9 minute read
acRoss canada
NOVEL APPROACHES TO CARTILAGE REGENERATION:
the future of joint repair
Advertisement
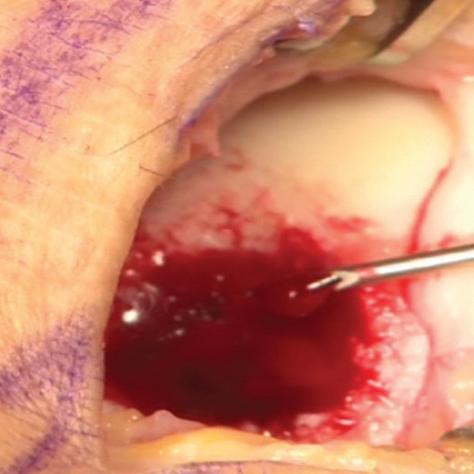
The arthroscopic images taken during a BST-CarGel surgery. The original lesion before BST-CarGel treatment.

The repaired lesion at the 13-month 2nd look arthroscopy. The delivery of BST-CarGel in a cartilage lesion.

Special to Biotechnology Focus
Osteoarthritis, a form of arthritis which occurs when cartilage becomes damaged or is worn away, is the most common kind of arthritis, affects one in eight Canadians and occurs as a result of physical trauma or excessive wear and tear which can cause chronic and debilitating pain.
Cartilage is a tissue in the body which provides structure and support to the body’s other tissues. In the knees and other joints, it is found at the end of bone where it provides a cushioning effect and helps joints to articulate smoothly. Once damaged, cartilage cannot heal on its own, and yet, if it is not treated, it can lead to arthritis or even total joint replacement.
Repairing damaged cartilage is one way to slow down or prevent knee joints from developing osteoarthritis – but the body does not do this well on its own, and even less so as we age. Despite the daunting hurdle, a group of Canadian scientists were inspired over 12 years ago to work together to address the challenge of repairing cartilage. The team of multi-disciplinary researchers led by Dr. Caroline Hoemann and Dr. Michael Buschmann from École Polytechnique in Montréal, in collaboration with the company BioSyntech (now Piramal Life Sciences�Bio-Orthopaedics Division), developed an entirely new therapy that uses biopolymer technology for enhancing cartilage regeneration which is already being marketed in Europe and also undergoing further study to expand its potential use.
The technology itself is simple: an implant, made of a chitosan solution and buffer, is mixed with autologous blood and applied to lesions. What makes it unique is that this combination stimulates the body to regenerate its own cartilage, and may indeed be pioneering an entirely new way to treat damaged joints.
Nurturing talent and collaboration: the key to discovery

setting.” — Dr. Caroline Hoemann
from industry to academia, funding was crucial so that I could ensure that my research on chitosan would continue,” says Dr. Hoemann whose research in this area was initially funded through the Canadian Arthritis Network, or CAN - a national research infrastructure, supporting arthritis research and development through multiinstitutional, trans-disciplinary partnerships between researchers, industry, government and consumers. “The support was crucial in terms of establishing my career and nurturing all of the fruitful collaborations that still continue to this day.”
At one of CAN’s annual meetings, Dr. Hoemann was inspired by a presentation on proteomics and blood serum from University of Manitoba researcher Dr. Hani El-Gabalawy (who is now scientific director of CIHR’s Institute of Musculoskeletal Health and Arthritis), and later worked with him and a hematologist on a related study.
“There are four key players that are involved in this research and CAN brought them all together,” says Dr. Hoemann, about the connections she made with scientists, clinicians, industry and also patients – known now in the arthritis community as ‘consumers’. “What really made a lasting impression on me was bringing in a consumer to contribute to my research, which really shaped a broader vision on how to translate my investigation to the clinical setting.”
Helping the body heal itself
Dr. Hoemann sees the work that she and her colleagues are doing in this area as important for several reasons.
First, the treatment is used in conjunction with microfracture surgery, the gold standard in cartilage repair therapy, and enhances its effectiveness. Second, following its primary function of stabilizing the blood clot in the cartilage lesion, the treatment encourages a natural healing effect by encouraging stem cells to be drawn to the lesion to naturally repair itself – which is less risky than taking stem cells from the patient themselves and reintroducing the cells directly into the lesion. Third, the treatment takes advantage of the body’s own mechanisms to regenerate cartilage by stimulating the cells that are already there to regenerate tissue.
The clinical turning point
Dr. Pierre Ranger is an orthopaedic surgeon who works in several clinics across Montréal and Laval, and has been practicing for more than 30 years. He operates on about 20 to 25 knees a week.
“I said: I have to use that,” Dr. Ranger recalls of the 2001 meeting when he first saw slides of repaired cartilage that resulted from implants that Dr. Hoemann had inserted into animal models. “It’s still the best result I’ve seen on cartilage – there’s nothing else like it.”
Dr. Ranger was later involved in Health Canada’s Special Access Program where 33 patients received the new treatment, and in the international randomized controlled trial of 80 patients, Dr. Ranger operated on several patients and the effects he was able to see through arthroscopy were the highest quality of cartilage repair that he had seen in his career. He believes the momentum that Dr. Hoemann and her team have created with their research is where the future of knee damage repair lies.
“Eventually I think we’ll be able to completely regenerate cartilage. Knees are the easiest joint to get into and to treat, so it’s the first joint where we’ll be doing something different than we are now.”
In-Market Success
Europe (it is not yet approved in Canada). It is applied as a one-step procedure by combining two components — a chitosan solution and a buffer which are then mixed with fresh autologous whole blood - just before its application to a lesion surgicallyprepared by bone marrow stimulation, such as microfracture.
A randomized clinical trial showed that BST-CarGel® treatment at 12 months was significantly superior to microfracture alone which is currently the standard of care in cartilage repair. The results showed consistently greater volume of repair tissue with highly improved tissue quality, more closely resembling the patient’s own cartilage. The clinical benefit to patients in terms of pain, stiffness, and ability to function were equivalent to microfracture at the 12 month mark with a similar safety profile. These results were recently published in the American Journal of Bone and Joint Surgery (Stanish, 2013).
The patients in this trial are part of a fiveyear follow-up study conducted by Piramal Life Sciences. It is well-documented that early improvement in structure (as observed following BST-CarGel® treatment) is predictive of long-term durability (Eshed, 2012; Brun, 2008; Krishnan, 2008; Knutsen, 2007). Consequently, the researchers are hoping to find that over time it will have more sustained results than micro fracture alone.
“These results are impressive,” Dr. Hoemann notes, “considering that there have only been four large randomized controlled trials in cartilage repair – ever. Making progress in cartilage repair is a real struggle,

since each patient knee has a unique repair environment, and also because it can take up to two years to demonstrate that a treatment produces a more durable repair outcome than the standard-of-care. In many other types of disease, efficacy can be proven within several months of treatment.”
Expanding use – beyond the ideal patient
BST-CarGel® is indicated for the repair of grade 3 to 4 or “severely abnormal” cartilage lesions and was recently approved in Europe to be used in all joints. The treatment thus far has been found to be most effective in younger patients with less advanced levels of cartilage damage, however, Dr. Hoemann and her colleagues are already on the case for expanding this indication.
“The goal is to elicit cartilage repair in a wider range of patients with different levels of knee damage,” says Dr. Hoemann.
A recent study, published this year in the Osteoarthritis Research Society International Journal (Guzman-Morales, 2014) looked at cartilage repair in older knee models with more ‘challenging’ cartilage damage to see if micro-drill holes (similar to the ones done in microfracture surgery) could be stimulated to induce higher quality cartilage repair tissue when treated by presolidified chitosan/blood implants. The implants were found to induce a unique “wound bloom” reaction, characterized by bone resorption and woven bone repair around the top of the initial hole edge. Treated drill holes with robust bone repair were also found to have superior cartilage repair quality.
The burden and cost of OA pain and disability in Canada is rising – and solutions are needed now more than ever
According to the Canadian Arthritis Society, it is projected that within 30 years there will be a new diagnosis of Osteoarthritis every 60 seconds, resulting in almost 30 per cent of the employed labour force having difficulty working due to the fact it hurts to move around. The potential impact of adequate pain management strategies in osteoarthritis would result in cumulative savings of $488 billion over the next 30 years, which is a reduction of nearly $41 billion in direct costs and $447 billion in indirect costs. What new understandings have been revealed over the past decade are providing scientists, physicians, and most importantly patients with hope for people with osteoarthritis so that their treatments can be less invasive, offer greater outcomes and provide relief from symptoms for longer than ever before.
References
1. Brun P, Dickinson SC, Zavan B, Cortivo R, Hollander AP, Abatangelo G. Characteristics of repair tissue in second-look and third-look biopsies from patients
treated with engineered cartilage: relationship to symptomatology and time after implantation. Arthritis Res Ther 2008; 10: R132. 2. Eshed I, Trattnig S, Sharon M, Arbel
R, Nierenberg G, Konen E, et al. Assessment of cartilage repair after chondrocyte transplantation with a fibrin-hyaluronan matrix--correlation of morphological MRI, biochemical T2 mapping and clinical outcome. Eur J
Radiol 2012; 81: 1216-1223. 3. Guzman-Morales J, Lafantaisie-Favreau
CH, Chen G, Hoemann CD. Subchondral chitosan/blood implant-guided bone plate resorption and woven bone repair is coupled to hyaline cartilage regeneration from microdrill holes in aged rabbit knees. Osteoarthritis Cartilage 2014; 22(2): 323-33. 4. Knutsen G, Drogset JO, Engebretsen L,
Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 2007; 89: 2105-2112. 5. Krishnan SP, Skinner JA, Jagiello J, Carrington RWJ, Flanagan AM, Briggs TWR, et al. Durability of cartilage repair-does histology matter. J Bone Joint Surg Br 2008; 90-B Suppl II: 323-324. 6. Stanish WD, McCormack R, Forriol F,
Mohtadi N, Pelet S, Desnoyers J, et al. Novel Scaffold-Based BST-CarGel
Treatment Results in Superior Cartilage
Repair Compared with Microfracture in a Randomized Controlled Trial. J Bone
Joint Surg Am 2013; 95: 1640-1650.
To see this story online visit www.biotechnologyfocus.ca/ novel-approaches-to-cartilageregeneration-the-future-ofjoint-repair