
8 minute read
DIAGNOSTICS
Variable Barcodes to Boost COVID-19 Test Kit Experience
KEREN SOOKNE, DIRECTOR OF EDITORIAL CONTENT
1. Technology turns a standard color or line result into a scannable barcode, leaving less to consumer interpretation.
Many consumers who’ve had the experience of purchasing at-home health tests have been faced with “faint line” confusion. Does it count? Is it even a line? They purchase the device looking for answers and end up more unsure.
Israel-based Varcode is looking to improve lateral fl ow test kits with technology intended to take the guesswork out of reading results by off ering a scannable barcode. Though barcodes are typically static, Varcode, which also has offi ces in the U.S., has developed a method of changing a barcode based on a sensor, making it dynamic and scannable. (Varcode stands for “variable barcode”).
TOP THREE TAKEAWAYS
2. Tests can be recalibrated because there is a central database of results—artifi cial intelligence can be used to recalibrate the test.

↑ Anatomy of a simple lateral fl ow test kit. 3. The data is being used to learn about the pandemic.
Temperature Indication
Varcode also produces temperature indication tags using thermochromatic ink for temperature-sensitive lab samples and products. In most cases vaccines are extremely sensitive to temperature, and Varcode has been approached by large pharmaceutical companies looking to ensure temperature compliance of the COVID-19 vaccines they’re developing.
At Netherlands-based Labonovum, a medical diagnostics company, the indicators are used for incoming patient samples (not related to COVID-19). Dennis Poland, clinical biochemist, explains, “When a blood sample arrives in our lab, the Varcode tag is immediately scanned. All the data is uploaded to our system and we get immediate feedback on the quality and condition of the sample,” he explains. “All of this happens within a blink of an eye without generating additional work on the fl oor. Most important however, is that we can guarantee a correct diagnosis.”
START








Innovation from hand to health
TAG ID: 1900769080 MDL #: 11080-1
1 2 3
Remove backing on tag Apply tag to specimen vial Pull green START tab powered by:
SMART TAG
Yaron Nemet, chief technology offi cer and director of Israeli operations, explains, “It’s relatively easy to take a barcode and make it unreadable. But it’s diffi cult to take one readable state and to move it into a diff erent readable state. We’re accomplishing this using time and a temperature sensor to change the barcode.” Varcode is now at work on applying this technology in a rapid fl ow test (RFT) for a COVID-19 sensor.
Rapid fl ow test
The RFT kit consists of lateral fl ow assay (LFA) inside of a housing (see image, left). The assay contains a certain protein that demonstrates the virus painted with gold, and the existence of antibodies in the blood plasma will tie to the protein and cause a color change in the form of a line.
With Varcode’s Smart Tag technology, the LFA remains the same—the changes are the additional printed opaque layer added on top of the LFA and the viewer scanning experience. The actual test kit itself, approximately the size of a credit card, is composed of plastic and is similar to what you would see in a pregnancy test on the market today.
Once the sample is collected, the patient follows the prompts in the mobile app, scans the Smart Tag(s) when instructed, and the information is automatically sent to the secure cloud. The mobile app processes the data, shares it with approved health agencies and/ or doctors, and returns results and/or instructions to the patient.
The manufacturer can also choose to obscure results from patients to prevent self-diagnosis or self-treatment. In these cases, the test can be performed from the comfort of home with physician-defi ned automated return messaging sent to the patient through the app.
Adding patient information
Allowing for additional patient information through an app can improve test accuracy. Certain LFAs benefi t from a barcode more than others. Nemet says, “Let’s take a pregnancy test. You’ll have a visible state with one or two lines and you can see them, but sometimes it’s not very clear whether you have two lines or not.”
Another case may be that the manufacturer wants to add additional information to contribute to the test’s accuracy. “So it’s not only the line, it’s the line plus, ‘Do you have fever? When was the last period?’ And then we can take all this information—with the intensity of the line—to give an answer. It’s a complicated task made possible by using a computerized algorithm,” he explains.
Accuracy and recalibration
The option to recalibrate tests is another feature Varcode technology can accommodate. “Most pregnancy tests state they have a 99.X% accuracy rate. How do they know that this is the actual number? It’s based on tests they made in the lab,” Nemet says. But variations during assay production can potentially aff ect accuracy.
Too little gold color added to an LFA can cause a very light line to show, even if the patient has a positive result. Too much gold color added during production, and the consumer may see a false positive. Nemet says, “If you have big data—a large number of tests—you can recalibrate your test afterwards. This is critical when you’re dealing with new viruses such as COVID-19 or the next COVID-20 that we all hope doesn’t come.” The idea is that they are storing the results in a database. As an example, a light line is thought to be “negative” at the outset of a pandemic. Later on with more testing conducted, researchers fi nd the result was actually “positive,” and they can calibrate the app that even this light line is “positive.”
Currently, manufacturers are in a rush and building millions of tests. Nemet says that later on, manufacturers may want to recalibrate as the data sets become bigger, “and this is something you cannot do if you have a standard rapid fl ow test,” he notes.
Nemet says the added costs to manufacturing are nominal. “The manufacturing process is the same, we are just adding printing to
the packaging which causes the barcode change. And of course, when you have a database, there are some costs related to that,” but dividing it by the number of tests, it’s reported by the company to be very small.
New applications in COVID-19 testing
When the magnitude of the COVID-19 pandemic was understood, a company that developed an RFT approached Varcode to build a mechanism to convert the barcode based on results. Nemet explains, “They understood that they needed to have a sample database—they needed big data in order to recalibrate the tests and get
Privacy
There are issues that need to be addressed regarding privacy, and regulations and expectations differ between countries. “Of course, all of the information that goes into our system is highly confi dential and the manufacturer defi nes whether certain data is mandatory to enter or not. Everything is based on the FDA regulation, FDA CFR21 Part 11. We’re defi nitely giving lots of thought and attention to confi dentiality and the security of the data,” Nemet explains.

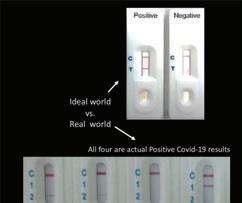
↑ Results can cause confusion for consumers when interpreting lines on traditional tests. The company is at work on FDA approval for its COVID-19 test kit.
GLASS AMPOULES Single-Use, Multiple Benefits

Prescription Oral Diagnostics Veterinary Dermatology
more accuracy, to reduce false positive and false negative results.”
The COVID-19 application is the first use of the Varocde technology in the lateral flow test market—they are now

working on FDA approval and they estimate that kits will hit the market before the end of the year based on current positive results.
“We are building a specific COVID-19 app because we want to get additional information

about the extra symptoms in order to combine to the results,” Nemet explains. They also want to collect information about the exposure of the patient to other people, such as where they were, if they were using a subway, and when. Of course, every country differs in privacy laws. In Israel, where Nemet is based, certain data is sent to the minister of health to contribute to public health decisions about offices, public spaces, etc. (See Privacy sidebar, left.)
A future for other applications
This system is not only applicable to COVID-19. “The method of using AI and big data in order to collect data and recalibrate our tests will enable us to use this technology for other RFT kits, with the goal of being more prepared for the next epidemic that everyone hopes will not happen,” says Nemet.
By offering RFTs with more intelligence, less is left to user interpretation. “If you have a very light line of IgM, the user may take whatever answer they want or expect. They may say, ‘It’s much lighter than the control line, therefore I’m not sick.’ Another patient may think, ‘I have a line, and although it’s very light, it’s not nothing. So maybe I am sick,’” Nemet notes. “So the idea is to give an answer to the patient that’s not solely based on their opinion and their way of analyzing the line. Of course, we are analyzing those lines, but we can compare the result to thousands of different lines.”
PACK EXPO Connects – November 9-13. Now more than ever, packaging and processing professionals need solutions for a rapidly changing world. The power of the PACK EXPO brand delivers the decision-makers you need to reach. For more information visit www.packexpoconnects.com.


