6 minute read
R&D LAB
from Beanscene Jun 2020
by Prime Group
Rather than sacrificing homecoffee to be the main culprits behind chemicals and sophisticated equipment? delivered specialty goodness for these coffee’s bitterness. Activated charcoal comes to mind, as experiments, I turned to supermarket CGAs are not bitter in their natural it’s commonly used for the removal of coffee. The description on the bag of forms. However, as the roast progresses, phenolic contaminants from wastewater. beans I’d bought described the coffee a chain of reactions convert CGAs to This is good news as CGAs and their as “medium roast”. I brewed the coffee chlorogenic acid lactones (a group of breakdown products are also phenolic with a chemex and tasted the whole bitter about 10 chemicals) responsible for some compounds. It’s even used in the Swiss segment of the coffee flavour wheel: harsh mild, but not unpleasant, bitterness. water process for removing caffeine from caustic bitterness combined with pungent Roasting the beans further breaks the green coffee. But what is it exactly and phenolic bitterness. There are some lactones down to a group of chemicals how does it work? pleasant nutty and malty aromas, but called phenylindanes that cause harsh Activated charcoal, or active carbon, the experience was dominated by those bitterness, a characteristic of dark roasted is made by heating plant materials, such persistent bitter flavours. coffee. In addition, according to the as coconut husks, to high temperatures in a low-oxygen environment, a process called pyrolysis. The charred material is then “activated” using oxygen or steam. Basically, the process creates tiny cracks and pores, dramatically increasing the surface area. Just one gram of activated carbon has a surface area of 500 to 3000 square metres, comparable to the size of a football field. That’s a lot of area for molecules to attach. Activated carbon acts like a sticky sponge for gases and chemicals, and it has wide usage from water purification to medical treatment for acute poisoning. Medical or food grade activated charcoal is safe to take in small amounts. It’s also safe to drink coffee filtered through it, as we will do in this experiment. However, it’s not recommended to ingest too much as it can strip food from its nutrients.
Dr. Monika Fekete shares a home experiment to demonstrate
how activated charcoal can remove the bitterness from coffee. I ’m taking these challenging times So where does bitterness in coffee authors of this study, caffeine accounts for as an excuse to indulge in online come from? According to a report in around 15 per cent of coffee’s bitter taste. shopping for locally crafted beans, Science magazine, food chemists at the But is there a way we could remove and to play with home-based Technical University of Munich discovered these bitter tasting compounds from coffee experiments. chlorogenic acids (CGA) present in green coffee at home without using laboratory
Dr Monika Fekete is the Founder of Coffee Science Lab. A bittersweet experiment
This leads us to another important point about activated charcoal: it’s not very selective. A large range of molecules Figure 1. USB microscope image comparing the grain size of “fishtank” and medical charcoal to coffee grinds. can stick to it. In the following experiment, I will show you how activated carbon can 74 beanscenemag.com.au
Figure 2. The TDS percentage of filter coffee was reduced using fishtank charcoal granules. The higher charcoal to coffee ratio removed the most dissolved material.
remove bitterness from coffee. However, we need to keep in mind that it might remove other, potentially desirable flavour compounds too. Let your tastebuds decide.
TRY THIS AT HOME Activated carbon is easy to come by. I bought Charcocaps capsules from the pharmacy and charcoal granules from the pet shop, used for purifying aquarium water. The two are very different, as Figure 1 shows. The “fishtank” carbon comes in larger beads, up to a few millimetres in size. The medical charcoal found inside the capsules is a very fine powder, finer than the smallest grains of ground coffee (shown in Figure 1 for comparison).
I brewed up a pot of coffee using my supermarket beans and a three-cup chemex, but you could use any filter preparation method. I used 20 grams of ground coffee to 320 grams of hot water with a brew time of three minutes, but feel free to use your own recipe.
First, let’s try removing bitter flavours with the fishtank carbon. Pre-soak the beads in water for a few hours so that water replaces air in the pores and any fine dust can be washed off.
Next, combine the charcoal granules with the brewed filter coffee in a plunger, changing the following variables: - Charcoal to coffee ratio. I expect that the more charcoal is used, the more bitterness will be removed. I prepared three different ratios: 1:1 (50 grams charcoal to 50 grams brewed coffee), 1:2 (25 grams charcoal to 50 grams coffee), and 1:0.5 (100 grams charcoal to 50 grams coffee). - Soaking time. The longer the coffee and charcoal are in contact, the more material the charcoal can adsorb.
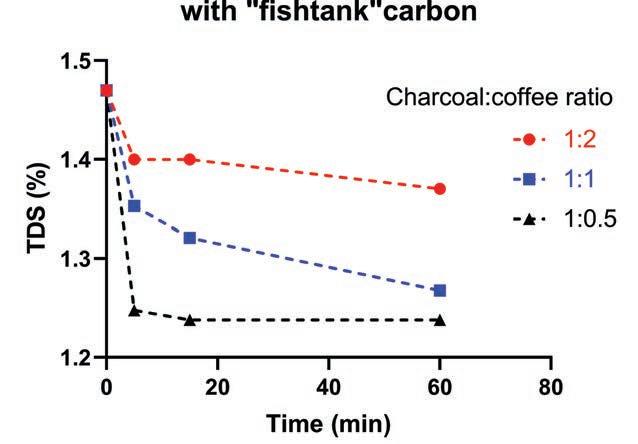

At the end of each treatment, the plunger filter can be used to easily separate the granular charcoal from the coffee. I tested the resulting total dissolved solids (TDS) percentage as an indication of how much dissolved material (therefore, flavour) was removed from the coffee. Figure 2 shows the reduction in TDS percentage observed after each treatment. It’s immediately clear that more carbon indeed removes more dissolved material. The lowest TDS percentage was measured with the highest (1:0.5) charcoal:coffee ratio. The charcoal works quickly. Most of the TDS percentage reduction occurs within the first five minutes of the treatment. Unfortunately, I found that the bitter taste still lingered on. After another 24 hours, the bitterness was finally gone, together with all other flavour. Activated charcoal can’t turn water to wine, but it can turn coffee into water. (Note: “fishtank” carbon is not rated as a food grade product. Even though we only use it for filtration, if you are concerned, just
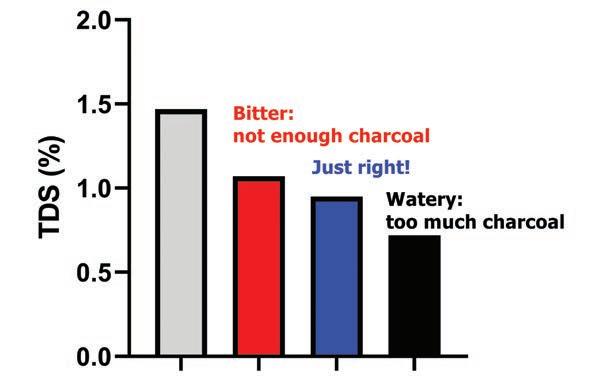
Figure 3. The TDS percentage of filter coffee was efficiently reduced in just one minute using small amounts of powdered charcoal.
follow this part as a demonstration).
Next, lets test the medical powdered carbon. Based on the first experiment, I decided to focus on the carbon:coffee ratio and fix the treatment time to just one minute. As the powder is finer, and therefore has a much larger surface area, I expected a smaller amount of carbon would be sufficient.
The carbon:coffee ratios I chose were 1:100, 1:50 and 1:25 (the last one had the most amount of carbon). When I mixed the powder with the coffee, I got something that resembled black sludge.
Separating the carbon from the liquid was quite difficult. As the grains are smaller than the coffee grinds, the pores of even a fine filter paper are too large to retain the carbon. Instead, I had to reach for syringe filters from my lab supplies. I first filtered out the larger grains using a 0.4 micrometer filter (the kind you might use with your TDS meter). Then, I filtered the resulting liquid again on a 0.22 micrometer syringe filter, which was fine enough to remove all the remaining carbon powder. A control experiment showed that the filters alone do not change TDS percentage.
The results are summarised in Figure 3. As visible in the graph, powdered carbon was much more efficient at reducing TDS percentage than the larger “fishtank” granules.
As for taste, the sample with the 1:100 ratio still had some residual bitterness. The 1:25 sample tasted watery and flat. The 1:50 sample, however, finally had what I was after: pure roasty, malty, somewhat savoury flavours with no hint of bitterness.
In conclusion, activated charcoal can be used to remove bitterness from coffee. Powdered charcoal worked better than granulated charcoal due to its enormous surface area available for the rapid adsorption of bitter flavours. Using an optimised carbon:coffee ratio, bitterness could be removed while a lot of desirable flavours remained in the resulting coffee beverage.