1 minute read
Level up & join the fight against counterfeiting
Similar to the requirements of Europe’s Falsified Medicines Directive, South Africa will be joining the global fight against counterfeit medicines with the promulgation of track and trace regulations, centred on product serialisation. These regulations will be phased in providing manufacturers with enough time for adequate preparation. Ultimately, all local pharma manufacturers should be compliant by 2022.
A look at the levels
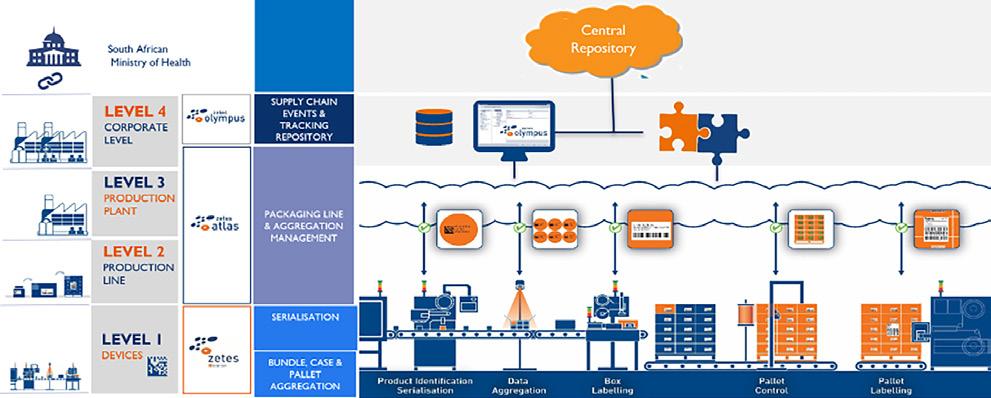
If you’re considering implementing a serialisation project and are unsure about what will meet your needs, Sébastien Sliski, Zetes’ serialisation expert, says the best way to understand serialisation and aggregation is to think in levels. This helps to break down and define the different components in line with your needs, whether you’re manufacturing medicines or dispensing them.
‘Zetes has been working on serialisation solutions for different markets for about 10 years. We are one of the actors working on all levels, from zero to four, and are now moving into working on level five,’ Sliski explains. ‘Levels zero to three are often for manufacturers to define their serialisation approach to be interoperable. Zero is the machine, one is the marking and verification technologies and two to three are the serialisation software levels on the line at factory level, linking to level 4 and integrated with the ERP. Level four is at the company level, which manages the status of the unique identifiers and their business evolution. It also collects and shares information concerning unique identifiers with other level fours and publishes the information to the government repository (level five). This is the repository where all the actors publish serialised numbers for controlling and checking.’
Agility and traceability guaranteed
Based on the global trend to secure markets such as pharmaceuticals, tobacco, agro-science and more recently dairy milk, Zetes provides a comprehensive range of solutions proven to ensure agility and traceability.
‘We are an ideal partner for pharma manufacturers to help secure and control their supply chains, while ensuring global visibility,’ adds Sliski. ‘With Zetes, you will be able to comply with all traceability regulations, combat counterfeiting, secure your distribution channels, achieve delivery service level agreements and in the event of a recall, you will be equipped to manage this efficiently.’








