops such as lettuce are very susceptible to Verticillium wilt.

for seed may exhibit Verticillium wilt symptoms when they begin to bolt, since bolting and seed production are physi ologically taxing. Fruit crops such as strawberry can show severe Verticillium wilt effects following heavy fruit set, since supporting many fruits is a stress factor for plants.

In late stages of disease, tissues of V. dahliae-infected row crop plants will become colonized by large numbers of the micro


sclerotia that function as survival structures. When disked and in corporated back into the soil, these crop residues return additional in oculum to the already-infested soil. Soil preparation steps with tractors and implements will spread the microsclerotia throughout the field; for tractors and equipment that are moved to other fields, contaminated dirt and mud on the equipment and tires can introduce V. dahliae micro sclerotia to clean fields.
For most plant diseases, it is risky to base a diagnosis on symptoms only. This risk applies very much to the diagnosis of Ver ticillium wilt. There are a number of other diseases that can affect water uptake by a plant and result in wilting and stunting of plants and yellowing and browning of plant foliage. Root rots, crown rots and especially Fusarium wilt diseases can all mimic the symptoms caused by Verticillium wilt. For example, in lettuce in California, a root rot disease (Pythium wilt), two crown rots (caused by Botrytis or Sclerotinia) and two wilt diseases (Fusarium and Verticillium wilts) share virtually the same aboveground symptom profile (Table 2). Therefore, accu rate confirmation of Verticillium wilt will require clinical lab tests.
Because of V. dahliae’s broad host range and long persistence in soil, this pathogen remains a challenge to manage despite the many advances in our knowledge of the fungus. Managing Verticillium wilt is dependent on integrated pest management practices.
First, confirm that the symptomatic plants have Verticillium wilt. Other diseases and conditions may manifest symptoms similar to those caused by V. dahliae. Confirmation will require laboratory testing.
If available, plant Verticillium wilt-resistant cultivars because this is the most efficient option in combatting the disease. Resistant cultivars that have excellent yields and

quality are highly valued assets for the grower.




In the cropping sequence, include crop plants that are not hosts of Verticillium Especially avoid planting Verticilli um-susceptible crops back-to-back. The use of broccoli in crop rotations has been demonstrated to help reduce V. dahliae soil inoculum levels.



















If susceptible crops and cultivars must be planted, as much as possible place these crops in fields that do not have a history of the disease. Sensitive DNAbased soil tests can sometimes be used to determine disease risk prior to planting.
For some annual row crops, Verticillium wilt tends to be more severe when soil temperatures warm up in the summer, so plant sensitive crops early in the

spring. Lettuce in coastal California, for example, may have fewer losses from Verticillium wilt if planted in February rather than in the summer through fall months.
As much as possible, prevent the move ment of infested soil to clean fields. Because Verticillium microsclerotia are so small, it is impossible to completely prevent the movement of contaminated soil between fields. However, washing equipment and other measures may slow down such spread.

Where economically feasible, with strawberries, for example, deploy preplant soil-applied fumigants which can significantly reduce soilborne inoculum. Conventional fungicides and biological products have not been effective at man aging Verticillium wilt.

Comments about this article? We want to hear from you. Feel free to email

at









One fortunate aspect of working in agriculture is that hot summer days are soon followed by harvest season and the onset of nicer weather. Now is the time to start thinking about postharvest soil fertility maintenance. Dry, granular phosphorus and potassium fertilizer products (e.g., monoammonium phosphorus (MAP: 11-52-0); sulfate of potash (SOP: 0-0-50); and muriate of potash (MOP: 0-0-60)) are pop ular choices for postharvest fertility work and a cornerstone of annual nutrient budgets for many crops.

These fertilizer formulations are popular with growers as they can apply bulk nutrients to help maintain soil fertility and get a jump start on supporting the next crop cycle. However, fertil izer prices, at the time of this writing, are expected to remain elevated due to a perfect storm of global events outside much of our control. With that in mind, there are various sources of

Figure 1. Estimates of soil-applied fertilizer efficiencies (amount used by crop relative to how much was applied total to the soil.) Phosphorus and potassium have a relatively lower use efficiency compared to nitrogen due to some of the factors listed in this article. Anticipating and counter acting soil “competition” can help push up fertilizer use efficiencies and drive a higher return on investment for the grower (Fixen et al. 2014).
“competition” that prevent the uptake of the nutrient by the plant, which leads to inefficiency in fertilizer use, reducing optimum fertilizer return on investment (Figure 1).
In a perfect world, 100% of your fertilizer application would be used by the crop to produce yield, return on investment would be maximized, and fertilizer losses would be minimal. How ever, as Figure 1 shows, use efficiency of fertilizers vary greatly by nutrient category. In this article, we will name the various efficiency loss pathways, or “competition”, for your phosphorus and potassium fertilizer applications so you can work with a crop advisor to try and work around them. Before we jump in, let’s start with some phosphorus and potassium 101.
Why Crops Need Phosphorus and Potassium Phosphorus and potassium are plant macronutrients that are required in large quantities by the crop to produce a high-qual ity yield. However, phosphorus and potassium play different roles in the plant. Phosphorus is a crucial component for con verting solar energy into food, fiber and other plant products. Furthermore, phosphorus also plays a key role in the metab olism of sugars, energy storage and transfer, cell growth and the transfer of genetic information. An interesting bit about phosphorus is that one can point out the element embedded in the actual chemical structures the plant is using to create yield. For example, you can point to the actual phosphorus that is used to build the biological currency plants use to “pay” for work (ATP) and the phosphorus backbone that all crops use to construct their genes (DNA).
On the other hand, potassium is much more difficult to point out in the crops’ molecular portfolio. However, we do know that potassium is still needed by the plant to support high yields. Briefly, potassium increases crop yield, improves crop quality, reduces disease and decreases risk of lodging and branch breakage, etc. Except for nitrogen, plants often require
Figure 2. Five competitors that reduce your phosphorus fertilizer use efficiency by the crop. A crop advisor can help you understand how to best work around the competition and get more phosphorus into the crop (Fixen et al. 2014).
more potassium than any other nutrient as it plays vital roles in crop growth and development.

With the basics out of the way, we can now turn our attention to the factors that reduce your fertilizer uptake efficiency by the crop. I will refer to the factors as “com petition” because these factors interfere with your best laid fertilizer plans and will reduce your crops’ ability to use the nutrient. This will impact the return on your fertilizer investment when harvest comes around.
Phosphorus can be a tricky nutrient to manage because it interacts with other soil elements that will reduce overall fertilizer use efficiency (Figure 2

Due to these interac
Figure 3. Most soil phosphorus is tied up by calcium in the Western U.S. due to elevated soil pH (>7 or alkaline soil). Note the small fraction of phosphorus that is available for plant uptake in alkaline soils.
Soils - Part 6: Phos phorus and Potassium in the Soil – University of Nebraska Extension Service.

tions, the fertilizer use efficiency range can be quite low. For example, only 5% to 30% of the fertilizer might get used by the crop (Figure 1, see page 10). The rest is lost to the competition.
Diffusion refers to the movement of

phosphorus from the bulk soil toward the roots by way of a concentration gradient. Other plant uptake pathways exist but diffusion is the major pathway for phosphorus. For example, research shows that corn acquires ~93% of its phosphorus needs for the season via diffusion (Barber 1995; Fernandez 2016). What’s the catch? The rate of diffusion is sensitive to several factors that reduce
the speed of how fast the process, or rate, can occur. These soil factors include cold temperatures, compaction, saturat ed or dry conditions. When these fac tors reduce the diffusion rate, they can compete with your fertilizer application by not allowing the crop to be adequate ly supplied with phosphate.

In some cases, the over application of other nutrients can prevent plant uptake of phosphorus, which will reduce fertil izer use efficiency. For example, research shows that excessive applications of calcium and magnesium can interfere with the crop’s ability to use phosphorus (Better Crop 1999).
Biologicals restore resilience against disease and pest pressure. Crop yield and quality are best when grown in biologically rich and nutritionally balanced soil. This balance and soil bio diversity help plants avoid the need for toxins and other silver bullets to rescue crops.
Pacific Gro helps growers improve both soil health and crop quality.


Provides plant available calcium, amino acid nitrogen and many carbon compounds that help mobilize nutrients through the plant. Fish oil feeds beneficial fungi which improve soil structure and crop resilience.
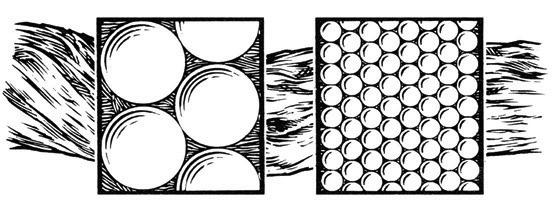
Competition for your phosphorus fertil izer efficiency can be influenced by soil pH and the conditions that result. For example, when soil calcium and phos phorus fertilizer come together under alkaline conditions (pH >7), they form a hard mineral called apatite, which is the same mineral that your bones and teeth are made of. Once this mineral fixation happens, the bound phospho rus is now unavailable for plant uptake, with reduces use efficiency. Figure 3 shows just how much soil phosphorus is made unavailable by the calcium complexes (up to 80% to 90% reduction in availability).
Phosphorus can bind to soil particles and move off the field during rainfall or during snowmelt. When soil leaves the field (erosion), it is more than likely car rying some phosphorus with it, reduc ing use efficiency. This impact is more pronounced on sloped fields or in areas where heavy rainfall occurs.
This might not be an obvious source of competition, but how your local ag lab analyzes the pool of soil phosphorus















matters. Briefly, soil phosphorus can be measured by three different methods: Bray, Mehlich and Olson. Your local soil pH should determine the extraction method as each works best within a cer tain pH range. A mismatch in method will overestimate or underestimate the phosphorus supply. The wrong test will lead to a bad fertilizer recommendation, which can be inefficient. In this case, your competition is bad information.
Now let’s turn our attention to the com petition for potassium. Crops are gen erally able to use more of the potassium provided by a fertilizer application rel ative to phosphorus. For example, 30% to 60% of potassium fertilizer might get used by the crop (Figure 1, see page 10). The rest is lost to the competition (Figure 4, see page 12).
Soils differ in their relative amounts of sand, silt and clay particles (e.g., tex ture), and this distribution impacts several important soil properties. For example, clay-dominated soils tend to have a high cation exchange capacity (CEC), which is the relative ability of a soil to store positively charged nutri ents. However, nutrient release rates are inversely related to the CEC value. In this sense, high clay soil will release stored potassium back to soil solution at a slower rate relative to sandy soil. Thus, clays can temporarily ‘tie up’ potassium fertilizer applications by storing it and then only slowly releasing it back to the soil for plant use, which can decrease efficiency.






This next competitor occurs under unique circumstances and has a longer lasting impact on your fertilizer pro gram. Certain clay minerals can scav enge potassium from the soil, including your potassium fertilizers, and render the nutrient unavailable for plant use. This process is called potassium fixation. Strong potassium fixation capacity is typically associated with the presence of vermiculite and mica-based materials in soil, in a specific age class, and from soils derived from granite parent ma terial (Pettygrove and Southard 2003). Fixed potassium, once tied up, is no longer available for plant use during the growing season and can have a serious impact on fertilizer use efficiency.

Sandy soils are poor at storing nutrients (e.g., low CEC). Furthermore, they also have a high nutrient release rate back to the soil. Under these conditions, po tassium fertilizers can leach out of the root zone, which reduces the fertilizer use efficiency. Thus, excessive rainfall or irrigation sets on sandy ground can compete with your crop for potassium.
Unlike phosphorus, ~20% of potassium is moved to the plant through the soil moisture in a process called mass flow (Barber 1995; Fernandez 2016). Exces sively dry soils at any depth throughout the rooting zone will prevent potassium from reaching the plant and reduce the
fertilizer use efficiency.
This section applies to those struggling with excessive sodium in the irrigation water or soil. In this case, the sodium can compete with your potassium fer tilizer plan by interfering with the crops’ ability to acquire the nutrient. Wakeel (2013) recently summarized the antag onistic effect of sodium on potassium uptake in detail. Briefly, excess sodium makes it difficult for potassium to be taken up by the crop and sodium also increases potassium leakage from the crop, which reduces use efficiency.
Now that we have named the competi tion, we can work to manage them to minimize their impact and get more of your applied nutrients into the crop where it belongs. Where to begin? Much of the competition mentioned above can be identified through routine soil testing and from sound advice from a local crop advisor. By using an updated soil report, you and your advisory team

can be on the lookout for conditions that might give your fertilizer compe tition a leg up in the field so you can manage them appropriately. For some other situations, a call to the local lab can confirm they are using the right extraction method and generating the correct fertilizer recommendation.
Local geological and soil series reports, if you suspect potassium fixation, can be invaluable for understanding conditions in your field that could be competing for your potassium.
As reviewed here, several competitors are working to reduce the use efficiency of fall fertilizer applications. If you are concerned about improving nutrient uptake in the crop and keeping the phosphorus and potassium out of the hands of the competition, consider contacting a local crop advisor to help develop a field-specific management plan. Once your competition is formally identified, using this article as a guide, it can be better managed on your farm and fertilizer use efficiency for phospho rus and potassium can be increased.
Dr. Karl Wyant serves as the Director of Agronomy at Nutrien and as the Board Chair for the Western Region Certified Crop Advisor group (www.wrcca.org). Please visit www.nutrien-ekonomics. com and www.smartnitrogen.com for the latest in nutrient management topics and other free resources.

Fixen et al. 2014. Nutrient/Fertilizer

Use Efficiency: Measurement, Current Situation and Trends
Better Crops/Vol. 83 (1999, No. 1). Phosphorus Interactions with Other Nutrients
Wakeel 2013. Potassium–sodium interactions in soil and plant under saline-sodic conditions
Comments about this article? We want to hear from you. Feel free to email us at article@jcsmarketinginc.com


 By MARK S. SISTERSON | USDA-ARS, San Joaquin Valley Agricultural Sciences Center
By MARK S. SISTERSON | USDA-ARS, San Joaquin Valley Agricultural Sciences Center

fastidiosa is a widespread bacterial plant pathogen. The pathogen is xylem-limited and is spread from plant-to-plant by xylem sap feeding insects, including several species of sharpshooters and spittlebugs. The bacterium is the causal agent of a wide variety of economically important plant diseases, including Pierce’s disease of grapevine and almond leaf scorch disease. From 2016 to 2018, USDA-ARS conducted a field study in Kern Coun ty during a Pierce’s disease epidemic associated with elevated populations of the glassy-winged sharpshooter, Homal odisca vitripennis (Sisterson et al. 2020). Removal of vines affected by Pierce’s disease is an important component of disease management, although identi fying infected vines for removal can be challenging as many of the symptoms associated with Pierce’s disease are generic, symptom expression varies with cultivar and symptom expression varies with time of year. In conjunction, leaf petiole samples from vines were collect ed monthly and subjected to diagnostic testing to confirm presence of X. fastid iosa. Optimal times to observe symp toms and collect samples for diagnostic testing are discussed. In addition, princi ples and concepts regarding effective use of roguing (removal of infected vines) to manage spread of insect-transmitted plant pathogens are presented.
X. fastidiosa in California Diseases caused by X. fastidiosa are a worldwide problem, with the geograph ic range of X. fastidiosa limited by low temperatures at higher latitudes. There are several recognized subspecies of

Figure 1. Epidemiology of Pierce’s disease varies with geographic region. Differences in epide miology among regions are driven by differences in key vectors, agroecosystem composition and climate. A) On the North Coast, the blue-green sharpshooter (Graphocephala atropunctata) is the primary vector. B) In the Central Valley, the primary vector is the green sharpshooter (Drae culacephala minerva). C) In Southern California and portions of the southern Central Valley, the glassy-winged sharpshooter (Homalodisca vitripennis) is the key vector. Purple shading in the map shows the location of vineyards in California.
X. fastidiosa, and the occurrence of disease depends on the host plant and X. fastidiosa subspecies combination. Pierce’s disease, caused by X. fastidiosa subspecies fastidiosa, is present in all grape-growing regions of California, although rates and patterns of pathogen spread vary with geographic region. Differences in rates of pathogen spread among regions may be attributed to three factors: behavior of the primary vector species, proximity of the vine yard to vector and/or inoculum source habitats and climate. As a result, Pierce’s disease is a minor problem in some geo
graphic areas, and in other areas, losses from Pierce’s disease can be devastating.
Within California there are three key vectors of X. fastidiosa, and each vector is associated with a geographic region (Figure 1). The blue-green sharpshoot er (Graphocephala atropunctata) is associated with Pierce’s disease on the North Coast of California, the green sharpshooter (Draeculacephala miner va) is associated with Pierce’s disease throughout much of the Central Valley

Only Radiant® SC insecticide controls worms, thrips and leafminers. (“3 Bugs. 1 Jug.”) And university trials in Arizona and California show that Radiant outperforms other commonly-used vegetable insecticides on all three of these pests. As a member of the spinosyn class of chemistry (IRAC Group 5), Radiant controls pests like no other class of chemistry used in vegetables. The Re-Entry Interval is only 4 hours, and the Pre-Harvest Interval is 1 day for most crops.

us at corteva.us
of Corteva Agriscience and its affiliated companies. Always read and follow label directions.
Corteva
and the glassy-winged sharpshooter is associated with Pierce’s disease in southern portions of the Central Valley and in Southern California. Vectors of minor importance include the red-headed sharpshooter (Xyphon fulgida) and the meadow spittle bug (Philaenus spumarius). Habitats outside of vineyards play an important role as vector sources for all three vector systems in California. The blue-green sharpshooter and the glassy-winged sharpshooter overwin ter in habitats outside of vineyards. The blue-green sharp shooter overwinters in riparian areas and the glassy-winged sharpshooter overwinters in citrus. Both vectors move out of overwintering habitats and into vineyards during spring,
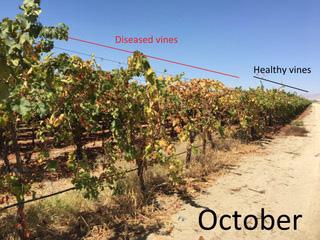
Figure 3. Vines chronically infected with Xylella fastidiosa display delayed budbreak in spring and lack vigor relative to healthy vines. The vine on the left is healthy and the vine on the right was confirmed positive for X. fastidiosa using diagnostic testing.



and both vectors will reside in vineyards throughout the growing season. In contrast, green sharpshooters build up large popula tions in irrigated pastures or poorly man aged alfalfa fields. As the green sharpshoot er is primarily a grass feeder, movement into vineyards is transient and populations rarely establish in vineyards unless irrigated grasses are present.

Pathogen spread can be divided into two categories: primary spread and secondary spread. Primary spread occurs when vectors acquire the pathogen outside of the vine yard and then move into the vineyard. In this case, the source of inoculum and the origin of the vector is outside the vineyard. Primary spread occurs in all three vector systems in California. Irrigated pastures and weeds found in riparian habitats and citrus orchards can serve as hosts for X. fastidiosa. Further, while the host range of the three sharpshooter species differs, each is known to feed on a wide variety of plant species. Secondary spread occurs when the source of the inoculum is present in the

vineyard. In this case, if a vine infected via primary spread remains in the vine yard, it is possible for vectors to acquire the pathogen from that vine and trans mit the pathogen to neighboring vines. In areas where the green sharpshooter is the primary vector, risk of secondary spread is low as movement of green sharpshooters into vineyards is tran sient and populations rarely establish. In contrast, in areas where the glassywinged sharpshooter and blue-green sharpshooter are the primary vectors, risk of secondary spread is higher as populations can establish in vineyards.
In systems with only primary spread, number of infected vines is expected to increase at a steady rate proportional to the number of inoculative insects that move into the vineyard from source habitats each year. With primary spread only, the spatial patten of infected vines is expected to appear random, with infected vines more likely to occur on vineyard edges that are in proximity to insect source habitats. In systems with primary and secondary spread, number of vines infected each year is expected to increase exponentially as each newly infected vine contributes to infecting


more vines. In systems with primary and secondary spread, spatial patterns of infected vines may initially appear random, with clusters of infected vines developing over time. In systems with secondary spread, prompt removal of infected vines is critical for preventing infected vines from serving as a source of inoculum for spread to neighbor ing vines. In contrast, in systems with only primary spread, infected vines do not contribute to in-field pathogen

spread. Accordingly, in systems with primary spread only, the decision to remove infected vines is based on the economic value of the infected vine. However, even in systems that appear to be dominated by primary spread, the only way to ensure that an infected vine does not serve as a source of inoculum is to remove it.
In areas with a history of Pierce’s disease and risk of secondary spread, vineyards should be surveyed to identify Pierce’s disease affected vines for remov al. Typical symptoms of Pierce’s disease include leaf scorching, match sticks (leafless petioles) and green islands (irregular lignification of canes). How ever, symptom expression varies with


cultivar and with time of year. Some cultivars display symptoms consistent to typical descriptions and others may de viate (compare Figure 2A and 2B, see page 18). Due to variation in symptom expression and similarities of Pierce’s disease symptoms with other diseases or drought-like responses, accurately iden tifying Pierce’s disease affected vines based solely on symptoms can be diffi cult. Ideally, a portion of vines identified during surveys should be subjected to diagnostic testing to confirm presence of X. fastidiosa. While it may not be necessary to test every candidate vine, testing a subset allows for determining accuracy of the surveyor and confirm presence of the suspected pathogen.
In the first year of infection, symptoms of Pierce’s disease may be mild. As a
result, vines showing clear symptoms of Pierce’s disease have likely been infected for more than one year. In the spring, vines with Pierce’s disease display delayed budbreak and a lack of vigor relative to neighboring healthy vines (Figure 3, see page 18 and 4A, see page 19). While a lack of vigor is noticeable throughout the season, foliage of infect ed vines did not display leaf scorching systems during April, May and June (Figure 5). Foliar scorching symptoms began to appear in July, getting progres sively worse as the season progressed. Canes from vigorously growing neigh boring healthy vines encroached on the canopies of infected vines (Figure 5). As a result, canopies of infected vines often appeared healthy as symptomatic leaves
Figure 5. Symptom progression in the cultivar Flame Seedless. Photos are of the same vine were taken on the date specified during 2018. Some photos were taken from the north side of the vine and others from the south side. Note the lack of vigor evident in April and May and that scorched leaves did not appear until July. The canopy of infected vines is encroached upon by the growth of canes from neighboring healthy vines. As a result, symptomatic leaves may be hidden behind what appears to be healthy foliage that originates from neighboring healthy vines. The vineyard was in a glassy-winged sharpshooter infested area in Kern County







Figure
Probability of detection of X. fastidiosa in known X. fastidiosa-positive vines via quantitative polymerase chain reaction (qPCR) based on month of sample collection. Petiole samples were most likely to test positive in late summer or early fall, following a pattern similar to that observed for the onset of symptomatic foliage observed in Figs. 4 and 5. Samples from the vines depicted in Figs. 4 and 5 were two of the 54 vines monitored to make this figure. Leaf petiole samples were collected from vineyards in a glassy-winged sharpshooter infested zone in Kern County. Cultivars sampled included Flame Seedless, Red Globe and Sugraone. For full methods see Sisterson et al. 2020.
Figure 7. Example of a severely pruned vine that pushed new growth that is positive for X. fastidiosa. For roguing to be effective, the entire vine must be removed. Photo taken during October 2016 in a vineyard in a glassy-winged sharpshooter infested zone in Kern County.

were hidden behind healthy foliage originating from neigh boring healthy vines.

During the 2016-18 field study in Kern County, leaf petioles were collected from vines monthly and subjected to quanti tative polymerase chain reaction (qPCR) to confirm presence of X. fastidiosa, with many of the vines monitored over two seasons. Likelihood of a vine testing positive depended on when the sample was collected and followed a pattern sim ilar to that of onset of leaf scorching symptoms (
Specifically, leaf petiole samples collected between budbreak and the beginning of August did not test positive. Thus, while delayed budbreak and a lack of early season vigor are useful symptoms for identifying candidate vines for diagnostic testing (Figure 3, see page 19 and 4A, see page 19), collection of foliage for diagnostic testing in spring is likely to result in a false negative. If diagnostic testing of vines displaying delayed budbreak is needed, such vines should be flagged and samples for diagnostic testing collected later in the year. Leaf petiole samples collected in August, September and October when foliar symptoms were present were most likely to test positive (Figure 6).
Simulation models of disease progression indicate that rogu ing can be an effective tactic for slowing pathogen spread in perennial cropping systems, provided several conditions are met (Sisterson and Stenger 2013). First, infected vines must be identified and removed promptly. As indicated earlier, identification of vines affected by Pierce’s disease in the first year of infection can be difficult. Thus, if infected vines are not recognized until they have been infected for one or more years, the vine may have already served as a source of inocu lum for further in-field spread. Second, the entire vine must be removed because Pierce’s disease cannot be eliminated by pruning (Figure 7). Third, roguing works best if coordinated on an areawide scale. Poorly managed vineyards may act as a source of vectors and inoculum for spread to neighboring vineyards. Finally, due to the difficulties associated with rapid identification of Pierce’s disease affected vines, roguing works
best if conducted in coordination with vector suppression.
Differences in Pierce’s disease epidemi ology among grape growing regions in California are also a result of differences in climate. Insect development rate is temperature-dependent, with insect populations developing more rapidly in hot compared to cool climates. Similarly, the growth rate of X. fastidiosa popula tions within plants increases with tem perature. As a result, vectors can acquire X. fastidiosa from infected vines earlier, and Pierce’s disease symptoms appear sooner in locations with hot compared to cool summers. It is important to recognize that the photographs present ed in Figures 4, see page 19 and 5, see page 20 were taken in a glassy-winged sharpshooter infested area in Kern County which has hot summers. There fore, symptom progression and optimal times for diagnostic testing (Figure 6, see page 22) may differ in areas with cooler or warmer summers.

Management of Pierce’s disease in glassy-winged sharpshooter infested areas has relied on an area-wide insect suppression program. Overreliance on a handful of active ingredients has result ed in elevated levels of resistance to in secticides commonly applied to control glassy-winged sharpshooter populations. While application of insecticides and roguing are important parts of a disease management program, incorporation of additional management approaches that do not rely on insecticides are needed. Recently, the University of California re leased five new Pierce’s disease resistant wine grape cultivars (Camminare Noir, Paseante Noir, Errante Noir, Ambulo Blanc and Caminante Blanc). In areas with a history of Pierce’s disease, re placement of susceptible cultivars with Pierce’s disease resistant cultivars would reduce the need to suppress vector populations and survey vineyards for diseased vines.
The author thanks Sean Uchima, Lind sey Burbank, Rodrigo Krugner, Drake

Stenger and David Haviland for their contributions to the study described here. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific informa tion and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity employer.
Sisterson, M. S. and Stenger, D. C. 2013. Roguing with replacement in perennial crops: conditions for successful disease management. Phytopa thology 103: 117-128.
Sisterson, M. S., Burbank, L. P., Krugner, R., Haviland, D., and Stenger, D. C. 2020. Xylella fastidiosa and glassy-winged sharpshooter population dynamics in the southern San Joaquin Valley of California. Plant Disease 104: 2994-3001.
Comments about this article? We want to hear from you. Feel free to email us at article@jcsmarketinginc.com



Drought and Climate Change Impacts on Tree Nut Pests
Jhalendra Rijal, UCCE Area IPM Advisor
Thursday, September 29
7:00 AM BREAKFAST Sponsored by ISCA, Inc.
7:30 AM TRADESHOW
DPR TRACK Weed and Disease Control in Lettuce with Band-Steam Mark Siemens, Associate Specialist U of A Cooperative Extension
Is Citrus Mealybug an Emerging Pest in San Joaquin Valley Citrus? Sandipa Gautum, UCCE Area Citrus IPM Advisor
Disease Management in Nut Crops Jim Adaskaveg, Plant Pathologist, UC Riverside
8:00 AM 8:30 AM 9:00 AM 9:30 AM
CCA TRACK Relationship between Non-structural Carbohydrates and Potassium Matt Comrey, Wilbur-Ellis, WRCCA
Improve Input Efficiency: Irrigation Strategy with a Short Supply Cory Broad, Territory Manager, Jain Irrigation, WRCCA
Improve Input Efficiency: Nutrient Use Efficiency as it pertains to Nitrogen Management JW Lemons, Verdesian Life Sciences, WRCCA
Industry Sponsored Talk: AGRO-K Sap Analysis as an Improved Test for Crop Nutrient Status Sean Jacobs, Technical Sales and Marketing Representative AGRO-K
10:00 AM BREAK AND TRADESHOW
11:00 AM
Improve Input Efficiency: Review of Sprayer Calibration Matt Comrey, Technical Nutrition Agronomist, Wilbur-Ellis, WRCCA
11:30 AM
Evaluation of mechanical and automated in-row cultivators for weed control in conventional processing tomatoes Amber Vinchesi-Vahl, UCCE Area Vegetables Crop Advisor
Challenge of Coverage: Improving NOW applications with Adjuvants and Lower Spray Volume
Joel Siegel, Research Entomologist, USDA-ARS
Grape Pests: Diagnosing Vineyard Pest Problems Steve Vasquez, Technical Viticulturist, Sun-Maid Growers of California, WRCCA
Responding to a Viral Epidemic in Lettuce in the Salinas Valley of California Daniel Hasegawa, Research Entomologist, USDA-ARS
Grapevine Disease Management during the Changing Environment George Zhuang, UCCE Viticulture Farm Advisor
Potassium Management Under Challenging Soil
Supply Conditions
Jerome Pier, Senior Agronomist, Quali-Tech, WRCCA
WRCCA Panel:
Fertilizer and Water Supply Outlook WRCCA Moderated by Karl Wyant, WRCCA Chair
Panel Continued
Industry Sponsored Talk: The ProFarm Group Managing Disease Using the Power of Biologicals Combined with the Performance of Chemistry Melissa O’Neal, PhD, Sr. Product Development Manager Western Region, The ProFarm Group


12:00 PM LUNCH Sponsored by The ProFarm Group

Presentation of WRCCA’s CCA of the Year award, Scholarships and Honorariums
1:00 PM
Walnut Scale Management Elizabeth Fichtner, UCCE Farm Advisor, Tulare County
1:30 PM Impact of Water Quality onPesticide Performance Karl Wyant, Director of Agronomy, Nutrien, Chair, WRCCA
2:00 PM Personal Protective Equipment Required by Labels for Employees and Owners Marianna Gentert, Deputy Ag Commissioner, Tulare County
Late Season Canopy Management to Improve Winegrapes and Wine in a Changing Climate
Luca Brillant, Bronco Viticulture Chair, CSU Fresno
 By ALEJANDRO HERNANDEZ | Graduate Student, Department of Plant Pathology, UC Davis and FLORENT TROUILLAS | Associate Professor of Cooperative Extension, Department of Plant Pathology, UC Davis
By ALEJANDRO HERNANDEZ | Graduate Student, Department of Plant Pathology, UC Davis and FLORENT TROUILLAS | Associate Professor of Cooperative Extension, Department of Plant Pathology, UC Davis
In spring 2022, our laboratory investigated a serious case of Phytophthora pruning wound cankers (PPWC) affecting two almond orchards in Solano County. Disease incidence (number of trees affected) in one of the orchards averaged 28%, with sections of the orchard reaching levels as high as 41% of trees showing infection. The length of cankers in this orchard averaged eight inches, and the longest canker measured was 16 inches. Most cankers occurred at thinning cuts in the tree crown that were made on November 1, 2021. The causal agent of PPWC was identified as Phytophthora syringae. Phytophtho ra pruning wound canker is relatively rare in almond orchards in California; however, it occurs sporadically during wet years, mainly in the Sacramento Valley, and can severely damage an orchard when conditions are favorable.
Early reports of this disease date back to 1982 affecting almond orchards throughout the Central Valley (Bostock and Doster 1985). Phytophthora pruning wound cankers are not to be confused with the perennial Phytophthora canker disease of almond, primar ily caused by Phytophthora cactorum and Phytophthora citricola. Both diseases can cause aerial infections of almond trees; however, pruning wound cankers are annual, nonlethal and associated with pruning wounds. On the other hand, perennial Phytophthora can kers are typically lethal and associated with water holding pockets and cracks at the tree crotch. Aside from almonds, pruning wound cankers caused by Phytophthora syringae have also been reported on apricots and French prunes in California (Doster and Bostock 1988d). Phytophthora syringae is also known to cause crown and collar rot of several stone fruit (including almond) and citrus brown rot in California. Aerial fruit rot of peaches and apple fruit rot caused by Phytophthora syringae have also been reported in Italy and England, respectively.
Phytophthora species were once considered fungi but are now categorized in a separate classification called oomy cetes, also known as “water molds”. Diseases caused by Phytophthora plant pathogens result in devastating losses to agriculture crops and native forests. They have a cell wall composition different than that of true fungi, so management of Phytophthora
species using fungicides that target true fungi are ineffective. Phytophthora soil-inhabiting pathogens that thrive under wet environmental conditions, hence the name water molds. thora species can survive dry periods through the formation of oospores (sex ual spores) or chlamydospores (asexual spores). These are hardy, thick-walled spores that are tolerant to unfavorable environmental conditions (i.e., drought, heat) and serve as survival structures for Phytophthora species. Under fa vorable environmental conditions (i.e., wet, cool), these survival structures can germinate and form sporangia (sac-like structure that contain spores), and these sporangia can then form zoospores (asexual swimming spores). Oospores, chlamydospores, sporangia and zoospores can all serve as infective propagules that cause plant diseases. Zoospore formation and release are favored when there is standing water in the orchard as a result of flooding, overwatering or significant rain events. Certain Phytophthora species may pro duce up to 68 zoospores from a single sporangium. Thus, under the right con ditions, zoospore disease inoculum can reach very high levels in orchards and increase the risk for disease to occur.






Cankers caused by Phytophthora syrin gae can easily be mistaken for common fungal canker diseases affecting al mond trees, such as Ceratocystis canker caused by Ceratocystis destructans and band canker caused by Botryosphae riaceae fungi. Phytophthora pruning wound cankers are typically associated with pruning wounds made during

the cool temperatures seen in fall, winter and early spring. Active cankers show profuse gumming around the affected pruning wound and limb (Figure 1, see page 26). Removal of the outer bark reveals distinct concentric canker margins of alternating light-brown to brown tissue (Figure 2, see page 27). Infections are generally restricted to the cambium tissue and do not cause internal wood discoloration as seen with some fungal cankers. Dieback of lateral branches can be
observed in orchards (Figure 3). However, trees typically do not die due to an infection by Phytophthora pruning wound canker.





Incidence of Phytophthora pruning wound canker is sporad ic; however, cool wet weather is thought to favor infection and disease development (Doster and Bostock 1988a). Thus, pruning wounds made during fall, winter and spring can be susceptible to infection, especially when weather condi tions are cool and rainy. At this time, the exact mechanism of inoculum dispersal to pruning wounds is not exactly known. However, it is presumed that oospores and eventu ally zoospores and sporangia of P. syringae occurring in the soil and leaf litter are blown up into the tree crown during high winds and heavy rain events where it can then infect fresh pruning wounds. It is important to note that pruning wounds can remain susceptible to infection by Phytoph thora syringae for about four weeks after pruning (Doster and Bostock 1988c). Following infection, canker expansion occurs relatively quickly and is favored by cool temperatures. Canker expansion slows down as temperatures begin to rise in late spring and eventually stops completely under hot summer weather (above 25 degrees C) when the pathogen dies. In the fall, fallen leaves on almond orchard floors have been found to contain oospores of P. syringae (Doster and Bostock 1988a). The pathogen’s ability to colonize and form oospore in the leaf litter can play a role in increasing and maintaining inoculum load present in the field and contrib ute to the spread of P. syringae to other parts of the orchard or to neighboring orchards.
Phytophthora pruning wound canker was observed more commonly in the late 1990s and early 2000s throughout the San Joaquin Valley (Greg Browne, Personnel Commu nication). High incidence of the disease generally has been correlated with heavy rainfalls typical of El Niño years in California. Strong El Niño events occurred in 1982-83 and 1997-98 in California and correlated with high incidence of pruning wound cankers in almond orchards. However, the general increase in temperatures and continuing drought conditions affecting California have created an environ ment that is less conducive to the disease. In addition, the growing adoption by almond growers of minimal pruning strategies have led to less pruning cuts made to almond trees. This may explain why phytophthora pruning wound has become more sporadic during the last decade in California. However, as illustrated by this year’s findings, Phytophthora syringae remains present in orchards, and periodic disease outbreaks can occur in California when conditions are favorable. During late October 2021, an atmospheric river (a narrow corridor of concentrated moisture in the atmosphere) brought historic strong rains to Northern California that may explain the recent localized outbreaks.

As previously mentioned, pruning wounds can remain susceptible to Phytophthora syringae infection for about four weeks after pruning if wounds are left unprotected. Previous research showed that the application of a phosphonate product (i.e., fosetyl-Al, which is currently registered for use in nonbearing almond trees only) directly to fresh pruning wounds was effective at preventing infection by Phytoph thora syringae for at least four weeks
following pruning (Doster and Bos tock 1988b). Cupric hydroxide was less effective than phosphonate and showed signs of phytotoxicity at the pruning wound: clear gumming, xylem discol oration, excessive inner bark dieback and abnormal lignification (Doster and Bostock 1988b). Aside from fungicides, certain cultural practices can be imple mented to manage the disease. Indeed, the disease can be avoided by pruning outside of rainy periods. Lignification and healing of pruning wounds occurs naturally, but it is faster under warm weather conditions. Accordingly, best
Fresh fruit and vegetables have more flavor when the nutrient pathways mobilize the full range of nutrients into the fruit.

And plants are more resilient to disease and pest pressure when the soil is biologically and nutritionally balanced.
Pacific Gro helps growers improve both soil health and crop quality.
Plant available calcium, amino acid nitrogen and various carbon compounds help mobilize nutrients through the plant. Fish oil feeds beneficial fungi which improve soil structure and crop resilience.
Pacific Gro is produced from salmon and enzymatically digested crab shell. Screened to 150 mesh (100 microns) for use in foliar, drip and pivot irrigation.

Contact us for ways to add Pacific Gro results to your program.


California distributor Deac Jones
timing for pruning is after harvest in the early fall or during dry weather in the late winter or early spring when a warmer weather favors the rapid healing of pruning wounds. There are no cultivars that are completely resistant or tolerant to PPWC; however, susceptibility trials conducted in 198486 showed that Ripon cultivar was the least susceptible when compared to Nonpareil, Butte, Mission, Fritz, Carmel, Ne Plus Ultra and Thompson cultivars.
Bostock, R.M., and Doster, M.A. 1985. Associa tion of Phytophthora syringae with pruning wound cankers of almond trees. Plant Disease 69: 568-571. apsnet.org/publications/plant disease/backissues/Documents/1985Articles/ PlantDisease69n07_568.PDF
Doster, M.A., and Bostock, R.M. 1988a. Incidence, distribution, and development of pruning wound cankers caused by Phytoph thora syringae in almond orchards in Califor nia. Phytopathology 78:468-472. apsnet.org/ publications/plantdisease/backissues/Docu ments/1985Articles/PlantDisease69n07_568.PDF
Doster, M.A., and Bostock, R. M. 1988b. Chemical protection of almond pruning wounds from infection by Phytophthora syringae. Plant Disease 72: 492-494. apsnet.org/ publications/plantdisease/backissues/Docu ments/1988Articles/PlantDisease72n06_492. PDF
Doster, M.A., and Bostock, R.M. 1988c. Effects of low temperature on resistance of almond trees to Phytophthora pruning wound cankers in relation to lignin and suberin formation in wounded bark tissue. Phytopathology 78:478-483. apsnet.org/publications/phytopa thology/backissues/Documents/1988Articles/ Phyto78n04_478.PDF
Doster, M.A., and Bostock, R.M. 1988d. Sus ceptibility of almond cultivars and stone fruit species to pruning wound cankers caused by Phytophthora syringae. Plant Disease 72: 490-492 apsnet.org/publications/PlantDisease/ BackIssues/Documents/1988Articles/PlantDis ease72n06_490.PDF
Comments about this article? We want to hear from you. Feel free to email us at article@jcsmarketinginc.com





 By MARK C. SIEMENS |Associate Specialist, Biosystems Engineering, University of Arizona
By MARK C. SIEMENS |Associate Specialist, Biosystems Engineering, University of Arizona
Use of steam heat is an effective tech nique for controlling soilborne pathogens and weeds. The method is commonly used in plant nurseries and greenhouses for soil sterilization and pas teurization. In these operations, the entire soil profile is heated which is too energy in tensive and slow to be feasible for field-scale agriculture. To reduce energy consumption and cost, we have been investigating the use of band-steaming where steam is applied only in the area where it is needed; in the plant root zone. In this method, only narrow strips of soil centered on the seed line are treated with steam rather than the whole bed.
Over the course of the last several years, we developed a novel band-steam applicator



and Steam Appl.
Steam rate: High 0 15 374,616 c1 97 9.6
Steam rate: Std 0.25 2,962,080 bc 81 27.8
Steam rate: Low 0.40 4,007,520 b 74 44.5
Non-treated 16,413,408 a 59.6
Romaine
Steam rate: High 0 15 287,496 c 100 10.5
Steam rate: Std 0.25 1,359,072 c 93 17 9
Steam rate: Low 0.40 5,932,872 b 75 37.0
Non-treated 23,365,584 a 90.4
wilt of lettuce control and crop yield. Steam was applied to a strip of soil four-inches-wide by three-inches-deep centered on the seedline prior to planting.
! Travel Speed Diseased Plants Head Weight Marketable Heads (mile hr-1) (no. ac-1) (lb head-1) (%)
Iceberg
Steam rate: High 0 15 2,116 b1 1 25 a 67 a
Steam rate: Avg 0.25 2,821 b 1 26 a 70 a
Steam rate: Low 0.40 3,153 b 1 12 b 55 a
Non-treated 6,347 a 0 90 c 23 b
Romaine
Steam rate: High 0 15 2,697 b 0.84 a 54 a
Steam rate: Avg 0.25 3,526 ab 0.86 a 50 a
Steam rate: Low 0.40 3,568 ab 0.80 ab 47 a
Non-treated 4,978 a 0.70 b 23 b
1Within columns and trial, means followed by the same letter are not different by Duncan s New Multiple Range Test.
designed to raise soil temperatures in a band four-inch-wide by three-inch-
deep” deep to levels sufficient to con trol weed seed and soilborne patho gens (140 degrees F for >20 minutes). The device is principally comprised of
a 35 BHP steam generator mounted on an elongated bed shaper (Figure 1, see page 32). The apparatus applies steam via shank injection and from
cone-shaped ports on top of the bed shaper while forming beds as it travels through the field. The operation is performed prior to planting. After the soil cools (<1 day), the crop is planted into the disinfested band.



Last fall, we established trials in vestigating the use of band-steam to control weeds and Fusarium wilt of lettuce in iceberg and romaine lettuce. In the study, the prototype band-steam applicator was configured to treat a fourinch-wide by three-inch-deep band of soil. Three rates of steam (Low, Standard, High) were ap plied by varying travel speed. The “Standard” rate was where steam was applied at rates needed to reach the target soil temperatures (>140 degrees F for >20 minutes). Higher and lower application rates were examined to ensure target temperatures were met/ exceeded to get a better under standing of the efficacy of steam treatment and to determine if higher travel speeds (less fuel consumption) could be used and still provide effective pest control.

Results showed that application of steam was highly effective at controlling weeds (nettleleaf

goosefoot predominant species) (Table 1, see page 34). At the Standard application rate, over 80% of the weeds were controlled. At High application rates, weed control approached 100%. What was particularly encouraging was that at the Low steam application rate where travel speeds were 60% faster, than Standard, and target temperatures were not met, weed control was still very good – about
75%. Hand weeding times were also significantly reduced in all steam-treated plots as compared to the control. An example of the effectiveness of band-steam-treat ment on seedline weed control is shown in Figure 2.
Effect on Disease and Yield in Iceberg and Romaine Steam treatment was also effective at controlling Fusarium wilt of let



tuce. Disease incidence in iceberg and romaine lettuce were reduced by more than 50% as compared to th control (Table 2, see page 34). Crop e untreated plants were noticeably larger and more vig orous throughout the growing season in all steam, treated plots (Figure 2 , see page 35). At the Standard and High application rates of steam, this translated into significant yield increases in iceberg (>300%) and romaine (>90%) lettuce. Significant yield increases at the Low application rate of steam were also found in iceberg (>200%) and romaine (>60%). Assuming a yield in crease of 50% and a crop value of $10,000/acre, farm revenues would increase by $5,000/acre.
The results are very promis ing, but it is important to note that steam treatment is not an end-all cure for Fusarium wilt disease. At the trial site, disease inoculum levels were considered moderate. However, when inoculum levels are very high, our trials have shown that a four-inches-wide by threeinches-deep band of soil is not effective at controlling the disease. We hypothesize that a wider and/or deeper band of treated soil is needed for effec tive control. This fall, we will be initiating trials to examine this.
Other considerations are fuel costs, water consumption and work rate. Although only a narrow band of soil was treated, fuel costs ($534/acre) and water use (1,268 gal/acre) were high, and work rate (0.08 acre/hr) was low. The reason for this is that the trials were conducted when initial soil temperatures were low (50 degrees F), and travel

speed had to be reduced to 0.15 mph in order to meet target temperatures (140 degrees F >20 minutes). When initial soil temperatures are signifi cantly higher, much less energy is needed to raise soil temperature to levels necessary for pest control. In separate trials, when initial tempera tures were high (110 degrees F), we were able to reach target tempera tures while traveling at speeds of 0.75 mph. In this scenario, fuel costs ($178/acre), water use (423 gal/acre) and work rate (0.26 acres/hour) were much improved.
In conclusion, our trials have shown that use of band-steam is an effec tive technique for controlling in-row weeds and can help control soilborne pathogens in lettuce crops. Although fuel costs are high and work rates are slow when soil temperatures are low, these drawbacks may be acceptable if the yield gains found in our trials can be realized.

The author would like to recognize and thank Victor Godinez, Jr. and Nicklaus Bahr, University of Arizona, and Steven A. Fennimore, UC Davis for their invaluable technical and sci entific contributions to this project.
This work is supported by the Arizo na Iceberg Lettuce Research Council, Arizona Specialty Crop Block Grant Program, and the Crop Production and Pest Management grant no. ARZT-3025630-G22-505 /project ac cession no. 1014065 from USDA-NIFA. We greatly appreciate their support. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
Comments about this article? We want to hear from you. Feel free to email us at article@jcsmarketinginc.com

 By PATRICIA LAZICKI |Ph.D., Postdoctoral Researcher, University of Tennessee, Knoxville
By PATRICIA LAZICKI |Ph.D., Postdoctoral Researcher, University of Tennessee, Knoxville
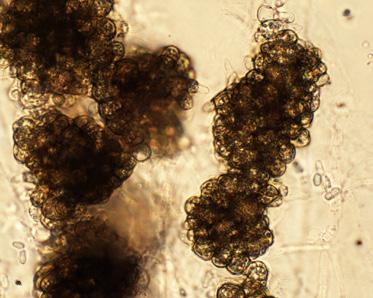
Vermicompost is an organic waste that has been digested by earthworms, altering its biological, physical and chemical properties. Earthworms are detritivores, meaning that they eat non-living organic materials. While ingesting the material, worms shred materials into small er pieces. This translates into more surface area which can be colonized by microbes, accelerating decomposition. As the worms move through the material, they mix and aerate it. During diges tion, these materials also come into close contact with the microbes in the earthworm’s guts. As the earthworms move on to consume fresh residues, the digested material “matures” as decomposi tion continues, and a new microbial community develops (Figure 1).
The result is a stable, uniform, small-grained, low-volume product with a diverse microbial community that is completely different from that of the feedstock. This can be used as a solid product or made into an extract or “tea” through a further aerated fermentation process.
Vermicompost and vermicompost extracts’ effects on plant growth are likely because they introduce a genetically and functionally diverse microbial community, carbon and nutrients, and a mix of potentially bioactive compounds8. To under stand vermicompost’s effect on plant growth and soil health, it’s necessary to recognize the complex interrela tionships between roots, microbes and the soil environment (Figure 2, see page 39).
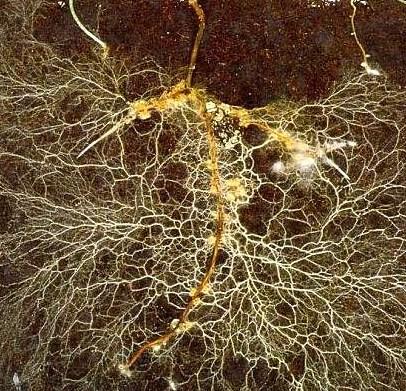
Stimulate root growth: Vermicompost and extracts can promote root growth, even in sufficiently fertilized plants5. For example, in one study, vermicompost extracts equaled or outperformed a commercial rooting hormone in stimulating rooting in cuttings3. This may have been due to the presence of plant hormones, including auxins and cytokinins, both of which are important in root development. Vermicompost is also high in humic substances, which can have a hor mone-like effect on plant growth3,7. The response and optimal application rates are both crop-dependent3,5 Increased root growth is thought to underlie or accentuate many of vermi
compost’s other observed benefits. In grapevines, this prop erty is likely to be especially important in promoting rapid establishment.

Improve abiotic stress resistance: Vermicomposts and extracts may help vines resist abiotic stresses like drought or salinity1,6. This effect is likely partly because increased root growth helps plants better access soil water as well as the nu trients they need to support coping mechanisms. Vermicom posts have also been observed to contain plant defense hor mones like salicylic acid8 and to stimulate plants to increase production of hormones like jasmonic acid as a protection against salinity stress6 .
Boost biotic stress resistance: Healthier plant roots, an active
 Figure 1. Vermicompost is organic waste that gains unique properties from being digested by earthworms (photo courtesy TerraVesco.)
Figure 1. Vermicompost is organic waste that gains unique properties from being digested by earthworms (photo courtesy TerraVesco.)
Figure 2. Complex feedbacks between roots, microbes and the soil envi ronment. Vermicompost likely has a triple mode of action: directly stimu lating roots, altering soil microbial communities and adding soil carbon and nutrients.
soils and plant nutrition at UC Davis. In her research, she has observed that vermicompost can improve total and soil active carbon and introduce better aggregate stability, especially when combined with cover crops. A healthier soil provides a better habitat for microbial communities and a better substrate for root growth. Improved struc ture can also improve drainage, which helps decrease salinity stress13
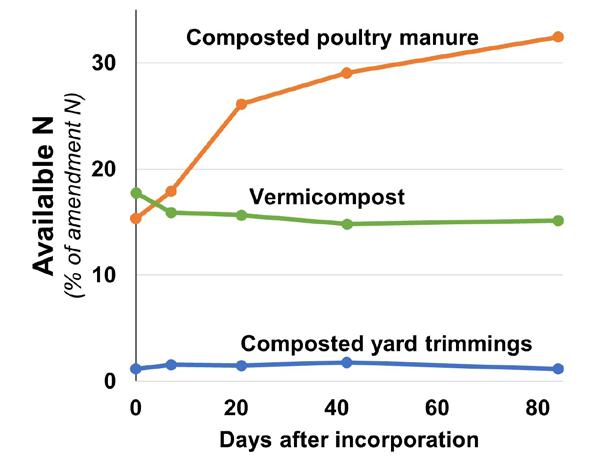
Add a predictable nutrient source: For wine grape growers, Lazcano notes a major benefit of vermicom posts is that they are a predictable, slow-release source of nutrients. A recent study comparing nitrogen release from different California organic materials demonstrated that while about 18% of the total N contained in vermi compost was immediately available for plant uptake, it released no additional N over the 12 weeks of incubation12 (Figure 3, see page 40). This predictability is particularly important for wine grapes, where N supplied in excess or at the wrong time can damage grape quality. Similarly, vermicompost has also been shown to be a slow-release source of phosphorus11. The nutritional content of vermi compost is influenced by the content of the feedstock. A reputable company can supply a guaranteed nutritional analysis.
and diverse microbiome, and stimulation of plant stress responses also likely underlie the increased resistance to biotic stresses which can occur in response to vermicompost application. For example, when vermicompost was mixed with soil at rates of 10% (by weight) or higher, it caused Agrobacterium tumefaciens (causal organism of crown gall) populations to decline in California walnut soils15. The suppressive effect was correlated to soil microbial abundance and diversity and didn’t occur when the vermicompost was heat-sterilized, suggesting the communities added with the vermicompost were responsible. Vermicompost microbial diversity was also credited with an observed reduction in root knot nematode reproduction factors and egg survival on tomato and pepper roots9. Tests on cucurbits and solanums also suggest vermicompost extracts have some efficacy in deterring insect pests like aphids, likely by improving plant vigor and stimulating production of secondary metabolites that make the plant less palatable4,16
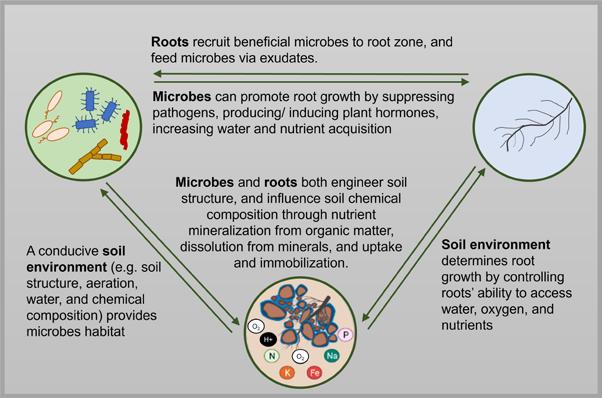
Improve soil health: Vermicompost can have a powerful effect on soil health, says Dr. Cristina Lazcano, professor of

Farms across the country are experiencing high fertilizer prices. Plants consume large amounts of N, P, and K for growth and crop production. Price ranges for some common inputs for N are between $800 - $1,500. P inputs are $1,100 - $1,500 and Potassium, K are right around $950 per ton.

Reduced fertilizer e ciency can be expected when the soil acidity is not adequately man aged. The routine addition of quality aglime products will help maintain a near neutral soil pH and increase the e ectiveness of fertilizers while reducing the amount of money wasted.
Check with your certi ed crop advisor for more information on fertilizer e ciency.
77% 48% 77% $940
89% 52% 100% $570
95% 63% 100% $399
For more information

In crease nutrient availability: Vermicom post applied near the root zone can also increase nutrient uptake and nutrient use efficiency, even in fertilized soil10. The links between vermicompost com munity composition and specific effects on plant growth are still not well under stood. However, recent work suggests that the changes to the microbiome during vermicomposting could help vermicompost to mobilize nutrients from the soil into plant-available forms. For example, one study found that microbial metabolic diversity increased during vermicomposting, suggesting that vermicompost microbes can use and break down more substances, po tentially increasing nutrient availability to plants8. Another study which exam ined the microbiome of the earthworm gut found it to be enriched in bacteria capable of solubilizing phosphorus2 . However, since the rhizospheres of healthy plants already select for bacteria which help them acquire nutrients, it’s not known which of vermicompost’s effects on nutrient uptake are due to the added microbial community and which come from other beneficial effects on soil structure, root growth and the existing microbiome14
In summary, vermicompost has poten tial as a tool for combatting abiotic and biotic stresses, both through its direct effects on the plant and indirect effects through altering soil biology, chemistry and structure. However, it’s important to note that its effects will vary with factors like crop, variety, soil type and weather.
Most formal research on vermicompost comes from annual crops and in nurs ery or greenhouse settings. However, several of the properties documented in the literature have been observed in Cal ifornia grapevines. TerraVesco has been supplying vermicompost to California vineyards since 1997. The Sonoma-based company uses a dual approach to ver micomposting by first pre-composting a mix of dairy manure with rice hulls in thermophilic piles and then feeding the compost to their red wigglers (Eisenia fetida; Figure 4, see page 40). In trials of



their two flagship products, TerraThrive Pro Vermicompost and Vermi-extract, on vineyards around California, they’ve observed that vermicompost consis tently stimulates root growth in wine grapes in both nursery and field settings (Figure 5, see page 40). Jack Chambers, TerraVesco’s founder and president, believes that vermicompost’s effect on roots is foundational to its success in vineyards.
“All vineyard managers are really trying to get that root ball established in the first couple years,” he said. They’ve also observed that the larger root mass then helps the vine to mine more water and nutrients. In a trial conducted with Purdue University, the grower was able to cut fertilizer by about 50%.
TerraVesco CEO Brian O’Toole main tains that the secret to their product’s effectiveness is in its rich microbial community. The Sacramento-based ag tech company BiomeMakers identified over 400 species of microbes in their vermicompost and vermi-extract, with a wide functional diversity. In the ver micompost, 24% of the bacterial types detected were capable of producing auxin and 5% were capable of produc ing cytokinin, two of the hormones implicated in root growth stimulation. Other potential functions included salt tolerance, exopolysaccharide production (important in stress tolerance and soil structural improvements) and ability to mobilize important plant nutrients.
Vermicomposts are more expensive than traditional composts and should be applied strategically. Given that their main modes of action are through the plant rhizosphere, it’s most effective to concentrate application in the root zone. For example, for vineyard replants, TerraVesco recommends putting 8 oz of solid vermicompost in the planting hole or 16 oz if it’s a hillside or rockier soil. For established vineyards, they recommend running 10 to 20 gallons per acre through the drip line at specific growth stages. Ideal times are budbreak, pre-veraison and postharvest. Howev er, what makes the most economic and
agronomic sense may change depending on the variety and the weather.
“It’s a good idea to evaluate what hap pened over the growing season, whether the vines need it, and what you can afford,” said Chambers.
In his experience, pre-veraison applica tions during July or August when the temperatures are getting hotter and
vines are experiencing heat and water stress have the most direct benefit to the plants. By contrast, a postharvest appli cation may only make economic sense if the vines have been stressed by drought or severe temperatures, or if a variety is particularly sensitive.
Not all vermicomposts are created equal,
and vermicompost expert Rhonda Sherman, an extension specialist at North Carolina State University, recommends that growers be careful when purchasing. A reputable com pany gives a lab analysis on request. Generally, a good compost should have a carbon percentage of less than 65%, a low respiration rate (≤8 mg CO2 per g organic matter per day) and have a neutral pH (6.0 to 8.5). These are all indicators that the material has been given enough time to mature. An immature compost may react with the soil when it is added, which can damage root growth by depleting soil oxygen, tying up nutrients and producing toxic compounds. Sherman recommends that if you’re unsure, before using a product for the first time, perform the standard test for compost maturity by doing a cucumber seed germination test. If more than 80% of the seeds germinate, the compost is considered safe for use on established vineyards. Also watch for herbicide residues, salinity and heavy metals. Guidelines for assessing com post safety are found in the ‘Resources’ section below.
And additionally, even the best vermi compost isn’t a silver bullet. “It’s part of a toolbox,” said O’Toole.
As with other interventions, it’s best to start on a trial block to test its effective ness, and to monitor its effectiveness by regular soil and plant testing.

1. Ahmad, A. et al. Soil application of wheat straw vermicompost enhances morpho-phys iological attributes and antioxidant defense in wheat under drought stress. Frontiers in Environmental Science 10, (2022).
2. Andleeb, S. et al. Molecular characterization of plant growth-promoting vermi-bacteria associated with Eisenia fetida gastrointestinal tract. PLOS ONE 17, e0269946 (2022).
3. Arancon, N., Cleave, J. V., Hamasaki, R., Nagata, K. & Felts, J. The influence of vermicompost water extracts on growth of plants propagat ed by cuttings. Journal of Plant Nutrition 43, 176–185 (2020).
4. Arancon, N. Q. et al. Suppression of two-spot ted spider mite (Tetranychus urticae), mealy bug (Pseudococcus sp) and aphid (Myzus persicae) populations and damage by vermi composts. Crop Protection 26, 29–39 (2007).
5. Bachman, G. R. & Metzger, J. D. Growth of bedding plants in commercial potting substrate amended with vermicompost. Bioresource Technology 99, 3155–3161 (2008).
6. Benazzouk, S., Dobrev, P. I., Djazouli, Z.E., Motyka, V. & Lutts, S. Positive impact of vermicompost leachate on salt stress resistance in tomato (Solanum lycopersicum L.) at the seedling stage: a phytohormonal approach. Plant Soil 446, 145–162 (2020).
7. Canellas, L. P., Olivares, F. L., Okoroko va-Façanha, A. L. & Façanha, A. R. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant Physiol 130, 1951–1957 (2002).
8. Domínguez, J., Aira, M., Kolbe, A. R., Gó mez-Brandón, M. & Pérez-Losada, M. Changes in the composition and function of bacterial communities during vermicomposting may explain beneficial properties of vermicompost. Sci Rep 9, 9657 (2019).
9. dos Santos Pereira, T. et al. Water-extractable fraction of vermicomposts enriched with Trich oderma enhances the growth of bell pepper and tomato as well as their tolerance against Meloidogyne incognita. Scientia Horticulturae 272, 109536 (2020).
10. Ebrahimi, E., Ghorbani, R. & von Fragstein und Niemsdorff, P. Effects of vermicompost placement on nutrient use efficiency and yield of tomato (Lycopersicum esculentum). Biologi cal Agriculture & Horticulture 36, 44–52 (2020).
11. Lazcano, C., Gómez-Brandón, M. & Domín guez, J. Comparison of the effectiveness of composting and vermicomposting for the biological stabilization of cattle manure. Che mosphere 72, 1013–1019 (2008).
12. Lazicki, P., Geisseler, D. & Lloyd, M. Nitrogen mineralization from organic amendments is variable but predictable. Journal of Environ mental Quality 49, 483–495 (2020).
13. Liu, M., Wang, C., Liu, X., Lu, Y. & Wang, Y. Sa
line-alkali soil applied with vermicompost and humic acid fertilizer improved macroaggregate microstructure to enhance salt leaching and inhibit nitrogen losses. Applied Soil Ecology 156, 103705 (2020).
14. Readyhough, T., Neher, D. A. & Andrews, T. Organic amendments alter soil hydrology and belowground microbiome of tomato (Solanum lycopersicum). Microorganisms 9, 1561 (2021).
15. Strauss, S. L., Stover, J. K. & Kluepfel, D. A. Impact of biological amendments on Agrobac terium tumefaciens survival in soil. Applied Soil Ecology 87, 39–48 (2015).
16. Sugino Souffront, D. K., Salazar-Amoretti, D. & Jayachandran, K. Influence of vermicompost tea on secondary metabolite production in tomato crop. Scientia Horticulturae 301, 111135 (2022).

TerraVesco is a major producer of vermicompost in California. Their website provides information on products and soil health: www.terravesco. com/.
General vermicompost questions? Rhonda Sher man has the answers! composting.ces.ncsu.edu/
Thinking about making your own? The Worm Farmer’s Handbook details the ins and outs of vermicomposting for mid- to large- scale operations: bookstore.acresusa.com/products/ the-worm-farmers-handbook
Guidelines for assessing compost safety (ANR Publication 8514): anrcatalog.ucanr.edu/pdf/8514. pdf
Oregon State University instructions for per forming and interpreting a compost test: catalog. extension.oregonstate.edu/sites/catalog/files/ project/pdf/em9217.pdf
Comments about this article? We want to hear from you. Feel free to email us at article@jcsmarketinginc.com