
8 minute read
Synthesis and Characterization of Transition Metal Complexes of N2o2 Tetra Dentate Ligands Resembling Biological Catalysts
N2O2 tetra dentate ligands Resembling Biological Catalysts Sahar Fattani and Gina Chiarella, Ph.D. Department of Chemistry, Prairie View A&M University
Abstract In this research project we expect to prepare transition metal complexes that, structurally, resemble the active centers of enzymes, and study their chemical and structural properties and test their activity as a potential ecological catalyst for industrial, pharmaceutical and medicinal uses. We have synthesizedsixSchiff bases ligands by reacting salicylaldehyde with o-phenylenediamine, acetylacetone with mphenylenediamine, acetylacetone with o-phenylenediamine, salicylaldehyde with mdiaminobenzene di-hydrochloride, o-vanillin with o-phenylenediamine, and salicylaldehyde with bis(hexamethylene)triamine. The formed ligands were then isolated and characterized. We have prepared and characterized the Schiff bases N, N-bis(salicylaldehyde)-o-Phenylendiimine (A) and N, N-bis(acetylacetone)-mphenylendiimine (B). the two ligands were prepared to react with zinc, nickel (II) and copper (II) acetates in solution to produce the respective transition metal complexes. The preparation procedure differed according to the physical and chemical properties of the ligand. The synthesis of complexes using the ligand A took place in ethanol solution at room temperature. The preparations using ligand B were also done using ethanol and refluxing until completion. All compounds were characterized by infrared and UV-visible spectroscopy; 1H-NMR additionally characterized the ligands and the zinc complexes; structures were determined by x-ray single-crystal diffraction. Computation studies were also performed in one group of these compounds to support the spectrometric analysis. The electrochemical studies have been performed on the metal complexes by using cyclic voltammetry techniques to pre-evaluate the catalytic redox behavior of these compounds. In conclusion, from the experimental results, we can state that we succeeded in preparing ligands A and B and their transition metal complexes of copper (II), nickel (II) and zinc. The instrumental analysis underlines the physical and chemical differences between ligands and metal complexes and among the metal complexes of the same ligand; electrochemical measurements indicate the possible catalytic potential of some of these metal compounds.
Introduction
This project consists of the synthesis of transition metal complexes using as ligands Schiff bases, also called imines or azomethines. A metal complex is a compound or ion that contains a ce ntral metal atom or ion surrounded by groups of molecules or ions called ligands, which are attached to the metal. The imines are organic compounds showing a carbon-nitrogen double bond (C=N), they are usually made by reacting an amine (R x -NH y ) with an aldehyde or ketone, the imines are very alike to molecular groups fencing the metallic center in enzymes. The Schiff bases are very important ligands in synthetic chemistry because they resemble the structure of the metal bonding site in enzymes and can be used as catalyst in different asymmetric reactions; they are also key intermediates for the synthesis of various bioactive compounds. They show variety of biological activities including antibacterial, antifungal, and anti cancer activities.
Objective The objective of this research is to prepare and characterize metal-compounds that, structurally, resemble the active centers of enzymes and also to study the chemical properties and test the redox activity of these compounds.
Hypothesis
To prepare the ligands using an amine and aldehyde group to form the Schiff bases that will react with three metal ions [Zn, Ni(II), Cu(II)]. To characterize the ligands and metal complexes to ensure successful metal-ligand reaction.
Methods
Synthetic Procedures Preparation of the N,N-bis (salicylaldehyde) oPhenylenediimine (A) and its metal complexes Study of the Synthesized Compounds The synthesized compounds were analyzed using spectroscopic methods as Infrared and UV- visible techniques. Analysis of the ligand A and its metal -complexes

Infrared spectra The IR spectra was taken in solid state at room temperature. UV-Visible spectra All the spectra were taken in methylene chloride solution.

Preparation of the N,N-bis (acetylacetone) m Phenylenediimine (B) and its metal complexes

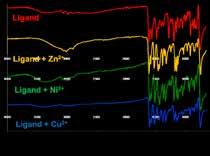

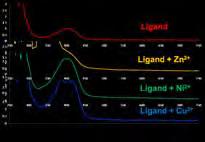
Analysis of the ligand B and its metal -complexes
Infrared spectra The IR spectra was taken in solid state at room temperature. UV-Visible spectra All the spectra were taken in ethanol solution.
Ligand A and Cu 2+ Crystal structure

Crystal System = Monoclinic Space group = Ia Z = 8 Goodness-of-fit on F2 = 0.938 Final R indexes [I>=2σ (I)]
R1 = 0.0426, wR2 = 0.1145

Conclusion • The project has achieved the synthesis of two Schiff base ligand: N, N-bis (salicylaldehyde) o- Phenylenediimine (A) and N, N-bis (acetylacetone) m-phenylenediimine (B). • A protocol for the preparation of ligands and metal complexes with zinc, nickel (II) and copper (II) acetate salts were obtained. • All compounds were successfully characterized by infrared and UV-visible spectroscopy. • The transition metal complexes that were formed, exhibited different chemical and physical characteristics from the ligands, confirming their synthesis.
Future Studies . The determination of the detailed chemical structure of these compounds. The testing of redox behavior and catalytic test of these compounds. The studies of additional properties (such as luminescence and conductivity) on these compounds. The search of alternative preparation methods such as the use of microwave irradiation as a source for synthesis for the Schiff bases.
References Ray, S., Jana, S., Jana, A., Konar, S., Das, K., Chatterjee, S.,
Butcher, R.J., Kar, S .K. Dicopper (II) complexes of a tridentate pyrimidine derived Schiff base ligand: Syntheses, crystal structures and catalytic reactions. Polyhedrom. 2012, 46, I 74. Zaithun, B. S., Emilia. A. M., Mohamed, T. Mohamed, I., Anneb, C.
K., Basyaruddin. A. R. M. Histidine-based copper tetrapeptides as enantioselective catalysts for aldol reactions. RSC Adv. 2018, 8, 34004. Lewing, D., Koppetz, H. and Hahn, F. E. Reversible Formation and
Transmetalation of Schiff-Base Complexes in Subcomponent
Self-Assembly Reactions. Inorg. Chem. 2015, 54, 7653.
Acknowledgements
R&I’s Office of Undergraduate Research (OUR, Prairie View A&M University.
SYNTHESIS AND CHARACTERIZATION OF TRANSITION METAL COMPLEXES OF N2O2 TETRA DENTATE LIGANDS RESEMBLING BIOLOGICAL CATALYSTS
Introduction
The objective of this research is to prepare and characterize metal-compounds that, structurally, resemble the active centers of enzymes and study the chemical properties and test the redox activity of these compounds. The hypothesis was to prepare the ligands using an amine and aldehyde group to form the Schiff bases that will react with three metal ions [Zn, Ni(II), Cu(II)] and to characterize the ligands and metal complexes to ensure successful metalligand reaction. This project consists of transition metal complexes synthesis using ligands Schiff bases, also called imines or azomethines. The Schiff bases are important ligands in synthetic chemistry because they resemble the structure of the metal-binding site in enzymes and can be used as catalysts in different asymmetric reactions; they are also vital intermediates for the synthesis of various bioactive compounds. They show a variety of biological activities, including antibacterial, antifungal, and anticancer activities.
Materials and Methods
We have synthesized six Schiff bases ligands by reacting salicylaldehyde with o-phenylenediamine, acetylacetone with m-phenylenediamine, acetylacetone with o-phenylenediamine, salicylaldehyde with m-diaminobenzene di-hydrochloride, o-vanillin with o-phenylenediamine, and salicylaldehyde with bis(hexamethylene)triamine. The formed ligands were then isolated and characterized. We have prepared and characterized the Schiff bases N, N-bis(salicylaldehyde)- o-Phenylendiimine (A) and N, N-bis(acetylacetone)-mphenylenediamine (B). The two synthesized ligands were reacted with metal ions such as Zn2+, Ni2+, and Cu2+.
Results and Discussion
The identification of all these compounds was made by IR and UV-Vis spectroscopy. The electrochemical studies have been performed on the metal complexes by using cyclic voltammetry techniques to pre-evaluate the catalytic redox behavior of these compounds. The Infrared spectrum displayed the stretching frequencies of the expected functional groups present in the compounds; the difference between the spectra of the ligand and the metal compounds confirmed the formation of the complexes and showed new stretching peaks due to the new interactions in the metal complex. The Ultraviolet, visible spectrometry tells us about the electronic transition in atoms and molecules. Transition metal complexes usually produce broad peaks of low intensity whereas most organic compounds as the Schiff base ligands, yield sharp and high-intensity peaks.
Study of the Synthesized Compounds
The synthesized compounds were analyzed using spectroscopic methods as Infrared and UV- visible. The crystal structure of Ligand A and Cu2+ was also taken.

Conclusion(s) or Summary
The project has achieved the synthesis of six Schiff base ligands but the characterization of two Schiff base ligands: N, N-bis(salicylaldehyde)-o-Phenylenediimine (A) and N, N-bis(acetylacetone)-m-phenylenediamine (B). A protocol for the preparation of ligands and metal complexes with zinc, nickel (II) and copper (II) acetate salts were obtained. All compounds were successfully characterized by infrared and UV-visible spectroscopy. The transition metal complexes that were formed exhibited different chemical and physical characteristics from the ligands, confirming their synthesis. The next step in this research is to test the redox behavior of the synthesized compounds to study their capability as potential catalysts contrasting with the enzymes that these compounds resemble. Additionally, we expect to study other physical and chemical properties of these compounds as magnetic properties, luminescence, and molecular structure.
References
Ray, S., Jana, S., Jana, A., Konar, S., Das, K., Chatterjee, S., Butcher, R.J., Kar, S.K. Dicopper (II) complexes of a tridentate pyrimidine derived Schiff base ligand: Syntheses, crystal structures, and catalytic reactions. Polyhedron. 2012, 46, I74. Zaithun, B. S., Emilia. A. M., Mohamed, T. Mohamed, I., Anneb, C. K., Basyaruddin. A. R. M. Histidine-based copper tetrapeptides as enantioselective catalysts for aldol reactions. RSC Adv. 2018, 8, 34004. Lewing, D., Koppetz, H. and Hahn, F. E. Reversible Formation and Transmetalation of Schiff-Base Complexes in Subcomponent Self-Assembly Reactions. Inorg. Chem. 2015, 54, 7653.
Sahar Fattani is a sophomore student majoring in Biology with a minor(s) in Chemistry and Psychology. Dr. Gina M. Chiarella is an Assistant Professor with research interests in bioinorganic chemistry, sustainability, catalysis, and synthetic chemistry.









