4 minute read
Diabetes and fasting
CPD | DIABETES Original paper: Efficacy and safety analysis of insulin degludec/insulin aspart compared with biphasic insulin aspart 30: A phase 3, multicentre, international, *Supplied content
open-label, randomised, treat-to-target trial in patients with type 2 diabetes fasting during Ramadan
Advertisement
Mohamed Hassanein, Akram Salim Echtay, Rachid Malek, Mahomed Omard, Shehla Sajid Shaikh, Magnus Ekelund, Kadriye Kaplan, Nor Azmi Kamaruddin. Diabetes Res Clin Pract 2017; https://doi.org/10.1016/j.diabres.2017.11.027
AIM:
To compare the efficacy and safety of insulin degludec/insulin aspart (IDegAsp) and biphasic insulin aspart 30 (BIAsp 30) before, during and after Ramadan in patients with type 2 diabetes mellitus (T2DM) who fasted during Ramadan.
Approximately 1.6 billion people worldwide are Muslims. Ramadan is the ninth month of the Islamic calendar, and fasting (total abstinence from food or drink) from dawn to sunset during Ramadan month is required. Each period of fasting may last up to 20 hours. The daily Ramadan fast starts after the pre-dawn meal – suhur, which is normally a small meal, and is concluded with a meal at sunset (iftar), which often includes a large amount of high-carbohydrate foods. In addition, at the end of Ramadan, there is a 3-day festival – Eid ul-Fitr, during which, overindulgence in food consumption is common even among patients with diabetes. Fasting during Ramadan is associated with an increased risk of dehydration and hypoglycaemia. In particular, patients with diabetes are at high risk of postprandial hyperglycaemia, diabetic ketoacidosis and thrombosis. Patients with very high or high risk include those with type 2 diabetes mellitus (T2DM) treated with premixed insulin or multiple daily insulin injections, many of whom choose to fast during Ramadan irrespective of knowing their risk of diabetic complications.
This is a CPD-accredited article, to do the questionnaire and earn points go to www.medicalacademic.co.za
METHODS:
In this multinational, randomised, treatto-target trial, patients with T2DM who intended to fast and were on basal, pre- or self-mixed insulin +- oral antidiabetic drugs (OADs) for ≥ 90 days were randomised (1:1) to IDegAsp twice daily (BID) or BIAsp 30 BID. Treatment period included preRamadan treatment initiation (with insulin titration for 8 - 20 weeks), Ramadan (4 weeks) and post-Ramadan (4 weeks). Insulin doses were reduced by 30 - 50 % for the pre-dawn meal (suhur) on the first day of Ramadan, and readjusted to the preRamadan levels at the end of Ramadan.
ENDPOINTS:
The efficacy endpoints were change in HbA 1c and fructosamine levels from baseline to the end of Ramadan (12 - 24 weeks) and from baseline to the end of post Ramadan (16 - 28 weeks), HbA 1c responders (< 7 %) and 8-point self-measured plasma glucose (SMPG) profile at the end of Ramadan and post Ramadan. Hypoglycaemia was analysed as overall (severe or plasma glucose < 3.1 mmol/L), nocturnal (00:01 - 05:59) or severe (requiring assistance of another person).

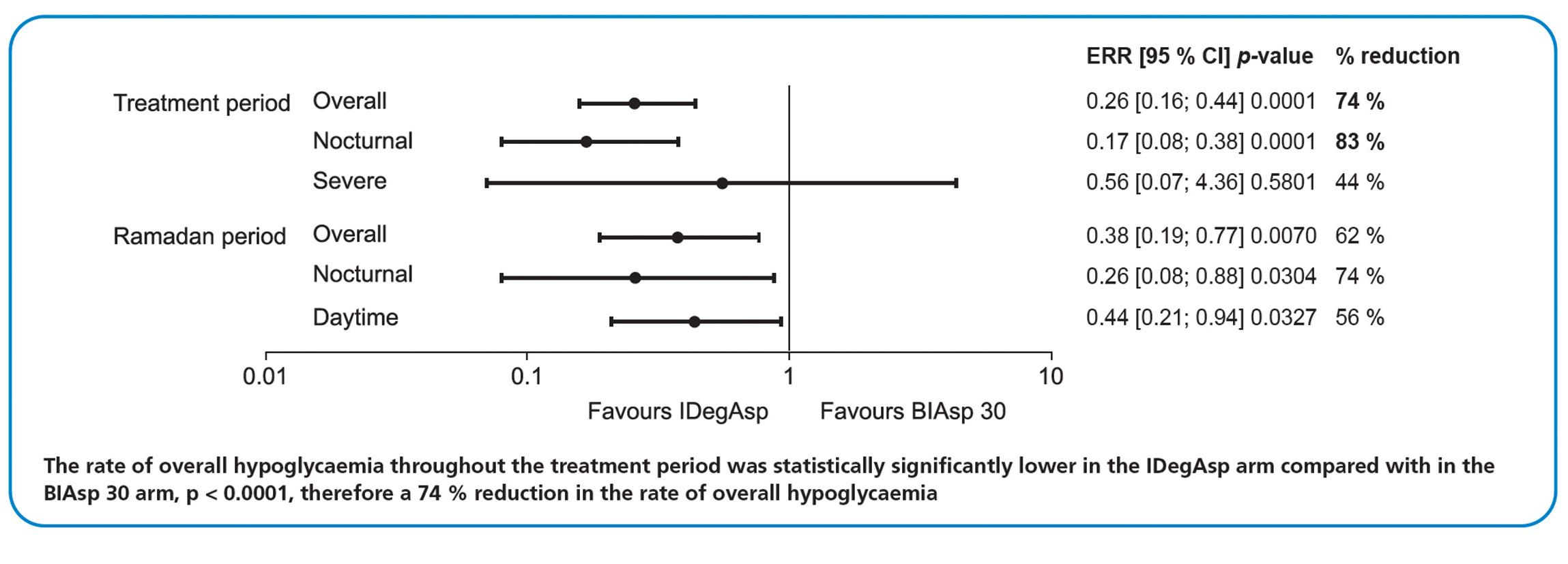
HYPOGLYCAEMIA: Hypoglycaemia rate ratios of the IDegAsp arm compared with the BIAsp 30arm during the 16- to 28-week treatment period and 4-week Ramadan period.
The rate of nocturnal hypoglycaemia was also statistically significantly lower in the IDegAsp arm compared with the BIAsp 30 arm (p < 0.0001), which translated into an 83 % reduction in the rate of nocturnal hypoglycaemia in patients receiving IDegAsp.
period, despite the reduction in insulin doses during Ramadan. The mean HbA 1c values fell from 8.5 % at baseline to 7.4 % at end of Ramadan and to 7.5 % at the end of 4 weeks post-Ramadan in both treatment arms, with almost all of the reduction occurring during the pre-Ramadan period. Similarly, there was no significant difference between IDegAsp and BIAsp 30 arms in terms of the change of fructosamine levels from baseline to end of Ramadan (p = 0.9382) or end of 4 weeks post-Ramadan (p = 0.8893). During the 4-week Ramadan period, the mean pre-iftar SMPG values were significantly lower in the IDegAsp arm compared with the BIAsp 30 arm (ETD: -0.54 mmol/L; [-1.02; -0.07] 95% CI, p = 0.0247). C M Y CM MY CY
DISCUSSION: The data indicate that glycaemic control was maintained in both treatment arms across the whole study period. The insulin dose was reduced at the beginning of Ramadan, and reverted to the pre-Ramadan dose at the end of Ramadan, in accordance with the IDF-DAR Practical Guidelines. During the treatment period, IDegAsp had significantly lower overall and nocturnal hypoglycaemia rates with similar glycaemic efficacy, versus BIAsp 30. As demonstrated by the results of this trial, at least 4-month preparation prior to Ramadan fasting may be beneficial. CMY K
CONCLUSION:
IDegAsp is a suitable therapeutic agent for patients who need insulin for sustained glucose control before, during and after Ramadan fasting, with a significantly lower risk of hypoglycaemia, versus BIAsp 30.
Sadad_ad.pdf 1 2014/05/22 12:41 PM