


Latest update on how the Professional Nurse Advocate (PNA) and Professional Midwifery Advocate (PMA) teams support our people
your work in the next edition of Our Journal Electronic prescribing and medicines administration (EPMA) team focus




Latest update on how the Professional Nurse Advocate (PNA) and Professional Midwifery Advocate (PMA) teams support our people
your work in the next edition of Our Journal Electronic prescribing and medicines administration (EPMA) team focus
We are pleased to share the fifth edition of Our Journal - a publication written by our clinical people, for our clinical people, at PAHT.
Our Journal includes the below, with reference to the articles in this edition:
y Focus features on clinical issues/ improvements and the impact on patient care: the new Butterfly Hub and developing the Butterfly service; and the Trauma Network visit showcasing our success
y Quality and safety agenda: Our patient centred anticoagulation service have been shortlisted for a prestigious award
y Conference and event reviews/updates: Biomedical scientists to present at leading conference; and research team conferences update
y Clinical transformation updates: Our electronic prescribing and medicines administration (EPMA) team focus on a project to improve the treatment of cancer
y Awards/clinical recognition: Medical education annual thank you and awards celebrations
y Clinical leadership successes: How the Professional Nurse Advocate (PNA) and Professional Midwifery Advocate (PMA) teams support our people
y PAHT 2030
y Research updates
y Input from external contributors
y Summary of research contributions and papers published by PAHT clinicians

Our Journal provides an amazing opportunity for us to showcase the wonderful work we do – please share it widely and let the communications team know which developments we can profile in the next edition of Our Journal at paht.communications@nhs.net
Best wishes
Dr Fay Gilder Medical director
 Sharon McNally Chief nurse and deputy chief executive
Sharon McNally Chief nurse and deputy chief executive
The anticoagulation team is absolutely delighted to have been shortlisted for an HSJ Patient Safety Award in the category of 'Improving Medicines Safety'.
We have been recognised for our work to move the monitoring of anticoagulant medication care from a traditional hospital-based service to an approach where patients are cared for in the community, in line with the NHS Long Term Plan.
Most patients taking anticoagulant medication (to reduce the risk of blood clots forming) need to do so for life and require regular blood tests to check their levels, which can have a great impact on their lives.
Our anticoagulation team (some of whom are pictured above) began work in 2019 to establish multi-disciplinary teams so that patients could have their regular checks in community based-settings, avoiding the need to travel to the hospital and wait there for test results.

The first session was held in Harlow in March 2020 and has since been extended across Bishop’s Stortford and Epping and surrounding communities. The teams have also introduced home visits for patients who are unable to travel to community clinics, which has improved patient safety and reduced the burden on district nurse teams.
There are now 12 dedicated point-of-care clinics held across different locations every week and since April 2020 over 50,000 tests have been carried out.
I am enormously proud of the the whole team, as they work extremely hard and always put
the patient at the centre of everything they do.
My thanks also go to everyone involved across the multidisciplinary teams for their engagement and support, which has been pivotal to the success of the service implementation.
We are looking forward to the awards ceremony in September, where the winners will be announced.
You can see some of the key data demonstrating the improvements to patient safety overleaf. For more information, please contact me at claire. gibson12@nhs.net
We have been able to demonstrate an improvement in patient safety by showing improvements to patients’ time in range and reduction in adverse events as well as other key metrics. This has been achieved by participating in a national and international benchmarking scheme for those centres who use the DAWN anticoagulation system. This is benchmarked against 79 national and 14 international sites. We use various metrics to monitor our performance, all of which are a measure of safety.
Time in range (all ranges)
Data from the previous model has been compared to the new patient-centred model:
y % time in range (tir) comparison by site

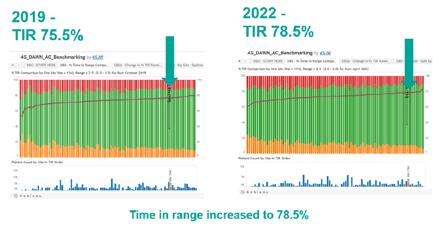
y % time in range comparison compared to previous model
y % time below range
y % time above range
y % INRs above 5.0
y % INRs above 8.0
y % DNAs

Coagulopathy has been shown to play an important role in COVID-19 infection. Reported abnormal coagulation laboratory values in severe COVID-19 are most notably a three-to four-fold increase in D-Dimer (Yao et al., 2020).
Several studies showed that elevated D-dimer in COVID-19 patients is associated with higher mortality. A retrospective cohort study carried out by Zhou, et al. (2020) also associated D-Dimer > 1000ng/ ml with higher odds of inpatient death. At the onset of the COVID 19 pandemic, there was no consensus as to how D-Dimer levels should be used for management of COVID-19 patients. In April 2020, PAHT decided to use a D-Dimer cutoff of 1000 ng/ml to determine anticoagulation dosage.
The source of reference used by PAHT in their decision making employed a D-Dimer assay with a cut off value of 500 ng/ml for the exclusion of venous thromboembolism (VTE). This raised an issue as the source of reference used by PAHT at the time employed a D Dimer assay with a cut off value of 230 ng/ml for the exclusion of VTE. This meant the Trust was unable to implement the new COVID-19
care bundle at the time. The haematology laboratory wished to do whatever possible to assist patient care and agreed to change the current D-Dimer methodology immediately to support the Trust's COVID-19 care bundle. The verification and implementation of the new D-Dimer assay with cut off at 500 ng/ml for the exclusion of VTE was completed within four weeks. A risk assessment and change control was put in place to ensure users were made aware of the change in cut off limit. This was made possible through the hard work and collaborative work of the haematology technical team, shared vision, and motivation, coupled with team engagement.
The Trust was able to implement the new COVID-19 care bundle with Dimer cut-off of 1000 ng/ml to determine anticoagulation dosage within four weeks. This led to significantly improved prognosis and a better
outcome for COVID-19 patients. The project was one of the case studies submitted to the Institute of Biomedical Science (IBMS) as part of my Higher Specialist Diploma (HSD) in Haematology. I sat the exams and successfully passed in November 2021. I have now been invited to attend the Institute of Biomedical Science (IBMS) Congress on 27 September at the International Convention Centre (ICC), Birmingham, to present my work. I feel honoured to have the opportunity to present this piece of work completed by the haematology laboratory during one of the most challenging periods in the history of the laboratory at the IBMS Congress.
I am proud that the positive impact this project made to the management of COVID-19 patients has been acknowledged by the IBMS. For more information, please contact me at eva.
nkansah@nhs.net.
I studied for an MSc in Biomedical Science at Greenwich University. This was funded by PAHT and I was able to complete this whilst still performing my normal job role. Since being awarded a Distinction, I was promoted to a Band 7 senior biomedical scientist, and I believe that the skills that I learnt on this course played a big role in this. In particular, I studied quality and learning and development modules –the latter has been particularly useful considering I am now working as the training officer for the biochemistry department.
My research piece was titled “The optimisation of a nearpatient sampling device for collection of samples for HbA1c measurement using an enzymatic methodology”. HbA1c is glycated haemoglobin – this is haemoglobin which has undergone an irreversible structural change as a result of sustained increased blood sugar levels. The proportion of HbA1c present in the blood, as compared to nonglycated haemoglobin, is thus indicative of the glycaemic control of the patient over the preceding 120 days (the life span of a red blood cell). This makes the HbA1c test an ideal marker for diabetes
monitoring. At the time that the project commenced, the COVID-19 pandemic had just begun. Diabetes mellitus (DM) affects a large subsection of the population and individuals with DM are more susceptible to COVID-19, and associated disease prognoses are worse than in non-diabetic populations. In 2011 the WHO approved HbA1c to be used for diagnosis for new DM patients.
Volumetric absorptive microsampling (VAMS) is a method of collecting whole blood and storing the sample, which can then be transported to a laboratory for later testing. Usually this is done using a dried paper spot (think the blood spot test performed on newborn babies); my project used a Neoteryx swab which was a swab that could be applied to a blood spot taken from a finger prick sample. Once a VAMS specimen is received by the laboratory, it must be extracted to be able to be tested. VAMS had previously been shown to have unsatisfactory performance when using the high performance liquid chromatography method (HPLC) – however I was able to validate the new enzymatic methodology.
One of the reasons that VAMS was so unsuccessful in previous work was that the process of drying the blood sample and 'rehydrating' it led to degradation and structural changes to the haemoglobin – this is not ideal when the HPLC relies on the structure to provide a HbA1c measurement. However, the

enzymatic method does not rely on the structure of haemoglobin for HbA1c results. Verifying this method made the VAMS suitable. Another bonus to using the enzymatic methodology is that for people with haemoglobin variants, such as those with sickle cell anaemia, it provides less interference with the test than HPLC methodology.
Although the VAMS method never went live in the Trust, the work involved in validating the enzymatic HbA1c method was used to put this into routine use. Now, the enzymatic HbA1c method is our first-line for testing, and only results in the diagnostic cut-off ranges of 42-48 mmol/ mol, or with values above the linear range (>129 mmol/ mol) are run using HPLC. Previously, patients who had variants identified from the chromatogram on HPLC were sent away to another hospital at a cost of £5 per sample – this is in addition to the cost of performing the HPLC analysis. The enzymatic method negates this – a £1.34 test that is minimally affected by haemoglobin variants. I feel a bit nervous, a bit excited, but most of all quite proud of myself to give this poster presentation at the IBMS congress
I am proud of the team for their commitment to continually growing the research activity taking place at PAHT.
Recently, three members of the research nursing team attended national events. Lily Robinson and Amara Benson, both research nurses, attended the Research and Development Forum from 21-23 May in Newcastle and in association with the Health Research Authority. This event was the first to connect, learn and share together again since the 2020 event was cancelled due to the COVID-19 pandemic, with more than 800 delegates in attendance.

The conference programme group pulled together speakers from across the UK, with the selected abstracts (from the hundreds submitted by delegates) around the central themes of research being embedded, inclusive and sustainable. Also showcased were the expanded Forum Working Groups, and the work they have been doing over the last three years, reaching out of the NHS into other settings such as prisons, schools, social care settings and commissioning organisations.
The Research and Development Forum continues to work collectively to advance research practice across
the UK, through sharing and developing resources, creating standards and providing a quality learning and development programme.
On 24 May, Rezma Miah, research nurse, attended the National Institute for Health and Care Research Mulsculoskeletal Trauma Trials Annual Meeting in the Assembly Room, Edinburgh. This was due to the research activity and engagement currently in trauma and orthopaedics at PAHT, in particular our involvement with the Ankle Fracture Treatment: Enhancing Rehabilitation (AFTER) Study – led by Mr Kar Teo as the principal investigator.
After a broken ankle, the lower leg is usually placed in a cast or boot for a number of weeks so the broken bone can heal. When the cast or boot is removed, the ankle initially feels stiff and sore. At this time, patients are given advice by health professionals on how to gradually get back to their usual activities and are given exercises to do at home. In some hospitals, patients are asked to attend physiotherapy sessions. In other hospitals, patients will
just receive advice. There is currently no scientific evidence showing that seeing a physiotherapist after an ankle fracture improves recovery. As physiotherapy appointments aren’t always convenient for patients, and because it’s important to make the best use of NHS time and resources, we want to find out if attending physiotherapy after an ankle fracture improves recovery. In the AFTER study, all participants will receive high quality information that will guide them with their recovery, with half of the participants also being asked to attend four-six physiotherapy sessions. Information on how quickly and how well participants are able to return to the majority of their usual activities will be used to make a comparison between the two treatments. The AFTER study has been developed by patients and healthcare professionals, including physiotherapists and doctors. Their experience and knowledge have been used to plan this study which could provide an answer as to what type of rehabilitation is best.
Being part of events such as these is a great opportunity to learn from the experts, from each other and to recognise the research activity going on across the NHS, with a view to developing further individuals, teams and healthcare organisations. For more information, please contact me at chris.cook6@nhs.net
I am delighted to share that we have now introduced a Butterfly Hub at The Princess Alexandra Hospital, a comfortable environment for loved ones of patients who are at the end of life. I would also like to highlight to colleagues the importance of an individualised care plan (ICP) for the anticipated last days of a patient's life being completed. The Butterfly service will be alerted once an ICP is in place.

The patient will appear on the site team’s end of life list, as well as the end of life category on Nervecentre. This reassures us that a conversation has taken place between the clinician and the patient/ their next of kin and we can be assured that we are not disclosing any confidential information by visiting the patient.
We are able to support both the patient, the family and help relieve busy ward staff. Please note that we can receive direct referrals from clinicians and nursing staff if the conversation has taken place but the
paperwork has not yet been completed.
Introducing the new Butterfly Hub
The need to go above and beyond statutory provision for end of life patients and their relatives is urgent and transcends localities. Families are getting smaller and more geographically disparate, society is ageing rapidly, and older people are increasingly living alone.
People are eight times more likely to die in a hospital than a hospice (Office for National Statistics), with hospital deaths making up nearly half of all deaths annually. Despite this, the disparity between the two is widely accepted to be significant. We can make further progress in enhancing the experience for those in hospital care.
For bereaved patients experiencing complicated grief, early intervention has been well-evidenced. Successful early intervention also reduces pressure on statutory services, primary care in particular. Increased public understanding of the pressures on clinical staff means that many relatives may not want to call on these teams for support. This can lead to
their own health and wellbeing needs being unmet. Moreover, clinical staff often do not have the capacity to go above and beyond for patients at the end of life and their families.
We have identified a need for support for carers of end of life patients through our close partnership working with The Anne Robson Trust. Since we launched the Butterfly Volunteer service, supporting patients at the end of life in early 2018, just over 5,000 visits have been conducted –for many, these visits were the only non-clinical contact during their final days of life. This year alone, the Butterfly Volunteers have supported nearly 300 family members/visitors by the bedside.
After three years of planning, the new The Princess Alexandra Hospital Butterfly
Hub opened at the end of May. We secured £50,000 of funding for the Butterfly Hub through a bid to NHS Charities Together, written by the original developer of the Butterfly Volunteer project at PAHT, Shahid Sardar, associate director of patient engagement and experience, working in partnership with the Anne Robson Trust, led by Liz Pryor MBE.
The furnishings were funded by kind donations from grateful families and from Butterfly Volunteer team fundraising events.
In summary:
What is the Butterfly Hub?
The hub has been created to provide families, whose loved one is nearing the end of life or who has just died, with:
y A peaceful, quiet, homely space to take a break from the busy ward environment

y Privacy and time to be alone
y Support from staff or the Butterfly service if needed
Who can use the hub?
Any team can use the hub to support a family whose loved one is dying or who has just died. The space is also available to assist families who have been bereaved and may be returning to the hospital to collect belongings, or who may need additional assistance or information.
How can the hub be accessed?
Swipe access is required and can be obtained from me on x 3805 or at nicki.harris1@ nhs.net - I am based in
the Alex Agile Zone at The Princess Alexandra Hospital.
Where is the hub located?
The Butterfly Hub is on the lower ground floor of The Princess Alexandra Hospital, opposite the sanctuary and next to the cashiers’ office.
What facilities are in the hub?
The hub offers comfortable seating for up to 10 people, a kitchenette and toilet facilities (including disabled).
Who maintains the space?
The hub is maintained by the Butterfly Volunteer team.
How can I book the hub?
A booking system will be available on AlexNet for appropriate teams to access in due course, in the meantime, please contact me on x 3805 or at nicki.harris1@nhs.net.
How can I access the hub out of hours?
Please contact the site team who hold swipe access to the hub out of hours on x 8213
y Weekdays, 4pm – 8am
y Weekends
y Bank Holidays
The site team are responsible for permitting access to the hub only. A member of ward staff will be required to accompany the family to the hub and in the event that the family would like time alone, they will need to be settled in by the member of staff and checked on periodically. The family will need to be made aware that once they leave the room they will not have access to re-enter it again, as swipe access will remain with the site team. It will be the responsibility of the staff member supporting the family to ensure that when the family are ready to leave, the hub is securely closed.
We will report the impact against the following outcomes in a future edition of Our Journal:
y Families and carers of end-of-life patients feel supported during their visits to the Butterfly Hub
y Families and carers have increased knowledge of long-term specialist support available to them
These outcomes will be measured through the Butterfly Volunteer coordinator and Hub manager collecting the following:
y Number of families visiting the Butterfly Hub
y Referrals made to community services
y Case studies
y Together with The Anne Robson Trust, we will review our progress against outcomes quarterly
We are also aware that there is considerable interest from other NHS Trusts in this project and expect to host visiting professionals over the coming months.
Thank you to everyone involved in this fantastic project for the loved ones of patients who are at the end of life.
Healthcare staff are often pleased to know that there is a specialist end of life volunteer available to offer companionship and comfort to a dying patient, especially if they themselves are unable to spend the time with the patient that they would wish to.
Our team of 20 Butterfly Volunteers have the gift of time to:
y Keep patients company by talking, reading or simply sitting quietly next to them
y Prioritise patients who would otherwise be alone
y Listen
y Inform nursing staff if a patient's level of comfort changes
y Support families/visitors by offering respite or simply by listening to their concerns
y Make refreshments for families/visitors
y Organise concessionary parking
y Organise beds and care packs for visitors staying overnight
y Signpost families/visitors to other support functions including chaplaincy, Carers First, and bereavement services
Patient visits since the service began
Year Total number of patients supported
Total number of patient visits made
2017 31 42 2018 412 1234
2019 461 1623
2020 Volunteers stood down during COVID-19 pandemic
2021 176 469 2022 323 856 Total 1,403 4224

The Butterfly Volunteers are currently available MondayFriday, from 10am-4pm.
Thank you to all of our Butterfly Volunteers. For more information, please contact me at nicki.harris1@nhs.net

 By Silpa Dhaneesh, lead professional
By Silpa Dhaneesh, lead professional
As you may be aware, I was appointed as lead professional nurse advocate/professional midwifery advocate (PNA/ PMA) and we brought all the PNAs and PMAs together as a team in August 2022, to support nurses and midwives across PAHT.
We currently have 19 PNAs and 9 PMAs, who are providing the A-EQUIP (Advocating for Education and Quality Improvement) model of supervision to nurses and midwives. This facilitates a continuous improvement process that values nurses and midwives, builds their personal and professional resilience and contributes to the provision of high-quality care. A-EQUIP (Advocating and Educating for Quality Improvement) is a model of clinical supervision that has four distinct functions: normative; formative; restorative; and
personal action and quality improvement.
y Normative: managerial aspects concerning practice, learning and core mandatory training
y Formative: educational aspects - developing knowledge and skills in professional development and self-reflection
y Restorative: supportive aspects including personal development, improving stress management and
mitigating burnout
y Personal action and quality improvement: to enable nurses to contribute to quality improvement
Our key roles are to:
y Support nurses/midwives in their career progression. We run a career clinic every first Wednesday of the month, offering career coaching
y Provide restorative clinical supervision (single or group) to our nurses/ midwives, which gives a
safe space for them to have confidential conversations about topics such as work life stress, conflicts and burnout. Restorative clinical supervision supports our people to have reflective conversations and open feedback
y Staff wellbeing is one of our primary responsibilities. We arrange many projects to support staff wellbeing. This includes Wellbeing Week, a four-day programme which brings all wellbeing services together to raise awareness of the support available, and with activities including team-building for all inpatient/outpatient departments
y We provide Three Step Support, with a programme of supportive sessions and resilience activities for newly recruited nurses, and a PNA Forum once a month where people can walk in for Restorative Clinical Supervision (RCS)
y In the maternity team, we run quarterly open days to raise awareness about PMA services, which also feature team building activities and fun games
y Empower our nurses/ midwives to advocate for themselves, their colleagues and their patients
y Support and encourage change and service improvements
Benefits for our people:
y Feeling supported
y Experiencing less stress, burnout and sickness absence
y Developing personally and professionally
y Being less inclined to leave the profession
y Increasing their confidence
y Feeling less isolated
y Developing clinical competence and knowledge
Benefits for the organisation
y Having a means of developing nursing practice to improve the quality of patient care
y Improved communication between professional groups
y Dissemination of good practice and shared learning
y Reduced turnover of staff/ sickness absence
y Innovation encouraged; more motivated colleagues with higher job satisfaction
Benefits for our patients
y Transforming our care is a key focus of our PAHT 2030 strategy. Quality patient care can only be delivered when our people feel valued and supported. It is important that our people are resilient, with the right support, and restorative clinical supervision plays an important role in this. It provides a safe and confidential place to share experiences and for the PNA/PMAs to signpost to support
We have made great progress as a team so far and look forward to continuing to provide these services to support nurses and midwives. We are also aiming to recruit more PNAs to provide the A-EQUIP model of supervision across the organisation. Our key areas of focus are quality improvement, the wellbeing of our people and career/ professional development.
If you would like to find out more and to get involved with Team PNA and PMA, please contact me at silpa.dhaneesh@nhs.net.
Thank you to everyone who has taken part in the first five editions of Our Journal. It has been excellent to showcase your work, with a broad range of articles written by our clinical people, for our clinical people.
Please contact us at paht.communications@nhs.net to feature in the next edition - publishing October 2023.
The Electronic Prescribing and Medicines Administration (EPMA) team plays a pivotal role in modern healthcare systems, revolutionising the way medications are prescribed, administered, and managed. In an era of advancing technology and digitisation, our team uses the power of electronic systems to enhance patient safety, streamline medication processes, and improve overall healthcare outcomes.
Our EPMA team consists of a mixture of pharmacists, technicians and nurses who each bring their own unique experiences and expertise to create a well-balanced, diversified skill mix.

The main prescribing program in use at PAHT is JAC, which
is used by doctors, nurses and other healthcare specialists to prescribe, validate and administer patient medication safely and effectively. As well as looking after the day-to-day maintenance tasks of JAC, our team are also regularly involved in:
y Streamlining pharmacy medication related processes
y Creating and implementing training packages, including face-to-face as well as e-learning modules
y Working with multidisciplinary teams to develop new protocols to standardise prescribing
y Cost Improvement Plans
y Medicines management and optimisation audits
y Working towards a paper light environment by digitising paper-based work streams as per the PAHT2030 strategy
y Creating bespoke medication reports
y Developing Business Continuity Plans
Our team are currently working on a project to transfer complex, paper-based clinical trials onto a specialist chemotherapy prescribing system, called ChemoCare.
The goal of this project is to improve how trial medications for chemotherapy are prescribed and administered to patients. It involves working closely with the specialist clinical trials teams at the Trust, as well as Great Ormond Street Hospital (GOSH), which is well-known for its expertise in children's healthcare. By working with this respected hospital, our team can benefit from their experience and insights in treating children with cancer.
Our team has achieved a remarkable milestone as we are on our way to becoming one of the first UK Trusts
to transition this paediatric ALLTogether (A2G) clinical trial from traditional paper-based methods to an electronic prescribing platform. As we continue with this important initiative, we understand how it can greatly impact patient outcomes and the field of chemotherapy.
By overcoming the challenges of integrating clinical trials into chemotherapy prescribing systems, we are contributing to medical progress and making way for more effective and personalised treatments.
Our work will undoubtedly change how cancer is treated at PAHT, bringing new hope and better outcomes for patients now and in the future.
We will update you on our progress in a forthcoming edition of Our Journal.

For more information, please contact me at robert.hart@nhs.net
Together with members of our executive team, we hosted a visit with colleagues from the Trauma Network on 26 May.
The Trauma Network is responsible for improving care for major trauma patients across the east of England. The visit was an opportunity to showcase the hard work of the trauma multidisciplinary committee to improve the care that we provide to our patients across all trauma pathways.
A big thank you to Bianca Johnson, our trauma audit and research network (TARN) coordinator, who works behind the scenes to drive improvements forward with our clinicians.
We have improved our scores for each performance indicator, so much so that we are now the second best performing unit in the region for our support and provision of care.
This is a great achievement – well done to all involved.
You can see the key data demonstrating our achievements overleaf.
Ws (survival probability for the hospital) for 2022 is positive = + 2.31 i.e. more survivors than death (as of May 2023 on TARN analytics)
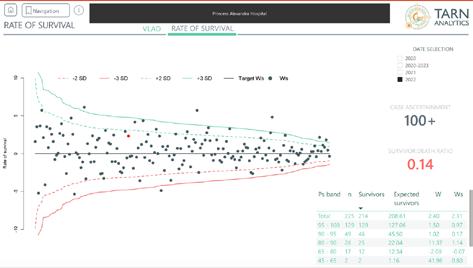
Improvement in Variable Life Adjusted Display (VLAD) upward trend again (as of May 2023 on TARN analytics)

TARN Ws (survival probability for the hospital) score for East of England trauma network - ranked second for all trauma units in the east of England

Well done to our medical colleagues who were recognised as part of the medical education annual thank you and awards celebrations on 9 June, held at Harlow Rugby and Football Club.

The event (pictured, right) aimed to recognise and thank our medical colleagues for their hard work and to celebrate their achievements, improvements and education
successes throughout the year. There were six award categories, including: the Outstanding Contribution Award, Clinical Excellence
Award, Quality Improvement Award, Research and Innovation Award, Leadership Award and E-portfolio Award.
19 junior medical colleagues were nominated:
y Dr Ben Chisnall
y Dr Elizabeth Winterton
y Dr Sarmad Mushtaq
y Dr Robert Chapman
y Mr Sameer Mansukhani
y Mr Narek Sargsyan
y Dr William Collier
y Dr Nisa Sekhon
y Dr Christos Dragonas
y Dr Husna Ali
y Dr Muhammad Shoaib Iqbal
y Dr Mohamed Marwa Bebars
y Dr Sajanee Samuel
y Dr Swati Jha
y Dr Irfan Saleem
y Dr Lucy Kinch
y Miss Gita Lingam
y Ms Rijitha Muthurajah
y Dr James Stevens
Please see the winners and runner-ups belowcongratulations all.
Outstanding Contribution Award Winner
Dr Ben Chisnall
Runner-up
Ms Rijitha Muthurajah
Clinical Excellence Award Winner
Dr Gita Lingam
Runner-up
Dr Robert Chapman
Quality Improvement Award Winner
Dr Husna Ali
Runner-up
Dr Irfan Saleem
Research and Innovation Award Winner
Dr Sameer Mansukhani
Leadership Award Winner
Dr Muhammad Shoaib Iqbal
E-portfolio Award Winner
Dr Elizabeth Winterton
Medical Education Educator Award
There were 30 nominees under the Medical Education Educator Award, including:
y Dr Kapila Gunasekera
y Mr Kar Teoh
y Dr Farshid Ejtehadi
y Mr Mohamed Osman
y Dr Anuvidya Reddy
y Mr Narek Sargsyan
y Dr Anna McCorquodale
y Mr Ali Gharib
y Dr Nicola Ray
y Dr Suboshini Kugaprasad
y Dr Fiona Hikmet
y Mr Naveed Kirmani
y Miss Vardhini Vijay
y Mr Phillip Chandler
y Mr Adam Hussein
y Mrs Rona Drumm
y Dr Ainkaran Muthiah
y Dr Deb Ghosh
y Dr Mawra Suzar
y Dr Sanath Reddy
y Dr Sreekanth Sakthi
y Dr Georgios Kounidas
y Dr Shavita Kuckreja
y Dr Galal Imtiaz
y Dr Rasim Chowdhury
y Dr Kylin Hu
y Dr Ahmed Alneshawy
y Dr Husna Ali
y Dr Sarah Gralton
y Mr Andrew Foster
The winner was: Dr Anuvidya Reddy
The joint runners-up were:
Mr Kar Teoh and Mr Andrew Foster
Thank you to all involved for their hard work, effort and support for a successful thank you and awards celebration.
y 6 July – one day event –Health Education England (HEE) Clinical Hub Day for Educators
y 7 July – two hour event –new consultant educator session
y 11 July – one day event
– Orientation to Clinical Practice Day
y 10 August - half day event
– Foundation Training Programme session
y 1 September – one day event - Courageous Conversations (consultants, staff and associate specialists (SAS) and locally employed doctors (LED)
y 14 September – 90 minute session – Providing and Receiving Feedback for Senior Leaders (via MST)
y 21 September - half day event – Foundation Training Programme session
y 11 October – three hours
- Faculty of Educators Evening Event
y 17 November – one day event – Bullying and Harassment (consultants, SAS and LED doctors
For more information, please contact me at preethi.
gopinath@nhs.net.
Take a look at some examples of the range of articles published and publications contributed to by our people since the last edition of Our Journal - a fantastic achievement. Full information of authors and articles are available from the library team: paht.lib.desk@nhs.net.
y Abbosh C., Frankell A.M., Harrison T., Kisistok J., Garnett A., Johnson L., et al (2023). "Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA."
Nature, no pagination. Approximately 300 authors, of which two are from PAHT: (L Robinson, P Russell)
y Al Bakir M., Huebner A., Martinez-Ruiz C., Grigoriadis K., Watkins T.B.K., Pich O., et al (2023). "The evolution of non-small cell lung cancer metastases in TRACERx." Nature, no pagination. Approximately 300 authors, of which two are from PAHT: (L Robinson, P Russell)
y Al-Jabri T, Ridha M, McCulloch RA, Kayani B, Arif A, Habad M, Kosuge
D, Jayadev C, Donaldson
J, Skinner JA. "Hip Resurfacing Arthroplasty: Past, Present and Future."
Orthop Rev (Pavia).
2023 Jul 1;15:77745. doi: 10.52965/001c.77745.
PMID: 37405271; PMCID: PMC10317512.10 authors, of which two are from PAHT: (Habad, M, Kosuge, D)
y Al-Janabi MM, Apostolides M, Southgate C, Dhinsa BS. "Early mobilization following elective ankle lateral collateral
ligament reconstruction in adults." Foot (Edinb). 2023 May;55:101988. doi: 10.1016/j.foot.2023.101988. Four authors, of which the lead author is from PAHT: Al-Janabi, M.M
y Al-Sawaf O., Weiss J., Skrzypski M., Lam J.M., Karasaki T., Zambrana F., et al (2023). "Body composition and lung cancer-associated cachexia in TRACERx." Nature Medicine, no pagination. Approximately 300 authors, of which two are from PAHT: (L Robinson, P Russell)
y Birkett L., Fowler T., Pullen S. "Performance of ChatGPT on a primary FRCA multiple choice question bank." Br J Anaesth 2023;no pagination. doi:10.1016/j. bja.2023.04.025. Three authors, of which one is from PAHT: (Pullen, S)
y Crabtree T.S.J., Sennik D., Bickerton A., et al. "The impact of diabetes duration on HbA1c and weight changes associated with injectable semaglutide: Subanalysis from the Association of British Clinical Diabetologist (ABCD) Semaglutide Audit." Diabet. Med. 2023;40(Supplement 1):131132. doi:10.1111/dme.15048.
10 authors, of which one is from PAHT: (Sennik, D)
y D'Angelo A., Chapman R., Sirico M., et al. "An update on antibody-drug conjugates in urothelial carcinoma: state of the art strategies and what comes next." Cancer Chemother. Pharmacol. 2022;90(3):191205. doi:10.1007/s00280022-04459-7. Seven authors, of which one is from PAHT: (Chapman, R)
y Deschepper M., Labeau S.O., Waegeman W., et al. "Pressure injury prediction models for critically-ill patients should consider both the case-mix and local factors." Intensive Crit. Care Nurs.. 2021;65:no pagination. doi:10.1016/j. iccn.2021.103033.
Hundreds of authors of which two are from PAHT: (Saha, Holmquist)
y Fennell D., Griffiths D., Eminton Z., et al. "Niraparib efficacy in patients with unresectable mesothelioma: a randomised phase II trial of niraparib versus active symptom control in patients with previously treated mesothelioma: NERO (TRIALS IN PROGRESS)." Lung Cancer 2023;178(Supplement 1):S85-S86.
doi:10.1016/S0169-
5002%2823%2900620-7. 28 authors, of which one is from PAHT: PapadatosPastos, D
y Fraser-Govil S., Akter N., Elangovan E., Winter H., Gopinath P. "Is nipple discharge cytology still a relevant tool in management of breast disease?". Eur. J. Surg. Oncol. 2023;49(5):e240. doi:10.1016/j. ejso.2023.03.096. All PAHT authors
y Griffiths, G.O. et al. (2023). "A randomised phase II trial of niraparib versus active symptom control in patients with previously treated mesothelioma: NERO." Journal of Clinical Oncology, 41, p. TPS8600. doi:10.1200/ JCO.2023.41.16_suppl. TPS8600. 15 authors, of which one is from PAHT: (Papadatos-Pastos, D)
y Hill W., Lim E.L., Weeden C.E., et al. "Lung adenocarcinoma promotion by air pollutants." Nature 2023;616(7955):159-167. doi:10.1038/s41586-02305874-3. Approximately 300 authors, of which two are from PAHT: (Robinson, L; Russell, P)
y Ho G.C., Samuel S.L., Haroon U., Drage M. "Youngest pancreas transplantation alone in the UK for type 1 diabetes and severe subcutaneous insulin resistance." BMJ Case Rep 2023;16(6):no pagination. doi:10.1136/ bcr-2023-255068. PAHT
author: S L Samuel
y Huber P.M., Afzal N., Arya M., et al. "Prostate Specific Antigen Criteria
to Diagnose Failure of Cancer Control following Focal Therapy of Nonmetastatic Prostate Cancer Using High Intensity Focused Ultrasound."
J. Urol. 2020;203(4):734742. doi:10.1097/ JU.0000000000000747. 20 authors, of which two are from PAHT: Arya, M; Virdi, J
y Javanmard-Emamghissi H., Doleman B., Kulkarni N., et al. "Author response to: Antibiotics as first-line alternative to appendicectomy in adult appendicitis: 90day follow-up from a prospective, multicentre cohort study." Br. J. Surg. 2022;109(4):E73-E74. doi:10.1093/bjs/znab456. Approximately 400 authors, of which five are from PAHT: Ejtehadi, F, Warrag, I, Ivanov, B, Refson, J, Boateng , C
y Karasaki T., Moore D.A., Veeriah S., NaceurLombardelli C., Toncheva A., Magno N., et al (2023). "Evolutionary characterization of lung adenocarcinoma morphology in TRACERx." Nature Medicine, no pagination. Approximately 300 authors, of which two are from PAHT: (L Robinson, P Russell)
y Leal G.C., Whitfield T., Praharaju J., Walker Z. & Oxtoby N.P. (2023). "Crop Filling: a pipeline for repairing memory clinic MRI corrupted by partial brain coverage." medRxiv, no pagination. Four authors, of which
one is from PAHT: (Janaki Praharaju)
y Light A, Peters M, Reddy D, et al. "External validation of a risk model predicting failure of salvage focal ablation for prostate cancer." BJU Int. 2023 doi:10.1111/bju.16102, 10.1111/bju.16102. 32 authors, of which one is from PAHT: Virdi, Jaspal
y Martinez-Ruiz C., Black J.R.M., Puttick C., et al.
"Genomic-transcriptomic evolution in lung cancer and metastasis." Nature 2023;616(7957):543-552. doi:10.1038/s41586-02305706-4. Approximately 200 authors, of which two are from PAHT: (Robinson, L; Russell, P)
y Nayar M., Varghese C., Kanwar A., et al. "SARS-CoV-2 infection is associated with an increased risk of idiopathic acute pancreatitis but not pancreatic exocrine insufficiency or diabetes: Long-term results of the COVIDPAN study." Gut 2023;no pagination. doi:10.1136/ gutjnl-2021-326218. 53 authors, of which one is from PAHT: (Ivanov, B)
y Ng K.W., Boumelha J., Enfield K.S.S., et al. "Antibodies against endogenous retroviruses promote lung cancer immunotherapy." Nature 2023;616(7957):563-573. doi:10.1038/s41586-02305771-9. Approximately 300 authors, of which two are from PAHT: (Robinson, L; Russell, P)
y Plagens-Rotman K., Jarzabek-Bielecka G.,
Lorkiewicz-Muszynska D., et al. "Outline of the Problem of Sexual Violence against Children Including STD." Clin. Exp. Obstet. Gynecol. 2023;50(6): no pagination. doi:10.31083/j.
ceog5006117. 17 authors, of which one is from PAHT: Drejza, M
y Robinson E, Smyth RL. "Preventing respiratory syncytial virus bronchiolitis in infants."
BMJ. . 2023381doi:10.1136/

bmj.p1023, 10.1136/bmj. p1023. Two authors, of which one is from PAHT: (Robinson, L; Russell, P)
The Princess Alexandra Hospital NHS Trust, Hamstel Road, Harlow, Essex, CM20 1QX 01279 44 44 55
NHSHarlow
@NHSHarlow
@PrincessAlexandraNHS
The Princess Alexandra Hospital NHS Trust