11 minute read
The Gastrointestinal Microbiome
An Evolving Understanding
By: Christi ne Stubbe, ND
The following arti cle is not endorsed and/or supported by The American Academy of Anti -Aging Medicine. The purposes of this publicati on do not imply endorsement and/or support of any author, company or theme related to this arti cle.
In this era of discovering the microbiome’s role in human health, we are only at the beginning of having a full understanding of the intricacies. What stories can the microbiome tell us about our health? Can measurement of these organisms serve as a gauge of how well we’re doing? Research continues to rapidly accumulate; however, it is challenging to piece together the entire puzzle with all the available information to tell a complete story. In the last decade, the amount of publications on the microbiome in general, and on the gastrointestinal microbiome has spiked signi cantly. e gastrointestinal microbiome is a complex and dynamic ecosystem that has both inter- and intrapersonal variation, and is in uenced by age, ethnicity, geographic location, diet, lifestyle, medications, and environmental factors. 1 It is unique, like a ngerprint. 2 If there are no two microbiomes alike, then how is it possible to draw conclusions? Translating the data to clinical application can be challenging; it takes time to arrive at meaningful conclusions. Pattern analysis is an essential part of having a greater understanding of how the microbiome a ects human health – and vice versa.
Microbes inhabit various places on and in the human body, with the vast majority concentrated in the gastrointestinal tract. ey in uence our health directly and indirectly via the production of postbiotic metabolites. e microbiome is responsible for immune and in ammation regulation, barrier function, short chain fatty acid production, vitamin synthesis, protection against pathogenic organisms and many other vital functions. 3-5 A sick microbiome can accelerate the aging and disease process. Some organisms are involved in the production of potentially harmful metabolites such as lipopolysaccharide (LPS) associated with liver disease, neurological degeneration, diabetes, and chronic gut in ammation, 6 and trimethylamine N-oxide (TMAO) involved in cardiovascular disease, diabetes, and obesity. 7,8 Additionally, there are also links to age-related changes such as loss of bone and muscle mass, as well as skin wrinkling. 9 Researchers are even discovering links between the microbiome and more abstract topics such as circadian rhythm. 10 We are discovering that the microbiome’s impact on human health is far more intricate than we’ve ever understood.
Since the inception of the Human Microbiome Project in 2007, research has come a long way with conducting a census and mapping the normal bacteria that live in and on the human body. 10 But, what is the ideal reference population for setting the optimal
healthy benchmark? e microbiome census shows us that microbiomes vary regionally, so what’s considered normal in one part of the world may be di erent from another part of the world. And is normal healthy? An unhealthy microbiome may precede chronic disease manifestation by years. 11-14 e distinction between normal/healthy versus diseased/unhealthy microbiome reference ranges is better determined in clinical studies of healthy versus diseased cohorts. Additionally, longitudinal studies help to determine what makes for an optimal microbiome.
Certain key commensal bacteria are known for their proor anti-in ammatory e ects. Most are familiar with the probiotics Lactobacillus and Bi dobacterium as having a bene cial impact on human health. Since the study of microbiome began, other bene cial key in uencers have emerged such as Faecalibacterium prausnitzii or Akkermansia muciniphila as having anti-in ammatory and gut membrane protective properties. 15 Other commensal species such as Fusobacterium spp. or Veillonella spp. are associated with in ammation. 16 However, the presence of these bacteria alone is not enough to determine whether a patient’s microbiome is in ammatory or not. While it is well-known that a single pathogen can promote infectious colitis; commensal bacteria do not necessarily regulate in ammation on their own. ey function in the context of a community. One paper discusses the concept of pathogenic communities:
“In addition to the classic pathogenic species, we propose that another kind of pathogenicity exists in the gut: one in which the whole community is ‘pathogenic’ when its emergent properties contribute to disease. In a ‘pathogenic community,’ no single microbe is pathogenic alone. Instead, the community assemblage is an environmental risk factor that contributes to a disease state. A microbial community will be pathogenic within the context of other risk factors, such as host genotype, diet, and behavior.” 17
It is important to identify pathogenic communities and patterns, versus placing importance on single organisms.
ASSESSING THE MICROBIOME
enterotypes. Enterotypes are clusters of organisms that form a community and people tend to be dominant in one of three enterotypes. Enterotypes are found in di erent regions of the world among di erent cultures with di erent diets and each enterotype has its own clinical relevance. However, the concept has been challenged in the literature due to lack of standardization. 18 Other attempts at nding microbiome patterns include analyzing ratios as they relate to health conditions. ere are phylum level assessments including the Firmicutes/Bacteroidetes and other phylum level ratios, as well as individual organism ratios. Literature suggests that a high Firmicutes/Bacteroidetes (F/B) ratio may be associated with a greater risk of metabolic syndrome, diabetes, and obesity. 19-21 However, the literature is mixed on this subject. Additionally, not all sources calculate the ratio using the same methodology.
e term microbiome includes the organisms (microbiota) and their genes. Whole genome sequencing helps to determine the individual organism’s functional roles, however, it is important to remember that not all genes are expressed. Assessing the direct metabolites shows their phenotypic expression versus only looking at genotypic potential.
Commercial laboratories measuring commensal bacteria are uniquely positioned to analyze data on a large scale, using their unique set of measured organisms. Certain types of analysis can provide meaningful data for the clinician that can help understand the patient’s microbiome patterns, assisting in treatment decisions.
Abundance
e total commensal abundance is a sum-total of the reported commensal bacteria compared to a healthy cohort. Low levels of commensal bacteria are often observed after antimicrobial therapy, or in diets lacking ber and/ or prebiotic-rich foods and may indicate the need for microbiome support. Conversely, higher total commensal abundance may indicate potential bacterial overgrowth or
probiotic supplementation.
Balance
Assessing overall balance is important to know how a patient’s composition of ora compares to a healthy cohort. In general, it is widely accepted that diversity of
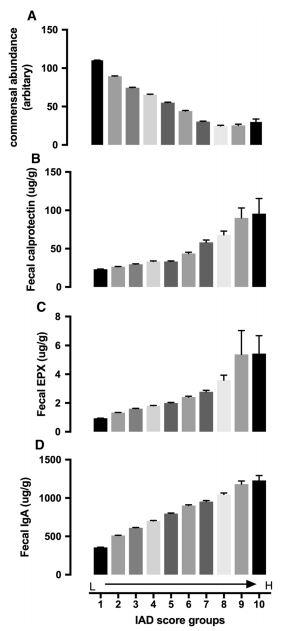
the microbiome is considered a marker of health. Decreased e creation of these types of scores are helpful because they can diversity has been associated with disease. 12,22 e presence of cause a clinician to change their treatment plan based on results. pathogens or potential pathogens can indicate imbalance and ere is no consensus in the literature on targeted strategies for may require treatment. correcting a certain pattern of dysbiosis. More outcome studies in a recently published paper. 16 e score of 24 commensal bacteria. e strength is unique because it was derived from It is not known whether dysbiosis is are needed. A unique aspect of the IAD score was a negative association with total commensal abundance. eoretically, if A NOVEL INFLAMMATION - ASSOCIATED the IAD score is elevated and abundance is low, antibiotics may DYSBIOSIS SCORE be detrimental to the already reduced abundance. However, if the IAD score is high and abundance is high, if antibiotics An In ammation-Associated option than if the abundance were low. Dysbiosis (IAD) score was introduced More studies are needed to con rm this. di erentiates patients with In ammatory is group also created another dysbiosis Bowel Disease (IBD) from those with condition score that is opposite the IAD other diagnoses including irritable score (unpublished data). e creation of bowel syndrome (IBS), celiac disease, these types of scores provides the clinician and healthy patients. e score is based with a meaningful synthesis of the data on a speci c pattern seen upon analysis that guides treatment. of this data set and score is that it is based on 173,221 stool tests. e score THERAPEUTIC OPTIONS the microbiome’s association with Rather than focusing on targeting in ammatory test biomarkers including individual organisms, it is important to fecal calprotectin, eosinophil protein X, treat the microbiome and human host as and secretory IgA. Additionally, it was a system. Simply identifying organisms validated in two separate studies that and reporting their amounts presents distinguish IBD from healthy and other a challenge for the clinician’s clinical diseases. utility. What does it mean when some is score is the rst of its kind that one require treatment? It is not possible incorporates stool biomarkers with Chen L, Reynolds C, David R, Peace Brewer A. to target only one commensal organism microbiome markers. Development of an Index Score for Intestinal In ammation-Associated Dysbiosis Using Real-World with a therapeutic since they function as Stool Test Results. Digestive diseases and sciences. 2019. a community. are warranted, it would likely be a safer are high, and some are low? Does each a cause or consequence of intestinal in ammation. It is likely It is important to remove the factors that are damaging or a vicious cycle. 23 e IAD score shows an association – not destabilizing to the microbiome while supplying the commensal necessarily causation, although the authors do discuss root cause. bacteria with what they need to thrive. Antibiotics, stress, Another interesting aspect of this study was that it reinforced environmental toxins, and a Western diet can all contribute to the pathogenic community concept discussed earlier. When the dysbiosis. Conversely, a ber-rich plant-based diet, prebiotics, 24 individual organisms were assessed individually, a correlation probiotics, physical activity, and stress management can promote with IBD could not be made – the score takes the community a healthy microbiome. 22 It is not surprising that the accumulating into account. data that links healthy diet and lifestyle to longevity and health also happens to be the factors that make for a healthy microbiome.
CONCLUSION
It is valuable for clinical laboratories to develop test-speci c algorithms that synthesize the clinical, as well as other biomarker data to provide a clinically meaningful microbiome evaluation. As data is assessed in this manner, we come closer to personalized treatment recommendations.
1. Rinninella E, Raoul P, Cintoni M, et al. What is the Healthy Gut Microbiota
Composition? A Changing Ecosystem across Age, Environment, Diet, and
Diseases. Microorganisms. 2019;7(1):14. 2. Franzosa EA, Huang K, Meadow JF, et al. Identifying personal microbiomes using metagenomic codes. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(22):E2930-2938. 3. Clemente JC, Manasson J, Scher JU. The role of the gut microbiome in systemic inflammatory disease. BMJ. 2018;360:j5145. 4. Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: friend of foe? World journal of gastroenterology. 2011;17(5):557-566. 5. Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. European journal of nutrition. 2018;57(1):1-24. 6. Wassenaar TM, Zimmermann K. Lipopolysaccharides in Food, Food
Supplements, and Probiotics: Should We be Worried? European journal of microbiology & immunology. 2018;8(3):63-69. 7. Dehghan P, Farhangi MA, Nikniaz L, Nikniaz Z, Asghari-Jafarabadi M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: An exploratory systematic review and dose-response meta- analysis. Obesity reviews : an official journal of the
International Association for the Study of Obesity. 2020. 8. Yang S, Li X, Yang F, et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical
Prognostic, and Potential as a Therapeutic Target. Frontiers in pharmacology. 2019;10:1360. 9. Finlay BB, Finlay JM. The Whole-Body Microbiome: How to Harness
Microbes—Inside and Out—for Lifelong Health. The Experiment; 2019. 10. Human Microbiome Project’s Health Relevance. Human Microbiome Project 2019; https://commonfund.nih.gov/hmp/public, 2020. 11. Ding S, Chi MM, Scull BP, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PloS one. 2010;5(8):e12191. 12. Wilkins LJ, Monga M, Miller AW. Defining Dysbiosis for a Cluster of
Chronic Diseases. Scientific reports. 2019;9(1):12918. 13. Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Frontiers in immunology. 2014;5:427. 14. Dutta SK, Verma S, Jain V, et al. Parkinson’s Disease: The Emerging Role of
Gut Dysbiosis, Antibiotics, Probiotics, and Fecal Microbiota Transplantation.
Journal of neurogastroenterology and motility. 2019;25(3):363-376. 15. Hiippala K, Jouhten H, Ronkainen A, et al. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation.
Nutrients. 2018;10(8). 16. Chen L, Reynolds C, David R, Peace Brewer A. Development of an Index
Score for Intestinal Inflammation-Associated Dysbiosis Using Real-World
Stool Test Results. Digestive diseases and sciences. 2019. 17. Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837-848. 18. Costea PI, Hildebrand F, Arumugam M, et al. Enterotypes in the landscape of gut microbial community composition. Nature microbiology. 2018;3(1):8-16. 19. Koliada A, Syzenko G, Moseiko V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC
Microbiol. 2017;17(1):120. 20. Indiani C, Rizzardi KF, Castelo PM, Ferraz LFC, Darrieux M, Parisotto
TM. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut
Microbiota: A Systematic Review. Childhood obesity (Print). 2018;14(8):501- 509. 21. Lyu M, Wang YF, Fan GW, Wang XY, Xu SY, Zhu Y. Balancing
Herbal Medicine and Functional Food for Prevention and Treatment of
Cardiometabolic Diseases through Modulating Gut Microbiota. Front
Microbiol. 2017;8:2146. 22. Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ (Clinical research ed). 2018;361:k2179. 23. Butto LF, Haller D. Dysbiosis in intestinal inflammation: Cause or consequence. International journal of medical microbiology : IJMM. 2016;306(5):302-309.

Christine Stubbe, ND
is a Medical Education specialist, GI product line specialist, and content writer for Genova Diagnostics. She participates in many teaching and speaking events, and has written for peer-reviewed journals and other publications.








