3 minute read
Our Best Tools Against COVID-19: Vaccines and Antivirals
by TEAM
megan mCgRaDy, mPh Cste aPPlIeD ePIDemIology
FelloW, allegheny County health DePaRtment
Advertisement
Bivalent Vaccine Data:
The COVID-19 bivalent vaccine booster protects against the virus and reduces the risk of severe outcomes, including hospitalization and death. In a study conducted by the National Institutes of Health from September to December 2022, the bivalent booster was found to be 62% effective against COVID-19, and 37% more effective in preventing severe outcomes when compared to the original booster.1
The bivalent vaccine has been recommended for people ≥ 12 years old since September 1, 2022, for those aged 5-11 years since October 12, 2022, and for those 6 months-4 years since December 9, 2022.
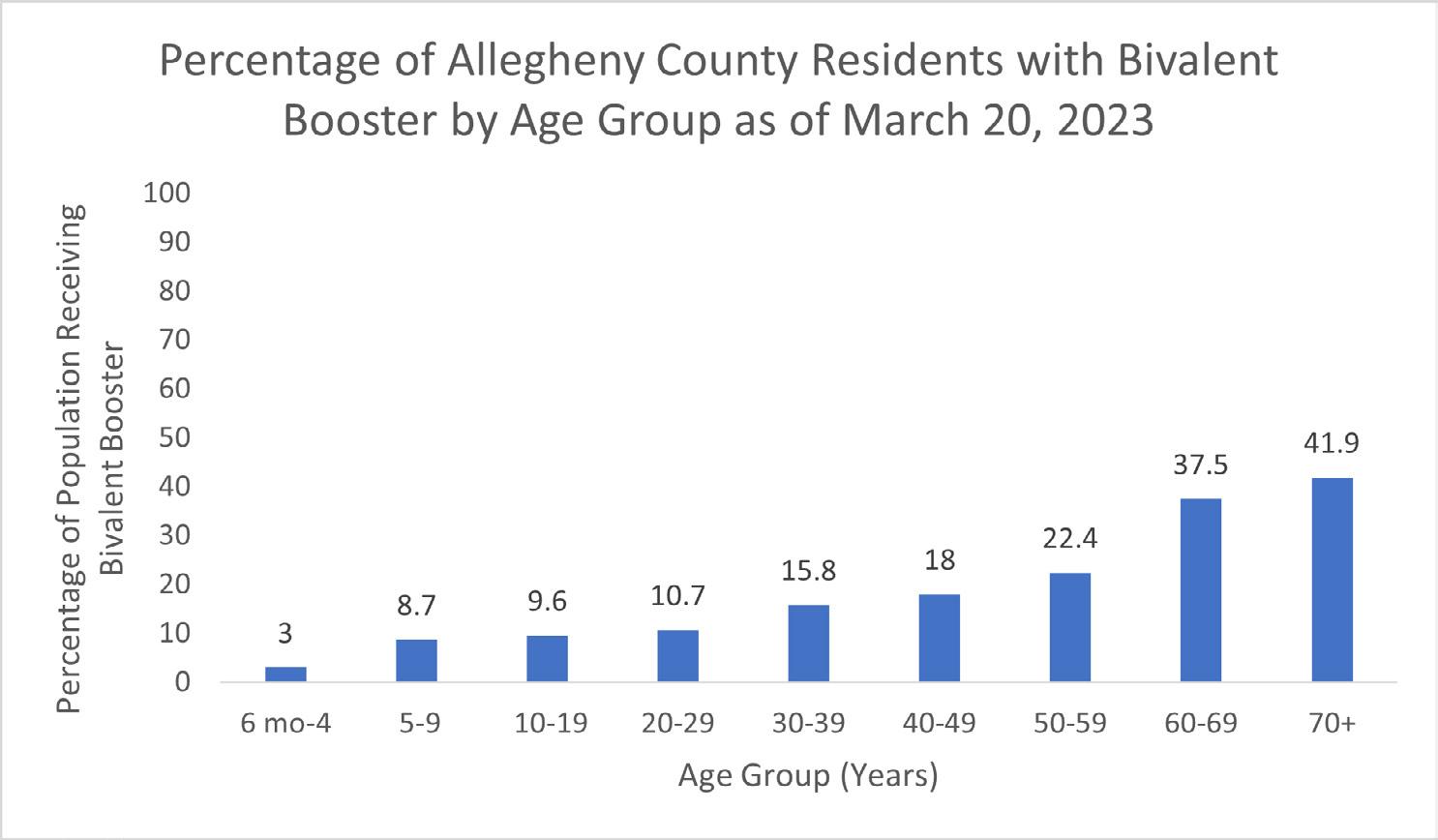
In Allegheny County, uptake of the bivalent vaccine has been low, even among seniors who are at risk for severe disease. Among those aged 70+ years, only about 42% have received the bivalent booster.
The National Immunization Survey (NIS) Adult COVID Module in 2021 found that adults who received a provider recommendation for a COVID-19 vaccine were more likely to be vaccinated, to be concerned about COVID-19 and to believe COVID-19 vaccines are important and safe.2 In 2022, the survey found that of eligible adults, 27.1% received a bivalent booster, 39.4% had not received a booster but were receptive to getting one, and the rest were unsure or reluctant.3 Among those receptive,
58.9% had not received a bivalent booster recommendation from their provider. In the NIS-Child COVID Module, 52.0% of parents were receptive to their adolescents receiving the booster, but 32.4% of them had not received any COVID-19 vaccine recommendation from their provider.3
Health care providers should continue to recommend the bivalent booster by informing adults and parents about COVID-19 related illness and the benefits and safety of the bivalent booster.
COVID-19 Outpatient Treatment:
Nirmatrelvir-ritonavir (Paxlovid), an outpatient antiviral medication given orally, is recommended for persons ≥ 12 years old, with mild-moderate COVID-19, who are at an increased risk for severe illness. Nirmatrelvir-ritonavir has been shown to be 80% effective in preventing COVID-19 hospitalization or death when given within five days of symptom onset.4 Possible side effects include dysgeusia, diarrhea, increased blood pressure and myalgia.5 COVID-19 rebound has occurred in a small percentage of those initially infected who take the antiviral medication, occurring
2-8 days after recovery or a negative test.6 Rebound symptoms are similar to the initial symptoms, but milder, and there are no recommendations for additional treatment. Rebound also occurs in persons with COVID-19 who do not take Paxlovid.7
Remdesivir (Veklury) and molnupiravir (Lagevrio) are additional COVID-19 antiviral medications. Remdesivir is an intravenous infusion administered at health care facilities for adults and children with a positive COVID-19 test who are at risk for severe disease. Remdesivir should be started as soon as possible, and definitely within seven days of symptom onset. Possible side effects include nausea and hypersensitivity. Remdesivir has been shown to reduce the risk of hospitalization and death by 87% among patients at high risk for severe disease.8 Molnupiravir is an oral antiviral medication authorized for adults ≥ 18 years of age. Molnupiravir should be given within five days of symptom onset. Possible side effects are nausea, vomiting and diarrhea. It is reported that molnupiravir reduces the risk of hospitalization or death due to COVID-19 by about 30% among unvaccinated adults at high risk for disease.9 Molnupiravir should not be given to anyone who is pregnant.
See the CDC’s COVID-19 treatment guidelines for health care providers to determine best treatment options for patients: COVID-19 Treatments and Medications | CDC. The infectious Disease Society of America has a quick reference guide for nirmatrelvir-ritonavir treatment: https://www.idsociety.org/ covid-19-real-time-learning-network/ therapeutics-and-interventions/ nirmatrelvir-ritonavir-paxlovid-point-ofcare-reference/
References:
1. NIH. Bivalent boosters provide better protection against severe COVID-19. US Department of Health and Human Services, NIH; 2023. Bivalent boosters provide better protection against severe COVID-19 | National Institutes of Health (NIH).
2. Nguygen KH, Yankey D, Lu P, et al. Report of Health Care Provider Recommendation for COVID-19 Vaccination Among Adults, by Recipient COVID-19 Vaccination Status and Attitudes – Unites States, April-September 2021. MMWR Morb Mortal Wkly Rep 2021;70:1723-1730. DOI: http://dx.doi.org/10.15585/ mmwr.mm7050a1.
3. Lu P, Zhou T, Santibanez TA, et al. COVID-9 Bivalent Booster Vaccination Coverage and Intent to Receive Booster Vaccination Among Adolescents and Adults – United States, November-December 2022. MMWR Morb Mortal Wkly Rep 2023;72:190-198. DOI: http://dx.doi. org/10.15585/mmwr.mm7207a5.
4. Lewnard JA, McLaughlin JM, Malden D, et al. Effectiveness of nirmatrelvirritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. Lancet Infect Dis. 2023 Mar 15:S14733099(23)00118-4. doi: 10.1016/ S1473-3099(23)00118-4. Epub ahead of print. PMID: 36933565.
5. FDA. Frequently asked questions on the emergency use authorization for Paxlovid for treatment of COVID-19. US Department of Health and Human Services, FDA; 2023. Paxlovid FAQs 02072023 (fda.gov).
6. CDC. Interim clinical considerations for COVID-19 treatment in outpatients. US Department of Health and Human Services, CDC; 2023. Interim Clinical Considerations for COVID-19 Treatment in Outpatients | CDC.
7. Deo R, Choudhary MC, Moser C, et al. Symptom and Viral Rebound in Untreated SARS-CoV-2 Infection. Ann Intern Med. 2023 Mar;176(3):348354. doi: 10.7326/M22-2381. Epub 2023 Feb 21. PMID: 36802755.
8. Gottlieb RL, Vaca CE, Paredes R, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med. 2022 Jan 27;386(4):305-315. doi: 10.1056/ NEJMoa2116846. Epub 2021 Dec 22. PMID: 34937145; PMCID: PMC8757570.
9. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022 Feb 10;386(6):509-520. doi: 10.1056/NEJMoa2116044. Epub 2021 Dec 16. PMID: 34914868; PMCID: PMC8693688.








